AI-Assistance Body Composition CT at T12 and T4 in Lung Cancer: Diagnosing Sarcopenia, and Its Correlation with Morphofunctional Assessment Techniques
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting of the Study
2.2. Anthropometric and Body Composition Assessments
2.2.1. Bioelectrical Impedance Vector Analysis
2.2.2. Nutritional Ultrasound®
2.2.3. Functional Assessment
2.2.4. Computed Tomography at T12 Level by FocusedON®
2.2.5. Assessment of Sarcopenia
2.2.6. Statistical Analysis
3. Results
3.1. Demographic, Clinicopathological and Body Composition Characteristics Between Sarcopenic and Non-Sarcopenic According to EWGSOP2 Criteria
3.2. Correlation Between Morphofunctional Parameters (Muscle and Fat Tissue) and Sarcopenia Criteria
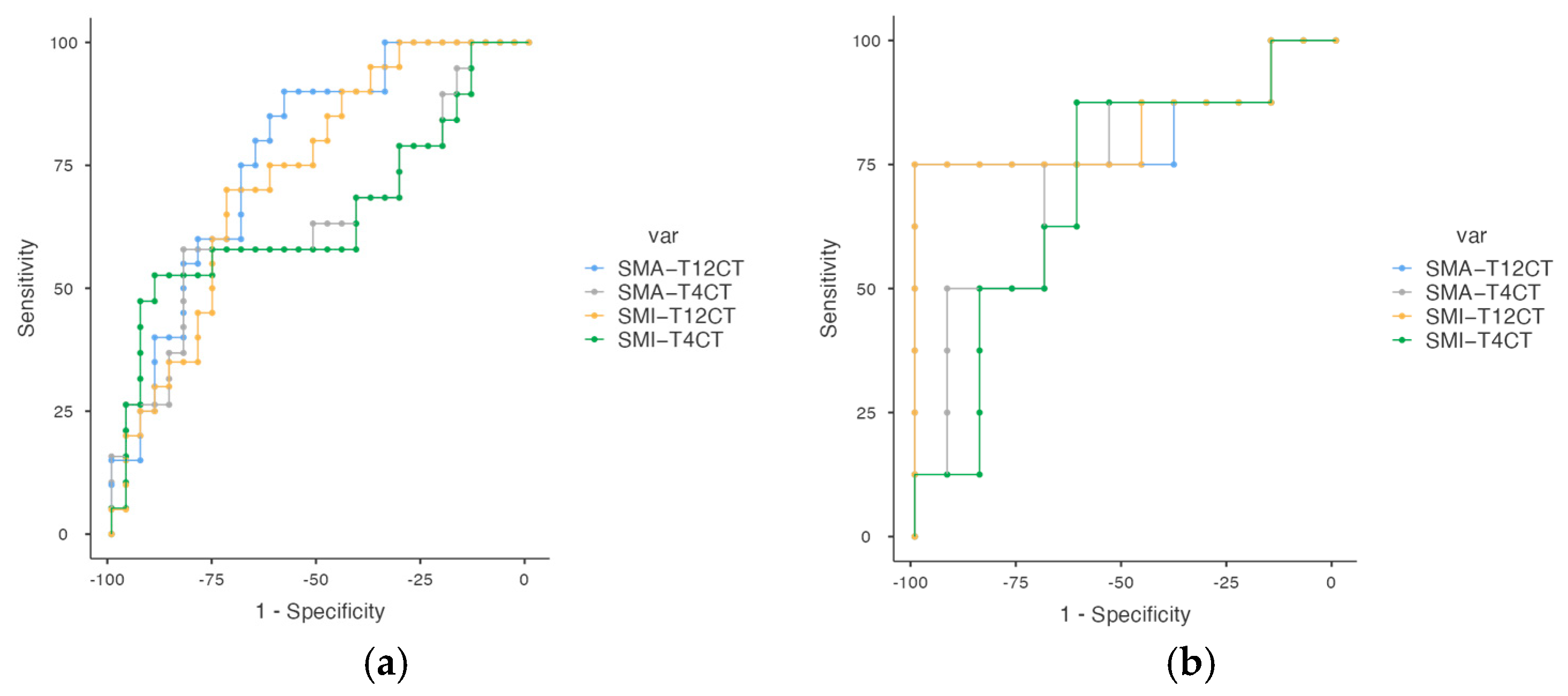
3.3. Cut-Off Points for Parameters of Low Muscle Mass and Sarcopenia Criteria
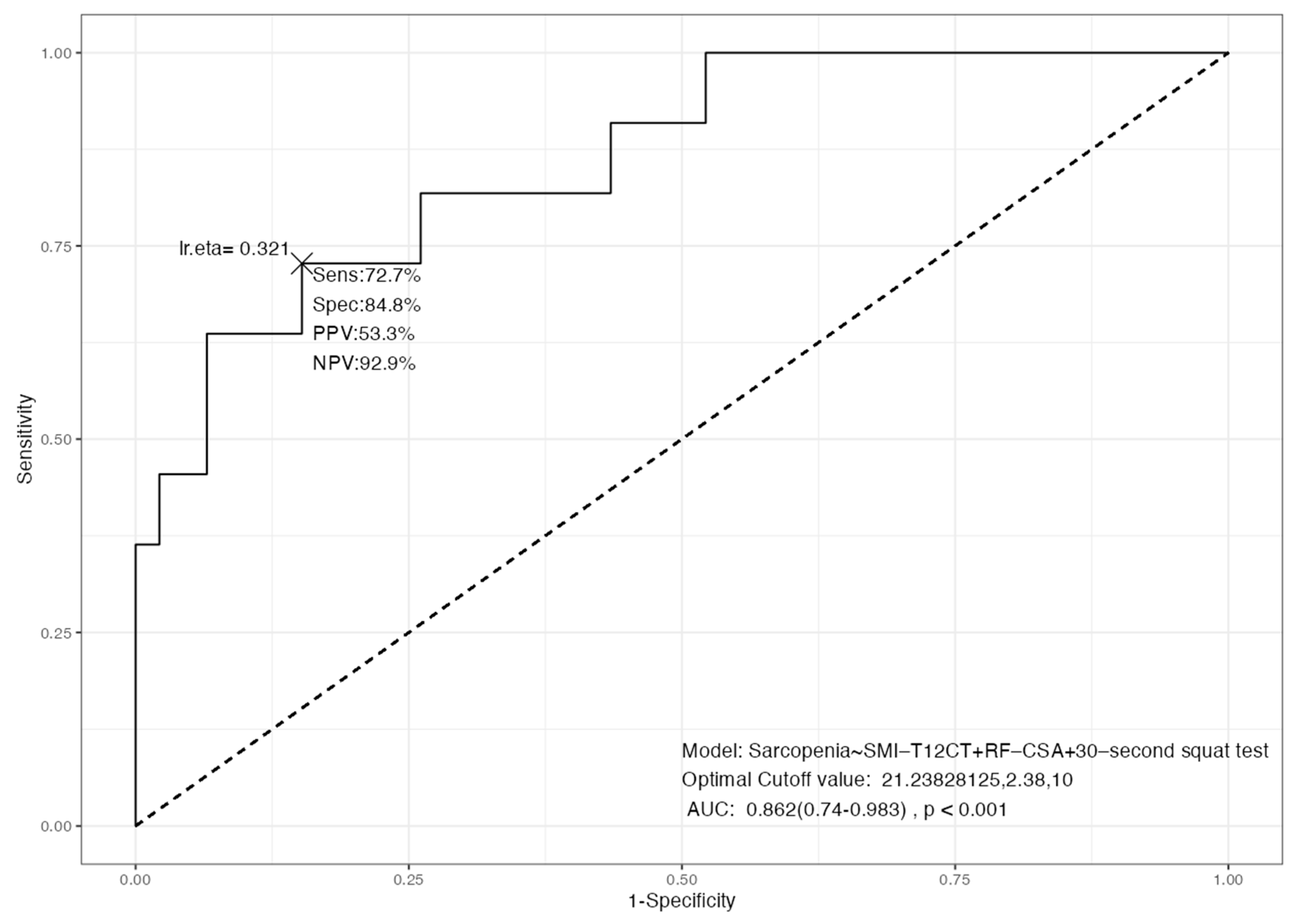
3.4. Integrated Diagnostic Models for Sarcopenia: Multimodal Performance and Independent Predictors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CT | Computed Tomography |
| Sc | Sarcopenia |
| SMI | Skeletal Muscle Index |
| SMA | Skeletal Muscle Area |
| ASMI | Appendicular Skeletal Muscle Index |
| BIVA | Bioelectrical Impedance Vector Analysis |
| NU | Nutritional Ultrasound |
| HGS | Handgrip Strength |
| RF_CSA | Rectus Femoris Cross-Sectional Area |
| RF_Y axis | Rectus Femoris Y-axis diameter |
| SAT | Subcutaneous Adipose Tissue |
| VAT | Visceral Adipose Tissue |
| IMAT | Intermuscular Adipose Tissue |
| ASMM | Appendicular Skeletal Muscle Mass |
| FFM | Fat-Free Mass |
| BCM | Body Cell Mass |
| FMI | Fat Mass Index |
| PA (º) | Phase Angle (degrees) |
| TUG | Timed Up and Go |
| EWGSOP2 | European Working Group on Sarcopenia in Older People, 2nd edition |
| OR | Odds Ratio |
| AUC | Area Under the Curve |
| PPV | Positive Predictive Value |
| NPV | Negative Predictive Value |
| ECOG | Eastern Cooperative Oncology Group |
References
- Strange, C.D.; Strange, T.A.; Erasmus, L.T.; Patel, S.; Ahuja, J.; Shroff, G.S.; Agrawal, R.; Truong, M.T. Imaging in Lung Cancer Staging. Clin. Chest Med. 2024, 45, 295–305. [Google Scholar] [CrossRef]
- Lin, T.Y.; Chen, Y.F.; Wu, W.T.; Han, D.S.; Tsai, I.C.; Chang, K.V.; Özçakar, L. Impact of sarcopenia on the prognosis and treatment of lung cancer: An umbrella review. Discov. Oncol. 2022, 13, 115. [Google Scholar] [CrossRef]
- Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 21 June 2025).
- Huang, Y.; Yuan, F.; Yang, L.; Guo, H.; Jiang, Y.; Cun, H.; Mou, Z.; Chen, J.; Li, C.; Zhang, Z.; et al. Computed tomography (CT)-based skeletal muscle vertebral-related index to assess low muscle mass in patients with non-small cell lung cancer. Quant. Imaging Med. Surg. 2024, 14, 5737–5747. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, D.; Abenavoli, E.M.; Beyer, T.; Gruenert, S.; Hacker, M.; Hesse, S.; Hofmann, L.; Pusitz, S.; Rullmann, M.; Sabri, O.; et al. Detection of cancer-associated cachexia in lung cancer patients using whole-body [18F]FDG-PET/CT imaging: A multi-centre study. J. Cachexia Sarcopenia Muscle 2024, 15, 2375–2386. [Google Scholar] [CrossRef]
- Williams, G.R.; Deal, A.M.; Muss, H.B.; Weinberg, M.S.; Sanoff, H.K.; Nyrop, K.A.; Pergolotti, M.; Shachar, S.S. Skeletal muscle measures and physical function in older adults with cancer: Sarcopenia or myopenia? Oncotarget 2017, 8, 33658–33665. [Google Scholar] [CrossRef]
- Kim, E.Y.; Kim, Y.S.; Park, I.; Ahn, H.K.; Cho, E.K.; Jeong, Y.M. Prognostic Significance of CT-Determined Sarcopenia in Patients with Small-Cell Lung Cancer. J. Thorac. Oncol. 2015, 10, 1795–1799. [Google Scholar] [CrossRef]
- He, S.; Zhang, G.; Huang, N.; Chen, S.; Ruan, L.; Liu, X.; Zeng, Y. Utilizing the T12 skeletal muscle index on computed tomography images for sarcopenia diagnosis in lung cancer patients. Asia-Pac. J. Oncol. Nurs. 2024, 11, 100512. [Google Scholar] [CrossRef] [PubMed]
- Kaltenhauser, S.; Niessen, C.; Zeman, F.; Stroszczynski, C.; Zorger, N.; Grosse, J.; Großer, C.; Hofmann, H.S.; Robold, T. Diagnosis of sarcopenia on thoracic computed tomography and its association with postoperative survival after anatomic lung cancer resection. Sci. Rep. 2023, 13, 18450. [Google Scholar] [CrossRef]
- Ishida, Y.; Maeda, K.; Yamanaka, Y.; Matsuyama, R.; Kato, R.; Yamaguchi, M.; Nonogaki, T.; Shimizu, A.; Ueshima, J.; Murotani, K.; et al. Formula for the Cross-Sectional Area of the Muscles of the Third Lumbar Vertebra Level from the Twelfth Thoracic Vertebra Level Slice on Computed Tomography. Geriatrics 2020, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.A.; Fantuzzi, G.; Stephan, J.T.; Kim, S.; Oddo, V.M.; Koh, T.J.; Gomez, S.L. Sarcopenia Identification Using Alternative Vertebral Landmarks in Individuals with Lung Cancer. Muscles 2024, 3, 121–132. [Google Scholar] [CrossRef]
- Dong, X.; Dan, X.; Yawen, A.; Haibo, X.; Huan, L.; Mengqi, T.; Linglong, C.; Zhao, R. Identifying sarcopenia in advanced non-small cell lung cancer patients using skeletal muscle CT radiomics and machine learning. Thorac. Cancer 2020, 11, 2650–2659. [Google Scholar] [CrossRef]
- Wakefield, C.J.; Lund, N.; Coughlin, J.; Karush, J.M.; Geissen, N.; Alex, G.; Liptay, M.J.; Borgia, J.A.; Shah, P.; Seder, C.W. The association between thoracic sarcopenia and survival is gender specific in early-stage lung cancer. J. Thorac. Dis. 2022, 14, 4256–4265. [Google Scholar] [CrossRef]
- Fernández-Jiménez, R.; Sanmartín-Sánchez, A.; Cabrera-César, E.; Espíldora-Hernández, F.; Vegas-Aguilar, I.; Amaya-Campos, M.D.M.; Palmas-Candia, F.X.; Claro-Brandner, M.; Olivares-Alcolea, J.; Simón-Frapolli, V.J.; et al. IA-Body Composition CT at T12 in Idiopathic Pulmonary Fibrosis: Diagnosing Sarcopenia and Correlating with Other Morphofunctional Assessment Techniques. Nutrients 2024, 16, 2885. [Google Scholar] [CrossRef]
- Piccoli, A.; Nigrelli, S.; Caberlotto, A.; Bottazzo, S.; Rossi, B.; Pillon, L.; Maggiore, Q. Bivariate normal values of the bioelectrical impedance vector in adult and elderly populations. Am. J. Clin. Nutr. 1995, 61, 269–270. [Google Scholar] [CrossRef]
- Piccoli, A.; Rossi, B.; Pillon, L.; Bucciante, G. A new method for monitoring body fluid variation by bioimpedance analysis: The RXc graph. Kidney Int. 1994, 46, 534–539. [Google Scholar] [CrossRef] [PubMed]
- De Palo, T.; Messina, G.; Edefonti, A.; Perfumo, F.; Pisanello, L.; Peruzzi, L.; Di Iorio, B.; Mignozzi, M.; Vienna, A.; Conti, G.; et al. Normal values of the bioelectrical impedance vector in childhood and puberty. Nutrition 2000, 16, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Collard, H.R.; Egan, J.J.; Martinez, F.J.; Behr, J.; Brown, K.K.; Colby, T.V.; Cordier, J.F.; Flaherty, K.R.; Lasky, J.A.; et al. An Official ATS/ERS/JRS/ALAT Statement: Idiopathic Pulmonary Fibrosis: Evidence-based Guidelines for Diagnosis and Management. Am. J. Respir. Crit. Care Med. 2011, 183, 788–824. [Google Scholar] [CrossRef] [PubMed]
- Cardinal, T.R.; Wazlawik, E.; Bastos, J.L.; Nakazora, L.M.; Scheunemann, L. Standardized phase angle indicates nutritional status in hospitalized preoperative patients. Nutr. Res. 2010, 30, 594–600. [Google Scholar] [CrossRef]
- Norman, K.; Stobäus, N.; Gonzalez, M.C.; Schulzke, J.D.; Pirlich, M. Hand grip strength: Outcome predictor and marker of nutritional status. Clin. Nutr. 2011, 30, 135–142. [Google Scholar] [CrossRef]
- Celis-Morales, C.A.; Welsh, P.; Lyall, D.M.; Steell, L.; Petermann, F.; Anderson, J.; Iliodromiti, S.; Sillars, A.; Graham, N.; Mackay, D.F.; et al. Associations of grip strength with cardiovascular, respiratory, and cancer outcomes and all cause mortality: Prospective cohort study of half a million UK Biobank participants. BMJ 2018, 361, k1651. [Google Scholar] [CrossRef]
- Park, T.S.; Shin, M.J. Comprehensive Assessment of Lower Limb Function and Muscle Strength in Sarcopenia: Insights from the Sit-to-Stand Test. Ann. Geriatr. Med. Res. 2024, 28, 1–8. [Google Scholar] [CrossRef]
- Mesquita, R.; Wilke, S.; Smid, D.E.; Janssen, D.J.; Franssen, F.M.; Probst, V.S.; Wouters, E.F.; Muris, J.W.; Pitta, F.; Spruit, M.A. Measurement properties of the Timed Up & Go test in patients with COPD. Chronic Respir. Dis. 2016, 13, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Derstine, B.A.; Holcombe, S.A.; Ross, B.E.; Wang, N.C.; Su, G.L.; Wang, S.C. Skeletal muscle cutoff values for sarcopenia diagnosis using T10 to L5 measurements in a healthy US population. Sci. Rep. 2018, 8, 11369. [Google Scholar] [CrossRef] [PubMed]
- Ying, L.; Xu, L.; Yang, J.; Zhang, Q. Prognostic significance of CT-determined sarcopenia in older patients with advanced squamous cell lung cancer treated with programmed death-1 inhibitors. Sci. Rep. 2024, 14, 12025. [Google Scholar] [CrossRef] [PubMed]
- Brath, M.S.G.; Sahakyan, M.; Mark, E.B.; Frøkjær, J.B.; Rasmussen, H.H.; Østergaard, L.R.; Weinreich, U.M. Association between thoracic and third lumbar CT-derived muscle mass and density in Caucasian patients without chronic disease: A proof-of-concept study. Eur. Radiol. Exp. 2023, 7, 26. [Google Scholar] [CrossRef]
- Hong, J.H.; Hong, H.; Choi, Y.R.; Kim, D.H.; Kim, J.Y.; Yoon, J.H.; Yoon, S.H. CT analysis of thoracolumbar body composition for estimating whole-body composition. Insights Imaging 2023, 14, 69. [Google Scholar] [CrossRef]
- Herault, A.; Lévêque, E.; Draye-Carbonnier, S.; Decazes, P.; Zduniak, A.; Modzelewski, R.; Libraire, J.; Achamrah, N.; Ménard, A.L.; Lenain, P.; et al. High prevalence of pre-existing sarcopenia in critically ill patients with hematologic malignancies admitted to the intensive care unit for sepsis or septic shock. Clin. Nutr. ESPEN 2023, 55, 373–383. [Google Scholar] [CrossRef]
- Timsina, S.R.; Tanomkiat, W.; Geater, S.L.; Ina, N. Exploring previously used thresholds for computed tomography-defined low skeletal muscle mass in predicting functional limitations among lung cancer patients. Thorac. Cancer 2024, 15, 1287–1295. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Kim, E.Y.; Lee, H.Y.; Kim, K.W.; Lee, J.I.; Kim, Y.S.; Choi, W.J.; Kim, J.H. Preoperative Computed Tomography-Determined Sarcopenia and Postoperative Outcome After Surgery for Non-Small Cell Lung Cancer. Scand. J. Surg. 2018, 107, 244–251. [Google Scholar] [CrossRef]
- Shoji, F.; Matsubara, T.; Kozuma, Y.; Haratake, N.; Akamine, T.; Takamori, S.; Katsura, M.; Toyokawa, G.; Okamoto, T.; Maehara, Y. Relationship Between Preoperative Sarcopenia Status and Immuno-nutritional Parameters in Patients with Early-stage Non-small Cell Lung Cancer. Anticancer Res. 2017, 37, 6997–7003. [Google Scholar] [CrossRef]
- Vegas-Aguilar, I.M.; Guirado-Peláez, P.; Fernández-Jiménez, R.; Boughanem, H.; Tinahones, F.J.; Garcia-Almeida, J.M. Exploratory Assessment of Nutritional Evaluation Tools as Predictors of Complications and Sarcopenia in Patients with Colorectal Cancer. Cancers 2023, 15, 847. [Google Scholar] [CrossRef]
- García-García, C.; Vegas-Aguilar, I.M.; Rioja-Vázquez, R.; Cornejo-Pareja, I.; Tinahones, F.J.; García-Almeida, J.M. Rectus Femoris Muscle and Phase Angle as Prognostic Factor for 12-Month Mortality in a Longitudinal Cohort of Patients with Cancer (AnyVida Trial). Nutrients 2023, 15, 522. [Google Scholar] [CrossRef] [PubMed]
- López-Gómez, J.J.; Benito-Sendín Plaar, K.; Izaola-Jauregui, O.; Primo-Martín, D.; Gómez-Hoyos, E.; Torres-Torres, B.; De Luis-Román, D.A. Muscular Ultrasonography in Morphofunctional Assessment of Patients with Oncological Pathology at Risk of Malnutrition. Nutrients 2022, 14, 1573. [Google Scholar] [CrossRef]
- Simón-Frapolli, V.J.; Vegas-Aguilar, I.M.; Fernández-Jiménez, R.; Cornejo-Pareja, I.M.; Sánchez-García, A.M.; Martínez-López, P.; Nuevo-Ortega, P.; Reina-Artacho, C.; Estecha-Foncea, M.A.; Gómez-González, A.M.; et al. Phase angle and rectus femoris cross-sectional area as predictors of severe malnutrition and their relationship with complications in outpatients with post-critical SARS-CoV2 disease. Front. Nutr. 2023, 10, 1218266. [Google Scholar] [CrossRef]
- Souza, N.C.; Avesani, C.M.; Prado, C.M.; Martucci, R.B.; Rodrigues, V.D.; de Pinho, N.B.; Heymsfield, S.B.; Gonzalez, M.C. Phase angle as a marker for muscle abnormalities and function in patients with colorectal cancer. Clin. Nutr. 2021, 40, 4799–4806. [Google Scholar] [CrossRef]
- Derstine, B.A.; Holcombe, S.A.; Goulson, R.L.; Ross, B.E.; Wang, N.C.; Sullivan, J.A.; Su, G.L.; Wang, S.C. Quantifying Sarcopenia Reference Values Using Lumbar and Thoracic Muscle Areas in a Healthy Population. J. Nutr. Health Aging 2017, 21, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Takamori, S.; Toyokawa, G.; Okamoto, T.; Shimokawa, M.; Kinoshita, F.; Kozuma, Y.; Matsubara, T.; Haratake, N.; Akamine, T.; Takada, K.; et al. Clinical Impact and Risk Factors for Skeletal Muscle Loss After Complete Resection of Early Non-small Cell Lung Cancer. Ann. Surg. Oncol. 2018, 25, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.H.; Do, K.H.; Chae, E.J.; Choi, S.H.; Jo, K.W.; Lee, S.O.; Hong, S.B. Association of Chest CT-Based Quantitative Measures of Muscle and Fat with Post-Lung Transplant Survival and Morbidity: A Single Institutional Retrospective Cohort Study in Korean Population. Korean J. Radiol. 2019, 20, 522–530. [Google Scholar] [CrossRef] [PubMed]
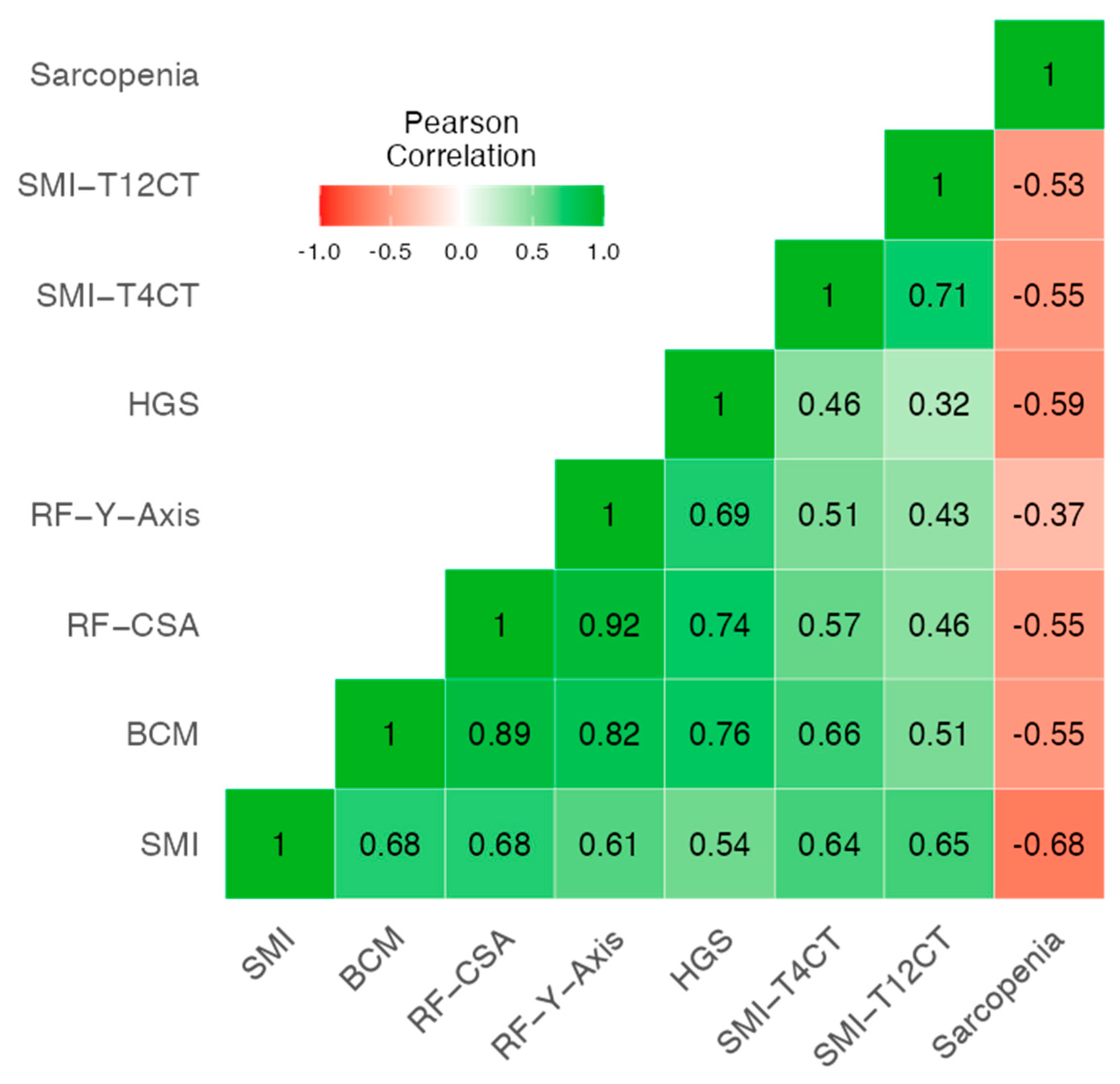


| Variable | Category | Non-Sarcopenia (N = 64) | Sarcopenia (N = 16) | p-Value |
|---|---|---|---|---|
| Demographic | ||||
| Age | Mean ± SD | 64.8 ± 9.58 | 71.8 ± 7.90 | p = 0.009 |
| Gender (Male) | 71.9% | 62.5% | p = 0.464 | |
| Clinicopathological | ||||
| TNM_T | T4 | 35.2% | 11.1% | p = 0.48 |
| TNM_N | N0 | 33.3% | 5.6% | p = 0.68 |
| TNM_M | M0 | 47.3% | 14.5% | p = 0.88 |
| Tumor Stage | ||||
| Non-stage | 14.0% | 6.2% | p = 0.19 | |
| Stage I | 31.2% | 43.7% | p = 0.54 | |
| Stage II | 6.2% | 0.0% | p = 0.57 | |
| Stage III | 20.3% | 25.0% | p = 0.73 | |
| Stage IV | 26.5% | 31.2% | p = 0.97 | |
| Surgery | Yes | 27.5% | 5.0% | p = 0.15 |
| Radiotherapy (RT) | Yes | 26.6% | 5.1% | p = 0.67 |
| Chemotherapy (QT) | Yes | 40.5% | 5.1% | p = 0.12 |
| Immunotherapy | Yes | 22.8% | 3.8% | p = 0.55 |
| Tumor Classification | NSCLC | 54.4% | 12.7% | p = 0.35 |
| SCLC | 2.5% | 3.8% | ||
| Mesotelioma | 3.8% | 0.0% | ||
| NP | 15.2% | 3.8% | ||
| Neuroendocrino | 2.5% | 0.0% | ||
| ECOG | 0 | 42.3% | 0.0% | p < 0.001 |
| 1 | 31.0% | 9.9% | ||
| 2 | 8.5% | 5.6% | ||
| 3 | 0.0% | 2.8% |
| N = 80 | p-Value | ||
|---|---|---|---|
| Handgrip strength (kg) | Mean ± SD | 30.1 ± 11.6 | <0.001 * |
| Men | Mean ± SD | 34.1 ± 10.2 | |
| Women | Mean ± SD | 20.8 ± 9.18 | |
| ASMM (kg) | 19.9 ± 4.92 | <0.001 * | |
| Men | Mean ± SD | 21.9 ± 4.14 | |
| Women | Mean ± SD | 15.2 ± 2.9 | |
| Low ASMM | N (%) | 18 (22.5%) | <0.001 ** |
| ASMI (kg/talla) | Mean ± SD | 7.04 ± 1.34 | <0.001 * |
| Men | Mean ± SD | 7.46 ± 1.22 | |
| Women | Mean ± SD | 6.06 ± 1.09 | |
| Low ASMI | N (%) | 39 (48.8%) | 0.182 ** |
| Low muscle (Low ASMM/ASMI) | N (%) | 43 (53.8%) | 0.96 ** |
| Dynapenia | N (%) | 19 (23.8%) | <0.87 ** |
| Sarcopenia (dynapenia + low muscle) | N (%) | 16 (20%) | 0.47 ** |
| Variable | Non-Sarcopenia (N = 64) | Sarcopenia (N = 16) | p-Value |
|---|---|---|---|
| BIVA | Mean ± SD | Mean ± SD | |
| BMI (kg/m2) | 27.4 ± 4.93 | 23.2 ± 2.96 | 0.002 |
| PA (º) | 4.72 ± 0.86 | 4.06 ± 0.93 | 0.009 |
| SPA | −1.39 ± 0.90 | −1.86 ± 0.89 | 0.086 |
| Rz | 516.5 ± 92.3 | 573.8 ± 70.8 | 0.023 |
| Xc | 42.5 ± 9.92 | 40.8 ± 10.4 | 0.533 |
| BCM (kg) | 25.2 ± 6.65 | 19.4 ± 5.83 | 0.002 |
| ASMM (kg) | 20.7 ± 4.89 | 16.7 ± 3.69 | 0.003 |
| FFM (kg) | 54.2 ± 10.19 | 46.4 ± 8.00 | 0.005 |
| TBW (kg) | 41.2 ± 8.48 | 35.1 ± 5.65 | 0.009 |
| ECW (kg) | 21.7 ± 4.69 | 20.1 ± 3.36 | 0.238 |
| FM (kg) | 23.1 ± 8.73 | 16.7 ± 5.94 | 0.007 |
| NAK | 1.24 ± 0.26 | 1.47 ± 0.40 | 0.006 |
| Hydration (%) | 75.7 ± 3.99 | 76.1 ± 5.11 | 0.814 |
| Nutrition | 712.1 ± 213.0 | 584.4 ± 161.6 | 0.028 |
| SMI (kg) | 9.16 ± 1.87 | 7.94 ± 1.25 | 0.016 |
| Echography exploration | |||
| RF_CSA | 3.83 ± 1.30 | 2.65 ± 0.73 | 0.002 |
| RF_Cir | 9.01 ± 1.34 | 8.14 ± 1.04 | 0.030 |
| RF_X_Axis | 3.59 ± 0.59 | 3.37 ± 0.50 | 0.224 |
| RF_Y_Axis | 1.19 ± 0.35 | 0.84 ± 0.18 | <0.001 |
| L_SAT (cm) | 0.83 ± 0.53 | 0.53 ± 0.28 | 0.048 |
| RF_Cont (cm) | 1.47 ± 1.43 | 1.08 ± 0.29 | <0.001 |
| T-SAT (cm) | 1.53 ± 0.76 | 1.05 ± 0.46 | 0.027 |
| S-SAT (cm) | 0.68 ± 0.39 | 0.51 ± 0.22 | 0.176 |
| VAT (cm) | 0.53 ± 0.32 | 0.34 ± 0.21 | 0.036 |
| Functional test | |||
| HGS (kg) | 33.6 ± 9.79 | 16.5 ± 7.69 | <0.001 |
| TUG (second) | 6.91 ± 2.66 | 6.28 ± 5.07 | 0.544 |
| 30 s squat test | 11.09 ± 6.83 | 6.5 ± 6.00 | 0.036 |
| Variable | Non-Sarcopenia (N = 52) Mean ± SD | Sarcopenia (N= 15) Mean ± SD | p-Value |
|---|---|---|---|
| Muscle_T4CT (%) | 28.00 ± 6.89 | 29.64 ± 7.90 | 0.433 |
| SMA_T4CT (cm2) | 147.7 ± 39.3 | 123.7 ± 31.1 | 0.033 |
| SMI_T4CT (cm2/m2) | 51.8 ± 11.3 | 46.6 ± 10.6 | 0.120 |
| Muscle_T4CT (UH) | 43.95 ± 10.43 | 40.04 ± 12.77 | 0.227 |
| IMAT_T4CT (%) | 2.05 ± 0.94 | 2.35 ± 1.02 | 0.318 |
| IMAT_T4CT (cm2) | 11.08 ± 5.78 | 10.69 ± 5.39 | 0.923 |
| IMAT_T4CT (UH) | −68.78 ± 8.05 | −68.00 ± 4.97 | 0.762 |
| VAT_T4CT (%) | 6.33 ± 3.36 | 7.41 ± 3.33 | 0.273 |
| VAT_T4CT (cm2) | 35.93 ± 22.93 | 35.37 ± 19.91 | 0.932 |
| VAT_T4CT (UH) | −94.79 ± 7.59 | −94.10 ± 5.54 | 0.743 |
| SAT_T4CT (%) | 28.13 ± 9.54 | 24.75 ± 9.05 | 0.224 |
| SAT_T4CT (cm2) | 153.90 ± 73.34 | 115.26 ± 52.38 | 0.092 |
| SAT_T4CT (UH) | −96.29 ± 13.50 | −92.13 ± 11.35 | 0.084 |
| Muscle_T12CT (%) | 11.63 ± 2.76 | 12.20 ± 4.20 | 0.533 |
| SMA_T12CT (cm2) | 82.64 ± 26.29 | 62.09 ± 12.65 | 0.008 |
| SMI_T12CT (cm2/m2) | 29.12 ± 8.48 | 23.03 ± 4.21 | 0.015 |
| Muscle_T12CT (UH) | 36.53 ± 16.00 | 32.74 ± 14.94 | 0.414 |
| IMAT_ T12CT (%) | 1.25 ± 0.70 | 1.26 ± 0.60 | 0.822 |
| IMAT_T12CT (cm2) | 9.38 ± 6.25 | 7.86 ± 5.44 | 0.356 |
| IMAT_T12CT (UH) | −64.02 ± 11.57 | −64.41 ± 6.26 | 0.517 |
| VAT_T12CT (%) | 15.17 ± 9.24 | 16.10 ± 8.96 | 0.730 |
| VAT_T12CT (cm2) | 120.79 ± 84.78 | 107.29 ± 80.41 | 0.583 |
| VAT_T12CT (UH) | −93.02 ± 8.42 | −92.45 ± 6.68 | 0.811 |
| SAT_T12CT (%) | 14.70 ± 7.04 | 11.96 ± 4.09 | 0.198 |
| SAT_T12CT (cm2) | 108.44 ± 68.13 | 71.59 ± 32.32 | 0.047 |
| SAT_T12CT (UH) | −93.55 ± 16.19 | −87.17 ± 14.36 | 0.040 |
| Variable | Cut-Off | AUC | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|
| SMA_T4CT (Men) | 169.89 | 0.644 | 57.89 | 82.76 | 68.75 | 75.00 |
| SMA_T4CT (Women) | 130.02 | 0.736 | 57.14 | 92.31 | 80.00 | 80.00 |
| SMA_T12CT (Men) | 80.34 | 0.772 | 90.00 | 58.62 | 60.00 | 89.47 |
| SMA_T12CT (Women) | 70.47 | 0.791 | 71.43 | 100.00 | 100.00 | 86.67 |
| SMI_T4CT (Men) | 59.05 | 0.650 | 52.63 | 89.66 | 76.92 | 74.29 |
| SMI_T4CT (Women) | 41.69 | 0.714 | 85.71 | 61.54 | 54.55 | 88.89 |
| SMI_T12CT (Men) | 31.98 | 0.733 | 70.00 | 72.41 | 63.64 | 77.78 |
| SMI_T12CT (Women) | 28.23 | 0.802 | 71.43 | 100.00 | 100.00 | 86.67 |
| Variable | Cut-Off | AUC | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|
| SMA_T4CT (Men) | 165.76 | 0.598 | 41.03 | 88.89 | 94.12 | 25.81 |
| SMA_T4CT (Women) | 105.27 | 0.631 | 83.33 | 64.29 | 50.00 | 90.00 |
| SMA_T12CT (Men) | 89.39 | 0.669 | 55.00 | 77.78 | 91.67 | 28.00 |
| SMA_T12CT (Women) | 54.37 | 0.595 | 83.33 | 64.29 | 50.00 | 90.00 |
| SMI_T4CT (Men) | 57.23 | 0.610 | 38.46 | 100.00 | 100.00 | 27.27 |
| SMI_T4CT (Women) | 49.35 | 0.643 | 66.67 | 71.43 | 50.00 | 83.33 |
| SMI_T12CT (Men) | 24.78 | 0.653 | 82.50 | 55.56 | 89.19 | 41.67 |
| SMI_T12CT (Women) | 21.24 | 0.583 | 83.33 | 57.14 | 45.45 | 88.89 |
| Dependent: Sarcopenia | No | Yes | OR (Univariable) | OR (Multivariable) | |
|---|---|---|---|---|---|
| SMA_T12CT | Mean ± SD | 83.8 ± 26.9 | 69.0 ± 23.6 | 0.98 (0.95–1.00, p = 0.106) | 0.96 (0.92–0.99, p = 0.022) |
| Age | Mean ± SD | 65.8 ± 9.5 | 72.5 ± 8.0 | 1.10 (1.01–1.23, p = 0.046) | 1.23 (1.07–1.47, p = 0.010) |
| 30 s squat test | Mean ± SD | 11.2 ± 6.9 | 6.0 ± 6.0 | 0.89 (0.80–0.98, p = 0.031) | 0.78 (0.63–0.91, p = 0.007) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montero-Benitez, M.Z.; Carmona-Llanos, A.; Fernández-Jiménez, R.; Román-Jobacho, A.; Gómez-Millán, J.; Modamio-Molina, J.; Cabrera-Cesar, E.; Vegas-Aguilar, I.; Amaya-Campos, M.d.M.; Tinahones, F.J.; et al. AI-Assistance Body Composition CT at T12 and T4 in Lung Cancer: Diagnosing Sarcopenia, and Its Correlation with Morphofunctional Assessment Techniques. Cancers 2025, 17, 3255. https://doi.org/10.3390/cancers17193255
Montero-Benitez MZ, Carmona-Llanos A, Fernández-Jiménez R, Román-Jobacho A, Gómez-Millán J, Modamio-Molina J, Cabrera-Cesar E, Vegas-Aguilar I, Amaya-Campos MdM, Tinahones FJ, et al. AI-Assistance Body Composition CT at T12 and T4 in Lung Cancer: Diagnosing Sarcopenia, and Its Correlation with Morphofunctional Assessment Techniques. Cancers. 2025; 17(19):3255. https://doi.org/10.3390/cancers17193255
Chicago/Turabian StyleMontero-Benitez, Maria Zhao, Alba Carmona-Llanos, Rocio Fernández-Jiménez, Alicia Román-Jobacho, Jaime Gómez-Millán, Javier Modamio-Molina, Eva Cabrera-Cesar, Isabel Vegas-Aguilar, Maria del Mar Amaya-Campos, Francisco J. Tinahones, and et al. 2025. "AI-Assistance Body Composition CT at T12 and T4 in Lung Cancer: Diagnosing Sarcopenia, and Its Correlation with Morphofunctional Assessment Techniques" Cancers 17, no. 19: 3255. https://doi.org/10.3390/cancers17193255
APA StyleMontero-Benitez, M. Z., Carmona-Llanos, A., Fernández-Jiménez, R., Román-Jobacho, A., Gómez-Millán, J., Modamio-Molina, J., Cabrera-Cesar, E., Vegas-Aguilar, I., Amaya-Campos, M. d. M., Tinahones, F. J., Molina-Montes, E., Cayón-Blanco, M., & García-Almeida, J. M. (2025). AI-Assistance Body Composition CT at T12 and T4 in Lung Cancer: Diagnosing Sarcopenia, and Its Correlation with Morphofunctional Assessment Techniques. Cancers, 17(19), 3255. https://doi.org/10.3390/cancers17193255







