Liver-Specific Nanoparticle-Mediated Delivery and MMP-Triggered Release of Veratridine to Effectively Target Metastatic Colorectal Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
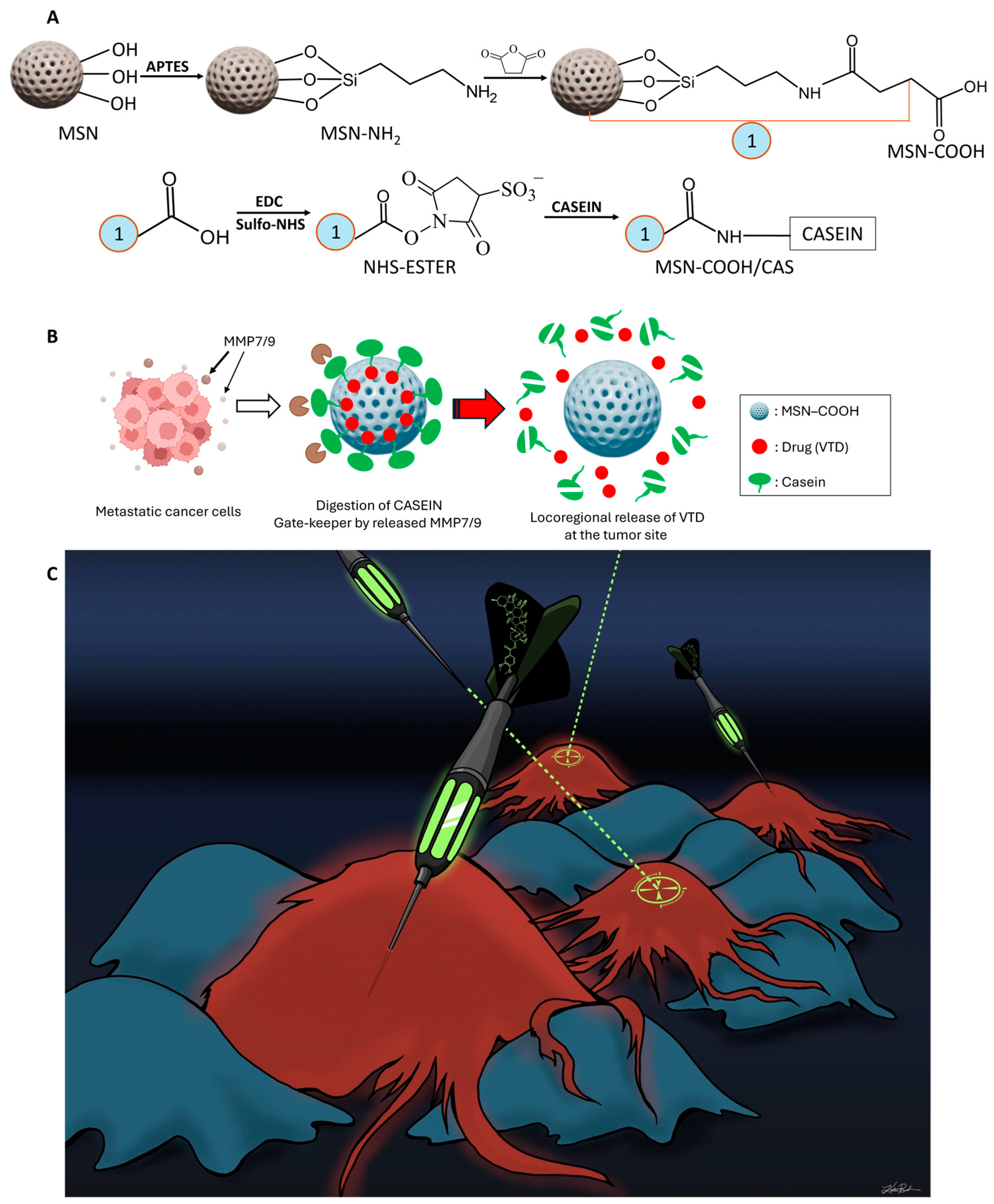
2.3. MSN Synthesis and Surface Functionalization
2.4. Drug Loading, Casein Attachment, and Stimuli-Triggered Drug Release with MSN-COOH
2.5. Synthesis of CaCO3 Particles, Drug Loading, Skim Milk Coating, and Drug Release
2.6. Cell Culture
2.7. Western Blot
2.8. Crystal Assay and Immunofluorescence Staining
2.9. Human Apoptosis Antibody Array
2.10. Assessment of Apoptosis
2.11. Nanoparticle Sterility
2.12. Animals and Preparation
2.13. Murine Mouse Model of Colorectal Cancer
2.14. Xenograft Mouse Model of Colorectal Cancer
2.15. Orthotopic Implantation of Cells for a Splenolepatic Mouse Model of Metastatic Colorectal Cancer (CRC)
2.16. Statistical Analysis
3. Results
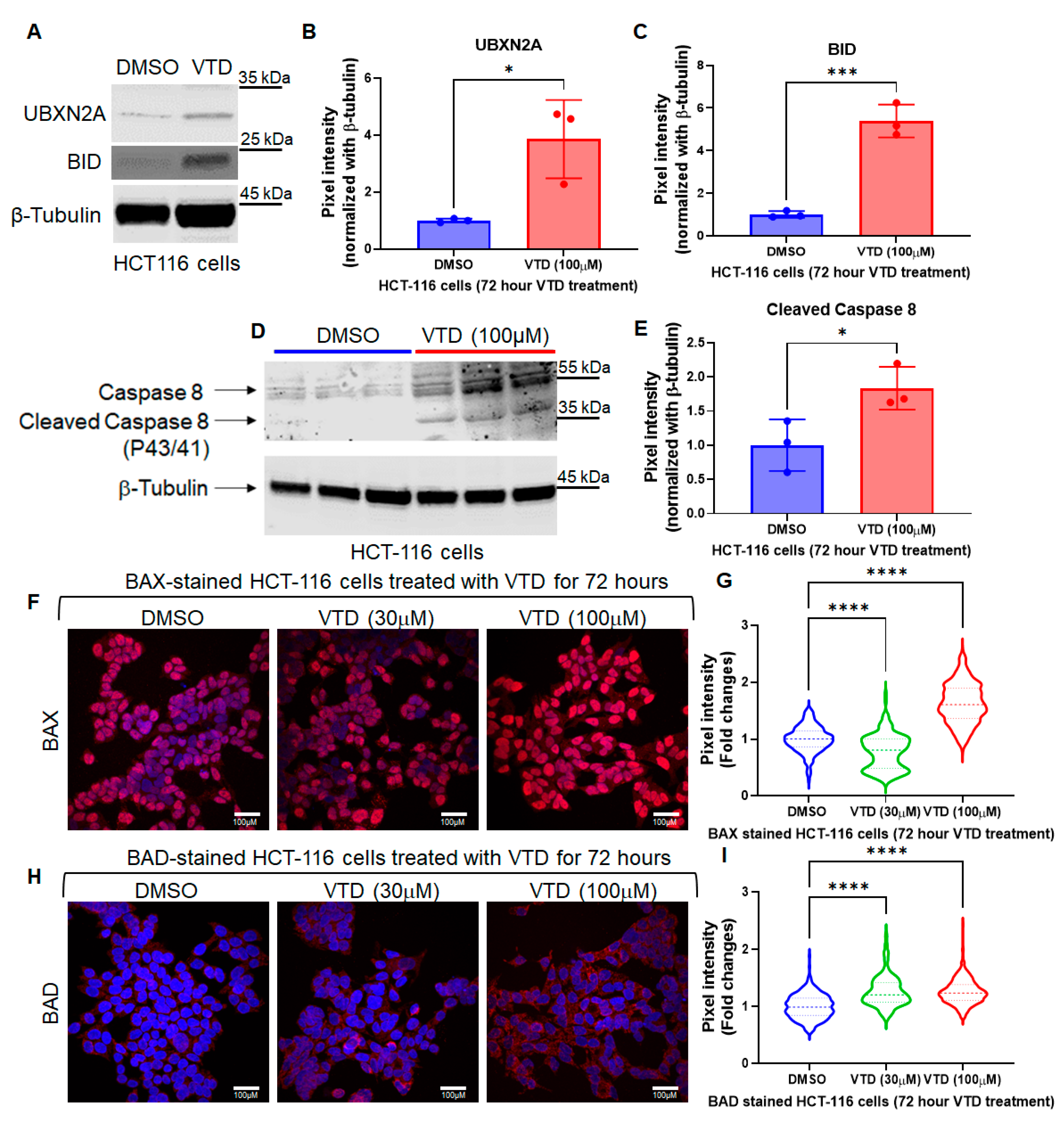
3.1. Veratridine (VTD), a Novel Small Anti-Cancer Molecule, Induces Apoptosis via Intrinsic and Extrinsic Pathways
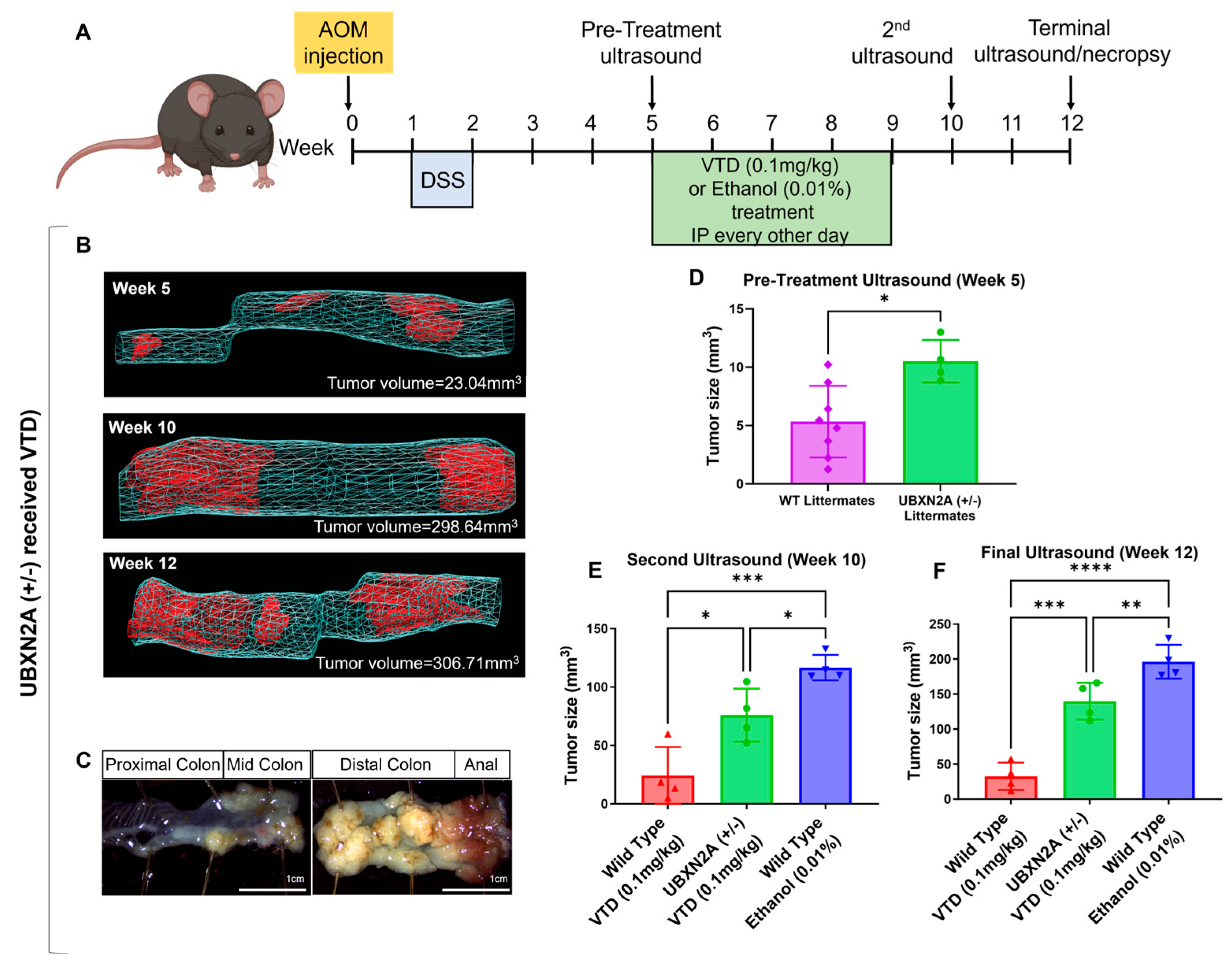
3.2. Veratridine Suppresses Tumor Progression in an AOM/DSS Mouse Colon Cancer Model in a UBXN2A-Dependent Manner
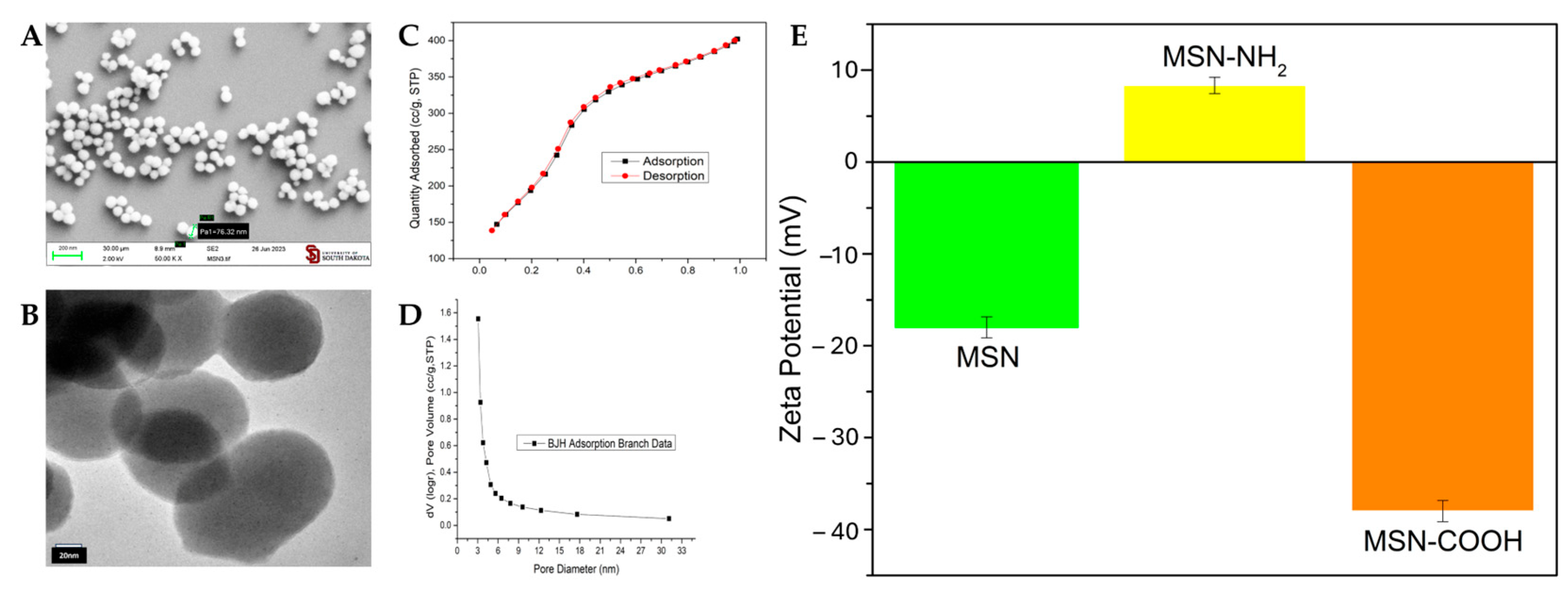
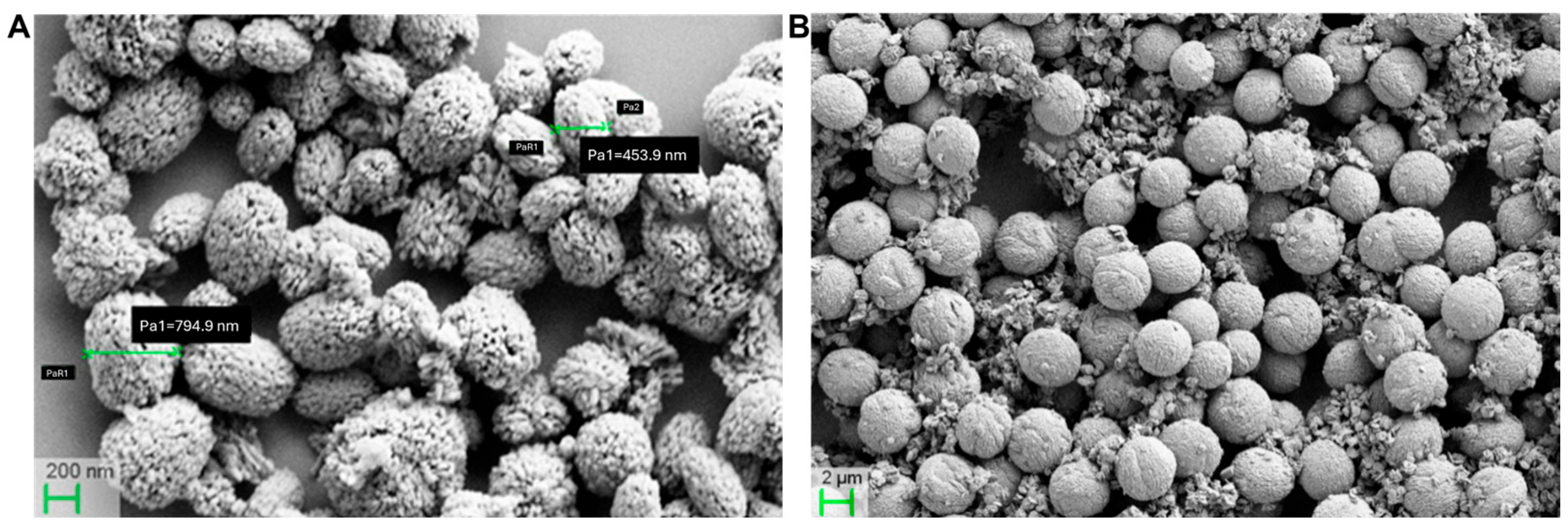
3.3. Pristine and Functionalized MSNs: Synthesis and Characterization
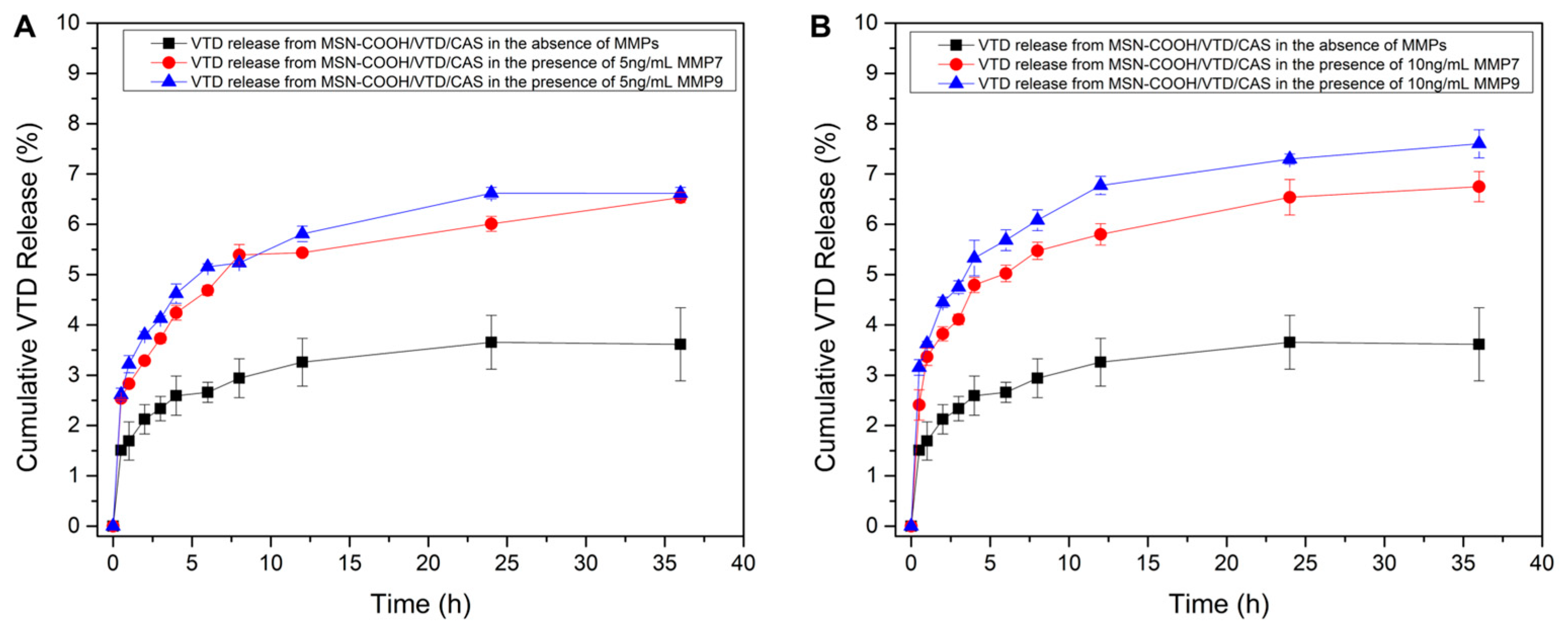
3.4. VTD Loading, Casein Coupling, and MMP-7 and 9-Responsive VTD Releasing
3.5. Tumor Suppressive Effect of MMP-Responsive Nanoparticles in a CRC Xenograft Model
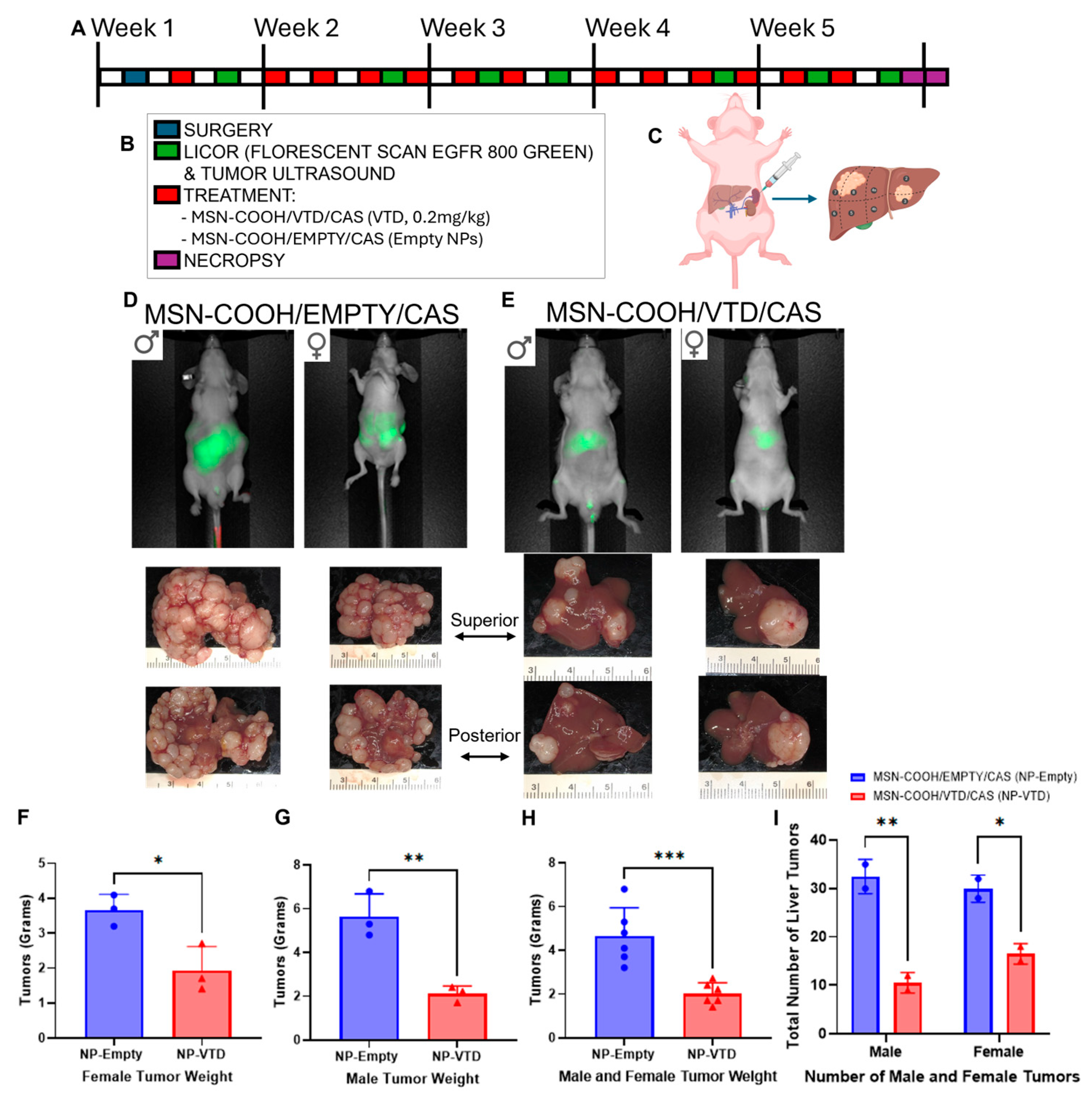
3.6. MSN-COOH/VTD/CAS (VTD, 0.2 mg/kg) Target and Suppress Metastatic Tumors in an Orthotopic Splenolepatic Mouse Model of CRC
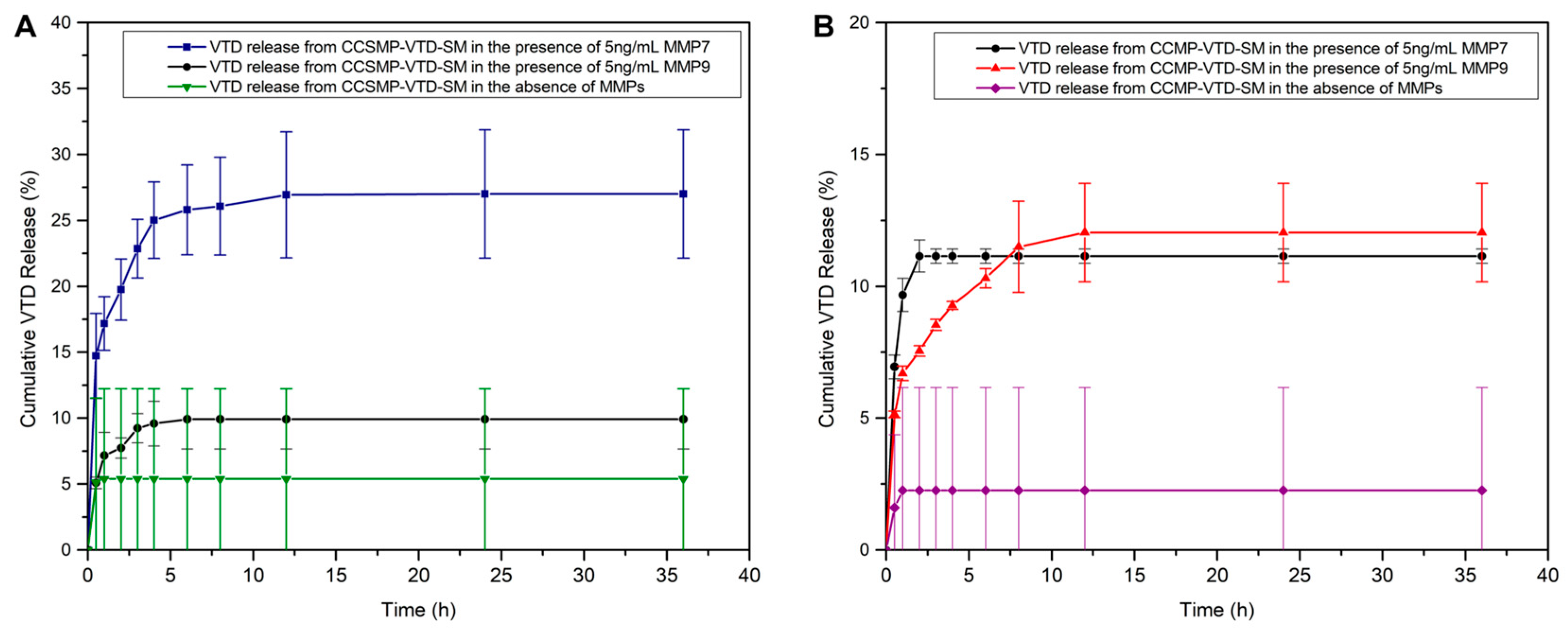
3.7. Calcium Carbonate Microparticles (CCMPs) and Calcium Carbonate Submicroparticles (CCSMPs): Improved Carriers for VTD Delivery
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VTD | Veratridine |
| CAS | Casein |
| MSNs | Mesoporous silica nanoparticles |
| CCMPs | Calcium carbonate microparticles |
| MMPs | Matrix Metalloproteinases |
| CRC | Colorectal cancer |
| NP-VTD | MSN-COOH/VTD/CAS |
| NP-Empty | MSN-COOH/EMPTY/CAS |
| CCSMPs | Calcium carbonate submicroparticles |
| AOM/DSS | azoxymethane/dextran sodium sulfate |
| UBXN2A (+/−) | heterozygous haploinsufficient UBXN2A |
| MSN-NH2 | Aminated mesoporous silica nanoparticles |
| MSN-COOH | Carboxylated mesoporous silica nanoparticles |
| APTES | 3-aminopropyltriethoxysilane |
| EDC | 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide |
| CTAB | Cetyltrimethylammonium bromide |
| TEOS | tetraethyl orthosilicate |
| DMF | Dimethy formamide |
| BSA | Bovine serum albumin |
| BET | Brunauer–Emmett–Teller |
| NIR | Near-infrared |
| BJH | Barrett-Joyner-Halenda |
| HPLC | High performance liquid chromatography |
References
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef]
- Hu, Z.; Ding, J.; Ma, Z.; Sun, R.; Seoane, J.A.; Scott Shaffer, J.; Suarez, C.J.; Berghoff, A.S.; Cremolini, C.; Falcone, A.; et al. Quantitative evidence for early metastatic seeding in colorectal cancer. Nat. Genet. 2019, 51, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.-H.; Chen, Y.-X.; Fang, J.-Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target. Ther. 2020, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.E.; Hu, C.-Y.; You, Y.N.; Bednarski, B.K.; Rodriguez-Bigas, M.A.; Skibber, J.M.; Cantor, S.B.; Chang, G.J. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975–2010. JAMA Surg. 2015, 150, 17–22. [Google Scholar] [CrossRef]
- Siegel, R.L.; Torre, L.A.; Soerjomataram, I.; Hayes, R.B.; Bray, F.; Weber, T.K.; Jemal, A. Global patterns and trends in colorectal cancer incidence in young adults. Gut 2019, 68, 2179–2185. [Google Scholar] [CrossRef]
- Yang, S.Y.; Sales, K.M.; Fuller, B.; Seifalian, A.M.; Winslet, M.C. Apoptosis and colorectal cancer: Implications for therapy. Trends Mol. Med. 2009, 15, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.; Sane, S.; Branick, K.A.; Freeling, J.L.; Wang, H.; Zhang, D.; Rezvani, K. A plant alkaloid, veratridine, potentiates cancer chemosensitivity by UBXN2A-dependent inhibition of an oncoprotein, mortalin-2. Oncotarget 2015, 16, 23561–23581. [Google Scholar] [CrossRef]
- Sane, S.; Abdullah, A.; Boudreau, D.A.; Autenried, R.K.; Gupta, B.K.; Wang, X.; Wang, H.; Schlenker, E.H.; Zhang, D.; Telleria, C.; et al. Ubiquitin-like (UBX)-domain-containing protein, UBXN2A, promotes cell death by interfering with the p53-Mortalin interactions in colon cancer cells. Cell Death Dis. 2014, 5, e1118. [Google Scholar] [CrossRef]
- Sane, S.; Abdullah, A.; Nelson, M.E.; Wang, H.; Chauhan, S.C.; Newton, S.S.; Rezvani, K. Structural studies of UBXN2A and mortalin interaction and the putative role of silenced UBXN2A in preventing response to chemotherapy. Cell Stress Chaperones 2016, 2, 313–326. [Google Scholar] [CrossRef]
- Sane, S.; Hafner, A.; Srinivasan, R.; Masood, D.; Slunecka, J.L.; Noldner, C.J.; Hanson, A.D.; Kruisselbrink, T.; Wang, X.; Wang, Y.; et al. UBXN2A enhances CHIP-mediated proteasomal degradation of oncoprotein mortalin-2 in cancer cells. Mol. Oncol. 2018, 12, 1753–1777. [Google Scholar] [CrossRef]
- Sane, S.; Srinivasan, R.; Potts, R.A.; Eikanger, M.; Zagirova, D.; Freeling, J.; Reihe, C.A.; Antony, R.M.; Gupta, B.K.; Lynch, D.; et al. UBXN2A suppresses the Rictor-mTORC2 signaling pathway, an established tumorigenic pathway in human colorectal cancer. Oncogene 2023, 42, 1763–1776. [Google Scholar] [CrossRef]
- Esfahanian, N.; Knoblich, C.D.; Bowman, G.A.; Rezvani, K. Mortalin: Protein partners, biological impacts, pathological roles, and therapeutic opportunities. Front. Cell Dev. Biol. 2023, 11, 1028519. [Google Scholar] [CrossRef]
- Bian, Y.-H.; Xu, J.; Zhao, W.-Y.; Zhang, Z.-Z.; Tu, L.; Cao, H.; Zhang, Z.-G. Targeting mTORC2 component rictor inhibits cell proliferation and promotes apoptosis in gastric cancer. Am. J. Transl. Res. 2017, 9, 4317–4330. [Google Scholar]
- Baumann, K.N.; Schröder, T.; Ciryam, P.S.; Morzy, D.; Tinnefeld, P.; Knowles, T.P.; Hernández-Ainsa, S. DNA–liposome hybrid carriers for triggered cargo release. ACS Appl. Bio Mater. 2022, 5, 3713–3721. [Google Scholar] [CrossRef]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The history of nanoscience and nanotechnology: From chemical–physical applications to nanomedicine. Molecules 2019, 25, 112. [Google Scholar] [CrossRef]
- Haley, B.; Frenkel, E. Nanoparticles for drug delivery in cancer treatment. In Urologic Oncology: Seminars and Original Investigations; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Bao, G.; Mitragotri, S.; Tong, S. Multifunctional nanoparticles for drug delivery and molecular imaging. Annu. Rev. Biomed. Eng. 2013, 15, 253–282. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.-J.; Sim, M.; Oh, L.; Lim, K.; Park, H. Carbon-based drug delivery carriers for cancer therapy. Arch. Pharmacal Res. 2014, 37, 43–52. [Google Scholar] [CrossRef]
- Nayl, A.; Abd-Elhamid, A.; Aly, A.A.; Bräse, S. Recent progress in the applications of silica-based nanoparticles. RSC Adv. 2022, 12, 13706–13726. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, T.; Shimizu, T.; Kuroda, K.; Kato, C. The preparation of alkyltrimethylammonium–kanemite complexes and their conversion to microporous materials. Bull. Chem. Soc. Jpn. 1990, 63, 988–992. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Rámila, A.; Del Real, R.; Pérez-Pariente, J. A new property of MCM-41: Drug delivery system. Chem. Mater. 2001, 13, 308–311. [Google Scholar] [CrossRef]
- Lérida-Viso, A.; Estepa-Fernández, A.; García-Fernández, A.; Martí-Centelles, V.; Martínez-Máñez, R. Biosafety of mesoporous silica nanoparticles; towards clinical translation. Adv. Drug Deliv. Rev. 2023, 201, 115049. [Google Scholar] [CrossRef] [PubMed]
- Giret, S.; Wong Chi Man, M.; Carcel, C. Mesoporous-silica-functionalized nanoparticles for drug delivery. Chem.—A Eur. J. 2015, 21, 13850–13865. [Google Scholar] [CrossRef] [PubMed]
- Godakhindi, V.; Tarannum, M.; Dam, S.K.; Vivero-Escoto, J.L. Mesoporous Silica Nanoparticles as an Ideal Platform for Cancer Immunotherapy: Recent Advances and Future Directions. Adv. Healthc. Mater. 2024, 13, 2400323. [Google Scholar] [CrossRef] [PubMed]
- Aznar, E.; Oroval, M.; Pascual, L.; Murguía, J.R.; Martinez-Manez, R.; Sancenon, F. Gated materials for on-command release of guest molecules. Chem. Rev. 2016, 116, 561–718. [Google Scholar] [CrossRef]
- Corma, A.; Botella, P.; Rivero-Buceta, E. Silica-based stimuli-responsive systems for antitumor drug delivery and controlled release. Pharmaceutics 2022, 14, 110. [Google Scholar] [CrossRef]
- Klupp, F.; Neumann, L.; Kahlert, C.; Diers, J.; Halama, N.; Franz, C.; Schmidt, T.; Koch, M.; Weitz, J.; Schneider, M.; et al. Serum MMP7, MMP10 and MMP12 level as negative prognostic markers in colon cancer patients. BMC Cancer 2016, 16, 494. [Google Scholar] [CrossRef]
- Polistena, A.; Cucina, A.; Dinicola, S.; Stene, C.; Cavallaro, G.; Ciardi, A.; Orlando, G.; Arena, R.; D’Ermo, G.; Cavallaro, A.; et al. MMP7 expression in colorectal tumours of different stages. In Vivo 2014, 28, 105–110. [Google Scholar]
- Chen, L.; Ke, X. MMP7 as a potential biomarker of colon cancer and its prognostic value by bioinformatics analysis. Medicine 2021, 100, e24953. [Google Scholar] [CrossRef]
- Liao, H.Y.; Da, C.M.; Liao, B.; Zhang, H.H. Roles of matrix metalloproteinase-7 (MMP-7) in cancer. Clin. Biochem. 2021, 92, 9–18. [Google Scholar] [CrossRef]
- Zeng, Z.-S.; Shu, W.-P.; Cohen, A.M.; Guillem, J.G. Matrix Metalloproteinase-7 Expression in Colorectal Cancer Liver Metastases. Clin. Cancer Res. 2002, 8, 144. [Google Scholar]
- Wijewantha, N.; Eikanger, M.M.; Antony, R.M.; Potts, R.A.; Rezvani, K.; Sereda, G. Targeting Colon Cancer Cells with Enzyme-Triggered Casein-Gated Release of Cargo from Mesoporous Silica-Based Nanoparticles. Bioconjug. Chem. 2021, 32, 2353–2365. [Google Scholar] [CrossRef]
- Scherer, R.L.; VanSaun, M.N.; McIntyre, J.O.; Matrisian, L.M. Optical imaging of matrix metalloproteinase-7 activity in vivo using a proteolytic nanobeacon. Mol. Imaging 2008, 7, 118–131. [Google Scholar] [CrossRef]
- Takeharu, H.; Yasukawa, K.; Inouye, K. Thermodynamic analysis of ionizable groups involved in the catalytic mechanism of human matrix metalloproteinase 7 (MMP-7). Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2011, 1814, 1940–1946. [Google Scholar] [CrossRef] [PubMed]
- Coll Merino, M.; Mondragón Martínez, L.; Martínez Mañez, R.; Sancenón Galarza, F.; Marcos Martínez, M.D.; Soto Camino, J.; Amoros del Toro, P.J.; Pérez Payá, E. Enzyme-mediated controlled release systems by anchoring peptide sequences on mesoporous silica supports. Angew. Chem. Int. Ed. 2011, 50, 2138–2140. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhong, S.; Xu, L.; He, S.; Dou, Y.; Zhao, S.; Chen, P.; Cui, X. Mesoporous silica nanoparticles capped with graphene quantum dots as multifunctional drug carriers for photo-thermal and redox-responsive release. Microporous Mesoporous Mater. 2019, 278, 130–137. [Google Scholar] [CrossRef]
- Klonoski, J.M.; Hurtig, H.R.; Juber, B.A.; Schuneman, M.J.; Bickett, T.E.; Svendsen, J.M.; Burum, B.; Penfound, T.A.; Sereda, G.; Dale, J.B. Vaccination against the M protein of Streptococcus pyogenes prevents death after influenza virus: S. pyogenes super-infection. Vaccine 2014, 32, 5241–5249. [Google Scholar] [CrossRef]
- Walker, J. The bicinchoninic acid (BCA) assay for protein quantitation. In The Protein Protocols Handbook; Humana Press: Totowa, NJ, USA, 2009; pp. 11–15. [Google Scholar]
- Sereda, G.; Ahammadullah, A.; Wijewantha, N.; Solano, Y.A. Acid-triggered release of eugenol and fluoride by desensitizing macro-and nanoparticles. J. Funct. Biomater. 2023, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, L.; Tang, X.; Zhang, X.; Qiao, Y.; Shi, Y.; Xu, Y.; Wang, Z.; Yu, Y.; Sun, F. Doxorubicin induces apoptosis by targeting Madcam1 and AKT and inhibiting protein translation initiation in hepatocellular carcinoma cells. Oncotarget 2015, 6, 24075–24091. [Google Scholar] [CrossRef]
- Yardeni, T.; Eckhaus, M.; Morris, H.D.; Huizing, M.; Hoogstraten-Miller, S. Retro-orbital injections in mice. Lab Anim. 2011, 40, 155–160. [Google Scholar] [CrossRef]
- Freeling, J.L.; Rezvani, K. Assessment of murine colorectal cancer by micro-ultrasound using three dimensional reconstruction and non-linear contrast imaging. Mol. Ther.—Methods Clin. Dev. 2016, 5, 16070. [Google Scholar] [CrossRef]
- Ganguly, K.K.; Pal, S.; Moulik, S.; Chatterjee, A. Integrins and metastasis. Cell Adhes. Migr. 2013, 7, 251–261. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, N.; Hadden, T.J.; Rishi, A.K. Akt, FoxO and regulation of apoptosis. Biochim. Biophys. Acta 2011, 1813, 1978–1986. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, J.; Bai, J.; Tong, R.; An, F.; Jiao, P.; He, L.; Zeng, D.; Long, E.; Yan, J.; et al. The Application of Natural Products in Cancer Therapy by Targeting Apoptosis Pathways. Curr. Drug Metab. 2018, 19, 739–749. [Google Scholar] [CrossRef]
- Rajabi, S.; Maresca, M.; Yumashev, A.V.; Choopani, R.; Hajimehdipoor, H. The Most Competent Plant-Derived Natural Products for Targeting Apoptosis in Cancer Therapy. Biomolecules 2021, 11, 534. [Google Scholar] [CrossRef] [PubMed]
- Freeling, J.L.; Scholl, J.L.; Eikanger, M.; Knoblich, C.; Potts, R.A.; Anderson, D.J.; Rower, J.E.; Farjoo, M.H.; Zhao, H.; Pillatzki, A.; et al. Pre-clinical safety and therapeutic efficacy of a plant-based alkaloid in a human colon cancer xenograft model. Cell Death Discov. 2022, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, P.J.; Van Assouw, H.P.; Peeters, L.; Leysen, J.E. Neurotoxic action of veratridine in rat brain neuronal cultures: Mechanism of neuroprotection by Ca++ antagonists nonselective for slow Ca++ channels. J. Pharmacol. Exp. Ther. 1990, 255, 1117–1122. [Google Scholar] [CrossRef]
- Ye, X.; Wang, Y.; Yang, M.; Wang, Q.; Liang, Q.; Ma, Z.; Zhang, B.; Gao, Y. Investigating the in vitro metabolism of veratridine: Characterization of metabolites and involved cytochrome P450 isoforms. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 141–148. [Google Scholar] [CrossRef]
- Liu, X.; Xin, Z.; Wang, K. Patient-derived xenograft model in colorectal cancer basic and translational research. Anim. Models Exp. Med. 2023, 6, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Jung, J. Sex-dependent liver cancer xenograft models for predicting clinical data in the evaluation of anticancer drugs. Lab. Anim. Res. 2021, 37, 10. [Google Scholar] [CrossRef]
- He, F.; Chen, H.; Yang, P.; Wu, Q.; Zhang, T.; Wang, C.; Wei, J.; Chen, Z.; Hu, H.; Li, W.; et al. Gankyrin sustains PI3K/GSK-3β/β-catenin signal activation and promotes colorectal cancer aggressiveness and progression. Oncotarget 2016, 7, 81156–81171. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Poon, W.; Tavares, A.J.; McGilvray, I.D.; Chan, W.C.W. Nanoparticle-liver interactions: Cellular uptake and hepatobiliary elimination. J. Control. Release 2016, 240, 332–348. [Google Scholar] [CrossRef]
- Walstra, P.; Walstra, P.; Wouters, J.T.; Geurts, T.J. Dairy Science and Technology; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Nouri, Z.; Fakhri, S.; Nouri, K.; Wallace, C.E.; Farzaei, M.H.; Bishayee, A. Targeting Multiple Signaling Pathways in Cancer: The Rutin Therapeutic Approach. Cancers 2020, 12, 2276. [Google Scholar] [CrossRef] [PubMed]
- Bordoloi, D.; Roy, N.K.; Monisha, J.; Padmavathi, G.; Kunnumakkara, A.B. Multi-Targeted Agents in Cancer Cell Chemosensitization: What We Learnt from Curcumin Thus Far. Recent Pat. Anti-Cancer Drug Discov. 2016, 11, 67–97. [Google Scholar] [CrossRef] [PubMed]
- Cevatemre, B.; Erkısa, M.; Aztopal, N.; Karakas, D.; Alper, P.; Tsimplouli, C.; Sereti, E.; Dimas, K.; Armutak, E.I.I.; Gurevin, E.G.; et al. A promising natural product, pristimerin, results in cytotoxicity against breast cancer stem cells in vitro and xenografts in vivo through apoptosis and an incomplete autopaghy in breast cancer. Pharmacol. Res. 2018, 129, 500–514. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Akash, S.; Rahman, M.M.; Nowrin, F.T.; Akter, T.; Shohag, S.; Rauf, A.; Aljohani, A.S.M.; Simal-Gandara, J. Colon cancer and colorectal cancer: Prevention and treatment by potential natural products. Chem. Biol. Interact. 2022, 368, 110170. [Google Scholar] [CrossRef]
- Kim, M.; Bakyt, L.; Akhmetkaliyev, A.; Toktarkhanova, D.; Bulanin, D. Re-Sensitizing Cancer Stem Cells to Conventional Chemotherapy Agents. Int. J. Mol. Sci. 2023, 24, 2122. [Google Scholar] [CrossRef]
- Wang, Q.; Shen, X.; Chen, G.; Du, J. Drug Resistance in Colorectal Cancer: From Mechanism to Clinic. Cancers 2022, 14, 2928. [Google Scholar] [CrossRef]
- Chiang, M.R.; Hsu, C.W.; Pan, W.C.; Tran, N.T.; Lee, Y.S.; Chiang, W.H.; Liu, Y.C.; Chen, Y.W.; Chiou, S.H.; Hu, S.H. Reprogramming Dysfunctional Dendritic Cells by a Versatile Catalytic Dual Oxide Antigen-Captured Nanosponge for Remotely Enhancing Lung Metastasis Immunotherapy. ACS Nano 2025, 19, 2117–2135. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, M.; Eikanger, M.; Sane, S.; Wijewardhane, K.S.K.; Slunecka, J.L.; Freeling, J.; Rezvani, K.; Sereda, G. Liver-Specific Nanoparticle-Mediated Delivery and MMP-Triggered Release of Veratridine to Effectively Target Metastatic Colorectal Cancer. Cancers 2025, 17, 3253. https://doi.org/10.3390/cancers17193253
Hasan M, Eikanger M, Sane S, Wijewardhane KSK, Slunecka JL, Freeling J, Rezvani K, Sereda G. Liver-Specific Nanoparticle-Mediated Delivery and MMP-Triggered Release of Veratridine to Effectively Target Metastatic Colorectal Cancer. Cancers. 2025; 17(19):3253. https://doi.org/10.3390/cancers17193253
Chicago/Turabian StyleHasan, Mahadi, Morgan Eikanger, Sanam Sane, Krishantha S. K. Wijewardhane, John L. Slunecka, Jessica Freeling, Khosrow Rezvani, and Grigoriy Sereda. 2025. "Liver-Specific Nanoparticle-Mediated Delivery and MMP-Triggered Release of Veratridine to Effectively Target Metastatic Colorectal Cancer" Cancers 17, no. 19: 3253. https://doi.org/10.3390/cancers17193253
APA StyleHasan, M., Eikanger, M., Sane, S., Wijewardhane, K. S. K., Slunecka, J. L., Freeling, J., Rezvani, K., & Sereda, G. (2025). Liver-Specific Nanoparticle-Mediated Delivery and MMP-Triggered Release of Veratridine to Effectively Target Metastatic Colorectal Cancer. Cancers, 17(19), 3253. https://doi.org/10.3390/cancers17193253





