Proline Metabolism in Cancer: Emerging Roles in Redox Homeostasis and Therapeutic Opportunities
Simple Summary
Abstract
1. Introduction
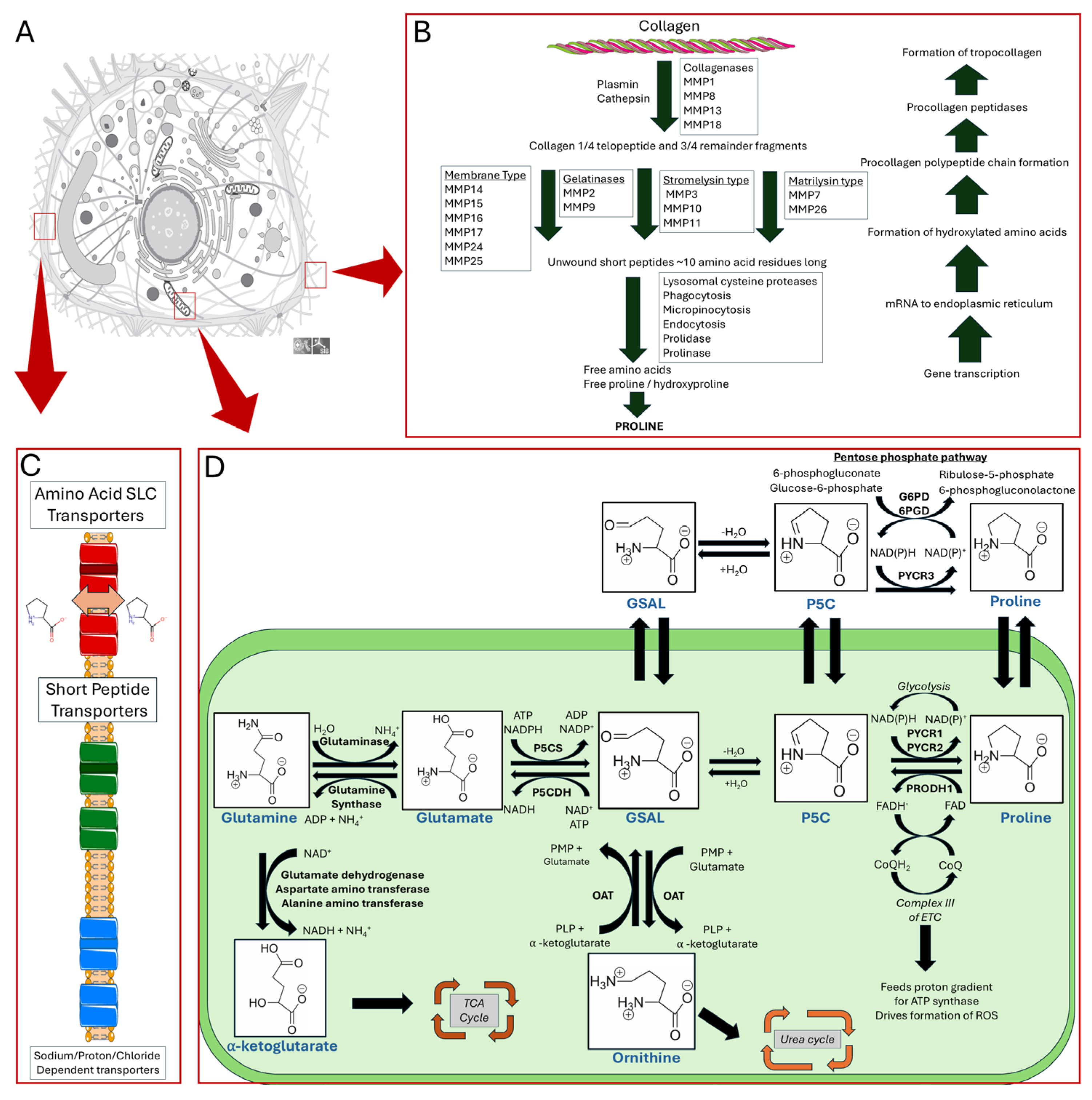
2. Review of Proline Metabolism
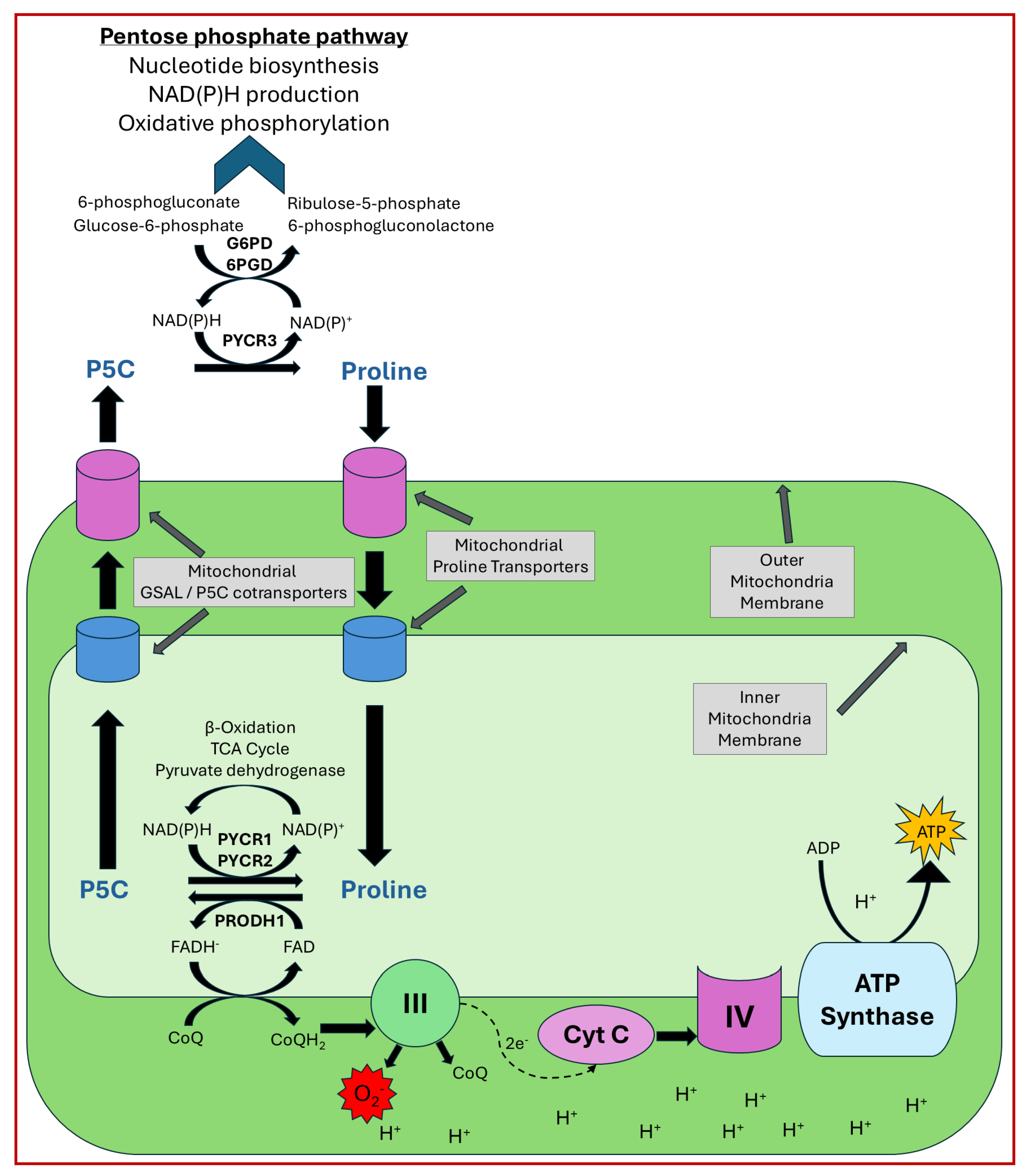
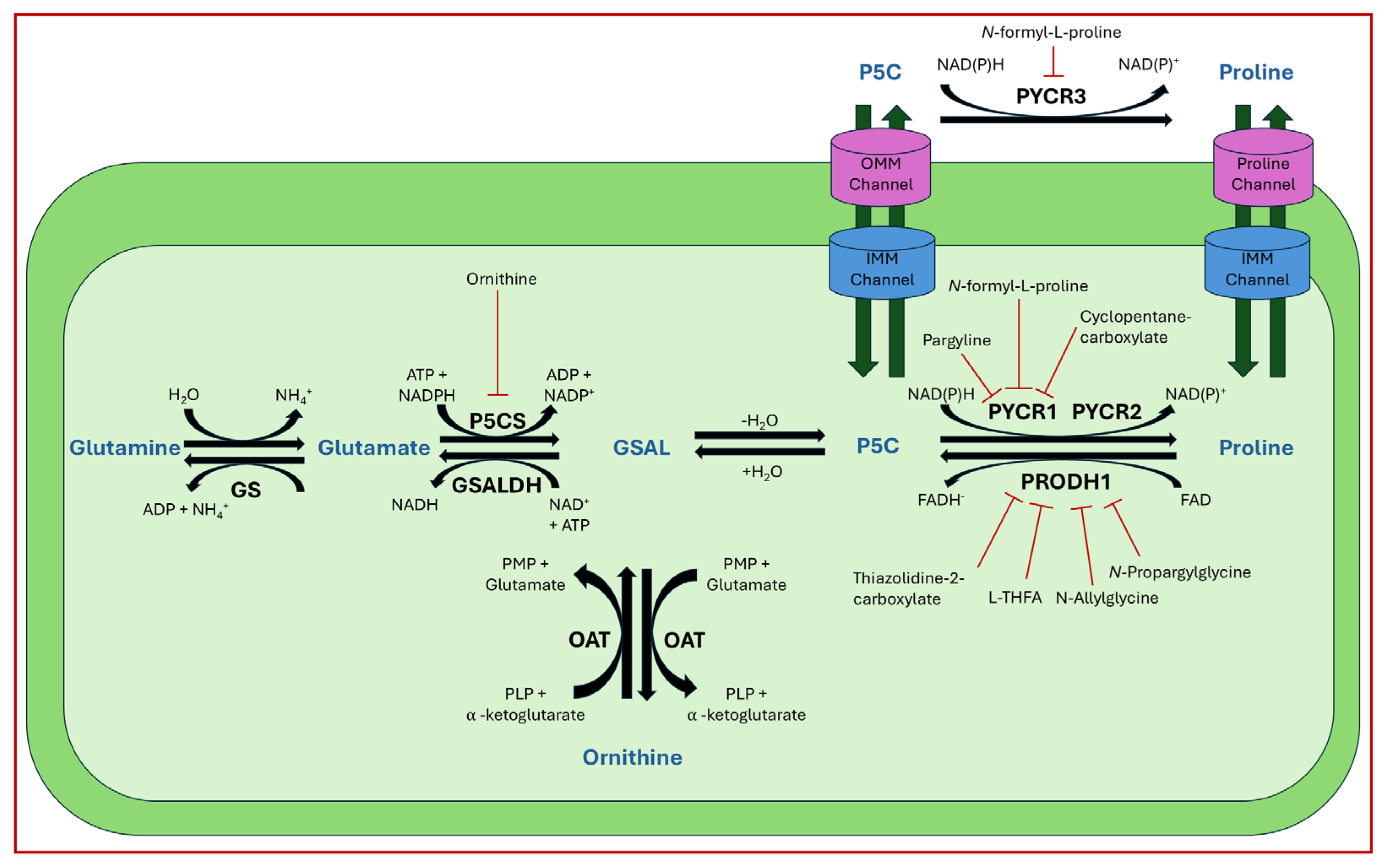
2.1. Proline Biosynthesis, Catabolism, and Transport
2.2. General Proline Function
3. Proline Metabolism in Cancer
3.1. Proline Enzyme Expression Levels in Cancer
3.2. Cell Line Requirements for Exogenous Proline
3.3. Regulation of Proline Metabolic Enzyme Expression
3.4. Enzyme Regulation
4. Proline Metabolism, Redox Homeostasis, and Hypoxia
4.1. Proline Cycle and Cancer
4.2. PRODH in ROS Signaling and Hypoxia
4.3. PYCR and Hypoxia
5. Collagen, Proline Biosynthesis, and Cell Proliferation
5.1. Collagen Synthesis, Degradation, and Interaction in the Tumor Niche
5.2. Regulation of Proline Biosynthesis in Different Cancers
6. Therapeutic Opportunities Targeting Proline Metabolism
6.1. Proline Enzyme Variants and Cancer
6.2. Inhibition of Proline Metabolic Enzymes
6.3. Alternative Targets of Proline Metabolism
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PRODH1 | Proline dehydrogenase isoform 1 |
| PRODH2 | Proline dehydrogenase isoform 2 |
| NEAA | Non-essential amino acid |
| GSAL | L-glutamate-γ-semialdehyde |
| P5C | L-Δ1-pyrroline-5-carboxylate |
| P5CS | L-Δ1-pyrroline-5-carboxylate synthase |
| PYCR | L-Δ1-pyrroline-5-carboxylate reductase |
| PLP | Pyridoxal-5-phosphate |
| OAT | Ornithine-γ-aminotransferase |
| PRODH | Proline dehydrogenase |
| FAD | Flavin adenine dinucleotide |
| P5CDH | L-Δ1-pyrroline-5-carboxylate dehydrogenase |
| SLC6A19 | Solute carrier family 6 member 19 |
| SLC6A7 | Solute carrier family 6 member 7 |
| PTM | Post translational modifications |
| GABA | Gamma-aminobutyric acid |
| GAD | Gamma-aminobutyric acid receptors |
| PIG6 | p53 inducible gene |
| ChIP | Chromatin immunoprecipitation assay |
| LDL | Low-density lipoprotein |
| oxLDL | oxidized low-density lipoprotein |
| KSHV | Kaposi’s sarcoma-associated herpesvirus |
| L-THFA | L-tetra-hydro-2-furoic acid |
| CBP | CREB binding protein |
| USP9x | Ubiquitin-specific peptidase 9 X-linked |
| UCP | Uncoupling proteins |
| VHL | Von-Hippel Lindau ubiquitin ligase |
| NFAT | Nuclear factors of activated T-Cells |
| RST | Redox stress signaling threshold |
| PEPCK-M | Mitochondrial phosphoenolpyruvate carboxykinase |
| ECM | Extra cellular matrix |
| P4H | Prolyl-4-hydroxlase |
| P3H | Prolyl-3-hydroxylase |
| MMP | Matrix metalloproteinases |
| TGFB | Transforming growth factor beta |
| P4HA1 | Prolyl 4 hydroxylase subunit alpha 1 |
| CAF | Cancer-associated fibroblast |
| TRAIL | TNF related apoptosis inducing ligand protein |
| MPC1 | Mitochondrial pyruvate carrier protein |
| BHELH41 | Basic helix-loop-helix family member e41 |
| CRC | Colorectal cancer |
| myCAF | myofibroblastic cancer-associated fibroblast |
| SASP | Senescence-associated secretory phenotype |
| TNBC | Triple negative breast cancer cells |
| THFA | S-(-)-tetrahydro-2-furoic acid |
| B32G | But-3-yn-2-ylglycine |
| NFLP | N-formyl-L-proline |
References
- Naser, R.; Dilabazian, H.; Bahr, H.; Barakat, A.; El-Sibai, M. A Guide through Conventional and Modern Cancer Treatment Modalities: A Specific Focus on Glioblastoma Cancer Therapy (Review). Oncol. Rep. 2022, 48, 190. [Google Scholar] [CrossRef]
- Atlihan-Gundogdu, E.; Ilem-Ozdemir, D.; Ekinci, M.; Ozgenc, E.; Demir, E.S.; Sánchez-Dengra, B.; González-Alvárez, I. Recent Developments in Cancer Therapy and Diagnosis. J. Pharm. Investig. 2020, 50, 349–361. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, X.; Liu, Z.; Cheng, L. Recent Progress of Chemodynamic Therapy-Induced Combination Cancer Therapy. Nano Today 2020, 35, 100946. [Google Scholar] [CrossRef]
- Kontomanolis, E.N.; Koutras, A.; Syllaios, A.; Schizas, D.; Mastoraki, A.; Garmpis, N.; Diakosavvas, M.; Angelou, K.; Tsatsaris, G.; Pagkalos, A.; et al. Role of Oncogenes and Tumor-Suppressor Genes in Carcinogenesis: A Review. Anticancer. Res. 2020, 40, 6009–6015. [Google Scholar] [CrossRef]
- Kaczmarek, M.; Poznańska, J.; Fechner, F.; Michalska, N.; Paszkowska, S.; Napierała, A.; Mackiewicz, A. Cancer Vaccine Therapeutics: Limitations and Effectiveness—A Literature Review. Cells 2023, 12, 2159. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Bhardwaj, A.; Gupta, S. Cancer Treatment Therapies: Traditional to Modern Approaches to Combat Cancers. Mol. Biol. Rep. 2023, 50, 9663–9676. [Google Scholar] [CrossRef]
- Vettore, L.; Westbrook, R.L.; Tennant, D.A. New Aspects of Amino Acid Metabolism in Cancer. Br. J. Cancer 2020, 122, 150–156. [Google Scholar] [CrossRef]
- Lunt, S.Y.; Fendt, S.-M. Metabolism—A Cornerstone of Cancer Initiation, Progression, Immune Evasion and Treatment Response. Curr. Opin. Syst. Biol. 2018, 8, 67–72. [Google Scholar] [CrossRef]
- Tanner, J.J.; Fendt, S.-M.; Becker, D.F. The Proline Cycle as a Potential Cancer Therapy Target. Biochemistry 2018, 57, 3433–3444. [Google Scholar] [CrossRef]
- Geck, R.C.; Toker, A. Nonessential Amino Acid Metabolism in Breast Cancer. Adv. Biol. Regul. 2016, 62, 11–17. [Google Scholar] [CrossRef]
- Tang, L.; Zeng, J.; Geng, P.; Fang, C.; Wang, Y.; Sun, M.; Wang, C.; Wang, J.; Yin, P.; Hu, C.; et al. Global Metabolic Profiling Identifies a Pivotal Role of Proline and Hydroxyproline Metabolism in Supporting Hypoxic Response in Hepatocellular Carcinoma. Clin. Cancer Res. 2018, 24, 474–485. [Google Scholar] [CrossRef]
- D’Aniello, C.; Patriarca, E.J.; Phang, J.M.; Minchiotti, G. Proline Metabolism in Tumor Growth and Metastatic Progression. Front. Oncol. 2020, 10, 776. [Google Scholar] [CrossRef]
- Mussini, E.; Hutton, J.J.; Udenfriend, S. Collagen Proline Hydroxylase in Wound Healing, Granuloma Formation, Scurvy, and Growth. Science 1967, 157, 927–929. [Google Scholar] [CrossRef]
- Cyran, A.M.; Zhitkovich, A. HIF1, HSF1, and NRF2: Oxidant-Responsive Trio Raising Cellular Defenses and Engaging Immune System. Chem. Res. Toxicol. 2022, 35, 1690–1700. [Google Scholar] [CrossRef]
- Struys, E.A.; Jansen, E.E.W.; Salomons, G.S. Human Pyrroline-5-carboxylate Reductase (PYCR1) Acts on Δ1 -piperideine-6-carboxylate Generating L-pipecolic Acid. J. Inher Metab. Disea 2014, 37, 327–332. [Google Scholar] [CrossRef] [PubMed]
- De Ingeniis, J.; Ratnikov, B.; Richardson, A.D.; Scott, D.A.; Aza-Blanc, P.; De, S.K.; Kazanov, M.; Pellecchia, M.; Ronai, Z.; Osterman, A.L.; et al. Functional Specialization in Proline Biosynthesis of Melanoma. PLoS ONE 2012, 7, e45190. [Google Scholar] [CrossRef]
- Christensen, E.M.; Patel, S.M.; Korasick, D.A.; Campbell, A.C.; Krause, K.L.; Becker, D.F.; Tanner, J.J. Resolving the Cofactor-Binding Site in the Proline Biosynthetic Enzyme Human Pyrroline-5-Carboxylate Reductase 1. J. Biol. Chem. 2017, 292, 7233–7243. [Google Scholar] [CrossRef]
- Bogner, A.N.; Stiers, K.M.; Tanner, J.J. Structure, Biochemistry, and Gene Expression Patterns of the Proline Biosynthetic Enzyme Pyrroline-5-Carboxylate Reductase (PYCR), an Emerging Cancer Therapy Target. Amino Acids 2021, 53, 1817–1834. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Hancock, C.N.; Fischer, J.W.; Harman, M.; Phang, J.M. Proline Biosynthesis Augments Tumor Cell Growth and Aerobic Glycolysis: Involvement of Pyridine Nucleotides. Sci. Rep. 2015, 5, 17206. [Google Scholar] [CrossRef] [PubMed]
- Becirovic, T.; Zhang, B.; Lindskog, C.; Norberg, E.; Vakifahmetoglu-Norberg, H.; Kaminskyy, V.O.; Kochetkova, E. Deubiquitinase USP9x Regulates the Proline Biosynthesis Pathway in Non-Small Cell Lung Cancer. Cell Death Discov. 2024, 10, 342. [Google Scholar] [CrossRef] [PubMed]
- Westbrook, R.L.; Bridges, E.; Roberts, J.; Escribano-Gonzalez, C.; Eales, K.L.; Vettore, L.A.; Walker, P.D.; Vera-Siguenza, E.; Rana, H.; Cuozzo, F.; et al. Proline Synthesis through PYCR1 Is Required to Support Cancer Cell Proliferation and Survival in Oxygen-Limiting Conditions. Cell Rep. 2022, 38, 110320. [Google Scholar] [CrossRef]
- Tanner, J.J. Structural Biology of Proline Catabolic Enzymes. Antioxid. Redox Signal. 2019, 30, 650–673. [Google Scholar] [CrossRef]
- Summitt, C.B.; Johnson, L.C.; Jönsson, T.J.; Parsonage, D.; Holmes, R.P.; Lowther, W.T. Proline Dehydrogenase 2 (PRODH2) Is a Hydroxyproline Dehydrogenase (HYPDH) and Molecular Target for Treating Primary Hyperoxaluria. Biochem. J. 2015, 466, 273–281. [Google Scholar] [CrossRef]
- Miner-Williams, W.M.; Stevens, B.R.; Moughan, P.J. Are Intact Peptides Absorbed from the Healthy Gut in the Adult Human? Nutr. Res. Rev. 2014, 27, 308–329. [Google Scholar] [CrossRef]
- Wu, C.C.; MacCoss, M.J.; Howell, K.E.; Yates, J.R. A Method for the Comprehensive Proteomic Analysis of Membrane Proteins. Nat. Biotechnol. 2003, 21, 532–538. [Google Scholar] [CrossRef]
- Patriarca, E.J.; Cermola, F.; D’Aniello, C.; Fico, A.; Guardiola, O.; De Cesare, D.; Minchiotti, G. The Multifaceted Roles of Proline in Cell Behavior. Front. Cell Dev. Biol. 2021, 9, 728576. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wu, G. Roles of Dietary Glycine, Proline, and Hydroxyproline in Collagen Synthesis and Animal Growth. Amino Acids 2018, 50, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Brandsch, M. Transport of L-Proline, L-Proline-Containing Peptides and Related Drugs at Mammalian Epithelial Cell Membranes. Amino Acids 2006, 31, 119–136. [Google Scholar] [CrossRef]
- Fremeau, R.T.; Caron, M.G.; Blakely, R.D. Molecular Cloning and Expression of a High Affinity L-Proline Transporter Expressed in Putative Glutamatergic Pathways of Rat Brain. Neuron 1992, 8, 915–926. [Google Scholar] [CrossRef]
- Block, S.; Chi, F.; Rosen, P.C.; Pineda, S.S.; Darnell, A.M.; Abbott, K.L.; Pena, I.A.; Heiman, M.; Yilmaz, Ö.H.; Kory, N.; et al. Sideroflexins Enable Mitochondrial Transport of Polar Neutral Amino Acids. bioRxiv 2025. bioRxiv: 2025.06.18.660357. [Google Scholar] [CrossRef] [PubMed]
- MacArthur, M.W.; Thornton, J.M. Influence of Proline Residues on Protein Conformation. J. Mol. Biol. 1991, 218, 397–412. [Google Scholar] [CrossRef]
- Signorelli, S.; Coitiño, E.L.; Borsani, O.; Monza, J. Molecular Mechanisms for the Reaction Between •OH Radicals and Proline: Insights on the Role as Reactive Oxygen Species Scavenger in Plant Stress. J. Phys. Chem. B 2014, 118, 37–47. [Google Scholar] [CrossRef]
- Ignatova, Z.; Gierasch, L.M. Inhibition of Protein Aggregation in Vitro and in Vivo by a Natural Osmoprotectant. Proc. Natl. Acad. Sci. USA 2006, 103, 13357–13361. [Google Scholar] [CrossRef]
- Kivirikko, K.I.; Myllylä, R.; Pihlajaniemi, T. Hydroxylation of Proline and Lysine Residues in Collagens and Other Animal and Plant Proteins. In Post-Translational Modifications of Proteins; CRC Press: Boca Raton, 2024; pp. 1–51. ISBN 978-1-003-57416-3. [Google Scholar]
- Savitski, M.M.; Nielsen, M.L.; Zubarev, R.A. ModifiComb, a New Proteomic Tool for Mapping Substoichiometric Post-Translational Modifications, Finding Novel Types of Modifications, and Fingerprinting Complex Protein Mixtures. Mol. Cell. Proteom. 2006, 5, 935–948. [Google Scholar] [CrossRef]
- Kubyshkin, V.; Rubini, M. Proline Analogues. Chem. Rev. 2024, 124, 8130–8232. [Google Scholar] [CrossRef]
- Gill, A.C. Post-Translational Hydroxylation at the N-Terminus of the Prion Protein Reveals Presence of PPII Structure in Vivo. EMBO J. 2000, 19, 5324–5331. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Y.; Yang, H.; Li, X.; Jie, M.; Hu, C.; Wu, Y.; Yang, S.; Yang, Y. Prolyl Isomerase Pin1: A Promoter of Cancer and a Target for Therapy. Cell Death Dis. 2018, 9, 883. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Ling, R.; Tang, X.; Yu, Y.; Zhou, Y.; Chen, D. Post-Translational Modifications of BRD4: Therapeutic Targets for Tumor. Front. Oncol. 2022, 12, 847701. [Google Scholar] [CrossRef] [PubMed]
- Schindler, L.; Dickerhof, N.; Hampton, M.B.; Bernhagen, J. Post-Translational Regulation of Macrophage Migration Inhibitory Factor: Basis for Functional Fine-Tuning. Redox Biology 2018, 15, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Kokkinidis, M.; Glykos, N.M.; Fadouloglou, V.E. Catalytic Activity Regulation through Post-Translational Modification: The Expanding Universe of Protein Diversity. In Advances in Protein Chemistry and Structural Biology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 122, pp. 97–125. ISBN 978-0-12-817762-4. [Google Scholar]
- Mailloux, R.J. Proline and Dihydroorotate Dehydrogenase Promote a Hyper-Proliferative State and Dampen Ferroptosis in Cancer Cells by Rewiring Mitochondrial Redox Metabolism. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2024, 1871, 119639. [Google Scholar] [CrossRef]
- Ferreira, A.G.K.; Biasibetti-Brendler, H.; Sidegum, D.S.V.; Loureiro, S.O.; Figueiró, F.; Wyse, A.T.S. Effect of Proline on Cell Death, Cell Cycle, and Oxidative Stress in C6 Glioma Cell Line. Neurotox. Res. 2021, 39, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Victor Nadler, J.; Wang, A.; Hakim, A. Toxicity of L-Proline toward Rat Hippocampal Neurons. Brain Res. 1988, 456, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, G.W.; Park, A.J.; Gordon, J.A.; Gogos, J.A. Cytosolic Accumulation of L-Proline Disrupts GABA-Ergic Transmission through GAD Blockade. Cell Rep. 2016, 17, 570–582. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, A.; Kunii, Y.; Hino, M.; Izumi, R.; Nagashima, C.; Takeshima, A.; Sainouchi, M.; Nawa, H.; Kakita, A.; Yabe, H. ALDH4A1 Expression Levels Are Elevated in Postmortem Brains of Patients with Schizophrenia and Are Associated with Genetic Variants in Enzymes Related to Proline Metabolism. J. Psychiatr. Res. 2020, 123, 119–127. [Google Scholar] [CrossRef]
- Das, A.; Gauthier-Coles, G.; Bröer, S.; Rae, C.D. L-Proline Alters Energy Metabolism in Brain Cortical Tissue Slices. Neurochem. Res. 2025, 50, 16. [Google Scholar] [CrossRef]
- Wyse, A.T.S.; Netto, C.A. Behavioral and Neurochemical Effects of Proline. Metab. Brain Dis. 2011, 26, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xue, Z.; Lin, J.; Wang, Y.; Ying, H.; Lv, Q.; Hua, C.; Wang, M.; Chen, S.; Zhou, B. Proline Improves Cardiac Remodeling Following Myocardial Infarction and Attenuates Cardiomyocyte Apoptosis via Redox Regulation. Biochem. Pharmacol. 2020, 178, 114065. [Google Scholar] [CrossRef]
- Jones, B.; Balasubramaniam, M.; Lebowitz, J.J.; Taylor, A.; Villalta, F.; Khoshbouei, H.; Grueter, C.; Grueter, B.; Dash, C.; Pandhare, J. Activation of Proline Biosynthesis Is Critical to Maintain Glutamate Homeostasis during Acute Methamphetamine Exposure. Sci. Rep. 2021, 11, 1422. [Google Scholar] [CrossRef]
- Liu, Y.; Mao, C.; Wang, M.; Liu, N.; Ouyang, L.; Liu, S.; Tang, H.; Cao, Y.; Liu, S.; Wang, X.; et al. Cancer Progression Is Mediated by Proline Catabolism in Non-Small Cell Lung Cancer. Oncogene 2020, 39, 2358–2376. [Google Scholar] [CrossRef]
- Maxwell, S.A.; Rivera, A. Proline Oxidase Induces Apoptosis in Tumor Cells, and Its Expression Is Frequently Absent or Reduced in Renal Carcinomas. J. Biol. Chem. 2003, 278, 9784–9789. [Google Scholar] [CrossRef]
- Polyak, K.; Xia, Y.; Zweier, J.L.; Kinzler, K.W.; Vogelstein, B. A Model for P53-Induced Apoptosis. Nature 1997, 389, 300–305. [Google Scholar] [CrossRef]
- Kay, E.J.; Paterson, K.; Riera-Domingo, C.; Sumpton, D.; Däbritz, J.H.M.; Tardito, S.; Boldrini, C.; Hernandez-Fernaud, J.R.; Athineos, D.; Dhayade, S.; et al. Cancer-Associated Fibroblasts Require Proline Synthesis by PYCR1 for the Deposition of pro-Tumorigenic Extracellular Matrix. Nat. Metab. 2022, 4, 693–710. [Google Scholar] [CrossRef]
- Du, S.; Sui, Y.; Ren, W.; Zhou, J.; Du, C. PYCR1 Promotes Bladder Cancer by Affecting the Akt/Wnt/β-Catenin Signaling. J. Bioenerg. Biomembr. 2021, 53, 247–258. [Google Scholar] [CrossRef]
- Wang, H.; Xu, M.; Zhang, T.; Pan, J.; Li, C.; Pan, B.; Zhou, L.; Huang, Y.; Gao, C.; He, M.; et al. PYCR1 Promotes Liver Cancer Cell Growth and Metastasis by Regulating IRS1 Expression through Lactylation Modification. Clin. Transl. Med. 2024, 14, e70045. [Google Scholar] [CrossRef]
- Zheng, K.; Sha, N.; Hou, G.; Leng, Z.; Zhao, Q.; Zhang, L.; He, L.; Xu, M.; Jiang, Y.; Chen, T. IGF1R-Phosphorylated PYCR1 Facilitates ELK4 Transcriptional Activity and Sustains Tumor Growth under Hypoxia. Nat. Commun. 2023, 14, 6117. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, X.; Wang, E.; Yang, Y.; Hu, L.; Xu, H.; Zhang, B. PYCR1 Promotes the Malignant Progression of Lung Cancer through the JAK-STAT3 Signaling Pathway via PRODH-Dependent Glutamine Synthesize. Transl. Oncol. 2023, 32, 101667. [Google Scholar] [CrossRef]
- PYCR1 Expresses in Cancer-Associated Fibroblasts and Accelerates the Progression of C6 Glioblastoma. Histol. Histopathol. 2024, 40, 89–100. [CrossRef]
- Li, Z.; Zhou, X.; Huang, J.; Xu, Z.; Xing, C.; Yang, J.; Zhou, X. MicroRNA Hsa-miR-150-5p Inhibits Nasopharyngeal Carcinogenesis by Suppressing PYCR1 (Pyrroline-5-Carboxylate Reductase 1). Bioengineered 2021, 12, 9766–9778. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; He, Y.; Lin, L.; Li, M.; Zhou, Z.; Yang, Y. MiR-1207-5p Targets PYCR1 to Inhibit the Progression of Prostate Cancer. Biochem. Biophys. Res. Commun. 2021, 575, 56–64. [Google Scholar] [CrossRef]
- Wang, H.; Mao, W.; Lou, W.; Jin, D.; Wu, W.; Wang, D.; Kuang, T.; Rong, Y.; Xu, X.; Zhang, L. PYCR1: A Potential Prognostic Biomarker in Pancreatic Ductal Adenocarcinoma. J. Cancer 2022, 13, 1501–1511. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Song, Y.; Ye, Y.; He, S.; Ma, X.; Zhang, M.; Ni, J.; Wang, J.; Xia, W. PYCR1 Interference Inhibits Cell Growth and Survival via C-Jun N-Terminal Kinase/Insulin Receptor Substrate 1 (JNK/IRS1) Pathway in Hepatocellular Cancer. J. Transl. Med. 2019, 17, 343. [Google Scholar] [CrossRef] [PubMed]
- Hollinshead, K.E.R.; Munford, H.; Eales, K.L.; Bardella, C.; Li, C.; Escribano-Gonzalez, C.; Thakker, A.; Nonnenmacher, Y.; Kluckova, K.; Jeeves, M.; et al. Oncogenic IDH1 Mutations Promote Enhanced Proline Synthesis through PYCR1 to Support the Maintenance of Mitochondrial Redox Homeostasis. Cell Rep. 2018, 22, 3107–3114. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Cui, C.; Zhang, K.; Wang, J.; Wang, Y.; Lu, Y.; Chen, K.; Yuan, J.; Xiao, G.; Tang, B.; et al. Kindlin-2 Links Mechano-Environment to Proline Synthesis and Tumor Growth. Nat. Commun. 2019, 10, 845. [Google Scholar] [CrossRef]
- Loayza-Puch, F.; Rooijers, K.; Buil, L.C.M.; Zijlstra, J.; Oude Vrielink, J.F.; Lopes, R.; Ugalde, A.P.; Van Breugel, P.; Hofland, I.; Wesseling, J.; et al. Tumour-Specific Proline Vulnerability Uncovered by Differential Ribosome Codon Reading. Nature 2016, 530, 490–494. [Google Scholar] [CrossRef]
- Park, J.M.; Su, Y.-H.; Fan, C.-S.; Chen, H.-H.; Qiu, Y.-K.; Chen, L.-L.; Chen, H.-A.; Ramasamy, T.S.; Chang, J.-S.; Huang, S.-Y.; et al. Crosstalk between FTH1 and PYCR1 Dysregulates Proline Metabolism and Mediates Cell Growth in KRAS-Mutant Pancreatic Cancer Cells. Exp. Mol. Med. 2024, 56, 2065–2081. [Google Scholar] [CrossRef]
- Fang, K.; Sun, M.; Leng, Z.; Chu, Y.; Zhao, Z.; Li, Z.; Zhang, Y.; Xu, A.; Zhang, Z.; Zhang, L.; et al. Targeting IGF1R Signaling Enhances the Sensitivity of Cisplatin by Inhibiting Proline and Arginine Metabolism in Oesophageal Squamous Cell Carcinoma under Hypoxia. J. Exp. Clin. Cancer Res. 2023, 42, 73. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; He, B.; Huang, Y.; Wang, C.; Luo, H.; Lu, J.; Su, K.; Zhang, X.; Luo, Y.; Zhao, Z.; et al. Pyrroline-5-Carboxylate Reductase 1 Reprograms Proline Metabolism to Drive Breast Cancer Stemness under Psychological Stress. Cell Death Dis. 2023, 14, 682. [Google Scholar] [CrossRef]
- Zeng, T.; Zhu, L.; Liao, M.; Zhuo, W.; Yang, S.; Wu, W.; Wang, D. Knockdown of PYCR1 Inhibits Cell Proliferation and Colony Formation via Cell Cycle Arrest and Apoptosis in Prostate Cancer. Med. Oncol. 2017, 34, 27. [Google Scholar] [CrossRef]
- Yan, K.; Xu, X.; Wu, T.; Li, J.; Cao, G.; Li, Y.; Ji, Z. Knockdown of PYCR1 Inhibits Proliferation, Drug Resistance and EMT in Colorectal Cancer Cells by Regulating STAT3-Mediated P38 MAPK and NF-κB Signalling Pathway. Biochem. Biophys. Res. Commun. 2019, 520, 486–491. [Google Scholar] [CrossRef]
- Wang, Q.-L.; Liu, L. PYCR1 Is Associated with Papillary Renal Cell Carcinoma Progression. Open Med. (Wars) 2019, 14, 586–592. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, X.; Wang, S.; Wang, Y.; Ji, C.; Yao, L.; Song, N. PYCR1 Regulates Glutamine Metabolism to Construct an Immunosuppressive Microenvironment for the Progression of Clear Cell Renal Cell Carcinoma. Am. J. Cancer Res. 2022, 12, 3780–3798. [Google Scholar]
- Wang, D.; Deng, Z.; Lu, M.; Deng, K.; Li, Z.; Zhou, F. Integrated Analysis of the Roles of Oxidative Stress Related Genes and Prognostic Value in Clear Cell Renal Cell Carcinoma. J. Cancer Res. Clin. Oncol. 2023, 149, 11057–11071. [Google Scholar] [CrossRef]
- Ding, J.; Kuo, M.-L.; Su, L.; Xue, L.; Luh, F.; Zhang, H.; Wang, J.; Lin, T.G.; Zhang, K.; Chu, P.; et al. Human Mitochondrial Pyrroline-5-Carboxylate Reductase 1 Promotes Invasiveness and Impacts Survival in Breast Cancers. Carcinogenesis 2017, 38, 519–531. [Google Scholar] [CrossRef]
- Weijin, F.; Zhibin, X.; Shengfeng, Z.; Xiaoli, Y.; Qijian, D.; Jiayi, L.; Qiumei, L.; Yilong, C.; Hua, M.; Deyun, L.; et al. The Clinical Significance of PYCR1 Expression in Renal Cell Carcinoma. Medicine 2019, 98, e16384. [Google Scholar] [CrossRef]
- Sang, S.; Zhang, C.; Shan, J. Pyrroline-5-Carboxylate Reductase 1 Accelerates the Migration and Invasion of Nonsmall Cell Lung Cancer In Vitro. Cancer Biother. Radiopharm. 2019, 34, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, L.; Zhang, Y.; Yan, Z.; Liu, L.; Chen, G. PYCR1 Promotes the Progression of Non-Small-Cell Lung Cancer under the Negative Regulation of miR-488. Biomed. Pharmacother. 2019, 111, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.; Miao, Y.; Liu, C.; Wu, T.; Shen, S.; Su, X.; Shi, Y. Pyrroline-5-carboxylate Reductase 1 Promotes Proliferation and Inhibits Apoptosis in Non-small Cell Lung Cancer. Oncol. Lett. 2017, 15, 731–740. [Google Scholar] [CrossRef]
- Ye, Y.; Wu, Y.; Wang, J. Pyrroline-5-Carboxylate Reductase 1 Promotes Cell Proliferation via Inhibiting Apoptosis in Human Malignant Melanoma. Cancer Manag. Res. 2018, 10, 6399–6407. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.-J.; Tong, Y.-Y.; Wu, L.-K.; Wang, Y.-C.; Teng, X.-S.; Yao, J. Circ_0000705 Facilitates Proline Metabolism of Esophageal Squamous Cell Carcinoma Cells by Targeting miR-621/PYCR1 Axis. Discov. Oncol. 2022, 13, 50. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J.; Bao, P.; Wei, Z.; Pan, L.; Zhou, J.; Wang, W. Survival and Clinicopathological Significance of PYCR1 Expression in Cancer: A Meta-Analysis. Front. Oncol. 2022, 12, 985613. [Google Scholar] [CrossRef]
- Chen, S.; Yang, X.; Yu, M.; Wang, Z.; Liu, B.; Liu, M.; Liu, L.; Ren, M.; Qi, H.; Zou, J.; et al. SIRT3 Regulates Cancer Cell Proliferation through Deacetylation of PYCR1 in Proline Metabolism. Neoplasia 2019, 21, 665–675. [Google Scholar] [CrossRef]
- Jariwala, U.; Prescott, J.; Jia, L.; Barski, A.; Pregizer, S.; Cogan, J.P.; Arasheben, A.; Tilley, W.D.; Scher, H.I.; Gerald, W.L.; et al. Identification of Novel Androgen Receptor Target Genes in Prostate Cancer. Mol. Cancer 2007, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-L.; Chou, H.-Y.; Chiu, Y.-C.; Cheng, A.N.; Fan, C.-C.; Chang, Y.-N.; Chen, C.-H.; Jiang, S.S.; Chen, N.-J.; Lee, A.Y.-L. Mitochondrial Oxidative Stress by Lon-PYCR1 Maintains an Immunosuppressive Tumor Microenvironment That Promotes Cancer Progression and Metastasis. Cancer Letters 2020, 474, 138–150. [Google Scholar] [CrossRef]
- Guo, L.; Cui, C.; Wang, J.; Yuan, J.; Yang, Q.; Zhang, P.; Su, W.; Bao, R.; Ran, J.; Wu, C. PINCH-1 Regulates Mitochondrial Dynamics to Promote Proline Synthesis and Tumor Growth. Nat. Commun. 2020, 11, 4913. [Google Scholar] [CrossRef]
- Oudaert, I.; Satilmis, H.; Vlummens, P.; De Brouwer, W.; Maes, A.; Hose, D.; De Bruyne, E.; Ghesquière, B.; Vanderkerken, K.; De Veirman, K.; et al. Pyrroline-5-Carboxylate Reductase 1: A Novel Target for Sensitizing Multiple Myeloma Cells to Bortezomib by Inhibition of PRAS40-Mediated Protein Synthesis. J. Exp. Clin. Cancer Res. 2022, 41, 45. [Google Scholar] [CrossRef]
- You, C.; He, J.; Cao, C.; Sheng, D.; Wang, L.; Huang, Z.; Zhang, X.; Yi, C.; Sun, Y.; Huang, Y. PYCR1 Regulates TRAIL-resistance in Non-small Cell Lung Cancer Cells by Regulating the Redistribution of Death Receptors. Oncol. Lett. 2024, 27, 216. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Huang, J.; Wu, H.; Li, Y.; Wang, Y. Pro-Tumorigenic Activity of PYCR1 in Gastric Cancer through Regulating the PI3K/AKT Signaling. Heliyon 2024, 10, e26883. [Google Scholar] [CrossRef]
- Xiao, S.; Chen, J.; Wei, Y.; Song, W. BHLHE41 Inhibits Bladder Cancer Progression via Regulation of PYCR1 Stability and Thus Inactivating PI3K/AKT Signaling Pathway. Eur. J. Med. Res. 2024, 29, 302. [Google Scholar] [CrossRef] [PubMed]
- Sawicka, M.M.; Sawicki, K.; Jadeszko, M.; Bielawska, K.; Supruniuk, E.; Reszeć, J.; Prokop-Bielenia, I.; Polityńska, B.; Jadeszko, M.; Rybaczek, M.; et al. Proline Metabolism in WHO G4 Gliomas Is Altered as Compared to Unaffected Brain Tissue. Cancers 2024, 16, 456. [Google Scholar] [CrossRef]
- Alaqbi, S.S.; Burke, L.; Guterman, I.; Green, C.; West, K.; Palacios-Gallego, R.; Cai, H.; Alexandrou, C.; Myint, N.N.M.; Parrott, E.; et al. Increased Mitochondrial Proline Metabolism Sustains Proliferation and Survival of Colorectal Cancer Cells. PLoS ONE 2022, 17, e0262364. [Google Scholar] [CrossRef]
- Choi, U.Y.; Lee, J.J.; Park, A.; Zhu, W.; Lee, H.-R.; Choi, Y.J.; Yoo, J.-S.; Yu, C.; Feng, P.; Gao, S.-J.; et al. Oncogenic Human Herpesvirus Hijacks Proline Metabolism for Tumorigenesis. Proc. Natl. Acad. Sci. USA 2020, 117, 8083–8093. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Q.; Chen, J.; Mao, Y.; Duan, L.; Ye, D.; Cheng, W.; Chen, J.; Gao, X.; Lin, R.; et al. Deciphering the Effects of the PYCR Family on Cell Function, Prognostic Value, Immune Infiltration in ccRCC and Pan-Cancer. Int. J. Mol. Sci. 2024, 25, 8096. [Google Scholar] [CrossRef] [PubMed]
- Pilley, S.E.; Hennequart, M.; Vandekeere, A.; Blagih, J.; Legrave, N.M.; Fendt, S.-M.; Vousden, K.H.; Labuschagne, C.F. Loss of Attachment Promotes Proline Accumulation and Excretion in Cancer Cells. Sci. Adv. 2023, 9, eadh2023. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, X.; Wei, X.; Feng, H.; Hu, B.; Deng, Z.; Liu, B.; Luan, Y.; Ruan, Y.; Liu, X.; et al. A Mitochondrial Dysfunction and Oxidative Stress Pathway-Based Prognostic Signature for Clear Cell Renal Cell Carcinoma. Oxid. Med. Cell Longev. 2021, 2021, 9939331. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Du, X.; Jia, W.; Feng, K.; Zhang, Y. Engineered Extracellular Vesicles for Targeted Reprogramming of Cancer-Associated Fibroblasts to Potentiate Therapy of Pancreatic Cancer. Sig Transduct. Target. Ther. 2024, 9, 151. [Google Scholar] [CrossRef]
- Wang, S.; Yi, W.; Xu, Z.; Shi, M. PYCR2 Promotes Growth and Aerobic Glycolysis in Human Liver Cancer by Regulating the AKT Signaling Pathway. Biochem. Biophys. Res. Commun. 2023, 680, 15–24. [Google Scholar] [CrossRef]
- Wang, S.; Gu, L.; Huang, L.; Fang, J.; Liu, Z.; Xu, Q. The Upregulation of PYCR2 Is Associated with Aggressive Colon Cancer Progression and a Poor Prognosis. Biochem. Biophys. Res. Commun. 2021, 572, 20–26. [Google Scholar] [CrossRef]
- Zhang, Q.; Luo, H.; Xun, J.; Ma, Y.; Yang, L.; Zhang, L.; Wang, X.; Yu, X.; Wang, B. Targeting PYCR2 Inhibits Intraperitoneal Metastatic Tumors of Mouse Colorectal Cancer in a Proline-Independent Approach. Cancer Sci. 2023, 114, 908–920. [Google Scholar] [CrossRef]
- Lama Tamang, R.; Kumar, B.; Patel, S.M.; Thapa, I.; Ahmad, A.; Kumar, V.; Ahmad, R.; Becker, D.F.; Bastola, D.; Dhawan, P.; et al. Pyrroline-5-Carboxylate Reductase-2 Promotes Colorectal Carcinogenesis by Modulating Microtubule-Associated Serine/Threonine Kinase-like/Wnt/β-Catenin Signaling. Cells 2023, 12, 1883. [Google Scholar] [CrossRef]
- Wu, G.; Qin, S.; Gu, K.; Zhou, Y. PYCR2, Induced by c-Myc, Promotes the Invasiveness and Metastasis of Breast Cancer by Activating AKT Signalling Pathway. Int. J. Biochem. Cell Biol. 2024, 166, 106506. [Google Scholar] [CrossRef]
- Li, L.; Yang, M.; Pu, X.; Tang, Y.; Fei, F.; Li, Z.; Hou, H.; Chen, Q.; Wang, Q.; Wu, Y.; et al. ALKBH5-PYCR2 Positive Feedback Loop Promotes Proneural-Mesenchymal Transition Via Proline Synthesis In GBM. J. Cancer 2023, 14, 1579–1591. [Google Scholar] [CrossRef]
- Ou, R.; Zhang, X.; Cai, J.; Shao, X.; Lv, M.; Qiu, W.; Xuan, X.; Liu, J.; Li, Z.; Xu, Y. Downregulation of Pyrroline-5-Carboxylate Reductase-2 Induces the Autophagy of Melanoma Cells via AMPK/mTOR Pathway. Tumour Biol. 2016, 37, 6485–6491. [Google Scholar] [CrossRef]
- Zhuang, F.; Huang, S.; Liu, L. PYCR3 Modulates mtDNA Copy Number to Drive Proliferation and Doxorubicin Resistance in Triple-Negative Breast Cancer. Int. J. Biochem. Cell Biol. 2024, 171, 106581. [Google Scholar] [CrossRef]
- Sahu, N.; Dela Cruz, D.; Gao, M.; Sandoval, W.; Haverty, P.M.; Liu, J.; Stephan, J.-P.; Haley, B.; Classon, M.; Hatzivassiliou, G.; et al. Proline Starvation Induces Unresolved ER Stress and Hinders mTORC1-Dependent Tumorigenesis. Cell Metab. 2016, 24, 753–761. [Google Scholar] [CrossRef]
- Xi, X.; Zhang, M.; Li, Y.; Wang, X. Identification of PRODH as a Mitochondria- and Angiogenesis-Related Biomarker for Lung Adenocarcinoma. Transl. Cancer Res. 2024, 13, 2073–2093. [Google Scholar] [CrossRef] [PubMed]
- Olivares, O.; Mayers, J.R.; Gouirand, V.; Torrence, M.E.; Gicquel, T.; Borge, L.; Lac, S.; Roques, J.; Lavaut, M.-N.; Berthezène, P.; et al. Collagen-Derived Proline Promotes Pancreatic Ductal Adenocarcinoma Cell Survival under Nutrient Limited Conditions. Nat. Commun. 2017, 8, 16031. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Glunde, K.; Bhujwalla, Z.M.; Raman, V.; Sharma, A.; Phang, J.M. Proline Oxidase Promotes Tumor Cell Survival in Hypoxic Tumor Microenvironments. Cancer Res. 2012, 72, 3677–3686. [Google Scholar] [CrossRef]
- Lee, W.-J.; Cheng, T.-C.; Yen, Y.; Fang, C.-L.; Liao, Y.-C.; Kuo, C.-C.; Tu, S.-H.; Lin, L.-C.; Chang, H.-W.; Chen, L.-C.; et al. Tea Polyphenol Epigallocatechin-3-Gallate Inhibits Cell Proliferation in a Patient-Derived Triple-Negative Breast Cancer Xenograft Mouse Model via Inhibition of Proline-Dehydrogenase-Induced Effects. J. Food Drug Anal. 2021, 29, 113–127. [Google Scholar] [CrossRef]
- Zabirnyk, O.; Liu, W.; Khalil, S.; Sharma, A.; Phang, J.M. Oxidized Low-Density Lipoproteins Upregulate Proline Oxidase to Initiate ROS-Dependent Autophagy. Carcinogenesis 2010, 31, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Chang, L.; Tian, H.; Wang, L.; Zhang, Y.; Yang, T.; Li, G.; Hu, W.; Shah, K.; Chen, G.; et al. 1-Pyrroline-5-Carboxylate Released by Prostate Cancer Cell Inhibit T Cell Proliferation and Function by Targeting SHP1/Cytochrome c Oxidoreductase/ROS Axis. J. Immunother. Cancer 2018, 6, 148. [Google Scholar] [CrossRef]
- Liu, W.; Le, A.; Hancock, C.; Lane, A.N.; Dang, C.V.; Fan, T.W.-M.; Phang, J.M. Reprogramming of Proline and Glutamine Metabolism Contributes to the Proliferative and Metabolic Responses Regulated by Oncogenic Transcription Factor C-MYC. Proc. Natl. Acad. Sci. USA 2012, 109, 8983–8988. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, F.; Liu, X.; Wu, X.; Hu, C.; Guo, J.; Yang, Q.; Xia, J.; He, Y.; An, G.; et al. Proline Promotes Proliferation and Drug Resistance of Multiple Myeloma by Downregulation of Proline Dehydrogenase. Br. J. Haematol. 2023, 201, 704–717. [Google Scholar] [CrossRef]
- Tołoczko-Iwaniuk, N.; Dziemiańczyk-Pakieła, D.; Celińska-Janowicz, K.; Zaręba, I.; Klupczyńska, A.; Kokot, Z.J.; Nowaszewska, B.K.; Reszeć, J.; Borys, J.; Miltyk, W. Proline-Dependent Induction of Apoptosis in Oral Squamous Cell Carcinoma (OSCC)-The Effect of Celecoxib. Cancers 2020, 12, 136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Qian, N.; Lai, H.; Chen, S.; Wu, K.; Luo, X.; Lei, B.; Liu, M.; Cui, J. PRODH Regulates Tamoxifen Resistance through Ferroptosis in Breast Cancer Cells. Genes 2024, 15, 1316. [Google Scholar] [CrossRef]
- Pandhare, J.; Cooper, S.K.; Phang, J.M. Proline Oxidase, a Proapoptotic Gene, Is Induced by Troglitazone. J. Biol. Chem. 2006, 281, 2044–2052. [Google Scholar] [CrossRef]
- Maxwell, S.A.; Davis, G.E. Differential Gene Expression in P53-Mediated Apoptosis-Resistant vs. Apoptosis-Sensitive Tumor Cell Lines. Proc. Natl. Acad. Sci. USA 2000, 97, 13009–13014. [Google Scholar] [CrossRef]
- Donald, S.P.; Sun, X.Y.; Hu, C.A.; Yu, J.; Mei, J.M.; Valle, D.; Phang, J.M. Proline Oxidase, Encoded by P53-Induced Gene-6, Catalyzes the Generation of Proline-Dependent Reactive Oxygen Species. Cancer Res. 2001, 61, 1810–1815. [Google Scholar] [PubMed]
- Liu, Y.; Borchert, G.L.; Donald, S.P.; Surazynski, A.; Hu, C.-A.; Weydert, C.J.; Oberley, L.W.; Phang, J.M. MnSOD Inhibits Proline Oxidase-Induced Apoptosis in Colorectal Cancer Cells. Carcinogenesis 2005, 26, 1335–1342. [Google Scholar] [CrossRef]
- Liu, Y.; Borchert, G.L.; Surazynski, A.; Hu, C.-A.; Phang, J.M. Proline Oxidase Activates Both Intrinsic and Extrinsic Pathways for Apoptosis: The Role of ROS/Superoxides, NFAT and MEK/ERK Signaling. Oncogene 2006, 25, 5640–5647. [Google Scholar] [CrossRef]
- Kim, K.Y.; Ahn, J.H.; Cheon, H.G. Apoptotic Action of Peroxisome Proliferator-Activated Receptor-Gamma Activation in Human Non Small-Cell Lung Cancer Is Mediated via Proline Oxidase-Induced Reactive Oxygen Species Formation. Mol. Pharmacol. 2007, 72, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Borchert, G.L.; Donald, S.P.; Diwan, B.A.; Anver, M.; Phang, J.M. Proline Oxidase Functions as a Mitochondrial Tumor Suppressor in Human Cancers. Cancer Res. 2009, 69, 6414–6422. [Google Scholar] [CrossRef] [PubMed]
- Kazberuk, A.; Chalecka, M.; Palka, J.; Surazynski, A. Nonsteroidal Anti-Inflammatory Drugs as PPARγ Agonists Can Induce PRODH/POX-Dependent Apoptosis in Breast Cancer Cells: New Alternative Pathway in NSAID-Induced Apoptosis. Int. J. Mol. Sci. 2022, 23, 1510. [Google Scholar] [CrossRef] [PubMed]
- Oscilowska, I.; Rolkowski, K.; Baszanowska, W.; Huynh, T.Y.L.; Lewoniewska, S.; Nizioł, M.; Sawicka, M.; Bielawska, K.; Szoka, P.; Miltyk, W.; et al. Proline Dehydrogenase/Proline Oxidase (PRODH/POX) Is Involved in the Mechanism of Metformin-Induced Apoptosis in C32 Melanoma Cell Line. Int. J. Mol. Sci. 2022, 23, 2354. [Google Scholar] [CrossRef]
- Elia, I.; Broekaert, D.; Christen, S.; Boon, R.; Radaelli, E.; Orth, M.F.; Verfaillie, C.; Grünewald, T.G.P.; Fendt, S.-M. Proline Metabolism Supports Metastasis Formation and Could Be Inhibited to Selectively Target Metastasizing Cancer Cells. Nat. Commun. 2017, 8, 15267. [Google Scholar] [CrossRef]
- Zareba, I.; Celinska-Janowicz, K.; Surazynski, A.; Miltyk, W.; Palka, J. Proline Oxidase Silencing Induces Proline-Dependent pro-Survival Pathways in MCF-7 Cells. Oncotarget 2018, 9, 13748–13757. [Google Scholar] [CrossRef]
- Zareba, I.; Surazynski, A.; Chrusciel, M.; Miltyk, W.; Doroszko, M.; Rahman, N.; Palka, J. Functional Consequences of Intracellular Proline Levels Manipulation Affecting PRODH/POX-Dependent Pro-Apoptotic Pathways in a Novel in Vitro Cell Culture Model. Cell Physiol. Biochem. 2017, 43, 670–684. [Google Scholar] [CrossRef]
- Scott, G.K.; Yau, C.; Becker, B.C.; Khateeb, S.; Mahoney, S.; Jensen, M.B.; Hann, B.; Cowen, B.J.; Pegan, S.D.; Benz, C.C. Targeting Mitochondrial Proline Dehydrogenase with a Suicide Inhibitor to Exploit Synthetic Lethal Interactions with P53 Upregulation and Glutaminase Inhibition. Mol. Cancer Ther. 2019, 18, 1374–1385. [Google Scholar] [CrossRef]
- Liu, Y.; Borchert, G.L.; Surazynski, A.; Phang, J.M. Proline Oxidase, a P53-Induced Gene, Targets COX-2/PGE2 Signaling to Induce Apoptosis and Inhibit Tumor Growth in Colorectal Cancers. Oncogene 2008, 27, 6729–6737. [Google Scholar] [CrossRef]
- Liu, W.; Zabirnyk, O.; Wang, H.; Shiao, Y.-H.; Nickerson, M.L.; Khalil, S.; Anderson, L.M.; Perantoni, A.O.; Phang, J.M. miR-23b Targets Proline Oxidase, a Novel Tumor Suppressor Protein in Renal Cancer. Oncogene 2010, 29, 4914–4924. [Google Scholar] [CrossRef]
- Kazberuk, A.; Chalecka, M.; Palka, J.; Bielawska, K.; Surazynski, A. NSAIDs Induce Proline Dehydrogenase/Proline Oxidase-Dependent and Independent Apoptosis in MCF7 Breast Cancer Cells. Int. J. Mol. Sci. 2022, 23, 3813. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Wang, J.; Guo, L.; Wu, C. PINCH-1 Promotes Δ1-Pyrroline-5-Carboxylate Synthase Expression and Contributes to Proline Metabolic Reprogramming in Lung Adenocarcinoma. Amino Acids 2021, 53, 1875–1890. [Google Scholar] [CrossRef]
- Hamanaka, R.B.; O’Leary, E.M.; Witt, L.J.; Tian, Y.; Gökalp, G.A.; Meliton, A.Y.; Dulin, N.O.; Mutlu, G.M. Glutamine Metabolism Is Required for Collagen Protein Synthesis in Lung Fibroblasts. Am. J. Respir. Cell Mol. Biol. 2019, 61, 597–606. [Google Scholar] [CrossRef]
- Linder, S.J.; Bernasocchi, T.; Martínez-Pastor, B.; Sullivan, K.D.; Galbraith, M.D.; Lewis, C.A.; Ferrer, C.M.; Boon, R.; Silveira, G.G.; Cho, H.M.; et al. Inhibition of the Proline Metabolism Rate-Limiting Enzyme P5CS Allows Proliferation of Glutamine-Restricted Cancer Cells. Nat. Metab. 2023, 5, 2131–2147. [Google Scholar] [CrossRef]
- Bai, J.; Liu, T.; Tu, B.; Yuan, M.; Shu, Z.; Fan, M.; Huo, S.; Guo, Y.; Wang, L.; Wang, H.; et al. Autophagy Loss Impedes Cancer-Associated Fibroblast Activation via Downregulating Proline Biosynthesis. Autophagy 2023, 19, 632–643. [Google Scholar] [CrossRef]
- Fang, Q.-Y.; Wang, Y.-P.; Zhang, R.-Q.; Fan, M.; Feng, L.-X.; Guo, X.-D.; Cheng, C.-R.; Zhang, X.-W.; Liu, X. Carnosol Ameliorated Cancer Cachexia-Associated Myotube Atrophy by Targeting P5CS and Its Downstream Pathways. Front. Pharmacol. 2023, 14, 1291194. [Google Scholar] [CrossRef] [PubMed]
- Kardos, G.R.; Wastyk, H.C.; Robertson, G.P. Disruption of Proline Synthesis in Melanoma Inhibits Protein Production Mediated by the GCN2 Pathway. Mol. Cancer Res. 2015, 13, 1408–1420. [Google Scholar] [CrossRef]
- Possemato, R.; Marks, K.M.; Shaul, Y.D.; Pacold, M.E.; Kim, D.; Birsoy, K.; Sethumadhavan, S.; Woo, H.-K.; Jang, H.G.; Jha, A.K.; et al. Functional Genomics Reveal That the Serine Synthesis Pathway Is Essential in Breast Cancer. Nature 2011, 476, 346–350. [Google Scholar] [CrossRef]
- Aken, B.L.; Achuthan, P.; Akanni, W.; Amode, M.R.; Bernsdorff, F.; Bhai, J.; Billis, K.; Carvalho-Silva, D.; Cummins, C.; Clapham, P.; et al. Ensembl 2017. Nucleic Acids Res. 2017, 45, D635–D642. [Google Scholar] [CrossRef] [PubMed]
- Saha, M.; Deshpande, N.; Dubey, A.; Pal, D.; Atreya, H.S.; Rangarajan, A. Sustained AMPK Activation and Proline Metabolism Play Critical Roles in the Survival of Matrix-Deprived Transformed Cells. Front. Cell Dev. Biol. 2021, 9, 771366. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Tchernyshyov, I.; Chang, T.-C.; Lee, Y.-S.; Kita, K.; Ochi, T.; Zeller, K.I.; De Marzo, A.M.; Van Eyk, J.E.; Mendell, J.T.; et al. C-Myc Suppression of miR-23a/b Enhances Mitochondrial Glutaminase Expression and Glutamine Metabolism. Nature 2009, 458, 762–765. [Google Scholar] [CrossRef]
- Ryu, K.W.; Fung, T.S.; Baker, D.C.; Saoi, M.; Park, J.; Febres-Aldana, C.A.; Aly, R.G.; Cui, R.; Sharma, A.; Fu, Y.; et al. Cellular ATP Demand Creates Metabolically Distinct Subpopulations of Mitochondria. Nature 2024, 635, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Bogner, A.N.; Tanner, J.J. Structure-Affinity Relationships of Reversible Proline Analog Inhibitors Targeting Proline Dehydrogenase. Org. Biomol. Chem. 2022, 20, 895–905. [Google Scholar] [CrossRef]
- Smith, M.E.; Greenberg, D.M. Preparation and properties of partially purified glutamic semialdehyde reductase. J. Biol. Chem. 1957, 226, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Kowaloff, E.M.; Phang, J.M.; Granger, A.S.; Downing, S.J. Regulation of Proline Oxidase Activity by Lactate. Proc. Natl. Acad. Sci. USA 1977, 74, 5368–5371. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Gincherman, Y.; Docherty, P.; Spilling, C.D.; Becker, D.F. Effects of Proline Analog Binding on the Spectroscopic and Redox Properties of PutA. Arch. Biochem. Biophys. 2002, 408, 131–136. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Nadaraia, S.; Gu, D.; Becker, D.F.; Tanner, J.J. Structure of the Proline Dehydrogenase Domain of the Multifunctional PutA Flavoprotein. Nat. Struct. Biol. 2003, 10, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.A.; Lin, W.-W.; Obie, C.; Valle, D. Molecular Enzymology of Mammalian Δ1-Pyrroline-5-Carboxylate Synthase. J. Biol. Chem. 1999, 274, 6754–6762. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, X.; Shang, W.; Liu, Y.; Ji, J.-F.; Liu, J.-P.; Tong, C. Pyrroline-5-Carboxylate Synthase Senses Cellular Stress and Modulates Metabolism by Regulating Mitochondrial Respiration. Cell Death Differ. 2021, 28, 303–319. [Google Scholar] [CrossRef]
- Phang, J.M.; Downing, S.J.; Yeh, G.C. Linkage of the HMP Pathway to ATP Generation by the Proline Cycle. Biochem. Biophys. Res. Commun. 1980, 93, 462–470. [Google Scholar] [CrossRef]
- Hagedorn, C.H.; Phang, J.M. Transfer of Reducing Equivalents into Mitochondria by the Interconversions of Proline and Δ1-Pyrroline-5-Carboxylate. Arch. Biochem. Biophys. 1983, 225, 95–101. [Google Scholar] [CrossRef]
- Hagedorn, C.H.; Phang, J.M. Catalytic Transfer of Hydride Ions from NADPH to Oxygen by the Interconversions of Proline and Δ1-Pyrroline-5-Carboxylate. Arch. Biochem. Biophys. 1986, 248, 166–174. [Google Scholar] [CrossRef]
- Hancock, C.N.; Liu, W.; Alvord, W.G.; Phang, J.M. Co-Regulation of Mitochondrial Respiration by Proline Dehydrogenase/Oxidase and Succinate. Amino Acids 2016, 48, 859–872. [Google Scholar] [CrossRef]
- Phang, J.M.; Yeh, G.C.; Hagedorn, C.H. The Intercellular Proline Cycle. Life Sci. 1981, 28, 53–58. [Google Scholar] [CrossRef]
- Baffy, G.; Derdak, Z.; Robson, S.C. Mitochondrial Recoupling: A Novel Therapeutic Strategy for Cancer? Br. J. Cancer 2011, 105, 469–474. [Google Scholar] [CrossRef]
- Nagano, T.; Nakashima, A.; Onishi, K.; Kawai, K.; Awai, Y.; Kinugasa, M.; Iwasaki, T.; Kikkawa, U.; Kamada, S. Proline Dehydrogenase Promotes Senescence through the Generation of Reactive Oxygen Species. J. Cell Sci. 2017, 130, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, Q.; Chen, W.; Gong, Z.; Kang, B.; Sui, M.; Huang, L.; Wang, Y.-J. PRODH Safeguards Human Naive Pluripotency by Limiting Mitochondrial Oxidative Phosphorylation and Reactive Oxygen Species Production. EMBO Rep. 2024, 25, 2015–2044. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, S.K.; Zhu, W.; Liang, X.; Zhang, L.; Demers, A.J.; Zimmerman, M.C.; Simpson, M.A.; Becker, D.F. Proline Dehydrogenase Is Essential for Proline Protection against Hydrogen Peroxide-Induced Cell Death. Free. Radic. Biol. Med. 2012, 53, 1181–1191. [Google Scholar] [CrossRef]
- Phang, J.M. Proline Metabolism in Cell Regulation and Cancer Biology: Recent Advances and Hypotheses. Antioxid. Redox Signal. 2019, 30, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, R.L.S.; Rothschild, D.E.; Quinlan, C.L.; Scott, G.K.; Benz, C.C.; Brand, M.D. Sources of Superoxide/H2O2 during Mitochondrial Proline Oxidation. Redox Biol. 2014, 2, 901–909. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Chandel, N.S. We Need to Talk about the Warburg Effect. Nat. Metab. 2020, 2, 127–129. [Google Scholar] [CrossRef]
- Epstein, A.C.R.; Gleadle, J.M.; McNeill, L.A.; Hewitson, K.S.; O’Rourke, J.; Mole, D.R.; Mukherji, M.; Metzen, E.; Wilson, M.I.; Dhanda, A.; et al. C. Elegans EGL-9 and Mammalian Homologs Define a Family of Dioxygenases That Regulate HIF by Prolyl Hydroxylation. Cell 2001, 107, 43–54. [Google Scholar] [CrossRef]
- Pugh, C.W.; Ratcliffe, P.J. Regulation of Angiogenesis by Hypoxia: Role of the HIF System. Nat. Med. 2003, 9, 677–684. [Google Scholar] [CrossRef]
- Ray, R.; Chen, G.; Vande Velde, C.; Cizeau, J.; Park, J.H.; Reed, J.C.; Gietz, R.D.; Greenberg, A.H. BNIP3 Heterodimerizes with Bcl-2/Bcl-XL and Induces Cell Death Independent of a Bcl-2 Homology 3 (BH3) Domain at Both Mitochondrial and Nonmitochondrial Sites. J. Biol. Chem. 2000, 275, 1439–1448. [Google Scholar] [CrossRef]
- Krishnamachary, B.; Berg-Dixon, S.; Kelly, B.; Agani, F.; Feldser, D.; Ferreira, G.; Iyer, N.; LaRusch, J.; Pak, B.; Taghavi, P.; et al. Regulation of Colon Carcinoma Cell Invasion by Hypoxia-Inducible Factor 1. Cancer Res. 2003, 63, 1138–1143. [Google Scholar]
- Huynh, T.Y.L.; Zareba, I.; Baszanowska, W.; Lewoniewska, S.; Palka, J. Understanding the Role of Key Amino Acids in Regulation of Proline Dehydrogenase/Proline Oxidase (Prodh/Pox)-Dependent Apoptosis/Autophagy as an Approach to Targeted Cancer Therapy. Mol. Cell Biochem. 2020, 466, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Pandhare, J.; Donald, S.P.; Cooper, S.K.; Phang, J.M. Regulation and Function of Proline Oxidase under Nutrient Stress. J. Cell. Biochem. 2009, 107, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-Antioxidant Response Element Signaling Pathway and Its Activation by Oxidative Stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef] [PubMed]
- Hyroššová, P.; Aragó, M.; Moreno-Felici, J.; Fu, X.; Mendez-Lucas, A.; García-Rovés, P.M.; Burgess, S.; Figueras, A.; Viñals, F.; Perales, J.C. PEPCK-M Recoups Tumor Cell Anabolic Potential in a PKC-ζ-Dependent Manner. Cancer Metab. 2021, 9, 1. [Google Scholar] [CrossRef]
- Oldford, C.; Kuksal, N.; Gill, R.; Young, A.; Mailloux, R.J. Estimation of the Hydrogen Peroxide Producing Capacities of Liver and Cardiac Mitochondria Isolated from C57BL/6N and C57BL/6J Mice. Free. Radic. Biol. Med. 2019, 135, 15–27. [Google Scholar] [CrossRef]
- Lewoniewska, S.; Oscilowska, I.; Forlino, A.; Palka, J. Understanding the Role of Estrogen Receptor Status in PRODH/POX-Dependent Apoptosis/Survival in Breast Cancer Cells. Biology 2021, 10, 1314. [Google Scholar] [CrossRef]
- Lu, W.; Katzenellenbogen, B.S. Estrogen Receptor-β Modulation of the ERα-P53 Loop Regulating Gene Expression, Proliferation, and Apoptosis in Breast Cancer. Horm. Cancer 2017, 8, 230–242. [Google Scholar] [CrossRef]
- Milne, K.; Sun, J.; Zaal, E.A.; Mowat, J.; Celie, P.H.N.; Fish, A.; Berkers, C.R.; Forlani, G.; Loayza-Puch, F.; Jamieson, C.; et al. A Fragment-like Approach to PYCR1 Inhibition. Bioorganic Med. Chem. Lett. 2019, 29, 2626–2631. [Google Scholar] [CrossRef] [PubMed]
- Phang, J.M.; Liu, W.; Zabirnyk, O. Proline Metabolism and Microenvironmental Stress. Annu. Rev. Nutr. 2010, 30, 441–463. [Google Scholar] [CrossRef] [PubMed]
- Hamanaka, R.B.; Mutlu, G.M. The Role of Metabolic Reprogramming and de Novo Amino Acid Synthesis in Collagen Protein Production by Myofibroblasts: Implications for Organ Fibrosis and Cancer. Amino Acids 2021, 53, 1851–1862. [Google Scholar] [CrossRef]
- Gorres, K.L.; Raines, R.T. Prolyl 4-Hydroxylase. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 106–124. [Google Scholar] [CrossRef]
- Srivastava, M.; Steinwede, K.; Kiviranta, R.; Morko, J.; Hoymann, H.-G.; Länger, F.; Buhling, F.; Welte, T.; Maus, U.A. Overexpression of Cathepsin K in Mice Decreases Collagen Deposition and Lung Resistance in Response to Bleomycin-Induced Pulmonary Fibrosis. Respir. Res. 2008, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Everts, V.; Van Der Zee, E.; Creemers, L.; Beertsen, W. Phagocytosis and Intracellular Digestion of Collagen, Its Role in Turnover and Remodelling. Histochem. J. 1996, 28, 229–245. [Google Scholar] [CrossRef]
- Madsen, D.H.; Engelholm, L.H.; Ingvarsen, S.; Hillig, T.; Wagenaar-Miller, R.A.; Kjøller, L.; Gårdsvoll, H.; Høyer-Hansen, G.; Holmbeck, K.; Bugge, T.H.; et al. Extracellular Collagenases and the Endocytic Receptor, Urokinase Plasminogen Activator Receptor-Associated Protein/Endo180, Cooperate in Fibroblast-Mediated Collagen Degradation. J. Biol. Chem. 2007, 282, 27037–27045. [Google Scholar] [CrossRef]
- Eni-Aganga, I.; Lanaghan, Z.M.; Balasubramaniam, M.; Dash, C.; Pandhare, J. PROLIDASE: A Review from Discovery to Its Role in Health and Disease. Front. Mol. Biosci. 2021, 8, 723003. [Google Scholar] [CrossRef]
- Cunningham, D.F.; O’Connor, B. Proline Specific Peptidases. Biochim. Et Biophys. Acta (BBA)-Protein Struct. Mol. Enzymol. 1997, 1343, 160–186. [Google Scholar] [CrossRef]
- Arioz, D.T.; Camuzcuoglu, H.; Toy, H.; Kurt, S.; Celik, H.; Aksoy, N. Serum Prolidase Activity and Oxidative Status in Patients With Stage I Endometrial Cancer. Int. J. Gynecol. Cancer 2009, 19, 1244–1247. [Google Scholar] [CrossRef] [PubMed]
- Wilk, P.; Wątor, E.; Weiss, M.S. Prolidase—A Protein with Many Faces. Biochimie 2021, 183, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Celik, S.; Kızıltan, R.; Yılmaz, E.M.; Yılmaz, Ö.; Demir, H. Potential Diagnostic and Prognostic Significance of Plasma Prolidase Activity in Gastric Cancer. Biomark. Med. 2017, 11, 319–327. [Google Scholar] [CrossRef]
- Cechowska-Pasko, M.; Pałka, J.; Wojtukiewicz, M.Z. Enhanced Prolidase Activity and Decreased Collagen Content in Breast Cancer Tissue. Int. J. Exp. Path 2006, 87, 289–296. [Google Scholar] [CrossRef]
- Schwörer, S.; Berisa, M.; Violante, S.; Qin, W.; Zhu, J.; Hendrickson, R.C.; Cross, J.R.; Thompson, C.B. Proline Biosynthesis Is a Vent for TGFβ-induced Mitochondrial Redox Stress. EMBO J. 2020, 39, e103334. [Google Scholar] [CrossRef]
- Shergalis, A.G.; Hu, S.; Bankhead, A.; Neamati, N. Role of the ERO1-PDI Interaction in Oxidative Protein Folding and Disease. Pharmacol. Ther. 2020, 210, 107525. [Google Scholar] [CrossRef]
- Ponrasu, T.; Jamuna, S.; Mathew, A.; Madhukumar, K.N.; Ganeshkumar, M.; Iyappan, K.; Suguna, L. Efficacy of L-Proline Administration on the Early Responses during Cutaneous Wound Healing in Rats. Amino Acids 2013, 45, 179–189. [Google Scholar] [CrossRef]
- Thangavel, P.; Ramachandran, B.; Kannan, R.; Muthuvijayan, V. Biomimetic Hydrogel Loaded with Silk and L -proline for Tissue Engineering and Wound Healing Applications. J. Biomed. Mater. Res. 2017, 105, 1401–1408. [Google Scholar] [CrossRef]
- Mehl, A.A.; Damião, A.O.; Viana, S.D.; Andretta, C.P. Hard-to-Heal Wounds: A Randomised Trial of an Oral Proline-Containing Supplement to Aid Repair. J. Wound Care 2021, 30, 26–31. [Google Scholar] [CrossRef]
- Nigdelioglu, R.; Hamanaka, R.B.; Meliton, A.Y.; O’Leary, E.; Witt, L.J.; Cho, T.; Sun, K.; Bonham, C.; Wu, D.; Woods, P.S.; et al. Transforming Growth Factor (TGF)-β Promotes de Novo Serine Synthesis for Collagen Production. J. Biol. Chem. 2016, 291, 27239–27251. [Google Scholar] [CrossRef] [PubMed]
- Sporn, M.B.; Roberts, A.B.; Shull, J.H.; Smith, J.M.; Ward, J.M.; Sodek, J. Polypeptide Transforming Growth Factors Isolated from Bovine Sources and Used for Wound Healing in Vivo. Science 1983, 219, 1329–1331. [Google Scholar] [CrossRef]
- Ignotz, R.A.; Massagué, J. Transforming Growth Factor-Beta Stimulates the Expression of Fibronectin and Collagen and Their Incorporation into the Extracellular Matrix. J. Biol. Chem. 1986, 261, 4337–4345. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.B.; Sporn, M.B.; Assoian, R.K.; Smith, J.M.; Roche, N.S.; Wakefield, L.M.; Heine, U.I.; Liotta, L.A.; Falanga, V.; Kehrl, J.H. Transforming Growth Factor Type Beta: Rapid Induction of Fibrosis and Angiogenesis in Vivo and Stimulation of Collagen Formation in Vitro. Proc. Natl. Acad. Sci. USA 1986, 83, 4167–4171. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.J.; Phang, J.M. Proline Metabolism in Cartilage: The Importance of Proline Biosynthesis. Metabolism 1978, 27, 685–694. [Google Scholar] [CrossRef]
- Torrence, M.E.; MacArthur, M.R.; Hosios, A.M.; Valvezan, A.J.; Asara, J.M.; Mitchell, J.R.; Manning, B.D. The mTORC1-Mediated Activation of ATF4 Promotes Protein and Glutathione Synthesis Downstream of Growth Signals. eLife 2021, 10, e63326. [Google Scholar] [CrossRef]
- Li, Y.; Dai, C.; Wu, C.; Liu, Y. PINCH-1 Promotes Tubular Epithelial-to-Mesenchymal Transition by Interacting with Integrin-Linked Kinase. J. Am. Soc. Nephrol. 2007, 18, 2534–2543. [Google Scholar] [CrossRef]
- Farook, M.R.; Croxford, Z.; Morgan, S.; Horlock, A.D.; Holt, A.K.; Rees, A.; Jenkins, B.J.; Tse, C.; Stanton, E.; Davies, D.M.; et al. Loss of Mitochondrial Pyruvate Carrier 1 Supports Proline-Dependent Proliferation and Collagen Biosynthesis in Ovarian Cancer. Mol. Metab. 2024, 81, 101900. [Google Scholar] [CrossRef]
- Luo, Y.; Yu, J.; Lin, Z.; Wang, X.; Zhao, J.; Liu, X.; Qin, W.; Xu, G. Metabolic Characterization of Sphere-derived Prostate Cancer Stem Cells Reveals Aberrant Urea Cycle in Stemness Maintenance. Intl J. Cancer 2024, 155, 742–755. [Google Scholar] [CrossRef]
- Fan, G.; Yu, B.; Tang, L.; Zhu, R.; Chen, J.; Zhu, Y.; Huang, H.; Zhou, L.; Liu, J.; Wang, W.; et al. TSPAN8+ Myofibroblastic Cancer–Associated Fibroblasts Promote Chemoresistance in Patients with Breast Cancer. Sci. Transl. Med. 2024, 16, eadj5705. [Google Scholar] [CrossRef] [PubMed]
- Reversade, B.; Escande-Beillard, N.; Dimopoulou, A.; Fischer, B.; Chng, S.C.; Li, Y.; Shboul, M.; Tham, P.-Y.; Kayserili, H.; Al-Gazali, L.; et al. Mutations in PYCR1 Cause Cutis Laxa with Progeroid Features. Nat. Genet. 2009, 41, 1016–1021. [Google Scholar] [CrossRef]
- Bender, H.-U.; Almashanu, S.; Steel, G.; Hu, C.-A.; Lin, W.-W.; Willis, A.; Pulver, A.; Valle, D. Functional Consequences of PRODH Missense Mutations. Am. J. Hum. Genet. 2005, 76, 409–420. [Google Scholar] [CrossRef]
- Daudu, O.I.; Meeks, K.R.; Zhang, L.; Seravalli, J.; Tanner, J.J.; Becker, D.F. Functional Impact of a Cancer-Related Variant in Human Δ1 -Pyrroline-5-Carboxylate Reductase 1. ACS Omega 2023, 8, 3509–3519. [Google Scholar] [CrossRef]
- Buck, T.B.; Hall, A.L.; Sinha, C.C.; Bunce, O.R.; Thorgeirsson, U.P. Cis-Hydroxyproline Stimulates the Growth of Rat Mammary Carcinoma Cells. Vivo 2000, 14, 7–11. [Google Scholar]
- Tanner, J.J.; Ji, J.; Bogner, A.N.; Scott, G.K.; Patel, S.M.; Seravalli, J.; Gates, K.S.; Benz, C.C.; Becker, D.F. Noncovalent Inhibition and Covalent Inactivation of Proline Dehydrogenase by Analogs of N-Propargylglycine. Biochemistry 2024, 63, 2855–2867. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; White, T.A.; Schuermann, J.P.; Baban, B.A.; Becker, D.F.; Tanner, J.J. Structures of the Escherichia Coli PutA Proline Dehydrogenase Domain in Complex with Competitive Inhibitors. Biochemistry 2004, 43, 12539–12548. [Google Scholar] [CrossRef]
- Christensen, E.M.; Bogner, A.N.; Vandekeere, A.; Tam, G.S.; Patel, S.M.; Becker, D.F.; Fendt, S.-M.; Tanner, J.J. In Crystallo Screening for Proline Analog Inhibitors of the Proline Cycle Enzyme PYCR1. J. Biol. Chem. 2020, 295, 18316–18327. [Google Scholar] [CrossRef] [PubMed]
- Meeks, K.R.; Bogner, A.N.; Tanner, J.J. Screening a Knowledge-Based Library of Low Molecular Weight Compounds against the Proline Biosynthetic Enzyme 1-Pyrroline-5-Carboxylate 1 (PYCR1). Protein Sci. 2024, 33, e5072. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.C.; Becker, D.F.; Gates, K.S.; Tanner, J.J. Covalent Modification of the Flavin in Proline Dehydrogenase by Thiazolidine-2-Carboxylate. ACS Chem. Biol. 2020, 15, 936–944. [Google Scholar] [CrossRef]
- White, T.A.; Johnson, W.H.; Whitman, C.P.; Tanner, J.J. Structural Basis for the Inactivation of Thermus Thermophilus Proline Dehydrogenase by N-Propargylglycine. Biochemistry 2008, 47, 5573–5580. [Google Scholar] [CrossRef]
- Tritsch, D.; Mawlawi, H.; Biellmann, J.-F. Mechanism-Based Inhibition of Proline Dehydrogenase by Proline Analogues. Biochim. Et Biophys. Acta (BBA)-Protein Struct. Mol. Enzymol. 1993, 1202, 77–81. [Google Scholar] [CrossRef]
- Scarpulla, R.C.; Soffer, R.L. Membrane-Bound Proline Dehydrogenase from Escherichia Coli. Solubilization, Purification, and Characterization. J. Biol. Chem. 1978, 253, 5997–6001. [Google Scholar] [CrossRef] [PubMed]
- Meeks, K.R.; Ji, J.; Scott, G.K.; Campbell, A.C.; Nix, J.C.; Tadeo, A.; Ellerby, L.M.; Benz, C.C.; Tanner, J.J. Biochemical, Structural, and Cellular Characterization of S-but-3-Yn-2-Ylglycine as a Mechanism-Based Covalent Inactivator of the Flavoenzyme Proline Dehydrogenase. Arch. Biochem. Biophys. 2025, 765, 110319. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.C.; Prater, A.R.; Bogner, A.N.; Quinn, T.P.; Gates, K.S.; Becker, D.F.; Tanner, J.J. Photoinduced Covalent Irreversible Inactivation of Proline Dehydrogenase by S-Heterocycles. ACS Chem. Biol. 2021, 16, 2268–2279. [Google Scholar] [CrossRef]
- Srivastava, D.; Zhu, W.; Johnson, W.H.; Whitman, C.P.; Becker, D.F.; Tanner, J.J. The Structure of the Proline Utilization a Proline Dehydrogenase Domain Inactivated by N-Propargylglycine Provides Insight into Conformational Changes Induced by Substrate Binding and Flavin Reduction. Biochemistry 2010, 49, 560–569. [Google Scholar] [CrossRef]
- Scott, G.K.; Mahoney, S.; Scott, M.; Loureiro, A.; Lopez-Ramirez, A.; Tanner, J.J.; Ellerby, L.M.; Benz, C.C. N-Propargylglycine: A Unique Suicide Inhibitor of Proline Dehydrogenase with Anticancer Activity and Brain-Enhancing Mitohormesis Properties. Amino Acids 2021, 53, 1927–1939. [Google Scholar] [CrossRef] [PubMed]
- Bons, J.; Tadeo, A.; Scott, G.K.; Teramayi, F.; Tanner, J.J.; Schilling, B.; Benz, C.C.; Ellerby, L.M. Therapeutic Targeting of HYPDH/PRODH2 with N-Propargylglycine Offers a Hyperoxaluria Treatment Opportunity. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 166848. [Google Scholar] [CrossRef]
- Li, F.; Simon, M.C. Cancer Cells Don’t Live Alone: Metabolic Communication within Tumor Microenvironments. Dev. Cell 2020, 54, 183–195. [Google Scholar] [CrossRef]
- Sturm, D.; Maletzki, C.; Braun, D.; Emmrich, J. Cis-Hydroxyproline-Mediated Pancreatic Carcinoma Growth Inhibition in Mice. Int. J. Colorectal Dis. 2010, 25, 921–929. [Google Scholar] [CrossRef]
- Mueller, C. Cis-Hydroxyproline-Induced Inhibition of Pancreatic Cancer Cell Growth Is Mediated by Endoplasmic Reticulum Stress. WJG 2006, 12, 1569. [Google Scholar] [CrossRef]
- Bernard, K.; Logsdon, N.J.; Benavides, G.A.; Sanders, Y.; Zhang, J.; Darley-Usmar, V.M.; Thannickal, V.J. Glutaminolysis Is Required for Transforming Growth Factor-Β1–Induced Myofibroblast Differentiation and Activation. J. Biol. Chem. 2018, 293, 1218–1228. [Google Scholar] [CrossRef]
- Chen, W.W.; Freinkman, E.; Wang, T.; Birsoy, K.; Sabatini, D.M. Absolute Quantification of Matrix Metabolites Reveals the Dynamics of Mitochondrial Metabolism. Cell 2016, 166, 1324–1337.e11. [Google Scholar] [CrossRef] [PubMed]
| Proline Enzyme | Expression Level | Cancer Progression | References |
|---|---|---|---|
| PYCR1 | UP | PRO | [16,21,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94] |
| DOWN | PRO | [95,96] | |
| DOWN | ANTI | [97] | |
| PYCR2 | UP | PRO | [92,93,94] |
| UP | ANTI | [98,99,100,101,102,103] | |
| PYCR3 | DOWN | ANTI | [67,103,104] |
| UP | PRO | [105,106] | |
| PRODH1 | UP | PRO | [51,52,67,91,95,107,108,109,110,111,112,113,114,115,116] |
| UP | ANTI | [117,118,119,120,121,122,123,124,125] | |
| DOWN | ANTI | [125,126,127,128,129,130,131,132] | |
| PRODH2 | DOWN | PRO | [11] |
| P5CS | UP | PRO | [11,106,133,134] |
| DOWN | ANTI | [19,135,136,137,138] | |
| P5CDH | DOWN | PRO | [66] |
| Compound | Structure | Type of Inactivation | Target Enzyme | References |
|---|---|---|---|---|
| N-formyl-L-Proline | 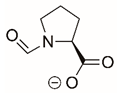 | Reversible | PYCR1 | [208] |
| Reversible | PYCR3 | [208] | ||
| Pargyline |  | Unreported | PYCR1 | [174] |
| Cyclopentanecarboxylate | 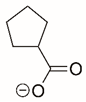 | Reversible | PYCR1 | [208] |
| L-thiazolidine-4-carboxylate | 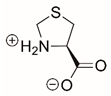 | Reversible | PYCR1 | [208] |
| N-(4-bromobenzyl)-N-methylprop-2-yn-1-amine |  | Unreported | PYCR1 | [174] |
| L-tetrahydro-2H-pyran-2-carboxylic acid | 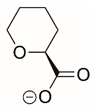 | Reversible | PYCR3 | [209] |
| Reversible | PYCR1 | [209] | ||
| ATP | 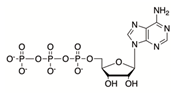 | Reversible | PYCR1-3 | [145] |
| Proline | 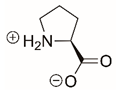 | Reversible | PYCR1 | [208] |
| L-thiazolidine-2-carboxylate | 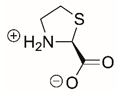 | Reversible | PYCR1 | [208] |
| Covalent | PRODH | [210] | ||
| L-tetrahydro-2-furoic acid |  | Reversible | PYCR1 | [208] |
| Reversible | PRODH1 | [147] | ||
| N-allylglycine | 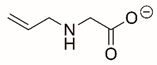 | Covalent | PRODH | [206] |
| 2-prop-2-enylsulfanylacetate | 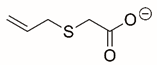 | Reversible | PRODH | [206] |
| N-propargylglycine | 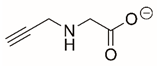 | Covalent | PRODH | [206,211] |
| 2-prop-2-ynylsulfanylacetate |  | Reversible | PRODH | [206] |
| Cyanomethyl sulfanylacetate | 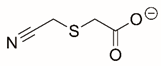 | Reversible | PRODH | [206] |
| S-5-oxo-2-tetrahydrofurancarboxylic acid | 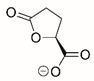 | Reversible | PRODH1 | [129] |
| 4-methylene-L-proline | 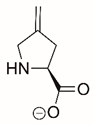 | Covalent | PRODH1 | [211,212] |
| L-lactate | 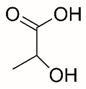 | Reversible | PRODH1 | [147,213] |
| (S)-but-3-yn-2-ylglycine |  | Covalent | PRODH | [214] |
| 1,3-dithiolane-2-carboxylate | 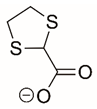 | Reversible and Covalent (photoinduced) | PRODH | [215] |
| Tetrahydrothiophene-2-carboxylate | 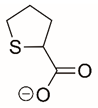 | Reversible and Covalent (photoinduced) | PRODH | [215] |
| Ornithine | 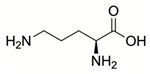 | Reversible | P5CS | [149] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossman, T.C.; Purohit, G.; Daudu, O.I.; Becker, D.F. Proline Metabolism in Cancer: Emerging Roles in Redox Homeostasis and Therapeutic Opportunities. Cancers 2025, 17, 3156. https://doi.org/10.3390/cancers17193156
Rossman TC, Purohit G, Daudu OI, Becker DF. Proline Metabolism in Cancer: Emerging Roles in Redox Homeostasis and Therapeutic Opportunities. Cancers. 2025; 17(19):3156. https://doi.org/10.3390/cancers17193156
Chicago/Turabian StyleRossman, Tyrell C., Gunjan Purohit, Oseeyi I. Daudu, and Donald F. Becker. 2025. "Proline Metabolism in Cancer: Emerging Roles in Redox Homeostasis and Therapeutic Opportunities" Cancers 17, no. 19: 3156. https://doi.org/10.3390/cancers17193156
APA StyleRossman, T. C., Purohit, G., Daudu, O. I., & Becker, D. F. (2025). Proline Metabolism in Cancer: Emerging Roles in Redox Homeostasis and Therapeutic Opportunities. Cancers, 17(19), 3156. https://doi.org/10.3390/cancers17193156





