Moderate Immune-Related Liver Injury Is a Good Factor in Patients with Hepatoma Under Atezolizumab Plus Bevacizumab
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients’ Enrollment
2.2. Treatment and Follow-Up Protocol
2.3. Definitions of Immune-Related Liver Injury
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics of Enrolled Patients
3.2. Baseline Characteristics by irLI Severity
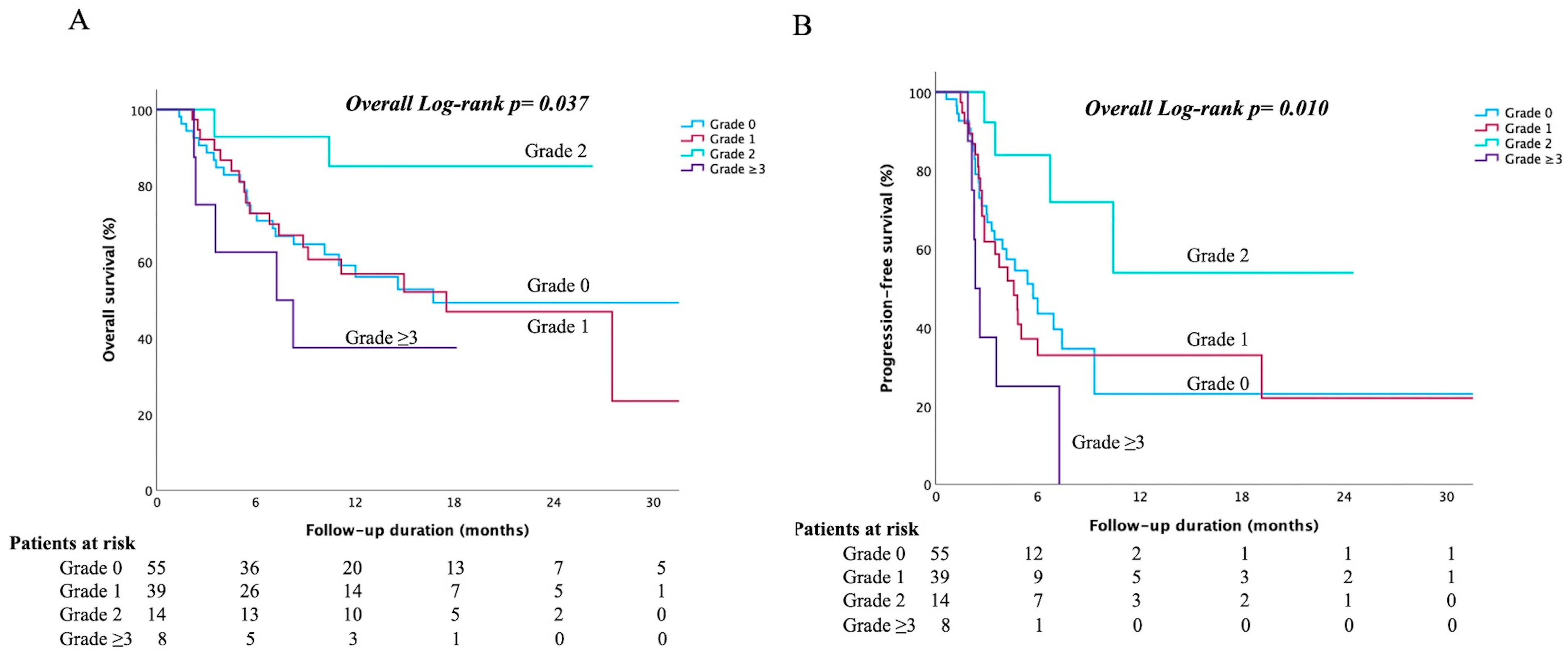
3.3. Treatment Responses and Survival Outcomes
3.4. Subgroup Analysis of Patients with irLI Severity
3.5. Corticosteroid Use and Outcomes
3.6. The Factors Determined Occurrence of Grade 2 irLI
3.7. Other Immune-Related Adverse Events
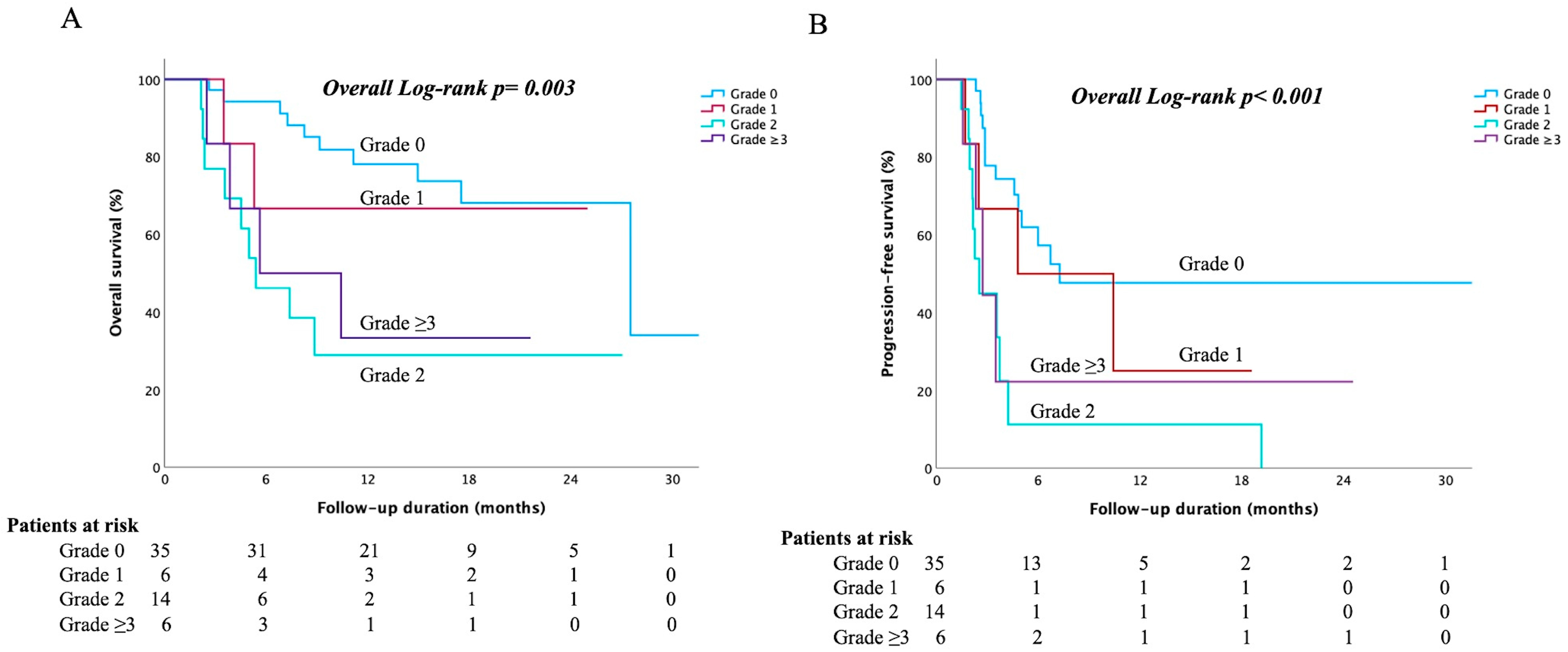
3.8. Comparison of Survival and Efficacy After Propensity Score Matching
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| BCLC | Barcelona Clinic Liver Cancer |
| CR | complete response; DCR, disease control rate |
| EHS | extrahepatic spread |
| HCC | Hepatocellular carcinoma |
| ICIs | immune checkpoint inhibitors |
| irLI | immune-related liver injury |
| LRT | locoregional therapy |
| ORR | objective response rate |
| OS | overall survival |
| PFS | progression-free survival |
| PVT | portal vein thrombosis |
| RFA | radiofrequency ablation |
| SD | stable disease |
| TACE | transarterial chemoembolization |
| ULN | upper limit of normal |
References
- Teng, W.; Wang, H.W.; Lin, S.M.; Diagnosis, G.; Diagnosis Group and Systemic Therapy Group of TLCA. Management Consensus Guidelines for Hepatocellular Carcinoma: 2023 Update on Surveillance, Diagnosis, Systemic Treatment, and Posttreatment Monitoring by the Taiwan Liver Cancer Association and the Gastroenterological Society of Taiwan. Liver Cancer 2024, 13, 468–486. [Google Scholar] [CrossRef]
- Singal, A.G.; Llovet, J.M.; Yarchoan, M.; Mehta, N.; Heimbach, J.K.; Dawson, L.A.; Jou, J.H.; Kulik, L.M.; Agopian, V.G.; Marrero, J.A.; et al. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology 2023, 78, 1922–1965. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Chan, S.L.; Dawson, L.A.; Kelley, R.K.; Llovet, J.M.; Meyer, T.; Ricke, J.; Rimassa, L.; Sapisochin, G.; Vilgrain, V.; et al. Hepatocellular carcinoma: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2025, 36, 491–506. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fabrega, J.; Burrel, M.; Garcia-Criado, A.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Gordan, J.D.; Kennedy, E.B.; Abou-Alfa, G.K.; Beal, E.; Finn, R.S.; Gade, T.P.; Goff, L.; Gupta, S.; Guy, J.; Hoang, H.T.; et al. Systemic Therapy for Advanced Hepatocellular Carcinoma: ASCO Guideline Update. J. Clin. Oncol. 2024, 42, 1830–1850. [Google Scholar] [CrossRef]
- Su, G.L.; Altayar, O.; O’Shea, R.; Shah, R.; Estfan, B.; Wenzell, C.; Sultan, S.; Falck-Ytter, Y. AGA Clinical Practice Guideline on Systemic Therapy for Hepatocellular Carcinoma. Gastroenterology 2022, 162, 920–934. [Google Scholar] [CrossRef]
- Lau, G.; Obi, S.; Zhou, J.; Tateishi, R.; Qin, S.; Zhao, H.; Otsuka, M.; Ogasawara, S.; George, J.; Chow, P.K.H.; et al. APASL clinical practice guidelines on systemic therapy for hepatocellular carcinoma-2024. Hepatol. Int. 2024, 18, 1661–1683. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. Electronic address, e.e.e.; European Association for the Study of the, L. EASL Clinical Practice Guidelines on the management of hepatocellular carcinoma. J. Hepatol. 2025, 82, 315–374. [Google Scholar] [CrossRef]
- Sangro, B.; Chan, S.L.; Meyer, T.; Reig, M.; El-Khoueiry, A.; Galle, P.R. Diagnosis and management of toxicities of immune checkpoint inhibitors in hepatocellular carcinoma. J. Hepatol. 2020, 72, 320–341. [Google Scholar] [CrossRef]
- Celsa, C.; Cabibbo, G.; Fulgenzi, C.A.M.; Scheiner, B.; D’Alessio, A.; Manfredi, G.F.; Nishida, N.; Ang, C.; Marron, T.U.; Saeed, A.; et al. Characteristics and outcomes of immunotherapy-related liver injury in patients with hepatocellular carcinoma versus other advanced solid tumours. J. Hepatol. 2024, 80, 431–442. [Google Scholar] [CrossRef]
- Zheng, C.; Huang, S.; Lin, M.; Hong, B.; Ni, R.; Dai, H.; Lin, X.; Yang, J. Hepatotoxicity of immune checkpoint inhibitors: What is Currently Known. Hepatol. Commun. 2023, 7, e0063. [Google Scholar] [CrossRef]
- Li, M.; Wong, D.; Sack, J.S.; Vogel, A.S.; Hodi, F.S.; Fong, L.; Lai, J.C.; Zucker, S.D.; Grover, S. Outcomes of High-Grade Immune Checkpoint Inhibitor Hepatitis in Hospitalized and Nonhospitalized Patients. Clin. Gastroenterol. Hepatol. 2024, 22, 1444–1452 e1444. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yao, Z.; Yang, H.; Liang, N.; Zhang, X.; Zhang, F. Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? A systematic review and meta-analysis. BMC Med. 2020, 18, 87. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.; Lee, J.; Han, J.W.; Lee, S.K.; Yang, H.; Lee, H.L.; Sung, P.S.; Kim, H.Y.; Kim, S.H.; Song, M.J.; et al. Analysis of Immune-Related Adverse Events of Atezolizumab and Bevacizumab in Patients with Hepatocellular Carcinoma: A Multicentre Cohort Study. Liver Cancer 2024, 13, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Pinato, D.J.; Marron, T.U.; Mishra-Kalyani, P.S.; Gong, Y.; Wei, G.; Szafron, D.; Sharon, E.; Saeed, A.; Jun, T.; Dharmapuri, S.; et al. Treatment-related toxicity and improved outcome from immunotherapy in hepatocellular cancer: Evidence from an FDA pooled analysis of landmark clinical trials with validation from routine practice. Eur. J. Cancer 2021, 157, 140–152. [Google Scholar] [CrossRef]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef]
- Terrault, N.A.; Lok, A.S.F.; McMahon, B.J.; Chang, K.M.; Hwang, J.P.; Jonas, M.M.; Brown, R.S., Jr.; Bzowej, N.H.; Wong, J.B. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018, 67, 1560–1599. [Google Scholar] [CrossRef]
- Ito, T.; Takeuchi, Y.; Mizuno, K.; Imai, M.; Yoshimaru, Y.; Abe, K.; Abe, M.; Matsuura, T.; Yokode, M.; Shiokawa, M.; et al. Diagnostic guide for immune checkpoint inhibitor-induced liver injury. Hepatol. Res. 2024, 54, 719–726. [Google Scholar] [CrossRef]
- Cheng, A.L.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Lim, H.Y.; Kudo, M.; Breder, V.; Merle, P.; et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 2022, 76, 862–873. [Google Scholar] [CrossRef]
- Hung, Y.P.; Lee, P.C.; Chang, Y.H.; Yang, M.H.; Chiu, C.H.; Chen, M.H.; Lan, K.H.; Lee, I.C.; Hou, M.C.; Chao, Y.; et al. Hepatic Events During Immune Checkpoint Inhibitor Treatment Between Liver and Non-Liver Malignancies in Hepatitis B Endemic Areas. Aliment. Pharmacol. Ther. 2025, 61, 501–512. [Google Scholar] [CrossRef]
- De Martin, E.; Michot, J.M.; Papouin, B.; Champiat, S.; Mateus, C.; Lambotte, O.; Roche, B.; Antonini, T.M.; Coilly, A.; Laghouati, S.; et al. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J. Hepatol. 2018, 68, 1181–1190. [Google Scholar] [CrossRef]
- Takaki, S.; Kurosaki, M.; Mori, N.; Tsuji, K.; Ochi, H.; Marusawa, H.; Nakamura, S.; Tada, T.; Narita, R.; Uchida, Y.; et al. Effects on survival of the adverse event of atezolizumab plus bevacizumab for hepatocellular carcinoma: A multicenter study by the Japan Red Cross Liver Study Group. Investig. New Drugs 2023, 41, 340–349. [Google Scholar] [CrossRef]
- Chang, W.T.; Lu, S.N.; Rau, K.M.; Huang, C.S.; Lee, K.T. Increased cumulative doses and appearance of hand-foot skin reaction prolonged progression free survival in sorafenib-treated advanced hepatocellular carcinoma patients. Kaohsiung J. Med. Sci. 2018, 34, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Torres, F.; Rodriguez-Lope, C.; Forner, A.; LLarch, N.; Rimola, J.; Darnell, A.; Rios, J.; Ayuso, C.; Bruix, J. Early dermatologic adverse events predict better outcome in HCC patients treated with sorafenib. J. Hepatol. 2014, 61, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Howell, J.; Pinato, D.J.; Ramaswami, R.; Bettinger, D.; Arizumi, T.; Ferrari, C.; Yen, C.; Gibbin, A.; Burlone, M.E.; Guaschino, G.; et al. On-target sorafenib toxicity predicts improved survival in hepatocellular carcinoma: A multi-centre, prospective study. Aliment. Pharmacol. Ther. 2017, 45, 1146–1155. [Google Scholar] [CrossRef]
- Su, C.W.; Teng, W.; Shen, E.Y.; Huang, B.S.; Lin, P.T.; Hou, M.M.; Wu, T.H.; Tsan, D.L.; Hsieh, C.H.; Wang, C.T.; et al. Concurrent Atezolizumab Plus Bevacizumab and High-Dose External Beam Radiotherapy for Highly Advanced Hepatocellular Carcinoma. Oncologist 2024, 29, e922–e931. [Google Scholar] [CrossRef]
- Duffy, A.G.; Ulahannan, S.V.; Makorova-Rusher, O.; Rahma, O.; Wedemeyer, H.; Pratt, D.; Davis, J.L.; Hughes, M.S.; Heller, T.; ElGindi, M.; et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J. Hepatol. 2017, 66, 545–551. [Google Scholar] [CrossRef]
- Pinato, D.J.; Murray, S.M.; Forner, A.; Kaneko, T.; Fessas, P.; Toniutto, P.; Minguez, B.; Cacciato, V.; Avellini, C.; Diaz, A.; et al. Trans-arterial chemoembolization as a loco-regional inducer of immunogenic cell death in hepatocellular carcinoma: Implications for immunotherapy. J. Immunother Cancer 2021, 9, e003311. [Google Scholar] [CrossRef]
- Li, M.; Bhoori, S.; Mehta, N.; Mazzaferro, V. Immunotherapy for hepatocellular carcinoma: The next evolution in expanding access to liver transplantation. J. Hepatol. 2024, 81, 743–755. [Google Scholar] [CrossRef]
- Sorup, S.; Darvalics, B.; Russo, L.; Oksen, D.; Lamy, F.X.; Verpillat, P.; Aa, K.; Ht, S.; Cronin-Fenton, D. High-dose corticosteroid use and risk of hospitalization for infection in patients treated with immune checkpoint inhibitors—A nationwide register-based cohort study. Cancer Med. 2021, 10, 4957–4963. [Google Scholar] [CrossRef] [PubMed]
| Variables | Overall (n = 116) | No irLI (n = 55) | Any Grade irLI (n = 61) | p Value |
|---|---|---|---|---|
| Age (year-old) | 64.1 (IQR 57.7–71.9) | 64.5 (IQR 57.8–72.3) | 64.0 (IQR 56.4–71.5) | 0.831 |
| Male gender, n (%) | 91 (78.4) | 38 (69.1) | 53 (86.9) | 0.020 |
| HBV/HCV/NBNC, n (%) | 73/22/21 (62.9/19.0/18.1) | 33/13/9 (60.0/23.6/16.4) | 40/9/12 (65.6/14.8/19.7) | 0.467 |
| Antiviral therapy, n (%) | 33/95 (34.7) | 20/46 (43.5) | 13/49 (26.5) | 0.043 |
| NLR | 3.40 (IQR 2.51–4.99) | 3.38 (IQR 2.28–4.66) | 3.49 (IQR 2.70–5.99) | 0.308 |
| AST (IU/mL) | 53 (IQR 36–82) | 54 (IQR 32–91) | 51 (IQR 37–82) | 0.936 |
| ALT (IU/mL) | 48 (IQR 30–73) | 50 (IQR 31–99) | 46 (IQR 27–68) | 0.180 |
| Baseline AST or ALT > ULN, n (%) | 97 (83.6) | 46 (83.6) | 51 (83.6) | 0.811 |
| Bilirubin total (mg/dL) | 0.80 (IQR 0.50–1.20) | 0.85 (IQR 0.50–1.28) | 0.80 (IQR 0.50–1.15) | 0.593 |
| Albumin (g/dL) | 3.78 (IQR 3.40–4.16) | 3.79 (IQR 3.29–4.15) | 3.76 (IQR 3.55–4.19) | 0.519 |
| Platelet (103/μL) | 188 (IQR 145–284) | 168 (IQR 134–245) | 195 (IQR 153–310) | 0.038 |
| ALBI grade I/II, n (%) | 57/59 (49.1/50.9) | 29/26 (52.7/47.3) | 28/33 (45.9/54.1) | 0.463 |
| Portal vein thrombosis, n (%) | 60 (51.7) | 26 (47.3) | 34 (55.7) | 0.362 |
| Esophageal varices, n (%) | 23 (19.8) | 10 (18.2) | 23 (21.3) | 0.673 |
| Extrahepatic metastasis, n (%) | 43 (37.1) | 19 (34.5) | 24 (39.3) | 0.593 |
| BCLC stage B/C, n (%) | 31/85 (26.7/73.3) | 17/38 (30.9/69.1) | 14/47 (23.0/77.0) | 0.333 |
| Target tumor size, cm | 9.1 (IQR 4.6–11.9) | 7.9 (IQR 3.9–10.5) | 9.7 (IQR 5.7–13.0) | 0.011 |
| Tumor number 1/2/ ≥ 3 | 35/16/65 (30.2/13.8/56.0) | 22/10/23 (40.0/18.2/41.8) | 13/6/42 (21.3/9.8/68.9) | 0.014 |
| Beyond up-to-seven, n (%) | 92 (79.3) | 38 (69.1) | 54 (88.5) | 0.010 |
| AFP (ng/mL) | 377 (IQR 23–5719) | 423 (IQR 16–11903) | 239 (IQR 28–3307) | 0.770 |
| Combination with LRT, n (%) | 49 (42.2) | 21 (38.2) | 28 (45.9) | 0.401 |
| Prior LRT, n (%) | 48 (41.4) | 25 (45.5) | 23 (37.7) | 0.397 |
| ICI treatment duration (months) | 2.5 (IQR 1.5–4.6) | 2.8 (IQR 1.4–4.7) | 2.3 (IQR 1.8–6.2) | 0.038 |
| Variables | Mild, Grade 1 (n = 39) | Moderate, Grade 2 (n = 14) | Severe, Grade ≥ 3 (n = 8) | p Value |
|---|---|---|---|---|
| Age (year-old) | 64.0 (IQR 59.3–71.6) | 69.1 (IQR 57.3–74.2) | 58.4 (IQR 42.3–69.5) | 0.389 |
| Male gender, n (%) | 32 (82.1) | 13 (92.9) | 8 (100) | 0.294 |
| HBV/HCV/NBNC, n (%) | 26/7/6 (66.7/17.9/15.4) | 9/1/4 (64.3/7.1/28.6) | 5/1/2 (62.5/12.5/25.0) | 0.752 |
| Antiviral therapy, n (%) | 10/33 (30.3) | 3/10 (30.0) | 0/6 (0) | 0.061 |
| NLR | 3.40 (IQR 2.76–5.91) | 3.13 (IQR 2.26–6.02) | 3.92 (IQR 2.49–7.89) | 0.890 |
| AST (IU/mL) | 57 (IQR 42–85) | 37 (IQR 29–55) | 60 (IQR 45–110) | 0.151 |
| ALT (IU/mL) | 49 (IQR 30–68) | 27 (IQR 21–43) | 56 (IQR 34–105) | 0.174 |
| Baseline AST or ALT > ULN, n (%) | 34 (89.5) | 9 (64.3) | 8 (100) | 0.035 |
| Bilirubin total (mg/dL) | 0.90 (IQR 0.60–1.30) | 0.55 (IQR 0.48–0.80) | 0.85 (IQR 0.53–1.50) | 0.368 |
| Albumin (g/dL) | 3.78 (IQR 3.49–4.22) | 3.88 (IQR 3.61–4.17) | 3.55 (IQR 2.94–3.96) | 0.193 |
| Platelet (103/μL) | 192 (IQR 159–295) | 249 (IQR 147–315) | 262 (IQR 147–361) | 0.803 |
| ALBI grade I/II, n (%) | 17/22 (43.6/56.4) | 9/5 (64.3/35.7) | 2/6 (25.0/75.0) | 0.183 |
| Portal vein thrombosis, n (%) | 26 (66.7) | 4 (28.6) | 4 (50.0) | 0.045 |
| Esophageal varices, n (%) | 9 (23.1) | 2 (14.3) | 2 (25.0) | 0.760 |
| Extrahepatic metastasis, n (%) | 18 (46.2) | 4 (28.6) | 2 (25.0) | 0.345 |
| BCLC stage B/C, n (%) | 6/33 (15.4/84.6) | 6/8 (42.9/57.1) | 2/6 (25.0/75.0) | 0.110 |
| Target tumor size, cm | 10.4 (IQR 6.7–13.0) | 7.8 (IQR 2.4–12.8) | 8.9 (IQR 7.5–9.9) | 0.304 |
| Tumor number 1/2/ ≥ 3 | 9/3/27 (23.1/7.7/69.2) | 4/2/8 (28.6/14.3/57.1) | 0/1/7 (0/12.5/87.5) | 0.507 |
| Beyond up-to-seven, n (%) | 37 (94.9) | 9 (64.3) | 8 (100) | 0.005 |
| AFP (ng/mL) | 584 (IQR 46–3961) | 76 (IQR 14–363) | 280 (IQR 50–3108) | 0.669 |
| Combination with LRT, n (%) | 16 (41.0) | 8 (57.1) | 4 (50.0) | 0.046 |
| Prior LRT, n (%) | 10 (25.6) | 8 (57.1) | 5 (62.5) | 0.034 |
| ICI treatment duration (months) | 3.2 (IQR 1.2–4.8) | 6.7 (IQR 2.1–10.3) | 2.2 (IQR 1.7–4.6) | 0.042 |
| Corticosteroid therapy, n (%) | 8 (20.5) | 2 (14.3) | 4 (50.0) | 0.043 |
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | p Value | HR | 95%CI | p Value | |
| Age ≥ 65 years old (vs. <65 years old) | 0.626 | 0.346–1.132 | 0.121 | |||
| Male (vs. female) | 0.604 | 0.318–1.146 | 0.123 | |||
| Viral infection (vs. others) | 1.338 | 0.600–2.984 | 0.477 | |||
| Antiviral therapy (vs. no) | 0.878 | 0.458–1.684 | 0.696 | |||
| ALBI grade II (vs. I) | 2.489 | 1.381–4.487 | 0.002 | 2.003 | 1.079–3.720 | 0.028 |
| Baseline AST or ALT > ULN (vs. No) | 1.315 | 0.589–2.938 | 0.504 | |||
| BCLC stage C (vs. stage A/B) | 4.137 | 1.634–10.47 | 0.003 | 3.876 | 1.288–11.66 | 0.016 |
| Tumor size | 1.027 | 0.967–1.091 | 0.386 | |||
| Tumor numbers ≥ 3 (vs. <3) | 2.354 | 1.283–4.320 | 0.006 | 2.379 | 0.984–4.781 | 0.075 |
| Portal vein thrombosis (vs. No) | 2.229 | 1.207–4.118 | 0.010 | 0.910 | 0.438–1.889 | 0.800 |
| Extrahepatic metastasis (vs. No) | 1.483 | 0.838–2.625 | 0.177 | |||
| AFP ≥ 400 ng/mL (vs. <400 ng/mL) | 1.249 | 0.702–2.219 | 0.449 | |||
| Prior LRT (vs. No) | 0.477 | 0.256–0.889 | 0.020 | 0.733 | 0.369–1.454 | 0.374 |
| Combination with LRT (vs. No) | 1.195 | 0.870–2.129 | 0.246 | |||
| IrLI (vs. No) | 0.227 | 0.055–0.939 | 0.041 | |||
| Grade 1 | 1.228 | 0.684–2.203 | 0.492 | |||
| Grade 2 | 0.227 | 0.055–0.939 | 0.041 | 0.223 | 0.051–0.974 | 0.046 |
| Grade ≥ 3 | 1.964 | 0.776–4.974 | 0.155 | |||
| Corticosteroid therapy (vs. No) | 0.878 | 0.347–2.220 | 0.783 | |||
| ICI treatment duration | 0.870 | 0.782–1.167 | 0.097 | |||
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | p Value | HR | 95%CI | p Value | |
| Age ≥ 65 years old (vs. <65 years old) | 0.545 | 0.323–0.919 | 0.023 | 0.553 | 0.310–1.089 | 0.076 |
| Male (vs. female) | 0.939 | 0.517–1.708 | 0.837 | |||
| Viral infection (vs. others) | 1.439 | 0.682–3.035 | 0.340 | |||
| Antiviral therapy (vs. no) | 0.591 | 0.327–1.068 | 0.081 | |||
| ALBI grade II (vs. I) | 1.765 | 1.059–2.943 | 0.029 | 1.327 | 1.063–2.308 | 0.046 |
| Baseline AST or ALT > ULN (vs. No) | 1.662 | 0.786–3.515 | 0.184 | |||
| BCLC stage C (vs. A/B) | 1.592 | 12845–2.999 | 0.040 | 1.790 | 1.115–3.503 | 0.039 |
| Tumor size | 1.011 | 0.959–1.065 | 0.690 | |||
| Tumor numbers ≥ 3 (vs. <3) | 3.529 | 1.981–6.286 | 0.001 | 4.579 | 0.997–9.128 | 0.061 |
| Portal vein thrombosis (vs. No) | 1.324 | 0.795–2.204 | 0.281 | |||
| Extrahepatic metastasis (vs. No) | 1.193 | 0.713–1.997 | 0.502 | |||
| AFP ≥ 400 ng/mL (vs. <400 ng/mL) | 1.759 | 1.053–2.936 | 0.031 | 1.017 | 0.583–1.775 | 0.953 |
| Prior LRT (vs. No) | 0.496 | 0.285–0.862 | 0.013 | 0.716 | 0.390–1.314 | 0.281 |
| Combination with LRT (vs. No) | 0.742 | 0.560–1.385 | 0.823 | |||
| IrLI (vs. No) | ||||||
| Grade 1 | 1.153 | 0.682–1.949 | 0.596 | |||
| Grade 2 | 0.319 | 0.115–0.884 | 0.028 | 0.244 | 0.082–0.727 | 0.011 |
| Grade ≥ 3 | 2.755 | 0.943–6.108 | 0.063 | |||
| Corticosteroid therapy (vs. No) | 0.930 | 0.422–2.049 | 0.857 | |||
| ICI treatment duration | 0.755 | 0.667–1.055 | 0.081 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.-C.; Lin, P.-T.; Teng, W.; Shen, E.Y.-L.; Su, C.-W.; Hsieh, Y.-C.; Chen, W.-T.; Wu, T.-H.; Lin, C.-C.; Lin, S.-M.; et al. Moderate Immune-Related Liver Injury Is a Good Factor in Patients with Hepatoma Under Atezolizumab Plus Bevacizumab. Cancers 2025, 17, 3157. https://doi.org/10.3390/cancers17193157
Wu T-C, Lin P-T, Teng W, Shen EY-L, Su C-W, Hsieh Y-C, Chen W-T, Wu T-H, Lin C-C, Lin S-M, et al. Moderate Immune-Related Liver Injury Is a Good Factor in Patients with Hepatoma Under Atezolizumab Plus Bevacizumab. Cancers. 2025; 17(19):3157. https://doi.org/10.3390/cancers17193157
Chicago/Turabian StyleWu, Tai-Chi, Po-Ting Lin, Wei Teng, Eric Yi-Liang Shen, Chung-Wei Su, Yi-Chung Hsieh, Wei-Ting Chen, Tsung-Han Wu, Chen-Chun Lin, Shi-Ming Lin, and et al. 2025. "Moderate Immune-Related Liver Injury Is a Good Factor in Patients with Hepatoma Under Atezolizumab Plus Bevacizumab" Cancers 17, no. 19: 3157. https://doi.org/10.3390/cancers17193157
APA StyleWu, T.-C., Lin, P.-T., Teng, W., Shen, E. Y.-L., Su, C.-W., Hsieh, Y.-C., Chen, W.-T., Wu, T.-H., Lin, C.-C., Lin, S.-M., & Lin, C.-Y. (2025). Moderate Immune-Related Liver Injury Is a Good Factor in Patients with Hepatoma Under Atezolizumab Plus Bevacizumab. Cancers, 17(19), 3157. https://doi.org/10.3390/cancers17193157





