Transcriptional Condensates: Epigenetic Reprogramming and Therapeutic Targets in Hematologic Malignancies
Simple Summary
Abstract
1. Introduction
2. Liquid-Liquid Phase Separation and Aberrant Biomolecular Condensates in Cancer
2.1. Liquid-Liquid Phase Separation and Aberrant Biomolecular Condensate Formation
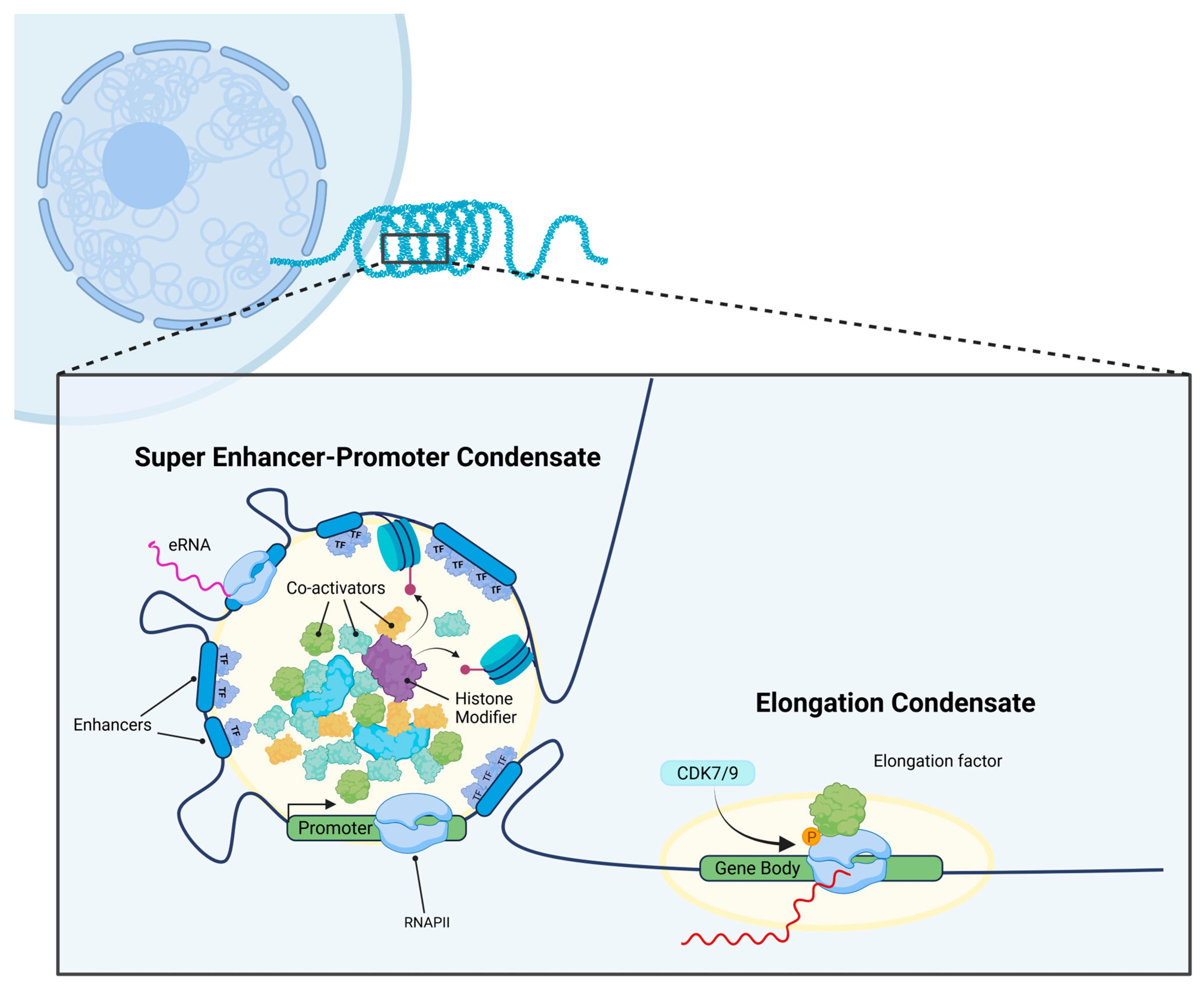
2.2. Nuclear Transcriptional Condensates in Transcriptional Regulation
2.3. Nuclear Transcriptional Condensates at SEs Drive Oncogenic Transcription
3. Nuclear Transcription Condensates Driving Transcription Regulation in Hematologic Malignancies
3.1. Aberrant LLPS and Biomolecular Condensates in Cancer
3.2. Multi-Omics Data Science Strategy to Inform Spatially Clustered Patterns of TF Binding and Phase-Separated TCs at SEs
| Method | Mechanism | Resolution | Advantages/Use Cases |
|---|---|---|---|
| FRAP [89] | Measure molecular dynamics via photobleaching recovery | ~250 nm | Quantitative dynamics, live cells |
| OptoDroplets/OptoIDR [90] | Light-controlled condensate formation | Single condensate | Precise temporal control, reversible |
| ChIP-seq/CUT&RUN [91] | Map genomic localization of TC components | ~200 bp | Genome-wide, quantitative |
| Hi-C/HiChIP [92,93] | 3D chromatin organization, loop detection | 1–10 kb | Links structure to function |
| Live super-resolution [63] | Visualize endogenous condensates | 20–50 nm | Native protein levels, dynamics |
| AICAP assay [86] | Screen for phase-separating proteins | N/A | Proteomics screening |
| Single-molecule tracking [94] | TF search kinetics and binding | ~30 nm | Direct mechanism, absolute measurements |
4. Transcriptional Condensates as a Novel Vulnerability in Hematologic Malignancies
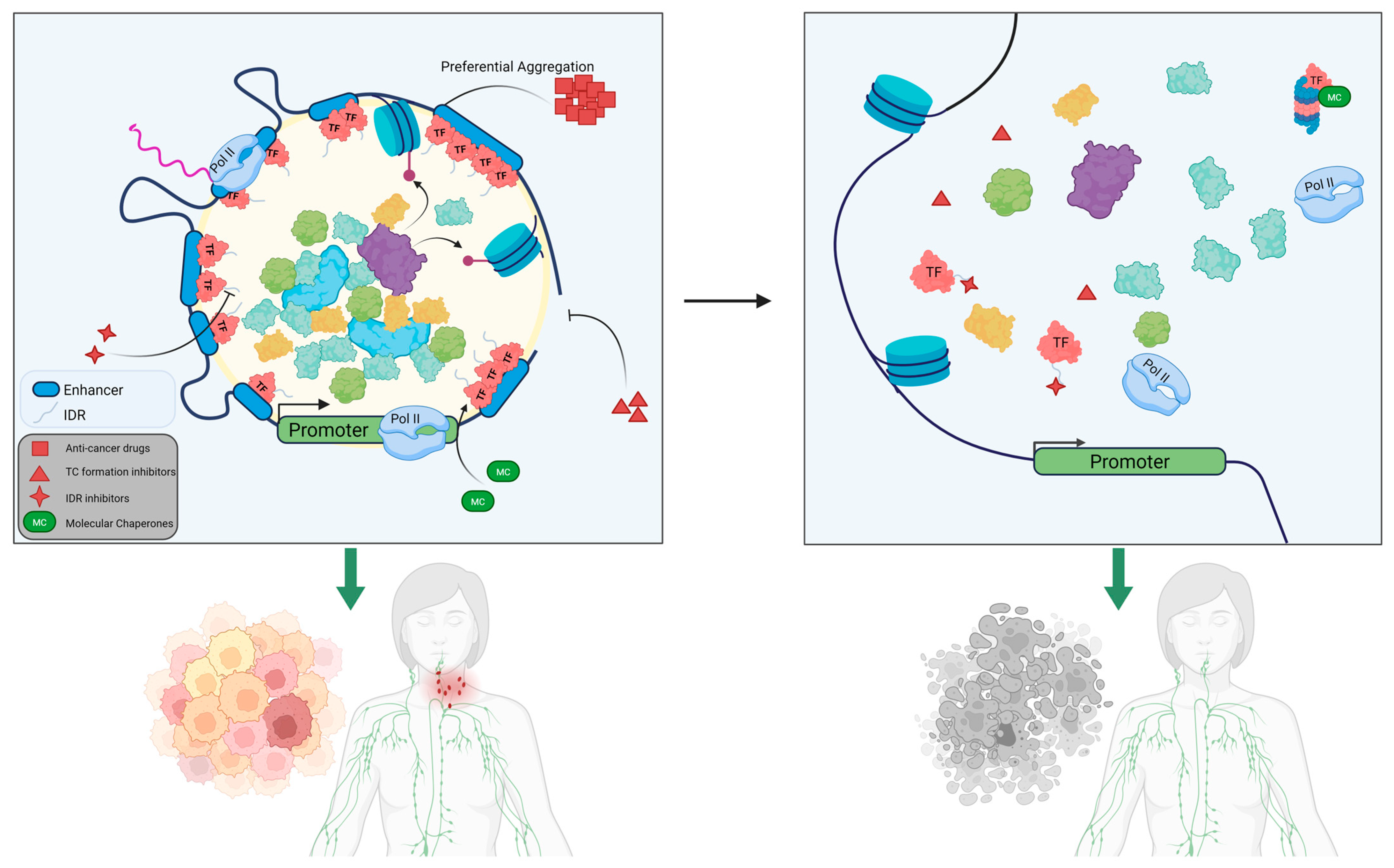
4.1. Targeting the SE Transcription Machinery of Transcriptional Condensates
4.2. Disruption of Transcriptional Condensates at Silent Enhancer Sites
4.3. Drugs Directly Interacting and Targeting IDRs
4.4. Degrading Biomolecular Condensate via Proteolysis
5. Concluding Remarks and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Kim, T.-K.; Shiekhattar, R. Architectural and Functional Commonalities between Enhancers and Promoters. Cell 2015, 162, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H.F.; Buecker, C. What is an enhancer? BioEssays News Rev. Mol. Cell. Dev. Biol. 2023, 45, e2300044. [Google Scholar] [CrossRef]
- Visel, A.; Rubin, E.M.; Pennacchio, L.A. Genomic views of distant-acting enhancers. Nature 2009, 461, 199–205. [Google Scholar] [CrossRef]
- Pobbati, A.V.; Hong, W. Emerging roles of TEAD transcription factors and its coactivators in c ancers. Cancer Biol. Ther. 2013, 14, 390–398. [Google Scholar] [CrossRef]
- Spiegelman, B.M.; Heinrich, R. Biological control through regulated transcriptional coactivators. Cell 2004, 119, 157–167. [Google Scholar] [CrossRef]
- Scholes, N.S.; Weinzierl, R.O.J. Molecular Dynamics of "Fuzzy" Transcriptional Activator-Coactivator Interactions. PLoS Comput. Biol. 2016, 12, e1004935. [Google Scholar] [CrossRef]
- Thomas, M.C.; Chiang, C.-M. The general transcription machinery and general cofactors. Crit. Rev. Biochem. Mol. Biol. 2006, 41, 105–178. [Google Scholar] [CrossRef]
- Hnisz, D.; Abraham, B.J.; Lee, T.I.; Lau, A.; Saint-André, V.; Sigova, A.A.; Hoke, H.A.; Young, R.A. Super-enhancers in the control of cell identity and disease. Cell 2013, 155, 934–947. [Google Scholar] [CrossRef]
- Bradner, J.E.; Hnisz, D.; Young, R.A. Transcriptional Addiction in Cancer. Cell 2017, 168, 629–643. [Google Scholar] [CrossRef]
- Lee, T.I.; Young, R.A. Transcriptional regulation and its misregulation in disease. Cell 2013, 152, 1237–1251. [Google Scholar] [CrossRef]
- Li, W.; Jiang, H. Nuclear Protein Condensates and Their Properties in Regulation of Gene Expression. J. Mol. Biol. 2022, 434, 167151. [Google Scholar] [CrossRef]
- Mann, R.; Notani, D. Transcription factor condensates and signaling driven transcription. Nucleus 2023, 14, 2205758. [Google Scholar] [CrossRef]
- Demmerle, J.; Hao, S.; Cai, D. Transcriptional condensates and phase separation: Condensing information across scales and mechanisms. Nucleus 2023, 14, 2213551. [Google Scholar] [CrossRef] [PubMed]
- Strom, A.R.; Brangwynne, C.P. The liquid nucleome—Phase transitions in the nucleus at a glance. J. Cell Sci. 2019, 132, jcs235093. [Google Scholar] [CrossRef]
- Sabari, B.R.; Dall’Agnese, A.; Boija, A.; Klein, I.A.; Coffey, E.L.; Shrinivas, K.; Abraham, B.J.; Hannett, N.M.; Zamudio, A.V.; Manteiga, J.C.; et al. Coactivator condensation at super-enhancers links phase separation and gene control. Science 2018, 361, eaar3958. [Google Scholar] [CrossRef]
- Cho, W.-K.; Spille, J.-H.; Hecht, M.; Lee, C.; Li, C.; Grube, V.; Cisse, I.I. Mediator and RNA polymerase II clusters associate in transcription-dep endent condensates. Science 2018, 361, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Feliciano, D.; Dong, P.; Flores, E.; Gruebele, M.; Porat-Shliom, N.; Sukenik, S.; Liu, Z.; Lippincott-Schwartz, J. Phase separation of YAP reorganizes genome topology for long-term YAP target gene expression. Nat. Cell Biol. 2019, 21, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wu, T.; Gutman, O.; Lu, H.; Zhou, Q.; Henis, Y.I.; Luo, K. Phase separation of TAZ compartmentalizes the transcription machinery to promote gene expression. Nat. Cell Biol. 2020, 22, 453–464. [Google Scholar] [CrossRef]
- Gibson, B.A.; Doolittle, L.K.; Schneider, M.W.G.; Jensen, L.E.; Gamarra, N.; Henry, L.; Gerlich, D.W.; Redding, S.; Rosen, M.K. Organization of Chromatin by Intrinsic and Regulated Phase Separation. Cell 2019, 179, 470–484.e421. [Google Scholar] [CrossRef]
- Larson, A.G.; Elnatan, D.; Keenen, M.M.; Trnka, M.J.; Johnston, J.B.; Burlingame, A.L.; Agard, D.A.; Redding, S.; Narlikar, G.J. Liquid droplet formation by HP1α suggests a role for phase separation in heterochromatin. Nature 2017, 547, 236–240. [Google Scholar] [CrossRef]
- Sanulli, S.; Trnka, M.J.; Dharmarajan, V.; Tibble, R.W.; Pascal, B.D.; Burlingame, A.L.; Griffin, P.R.; Gross, J.D.; Narlikar, G.J. HP1 reshapes nucleosome core to promote phase separation of heterochromatin. Nature 2019, 575, 390–394. [Google Scholar] [CrossRef]
- Liang, J.; Wang, Y.; Demmerle, J.; He, B.J.; Ricketts, C.J.; Linehan, W.M.; Zang, C.; Cai, D. TEAD1 condensates are transcriptionally inactive storage sites on the pericentromeric heterochromatin. bioRxiv 2025. [Google Scholar] [CrossRef] [PubMed]
- Taniue, K.; Akimitsu, N. Aberrant phase separation and cancer. FEBS J. 2022, 289, 17–39. [Google Scholar] [CrossRef] [PubMed]
- Wagh, K.; Garcia, D.A.; Upadhyaya, A. Phase separation in transcription factor dynamics and chromatin organization. Curr. Opin. Struct. Biol. 2021, 71, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; Zhang, J. Liquid-liquid phase separation drives cellular function and dysfunction in cancer. Nat. Rev. Cancer 2022, 22, 239–252. [Google Scholar] [CrossRef]
- Phair, R.D.; Misteli, T. High mobility of proteins in the mammalian cell nucleus. Nature 2000, 404, 604–609. [Google Scholar] [CrossRef]
- Snaar, S.; Wiesmeijer, K.; Jochemsen, A.G.; Tanke, H.J.; Dirks, R.W. Mutational analysis of fibrillarin and its mobility in living human cells. J. Cell Biol. 2000, 151, 653–662. [Google Scholar] [CrossRef]
- O’Connell, L.C.; Mowry, K.L. Regulation of spatially restricted gene expression: Linking RNA localization and phase separation. Biochem. Soc. Trans. 2021, 49, 2591–2600. [Google Scholar] [CrossRef]
- Zhang, H.; Ji, X.; Li, P.; Liu, C.; Lou, J.; Wang, Z.; Wen, W.; Xiao, Y.; Zhang, M.; Zhu, X. Liquid-liquid phase separation in biology: Mechanisms, physiological functions and human diseases. Sci. China Life Sci. 2020, 63, 953–985. [Google Scholar] [CrossRef]
- Fare, C.M.; Villani, A.; Drake, L.E.; Shorter, J. Higher-order organization of biomolecular condensates. Open Biol. 2021, 11, 210137. [Google Scholar] [CrossRef]
- Gomes, E.; Shorter, J. The molecular language of membraneless organelles. J. Biol. Chem. 2019, 294, 7115–7127. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Z.; Han, Q.; Maity, B.K.; Rodrigues, L.; Zboril, E.; Adhikari, R.; Ko, S.H.; Li, X.; Yoshida, S.R.; et al. Hormone-induced enhancer assembly requires an optimal level of hormone receptor multivalent interactions. Mol. Cell 2023, 83, 3438–3456.e12. [Google Scholar] [CrossRef]
- Fuxreiter, M. Fuzzy protein theory for disordered proteins. Biochem. Soc. Trans. 2020, 48, 2557–2564. [Google Scholar] [CrossRef]
- Iakoucheva, L.M.; Radivojac, P.; Brown, C.J.; O’Connor, T.R.; Sikes, J.G.; Obradovic, Z.; Dunker, A.K. The importance of intrinsic disorder for protein phosphorylation. Nucleic Acids Res. 2004, 32, 1037–1049. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Ward, P.S.; Thompson, C.B. Metabolic reprogramming: A cancer hallmark even warburg did not anticipate. Cancer Cell 2012, 21, 297–308. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef]
- Harlen, K.M.; Churchman, L.S. The code and beyond: Transcription regulation by the RNA polymerase II carboxy-terminal domain. Nat. Rev. Mol. Cell Biol. 2017, 18, 263–273. [Google Scholar] [CrossRef]
- Zaborowska, J.; Egloff, S.; Murphy, S. The pol II CTD: New twists in the tail. Nat. Struct. Mol. Biol. 2016, 23, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Boehning, M.; Dugast-Darzacq, C.; Rankovic, M.; Hansen, A.S.; Yu, T.; Marie-Nelly, H.; McSwiggen, D.T.; Kokic, G.; Dailey, G.M.; Cramer, P.; et al. RNA polymerase II clustering through carboxy-terminal domain phase separation. Nat. Struct. Mol. Biol. 2018, 25, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Hnisz, D.; Shrinivas, K.; Young, R.A.; Chakraborty, A.K.; Sharp, P.A. A Phase Separation Model for Transcriptional Control. Cell 2017, 169, 13–23. [Google Scholar] [CrossRef]
- Wu, Z.; Pope, S.D.; Ahmed, N.S.; Leung, D.L.; Hong, Y.; Hajjar, S.; Krabak, C.; Zhong, Z.; Raghunathan, K.; Yue, Q.; et al. Regulation of inflammatory responses by pH-dependent transcriptional condensates. Cell 2025. Online now. [Google Scholar] [CrossRef]
- Boija, A.; Klein, I.A.; Sabari, B.R.; Dall’Agnese, A.; Coffey, E.L.; Zamudio, A.V.; Li, C.H.; Shrinivas, K.; Manteiga, J.C.; Hannett, N.M.; et al. Transcription Factors Activate Genes through the Phase-Separation Capa city of Their Activation Domains. Cell 2018, 175, 1842–1855.e1816. [Google Scholar] [CrossRef]
- Chong, S.; Dugast-Darzacq, C.; Liu, Z.; Dong, P.; Dailey, G.M.; Cattoglio, C.; Heckert, A.; Banala, S.; Lavis, L.; Darzacq, X.; et al. Imaging dynamic and selective low-complexity domain interactions that control gene transcription. Science 2018, 361, eaar2555. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.-K.; Jayanth, N.; English, B.P.; Inoue, T.; Andrews, J.O.; Conway, W.; Grimm, J.B.; Spille, J.-H.; Lavis, L.D.; Lionnet, T.; et al. RNA Polymerase II cluster dynamics predict mRNA output in living cells. eLife 2016, 5, e13617. [Google Scholar] [CrossRef] [PubMed]
- Cisse, I.I.; Izeddin, I.; Causse, S.Z.; Boudarene, L.; Senecal, A.; Muresan, L.; Dugast-Darzacq, C.; Hajj, B.; Dahan, M.; Darzacq, X. Real-time dynamics of RNA polymerase II clustering in live human cells. Science 2013, 341, 664–667. [Google Scholar] [CrossRef]
- Cho, W.-K.; Jayanth, N.; Mullen, S.; Tan, T.H.; Jung, Y.J.; Cissé, I.I. Super-resolution imaging of fluorescently labeled, endogenous RNA Polymerase II in living cells with CRISPR/Cas9-mediated gene editing. Sci. Rep. 2016, 6, 35949. [Google Scholar] [CrossRef]
- Bulger, M.; Groudine, M. Functional and mechanistic diversity of distal transcription enhancers. Cell 2011, 144, 327–339. [Google Scholar] [CrossRef]
- Xie, W.; Ren, B. Developmental biology. Enhancing pluripotency and lineage specification. Science 2013, 341, 245–247. [Google Scholar] [CrossRef]
- Sabari, B.R.; Dall’Agnese, A.; Young, R.A. Biomolecular Condensates in the Nucleus. Trends Biochem. Sci. 2020, 45, 961–977. [Google Scholar] [CrossRef] [PubMed]
- Whyte, W.A.; Orlando, D.A.; Hnisz, D.; Abraham, B.J.; Lin, C.Y.; Kagey, M.H.; Rahl, P.B.; Lee, T.I.; Young, R.A. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell 2013, 153, 307–319. [Google Scholar] [CrossRef]
- Hnisz, D.; Schuijers, J.; Lin, C.Y.; Weintraub, A.S.; Abraham, B.J.; Lee, T.I.; Bradner, J.E.; Young, R.A. Convergence of developmental and oncogenic signaling pathways at trans criptional super-enhancers. Mol. Cell 2015, 58, 362–370. [Google Scholar] [CrossRef]
- Bahr, C.; Paleske, L.; Uslu, V.V.; Remeseiro, S.; Takayama, N.; Ng, S.W.; Murison, A.; Langenfeld, K.; Petretich, M.; Scognamiglio, R.; et al. A Myc enhancer cluster regulates normal and leukaemic haematopoietic stem cell hierarchies. Nature 2018, 553, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Scholz, B.A.; Sumida, N.; Lima, C.D.M.; Chachoua, I.; Martino, M.; Tzelepis, I.; Nikoshkov, A.; Zhao, H.; Mehmood, R.; Sifakis, E.G.; et al. WNT signaling and AHCTF1 promote oncogenic MYC expression through supe r-enhancer-mediated gene gating. Nat. Genet. 2019, 51, 1723–1731. [Google Scholar] [CrossRef]
- Lovén, J.; Hoke, H.A.; Lin, C.Y.; Lau, A.; Orlando, D.A.; Vakoc, C.R.; Bradner, J.E.; Lee, T.I.; Young, R.A. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell 2013, 153, 320–334. [Google Scholar] [CrossRef]
- Kwiatkowski, N.; Zhang, T.; Rahl, P.B.; Abraham, B.J.; Reddy, J.; Ficarro, S.B.; Dastur, A.; Amzallag, A.; Ramaswamy, S.; Tesar, B.; et al. Targeting transcription regulation in cancer with a covalent CDK7 inhibitor. Nature 2014, 511, 616–620. [Google Scholar] [CrossRef]
- Proudhon, C.; Snetkova, V.; Raviram, R.; Lobry, C.; Badri, S.; Jiang, T.; Hao, B.; Trimarchi, T.; Kluger, Y.; Aifantis, I.; et al. Active and Inactive Enhancers Cooperate to Exert Localized and Long-Range Control of Gene Regulation. Cell Rep. 2016, 15, 2159–2169. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; George, R.E. Super-Enhancer-Driven Transcriptional Dependencies in Cancer. Trends Cancer 2017, 3, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.E.; Manteiga, J.C.; Henninger, J.E.; Sabari, B.R.; Dall’Agnese, A.; Hannett, N.M.; Spille, J.-H.; Afeyan, L.K.; Zamudio, A.V.; Shrinivas, K.; et al. Pol II phosphorylation regulates a switch between transcriptional and splicing condensates. Nature 2019, 572, 543–548. [Google Scholar] [CrossRef]
- Kwon, I.; Kato, M.; Xiang, S.; Wu, L.; Theodoropoulos, P.; Mirzaei, H.; Han, T.; Xie, S.; Corden, J.L.; McKnight, S.L. Phosphorylation-regulated binding of RNA polymerase II to fibrous poly mers of low-complexity domains. Cell 2013, 155, 1049–1060. [Google Scholar] [CrossRef]
- Kroschwald, S.; Maharana, S.; Mateju, D.; Malinovska, L.; Nüske, E.; Poser, I.; Richter, D.; Alberti, S. Promiscuous interactions and protein disaggregases determine the material state of stress-inducible RNP granules. eLife 2015, 4, e06807. [Google Scholar] [CrossRef]
- Kroschwald, S.; Maharana, S.; Simon, A. Hexanediol: A chemical probe to investigate the material properties of membrane-less compartments. Matters 2017, 3, e201702000010. [Google Scholar] [CrossRef]
- Du, M.; Stitzinger, S.H.; Spille, J.H.; Cho, W.K.; Lee, C.; Hijaz, M.; Quintana, A.; Cissé, I.I. Direct observation of a condensate effect on super-enhancer controlled gene bursting. Cell 2024, 187, 331–344.e17. [Google Scholar] [CrossRef]
- Yamamoto, T.; Sakaue, T.; Schiessel, H. Slow chromatin dynamics enhances promoter accessibility to transcriptional condensates. Nucleic Acids Res. 2021, 49, 5017–5027. [Google Scholar] [CrossRef]
- Dean, A.; Larson, D.R.; Sartorelli, V. Enhancers, gene regulation, and genome organization. Genes Dev. 2021, 35, 427–432. [Google Scholar] [CrossRef]
- Heist, T.; Fukaya, T.; Levine, M. Large distances separate coregulated genes in living Drosophila embryo s. Proc. Natl. Acad. Sci. USA 2019, 116, 15062–15067. [Google Scholar] [CrossRef] [PubMed]
- Steensel, B.; Furlong, E.E.M. The role of transcription in shaping the spatial organization of the genome. Nat. Rev. Mol. Cell Biol. 2019, 20, 327–337. [Google Scholar] [CrossRef]
- Hua, P.; Badat, M.; Hanssen, L.L.P.; Hentges, L.D.; Crump, N.; Downes, D.J.; Jeziorska, D.M.; Oudelaar, A.M.; Schwessinger, R.; Taylor, S.; et al. Defining genome architecture at base-pair resolution. Nature 2021, 595, 125–129. [Google Scholar] [CrossRef]
- Liu, Y.; Wan, X.; Li, H.; Chen, Y.; Hu, X.; Chen, H.; Zhu, D.; Li, C.; Zhang, Y. CTCF coordinates cell fate specification via orchestrating regulatory hubs with pioneer transcription factors. Cell Rep. 2023, 42, 113259. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Kang, M.-K.; Kim, Y.-J.; Yang, B.; Shim, H.; Kim, S.; Kim, K.; Yang, C.M.; Min, B.-g.; Jung, W.-J.; et al. CTCF-mediated chromatin looping provides a topological framework for the formation of phase-separated transcriptional condensates. Nucleic Acids Res. 2022, 50, 207–226. [Google Scholar] [CrossRef] [PubMed]
- Kadoch, C.; Hargreaves, D.C.; Hodges, C.; Elias, L.; Ho, L.; Ranish, J.; Crabtree, G.R. Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes id entifies extensive roles in human malignancy. Nat. Genet. 2013, 45, 592–601. [Google Scholar] [CrossRef]
- Maneix, L.; Iakova, P.; Moree, S.E.; Hsu, J.I.; Mistry, R.M.; Stossi, F.; Lulla, P.; Sun, Z.; Sahin, E.; Yellapragada, S.V.; et al. Proteasome Inhibitors Silence Oncogenes in Multiple Myeloma through Localized Histone Deacetylase 3 (HDAC3) Stabilization and Chromatin Condensation. Cancer Res. Commun. 2022, 2, 1693–1710. [Google Scholar] [CrossRef] [PubMed]
- Boija, A.; Klein, I.A.; Young, R.A. Biomolecular Condensates and Cancer. Cancer Cell 2021, 39, 174–192. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.R.; Abraham, B.J.; Anders, L.; Berezovskaya, A.; Gutierrez, A.; Durbin, A.D.; Etchin, J.; Lawton, L.; Sallan, S.E.; Silverman, L.B.; et al. Oncogene regulation. An oncogenic super-enhancer formed through somatic mutation of a noncoding intergenic element. Science 2014, 346, 1373–1377. [Google Scholar] [CrossRef]
- Shrinivas, K.; Sabari, B.R.; Coffey, E.L.; Klein, I.A.; Boija, A.; Zamudio, A.V.; Schuijers, J.; Hannett, N.M.; Sharp, P.A.; Young, R.A.; et al. Enhancer Features that Drive Formation of Transcriptional Condensates. Mol. Cell 2019, 75, 549–561.e547. [Google Scholar] [CrossRef]
- Terlecki-Zaniewicz, S.; Humer, T.; Eder, T.; Schmoellerl, J.; Heyes, E.; Manhart, G.; Kuchynka, N.; Parapatics, K.; Liberante, F.G.; Müller, A.C.; et al. Biomolecular condensation of NUP98 fusion proteins drives leukemogenic gene expression. Nat. Struct. Mol. Biol. 2021, 28, 190–201. [Google Scholar] [CrossRef]
- Ahn, J.H.; Davis, E.S.; Daugird, T.A.; Zhao, S.; Quiroga, I.Y.; Uryu, H.; Li, J.; Storey, A.J.; Tsai, Y.-H.; Keeley, D.P.; et al. Phase separation drives aberrant chromatin looping and cancer development. Nature 2021, 595, 591–595. [Google Scholar] [CrossRef]
- Shi, B.; Li, W.; Song, Y.; Wang, Z.; Ju, R.; Ulman, A.; Hu, J.; Palomba, F.; Zhao, Y.; Le, J.P.; et al. UTX condensation underlies its tumour-suppressive activity. Nature 2021, 597, 726–731. [Google Scholar] [CrossRef]
- Andricovich, J.; Perkail, S.; Kai, Y.; Casasanta, N.; Peng, W.; Tzatsos, A. Loss of KDM6A Activates Super-Enhancers to Induce Gender-Specific Squamous-like Pancreatic Cancer and Confers Sensitivity to BET Inhibitors. Cancer Cell 2018, 33, 512–526.e518. [Google Scholar] [CrossRef]
- Wang, L.; Shilatifard, A. UTX Mutations in Human Cancer. Cancer Cell 2019, 35, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Chapuy, B.; McKeown, M.R.; Lin, C.Y.; Monti, S.; Roemer, M.G.M.; Qi, J.; Rahl, P.B.; Sun, H.H.; Yeda, K.T.; Doench, J.G.; et al. Discovery and characterization of super-enhancer-associated dependencies in diffuse large B cell lymphoma. Cancer Cell 2013, 24, 777–790. [Google Scholar] [CrossRef]
- Słabicki, M.; Yoon, H.; Koeppel, J.; Nitsch, L.; Roy Burman, S.S.; Di Genua, C.; Donovan, K.A.; Sperling, A.S.; Hunkeler, M.; Tsai, J.M.; et al. Small-molecule-induced polymerization triggers degradation of BCL6. Nature 2020, 588, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, M.Y.; Jiang, H.; Lwin, T.; Park, P.M.; Gao, J.; Meads, M.B.; Ren, Y.; Li, T.; Sun, J.; et al. Transcriptional programming drives Ibrutinib-resistance evolution in mantle cell lymphoma. Cell Rep. 2021, 34, 108870. [Google Scholar] [CrossRef]
- Long, H.K.; Prescott, S.L.; Wysocka, J. Ever-Changing Landscapes: Transcriptional Enhancers in Development and Evolution. Cell 2016, 167, 1170–1187. [Google Scholar] [CrossRef] [PubMed]
- Meeussen, J.V.W.; Pomp, W.; Brouwer, I.; Jonge, W.J.; Patel, H.P.; Lenstra, T.L. Transcription factor clusters enable target search but do not contribute to target gene activation. Nucleic Acids Res. 2023, 51, 5449–5468. [Google Scholar] [CrossRef]
- Shi, M.; You, K.; Chen, T.; Hou, C.; Liang, Z.; Liu, M.; Wang, J.; Wei, T.; Qin, J.; Chen, Y.; et al. Quantifying the phase separation property of chromatin-associated proteins under physiological conditions using an anti-1,6-hexanediol index. Genome Biol. 2021, 22, 229. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, Z.; Zang, C. Genomic clustering tendency of transcription factors reflects phase-separated transcriptional condensates at super-enhancers. Nucleic Acids Res. 2025, 53, gkaf015. [Google Scholar] [CrossRef]
- Fang, C.; Wang, Z.; Han, C.; Safgren, S.L.; Helmin, K.A.; Adelman, E.R.; Serafin, V.; Basso, G.; Eagen, K.P.; Gaspar-Maia, A.; et al. Cancer-specific CTCF binding facilitates oncogenic transcriptional dysregulation. Genome Biol. 2020, 21, 247. [Google Scholar] [CrossRef]
- Taylor, N.O.; Wei, M.T.; Stone, H.A.; Brangwynne, C.P. Quantifying Dynamics in Phase-Separated Condensates Using Fluorescence Recovery after Photobleaching. Biophys. J. 2019, 117, 1285–1300. [Google Scholar] [CrossRef]
- Shin, Y.; Berry, J.; Pannucci, N.; Haataja, M.P.; Toettcher, J.E.; Brangwynne, C.P. Spatiotemporal control of intracellular phase transitions using light-activated optoDroplets. Cell 2016, 168, 159–171.e14. [Google Scholar] [CrossRef]
- Skene, P.J.; Henikoff, S. An efficient targeted nuclease strategy for high-resolution mapping of DNA binding sites. eLife 2017, 6, e21856. [Google Scholar] [CrossRef] [PubMed]
- Belton, J.-M.; McCord, R.P.; Gibcus, J.; Naumova, N.; Zhan, Y.; Dekker, J. Hi-C: A comprehensive technique to capture the conformation of genomes. Methods 2012, 58, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Mumbach, M.R.; Rubin, A.J.; Flynn, R.A.; Dai, C.; Khavari, P.A.; Greenleaf, W.J.; Chang, H.Y.; Mumbach, M.R.; Rubin, A.J.; Flynn, R.A.; et al. HiChIP: Efficient and sensitive analysis of protein-directed genome architecture. Nat. Methods 2016, 13, 919–922. [Google Scholar] [CrossRef]
- Rhine, K.; Skanchy, S.; Myong, S. Single-molecule and ensemble methods to probe RNP nucleation and condensate properties. Methods 2022, 197, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Roe, J.-S.; Mercan, F.; Rivera, K.; Pappin, D.J.; Vakoc, C.R. BET Bromodomain Inhibition Suppresses the Function of Hematopoietic Transcription Factors in Acute Myeloid Leukemia. Mol. Cell 2015, 58, 1028–1039. [Google Scholar] [CrossRef]
- Bhagwat, A.S.; Roe, J.-S.; Mok, B.Y.L.; Hohmann, A.F.; Shi, J.; Vakoc, C.R. BET Bromodomain Inhibition Releases the Mediator Complex from Select c is-Regulatory Elements. Cell Rep. 2016, 15, 519–530. [Google Scholar] [CrossRef]
- Fong, C.Y.; Gilan, O.; Lam, E.Y.N.; Rubin, A.F.; Ftouni, S.; Tyler, D.; Stanley, K.; Sinha, D.; Yeh, P.; Morison, J.; et al. BET inhibitor resistance emerges from leukaemia stem cells. Nature 2015, 525, 538–542. [Google Scholar] [CrossRef]
- Rathert, P.; Roth, M.; Neumann, T.; Muerdter, F.; Roe, J.-S.; Muhar, M.; Deswal, S.; Cerny-Reiterer, S.; Peter, B.; Jude, J.; et al. Transcriptional plasticity promotes primary and acquired resistance to BET inhibition. Nature 2015, 525, 543–547. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, S.; Nie, D.; Lai, P.; Li, Y.; Li, Y.; Jin, Y.; Pan, J. Super-enhancer landscape reveals leukemia stem cell reliance on X-box binding protein 1 as a therapeutic vulnerability. Sci. Transl. Med. 2021, 13, eabh3462. [Google Scholar] [CrossRef]
- Zhao, X.; Ren, Y.; Lawlor, M.; Shah, B.D.; Park, P.M.C.; Lwin, T.; Wang, X.; Liu, K.; Wang, M.; Gao, J.; et al. BCL2 Amplicon Loss and Transcriptional Remodeling Drives ABT-199 Resistance in B Cell Lymphoma Models. Cancer Cell 2019, 35, 752–766.e759. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, S.; Zang, C. Spatially clustered pattern of transcription factor binding reveals phase-separated transcriptional condensates at super-enhancers. bioRxiv 2023. bioRxiv:18.545510. [Google Scholar] [CrossRef]
- Wang, C.; Lu, H.; Liu, X.; Gao, X.; Tian, W.; Chen, H.; Xue, Y.; Zhou, Q. A natural product targets BRD4 to inhibit phase separation and gene transcription. iScience 2022, 25, 103719. [Google Scholar] [CrossRef] [PubMed]
- Berg, T.; Cohen, S.B.; Desharnais, J.; Sonderegger, C.; Maslyar, D.J.; Goldberg, J.; Boger, D.L.; Vogt, P.K. Small-molecule antagonists of Myc/Max dimerization inhibit Myc-induced transformation of chicken embryo fibroblasts. Proc. Natl. Acad. Sci. USA 2002, 99, 3830–3835. [Google Scholar] [CrossRef] [PubMed]
- Klein, I.A.; Boija, A.; Afeyan, L.K.; Hawken, S.W.; Fan, M.; Dall’Agnese, A.; Oksuz, O.; Henninger, J.E.; Shrinivas, K.; Sabari, B.R.; et al. Partitioning of cancer therapeutics in nuclear condensates. Science 2020, 368, 1386–1392. [Google Scholar] [CrossRef]
- Yu, M.; Peng, Z.; Qin, M.; Liu, Y.; Wang, J.; Zhang, C.; Lin, J.; Dong, T.; Wang, L.; Li, S.; et al. Interferon-γ induces tumor resistance to anti-PD-1 immunotherapy by promoting YAP phase separation. Mol. Cell 2021, 81, 1216–1230.e1219. [Google Scholar] [CrossRef]
- Zhu, G.; Xie, J.; Fu, Z.; Wang, M.; Zhang, Q.; He, H.; Chen, Z.; Guo, X.; Zhu, J. Pharmacological inhibition of SRC-1 phase separation suppresses YAP on cogenic transcription activity. Cell Res. 2021, 31, 1028–1031. [Google Scholar] [CrossRef]
- Huang, J.; Wu, S.; Barrera, J.; Matthews, K.; Pan, D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell 2005, 122, 421–434. [Google Scholar] [CrossRef]
- Lopez-Hernandez, A.; Sberna, S.; Campaner, S. Emerging Principles in the Transcriptional Control by YAP and TAZ. Cancers 2021, 13, 4242. [Google Scholar] [CrossRef]
- Girdhar, A.; Bharathi, V.; Tiwari, V.R.; Abhishek, S.; Deeksha, W.; Mahawar, U.S.; Raju, G.; Singh, S.K.; Prabusankar, G.; Rajakumara, E.; et al. Computational insights into mechanism of AIM4-mediated inhibition of aggregation of TDP-43 protein implicated in ALS and evidence for in vitro inhibition of liquid-liquid phase separation (LLPS) of TDP-432C-A31 5T by AIM4. Int. J. Biol. Macromol. 2020, 147, 117–130. [Google Scholar] [CrossRef]
- Strzyz, P. Drugs enter a liquid phase. Nat. Rev. Mol. Cell Biol. 2020, 21, 419. [Google Scholar] [CrossRef]
- Wang, G.G.; Cai, L.; Pasillas, M.P.; Kamps, M.P. NUP98-NSD1 links H3K36 methylation to Hox-A gene activation and leukaemogenesis. Nat. Cell Biol. 2007, 9, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Jevtic, Z.; Matafora, V.; Casagrande, F.; Santoro, F.; Minucci, S.; Garre, M.; Rasouli, M.; Heidenreich, O.; Musco, G.; Schwaller, J.; et al. SMARCA5 interacts with NUP98-NSD1 oncofusion protein and sustains hematopoietic cells transformation. J. Exp. Clin. Cancer Res. CR 2022, 41, 34. [Google Scholar] [CrossRef] [PubMed]
- Csizmok, V.; Follis, A.V.; Kriwacki, R.W.; Forman-Kay, J.D. Dynamic Protein Interaction Networks and New Structural Paradigms in Signaling. Chem. Rev. 2016, 116, 6424–6462. [Google Scholar] [CrossRef] [PubMed]
- Dang, C.V.; Reddy, E.P.; Shokat, K.M.; Soucek, L. Drugging the ‘undruggable’ cancer targets. Nat. Rev. Cancer 2017, 17, 502–508. [Google Scholar] [CrossRef]
- Lee, R.; Buljan, M.; Lang, B.; Weatheritt, R.J.; Daughdrill, G.W.; Dunker, A.K.; Fuxreiter, M.; Gough, J.; Gsponer, J.; Jones, D.T.; et al. Classification of intrinsically disordered regions and proteins. Chem. Rev. 2014, 114, 6589–6631. [Google Scholar] [CrossRef]
- Uversky, V.N. Intrinsically disordered proteins from A to Z. Int. J. Biochem. Cell Biol. 2011, 43, 1090–1103. [Google Scholar] [CrossRef]
- Wright, P.E.; Dyson, H.J. Intrinsically disordered proteins in cellular signalling and regulation. Nat. Rev. Mol. Cell Biol. 2015, 16, 18–29. [Google Scholar] [CrossRef]
- Chen, J.; Kriwacki, R.W. Intrinsically Disordered Proteins: Structure, Function and Therapeutic s. J. Mol. Biol. 2018, 430, 2275–2277. [Google Scholar] [CrossRef]
- Metallo, S.J. Intrinsically disordered proteins are potential drug targets. Curr. Opin. Chem. Biol. 2010, 14, 481–488. [Google Scholar] [CrossRef]
- Martin, E.W.; Holehouse, A.S. Intrinsically disordered protein regions and phase separation: Sequence determinants of assembly or lack thereof. Emerg. Top. Life Sci. 2020, 4, 307–329. [Google Scholar] [CrossRef]
- Zhang, Z.; Boskovic, Z.; Hussain, M.M.; Hu, W.; Inouye, C.; Kim, H.-J.; Abole, A.K.; Doud, M.K.; Lewis, T.A.; Koehler, A.N.; et al. Chemical perturbation of an intrinsically disordered region of TFIID distinguishes two modes of transcription initiation. eLife 2015, 4, e07777. [Google Scholar] [CrossRef][Green Version]
- Lei, L.; Wu, Z.; Winklhofer, K.F. Protein quality control by the proteasome and autophagy: A regulatory role of ubiquitin and liquid-liquid phase separation. Matrix Biol. J. Int. Soc. Matrix Biol. 2021, 100–101, 9–22. [Google Scholar] [CrossRef]
- Ye, B.H. BCL-6 in the pathogenesis of non-Hodgkin’s lymphoma. Cancer Investig. 2000, 18, 356–365. [Google Scholar] [CrossRef]
- Mogk, A.; Bukau, B.; Kampinga, H.H. Cellular Handling of Protein Aggregates by Disaggregation Machines. Mol. Cell 2018, 69, 214–226. [Google Scholar] [CrossRef]
- Jiang, Y.; Rossi, P.; Kalodimos, C.G. Structural basis for client recognition and activity of Hsp40 chaperones. Science 2019, 365, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Faust, O.; Abayev-Avraham, M.; Wentink, A.S.; Maurer, M.; Nillegoda, N.B.; London, N.; Bukau, B.; Rosenzweig, R. HSP40 proteins use class-specific regulation to drive HSP70 functional diversity. Nature 2020, 587, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Mateju, D.; Franzmann, T.M.; Patel, A.; Kopach, A.; Boczek, E.E.; Maharana, S.; Lee, H.O.; Carra, S.; Hyman, A.A.; Alberti, S. An aberrant phase transition of stress granules triggered by misfolded protein and prevented by chaperone function. EMBO J. 2017, 36, 1669–1687. [Google Scholar] [CrossRef]
- Kampinga, H.H.; Andreasson, C.; Barducci, A.; Cheetham, M.E.; Cyr, D.; Emanuelsson, C.; Genevaux, P.; Gestwicki, J.E.; Goloubinoff, P.; Huerta-Cepas, J.; et al. Function, evolution, and structure of J-domain proteins. Cell Stress Chaperones 2019, 24, 7–15. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Greenwood, N.; Hernandez, J.; Cuperus, J.T.; Huang, B.; Ryder, B.D.; Queitsch, C.; Gestwicki, J.E.; Baker, D. De novo designed Hsp70 activator dissolves intracellular condensates. bioRxiv 2023. bioRxiv:18.558356. [Google Scholar] [CrossRef]
- Goloubinoff, P.; Sassi, A.S.; Fauvet, B.; Barducci, A.; De Los Rios, P. Chaperones convert the energy from ATP into the nonequilibrium stabili zation of native proteins. Nat. Chem. Biol. 2018, 14, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Z.; Lu, T.-W.; Stolerman, L.M.; Tenner, B.; Yang, J.R.; Zhang, J.-F.; Falcke, M.; Rangamani, P.; Taylor, S.S.; Mehta, S.; et al. Phase Separation of a PKA Regulatory Subunit Controls cAMP Compartmentation and Oncogenic Signaling. Cell 2020, 182, 1531–1544.e1515. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, M.C.; Burn, T.C.; Sparks, R.; Maduskuie, T.; Diamond, S.; Rupar, M.; Wen, X.; Volgina, A.; Zolotarjova, N.; Waeltz, P.; et al. The Novel Bromodomain and Extraterminal Domain Inhibitor INCB054329 Induces Vulnerabilities in Myeloma Cells That Inform Rational Combination Strategies. Clin. Cancer Res. Off. J. Am. Assoc. Ion Cancer Res. 2019, 25, 300–311. [Google Scholar] [CrossRef] [PubMed]

| Malignancy | Prevalence of TC Alterations | Condensate Components |
|---|---|---|
| AML [76] | 5–11% pediatric, 2–4% adult AML | NUP98 chimeras, EP300, CREBBP, BRD4, MED1 |
| DLBCL [81,82] | BCL6+ in 70%, BRD4+ in 85% | BCL6, BRD4, MED1, p300 |
| Multiple Myeloma [55,72] | MYC dysregulation in >40% | BRD4, CDK9, HDAC3 |
| T-ALL [74] | TAL1 alterations in 60% | MYB, TAL1 SE |
| CML [56] | CDK7-dependent in all cases | CDK7, BRD4, MED1 |
| MCL [83] | CDK9 addiction post-ibrutinib | CDK9, BRD4, P53 |
| Strategy | Anti-TC Mechanism of Action | Drug Examples | Resistance Mechanisms |
|---|---|---|---|
| BET Bromodomain Inhibition [55,97,98] | Displaces BRD4 from acetylated chromatin | JQ1, OTX015, INCB054329 | Wnt/β-catenin activation, PRC2 loss |
| CDK7 Inhibition [56,101] | Blocks Pol II CTD Ser5 phosphorylation | THZ1, SY-1365, CT7001 | MYC-independent programs |
| CDK9 Inhibition [83,100] | Prevents transcription elongation | Dinaciclib, AZD4573 | Alternative elongation factors |
| Direct IDR Targeting [102,103] | Binds IDR motifs, prevents LLPS | PCG, mycmycin-1/2 | Unknown |
| Induced Degradation [82] | Polymerization triggers proteasomal degradation | BI-3802 | Proteasome inhibition |
| Condensate Drug Partitioning [104] | Selective accumulation in TCs | Cisplatin (repurposing), tamoxifen (repurposing) | Condensate dissolution |
| YAP/TAZ Modulation [17,105,106] | Disrupts Hippo pathway condensates | Verteporfin (FDA approved), elvitegravir (repurposing) | YAP-independent pathways |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, K.; Yin, Q.; Zang, C.; Tao, J. Transcriptional Condensates: Epigenetic Reprogramming and Therapeutic Targets in Hematologic Malignancies. Cancers 2025, 17, 3148. https://doi.org/10.3390/cancers17193148
Qiu K, Yin Q, Zang C, Tao J. Transcriptional Condensates: Epigenetic Reprogramming and Therapeutic Targets in Hematologic Malignancies. Cancers. 2025; 17(19):3148. https://doi.org/10.3390/cancers17193148
Chicago/Turabian StyleQiu, Kevin, Qing Yin, Chongzhi Zang, and Jianguo Tao. 2025. "Transcriptional Condensates: Epigenetic Reprogramming and Therapeutic Targets in Hematologic Malignancies" Cancers 17, no. 19: 3148. https://doi.org/10.3390/cancers17193148
APA StyleQiu, K., Yin, Q., Zang, C., & Tao, J. (2025). Transcriptional Condensates: Epigenetic Reprogramming and Therapeutic Targets in Hematologic Malignancies. Cancers, 17(19), 3148. https://doi.org/10.3390/cancers17193148






