Unveiling the Mechanisms for the Development of Cardiotoxicity Following Chemotherapy Regimens Administration for Primary Colorectal Cancer: A Systematic Review
Simple Summary
Abstract
1. Introduction
Objective
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection—Eligibility Criteria
2.3. Inclusion Criteria
- Studies focusing on patients aged older than 19 years old diagnosed with primary CRC undergoing chemotherapy.
- Eligible interventions comprise commonly used chemotherapeutic agents such as fluoropyrimidines (5-fluorouracil and capecitabine), oxaliplatin, irinotecan, and regimens like FOLFOX, CAPEOX, FOLFIRI, and FOLFOXIRI.
- Studies must report cardiotoxicity outcomes including cardiac dysfunction, arrhythmias, myocarditis, QT prolongation, heart failure, or ischemic events in patients without a prior history of cardiovascular disease.
- Only clinical trials, cohort studies, case reports, cased series, case–control designs, and mechanistic studies published up to 2025 in English, with full-text availability, are considered for evaluation.
2.4. Exclusion Criteria
- Studies involving non-adult patients (under the age of 19 years old).
- Patients with metastatic CRC, other gastrointestinal malignancies, disease recurrence or non-cancer diagnoses.
- Interventions limited to radiotherapy or immunotherapy alone, or chemotherapy unrelated to CRC.
- Studies not reporting cardiovascular outcomes, or those involving patients with known cardiovascular disease.
- Additional filters must remove non-English studies without translation.
- Editorials, commentaries, abstracts, literature reviews and non-human or in vitro studies.
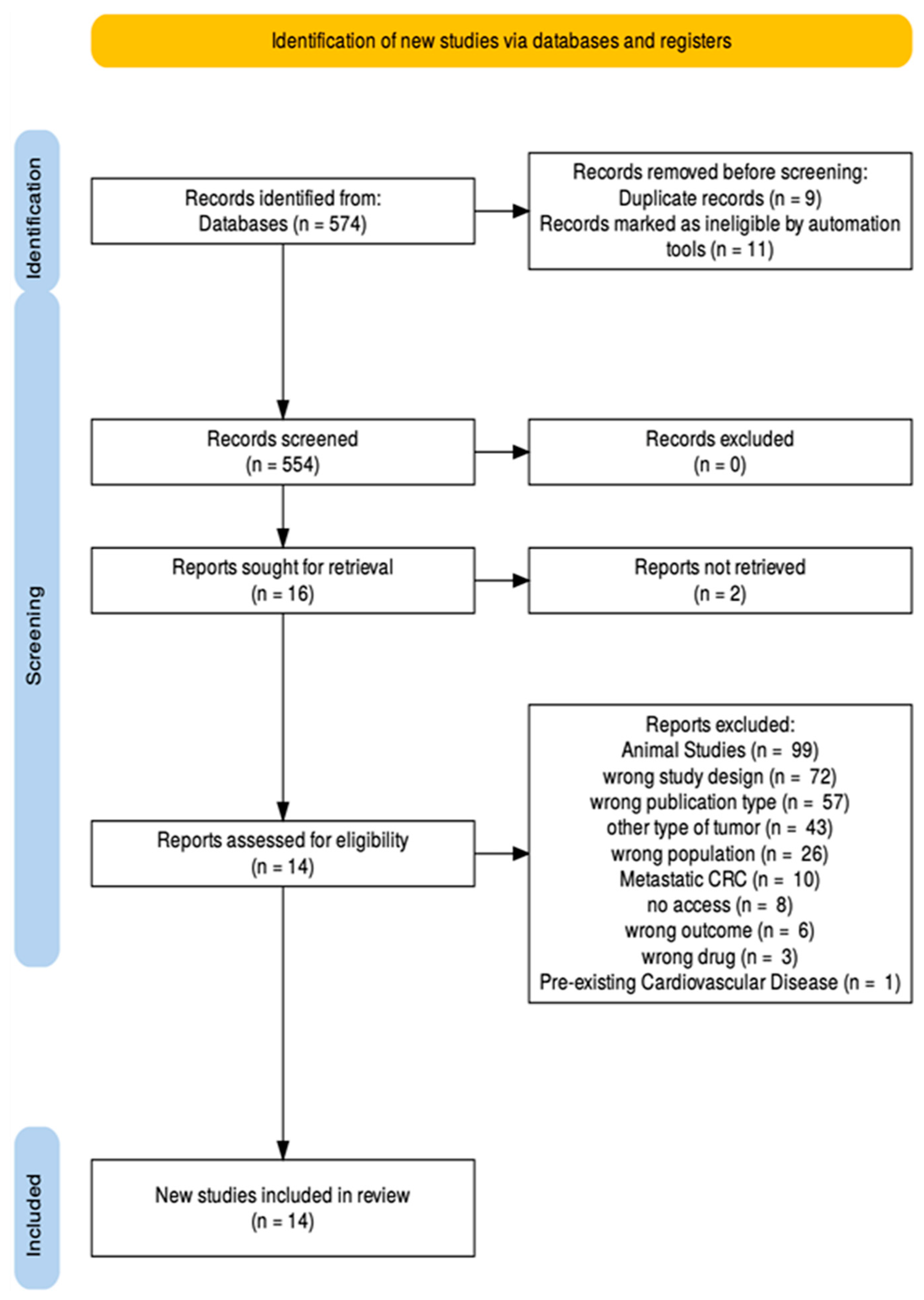
2.5. Screening Process
2.6. Quality Assessment
3. Results
3.1. Study Design and Geographical Scope of Included Research
3.2. Quality Assessment—Newcastle-Ottawa Scale (NOS)
3.3. Quality Assessment—JBI Checklist
4. Discussion
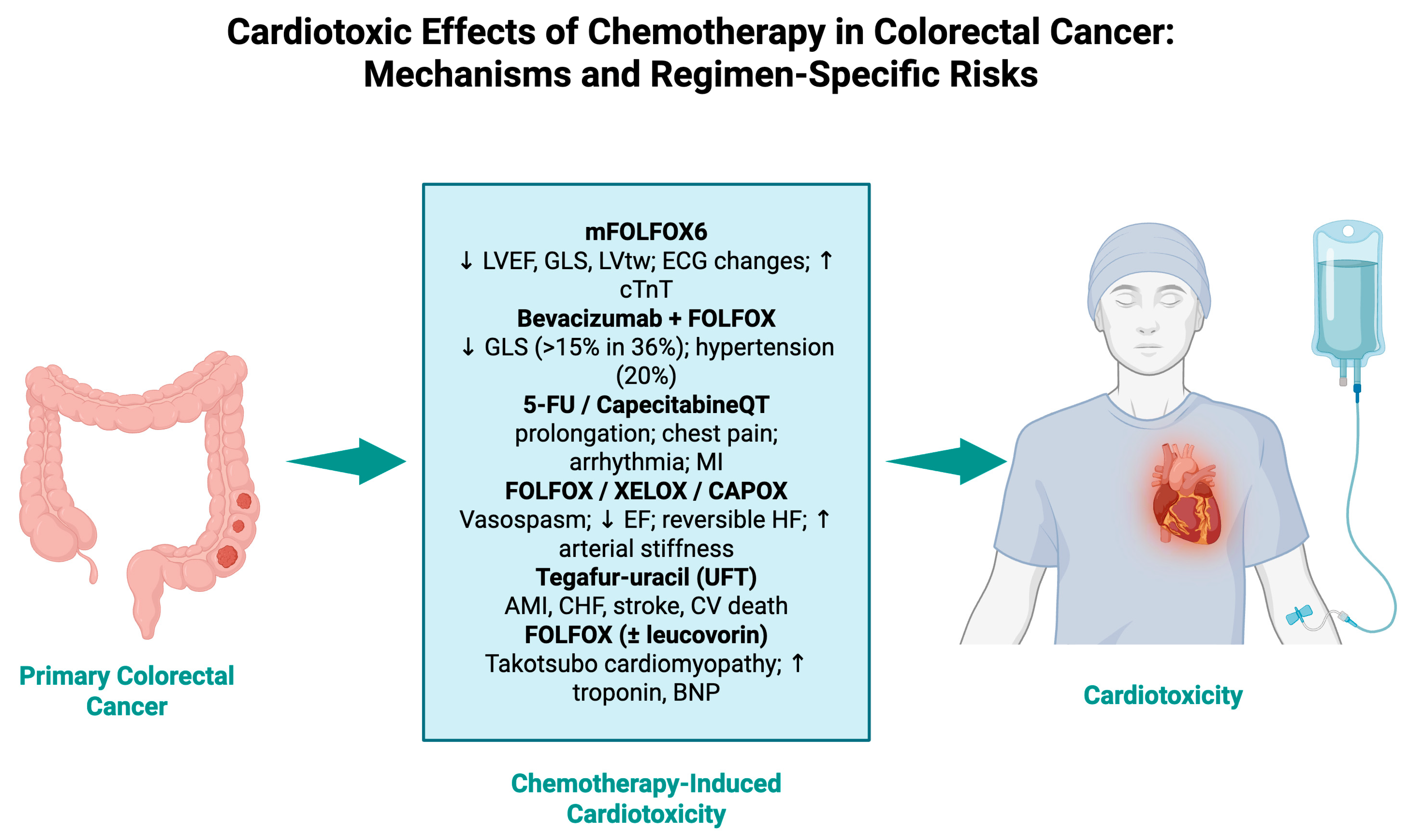
4.1. Chemotherapy Regimens and Treatment Combinations
4.2. Advanced Imaging Techniques for Detecting Chemotherapy-Induced Cardiotoxicity
4.3. Clinical Manifestations and Cardiotoxic Sequelae
4.4. Nanotechnology-Driven Innovations in Cardiotoxicity Prevention and Monitoring
4.5. Limitations
4.6. Prospective Research Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Gastroenterol. Rev. 2019, 14, 89–103. [Google Scholar] [CrossRef]
- Fadlallah, H.; El Masri, J.; Fakhereddine, H.; Youssef, J.; Chemaly, C.; Doughan, S.; Abou-Kheir, W. Colorectal cancer: Recent advances in management and treatment. World J. Clin. Oncol. 2024, 15, 1136–1156. [Google Scholar] [CrossRef]
- Sawicki, T.; Ruszkowska, M.; Danielewicz, A.; Niedźwiedzka, E.; Arłukowicz, T.; Przybyłowicz, K.E. A Review of Colorectal Cancer in Terms of Epidemiology, Risk Factors, Development, Symptoms and Diagnosis. Cancers 2021, 13, 2025. [Google Scholar] [CrossRef]
- Müller, M.F.; Ibrahim, A.E.K.; Arends, M.J. Molecular pathological classification of colorectal cancer. Virchows Arch. 2016, 469, 125–134. [Google Scholar] [CrossRef]
- Rebuzzi, F.; Ulivi, P.; Tedaldi, G. Genetic Predisposition to Colorectal Cancer: How Many and Which Genes to Test? Int. J. Mol. Sci. 2023, 24, 2137. [Google Scholar] [CrossRef] [PubMed]
- Dunne, P.D.; Arends, M.J. Molecular pathological classification of colorectal cancer—An update. Virchows Arch. 2024, 484, 273–285. [Google Scholar] [CrossRef]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Bretthauer, M.; Løberg, M.; Wieszczy, P.; Kalager, M.; Emilsson, L.; Garborg, K.; Rupinski, M.; Dekker, E.; Spaander, M.; Bugajski, M.; et al. Effect of Colonoscopy Screening on Risks of Colorectal Cancer and Related Death. N. Engl. J. Med. 2022, 387, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
- Shinji, S.; Yamada, T.; Matsuda, A.; Sonoda, H.; Ohta, R.; Iwai, T.; Takeda, K.; Yonaga, K.; Masuda, Y.; Yoshida, H. Recent Advances in the Treatment of Colorectal Cancer: A Review. J. Nippon Med. Sch. 2022, 89, 246–254. [Google Scholar] [CrossRef]
- Moertel, C.G.; Fleming, T.R.; Macdonald, J.S.; Haller, D.G.; Laurie, J.A.; Goodman, P.J.; Ungerleider, J.S.; Emerson, W.A.; Tormey, D.C.; Glick, J.H.; et al. Levamisole and Fluorouracil for Adjuvant Therapy of Resected Colon Carcinoma. N. Engl. J. Med. 1990, 322, 352–358. [Google Scholar] [CrossRef]
- Assed Bastos, D.; Coelho Ribeiro, S.; de Freitas, D.; Hoff, P.M. Review: Combination therapy in high-risk stage II or stage III colon cancer: Current practice and future prospects. Ther. Adv. Med. Oncol. 2010, 2, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.C.; Yang, A.S.H.; Fichera, A.; Tsai, M.-H.; Wu, Y.-H.; Yeh, Y.-M.; Shyr, Y.; Lai, E.C.-C.; Lai, C.-H. Neoadjuvant Radiotherapy vs Up-Front Surgery for Resectable Locally Advanced Rectal Cancer. JAMA Netw Open 2025, 8, e259049. [Google Scholar] [CrossRef]
- Body, A.; Prenen, H.; Latham, S.; Lam, M.; Tipping-Smith, S.; Raghunath, A.; Segelov, E. The Role of Neoadjuvant Chemotherapy in Locally Advanced Colon Cancer. Cancer Manag. Res. 2021, 13, 2567–2579. [Google Scholar] [CrossRef]
- Gosavi, R.; Chia, C.; Michael, M.; Heriot, A.G.; Warrier, S.K.; Kong, J.C. Neoadjuvant chemotherapy in locally advanced colon cancer: A systematic review and meta-analysis. Int. J. Color. Dis. 2021, 36, 2063–2070. [Google Scholar] [CrossRef]
- Herrmann, J.; Lenihan, D.; Armenian, S.; Barac, A.; Blaes, A.; Cardinale, D.; Carver, J.; Dent, S.; Ky, B.; Lyon, A.R.; et al. Defining cardiovascular toxicities of cancer therapies: An Inter-national Cardio-Oncology Society (IC-OS) consensus statement. Eur. Heart J. 2022, 43, 280–299. [Google Scholar] [CrossRef]
- Eaton, H.; Timm, K.N. Mechanisms of trastuzumab induced cardiotoxicity—Is exercise a potential treatment? Cardio-Oncology 2023, 9, 22. [Google Scholar] [CrossRef]
- Zeglinski Bsc, M.; Ludke Msc, A.; Jassal, D.S.; Singal, P.K. Trastuzumab-induced cardiac dysfunction: A “dual-hit”. Exp. Clin. Cardiol. 2011, 16, 70. [Google Scholar]
- Ling, G.; Ge, F.; Li, W.; Wei, Y.; Guo, S.; Zhang, Y.; Li, Y.; Zhang, Y.; Liu, H.; Wu, Y.; et al. Anthracycline-induced cardiotoxicity: Emerging mechanisms and therapies. Med. Plus 2025, 2, 100074. [Google Scholar] [CrossRef]
- Varga, Z.V.; Ferdinandy, P.; Liaudet, L.; Pacher, P. Drug-induced mitochondrial dysfunction and cardiotoxicity. Am. J. Physiol.-Heart Circ. Physiol. 2015, 309, H1453–H1467. [Google Scholar] [CrossRef]
- Bhutani, V.; Varzideh, F.; Wilson, S.; Kansakar, U.; Jankauskas, S.; Santulli, G. Doxorubicin-Induced Cardiotoxicity: A Comprehensive Update. J. Cardiovasc. Dev. Dis. 2025, 12, 207. [Google Scholar] [CrossRef] [PubMed]
- Sara, J.D.; Kaur, J.; Khodadadi, R.; Rehman, M.; Lobo, R.; Chakrabarti, S.; Herrmann, J.; Lerman, A.; Grothey, A. 5-fluorouracil and cardiotoxicity: A review. Ther. Adv. Med Oncol. 2018, 10, 1758835918780140. [Google Scholar] [CrossRef]
- Al-Hussaniy, H.A.; Alburghaif, A.H.; Alkhafaje, Z.; Al-Zobaidy, M.A.-H.J.; Alkuraishy, H.M.; Mostafa-Hedeab, G.; Azam, F.; Al-Samydai, A.M.; Al-Tameemi, Z.S.; Naji, M.A. Chemotherapy-induced cardiotoxicity: A new perspective on the role of Digoxin, ATG7 activators, Resveratrol, and herbal drugs. J. Med. Life 2023, 16, 491–500. [Google Scholar] [CrossRef]
- Wang, Z.; Qin, W.; Zhai, Z.; Huang, L.; Feng, J.; Guo, X.; Liu, K.; Zhang, C.; Wang, Z.; Lu, G.; et al. Use of spectral tracking technique to evaluate the changes in left ventricular function in patients undergoing chemotherapy for colorectal cancer. Int. J. Cardiovasc. Imaging 2021, 37, 1203–1213. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Albini, A.; Fossile, E.; Pessi, M.A.; Nicolosi, G.L.; Lombardo, M.; Anzà, C.; Ambrosio, G. Speckle-Tracking Echocardiography for Cardioncological Evaluation in Bevacizumab-Treated Colorectal Cancer Patients. Cardiovasc. Toxicol. 2020, 20, 581–592. [Google Scholar] [CrossRef]
- Płońska-Gościniak, E.; Różewicz, M.; Kasprzak, J.; Wojtarowicz, A.; Mizia-Stec, K.; Hryniewiecki, T.; Pysz, P.; Kułach, A.; Bodys, A.; Sulżyc, V.; et al. Tissue Doppler echocardiography detects subclinical left ventricular dysfunction in patients undergoing chemotherapy for Colon Cancer: Insights from ONCOECHO multicentre study. Kardiol. Pol. 2017, 75, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Dyhl-Polk, A.; Schou, M.; Vistisen, K.K.; Sillesen, A.-S.; Serup-Hansen, E.; Faber, J.; Klausen, T.W.; Bojesen, S.E.; Vaage-Nilsen, M.; Nielsen, D.L. Myocardial Ischemia Induced by 5-Fluorouracil: A Prospective Electrocardiographic and Cardiac Biomarker Study. Oncologist 2021, 26, e403–e413. [Google Scholar] [CrossRef]
- Visvikis, A.; Kyvelou, S.; Pietri, P.; Georgakopoulos, C.; Manousou, K.; Tousoulis, D.; Stefanadis, C.; Vlachopoulos, C.; Pektasides, D. Cardiotoxic profile and arterial stiffness of adjuvant chemotherapy for colorectal cancer. Cancer Manag. Res. 2020, 12, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Ben-Yakov, M.; Mattu, A.; Brady, W.J.; Dubbs, S.B. Prinzmetal angina (Coronary vasospasm) associated with 5-fluorouracil chemotherapy. Am. J. Emerg. Med. 2017, 35, 1038.e3–1038.e5. [Google Scholar] [CrossRef]
- Wong, C.-K.; Ho, I.; Choo, A.; Lau, R.; Ma, T.-F.; Chiu, A.C.H.-O.; Lam, T.-H.; Lin, M.; Leung, R.W.-H.; Tam, F.C.-C.; et al. Cardiovascular safety of 5-fluorouracil and capecitabine in colorectal cancer patients: Real-world evidence. Cardio-Oncology 2025, 11, 3. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Wang, W.; Dong, H.; Wang, G.; Chen, W.; Chen, J.; Chen, W. The association between systemic immune-inflammation index and cardi-otoxicity related to 5-Fluorouracil in colorectal cancer. BMC Cancer 2024, 24, 782. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Dong, H.; Wang, G.; Chen, W.; Chen, J.; Chen, W. Risk factors for fluoropyrimidine-induced cardiotoxicity in colorectal cancer: A retrospective cohort study and establishment of a prediction nomogram for 5-FU induced cardiotoxicity. Front. Oncol. 2023, 13, 1017237. [Google Scholar] [CrossRef]
- Huang, W.-K.; Ho, W.-P.; Hsu, H.-C.; Chang, S.-H.; Chen, D.-Y.; Chou, W.-C.; Chang, P.-H.; Chen, J.-S.; Yang, T.-S.; See, L.-C. Risk of cardiovascular disease among different fluoropyrimidine-based chemotherapy regimens as adjuvant treatment for resected colorectal cancer. Front. Cardiovasc. Med. 2022, 9, 880956. [Google Scholar] [CrossRef]
- Lee, S.F.; Yip, P.L.; Vellayappan, B.A.; Chee, C.E.; Wong, L.C.; Wan, E.Y.-F.; Chan, E.W.-Y.; Lee, C.-F.; Lee, F.A.-S.; Luque-Fernandez, M.A. Incident Cardiovascular Diseases among Survivors of High-Risk Stage II–III Colorectal Cancer: A Cluster-Wide Cohort Study. JNCCN J. Natl. Compr. Cancer Netw. 2022, 20, 1125–1133. [Google Scholar] [CrossRef]
- McAndrew, E.N.; Jassal, D.S.; Goldenberg, B.A.; Kim, C.A. Capecitabine-mediated heart failure in colorectal cancer: A case series. Eur. Heart J. Case Rep. 2021, 5, ytab079. [Google Scholar] [CrossRef]
- Sami, N.; Raizada, A.; Phillip, G.J.; Rana, D.; Alishetti, S. Takotsubo Cardiomyopathy in a Patient Undergoing 5-Fluorouracil Infusion: Considerations for Folinic Acid, 5-Fluorouracil, and Oxaliplatin (FOLFOX) Cardiotox-icity and Rechallenge. Cureus 2025, 17, e82145. [Google Scholar] [CrossRef]
- Vargo, C.A.; Blazer, M.; Reardon, J.; Gulati, M.; Bekaii-Saab, T. Successful completion of adjuvant chemotherapy in a patient with colon cancer experiencing 5-fluorouracil-induced cardiac vasospasm. Clin. Color. Cancer 2016, 15, e61–e63. [Google Scholar] [CrossRef]
- Liu, X.-X.; Su, J.; Long, Y.-Y.; He, M.; Zhu, Z.-Q. Perioperative risk factors for survival outcomes in elective colorectal cancer surgery: A retrospective cohort study. BMC Gastroenterol. 2021, 21, 169. [Google Scholar] [CrossRef] [PubMed]
- Farani, M.R.; Lak, M.; Cho, W.C.; Kang, H.; Azarian, M.; Yazdian, F.; Harirchi, S.; Khoshmaram, K.; Alipourfard, I.; Hushmandi, K.; et al. Carbon nanomaterials: A promising avenue in colorectal cancer treatment. Carbon Lett. 2024, 34, 2035–2053. [Google Scholar] [CrossRef]
- Narayana, S.; Gowda, B.J.; Hani, U.; Shimu, S.S.; Paul, K.; Das, A.; Ashique, S.; Ahmed, M.G.; Tarighat, M.A.; Abdi, G. Inorganic nanoparticle-based treatment approaches for colorectal cancer: Recent advancements and challenges. J. Nanobiotechnol. 2024, 22, 427. [Google Scholar] [CrossRef]
- Jiang, Y.; Jiang, Y.; Li, M.; Yu, Q. Will nanomedicine become a good solution for the cardiotoxicity of chemotherapy drugs? Front. Pharmacol. 2023, 14, 1143361. [Google Scholar] [CrossRef] [PubMed]
- Ammar, M.M.; Ali, R.; Elaziz, N.A.A.; Habib, H.; Abbas, F.M.; Yassin, M.T.; Maniah, K.; Abdelaziz, R. Nanotechnology in oncology: Advances in biosynthesis, drug delivery, and theranostics. Discov. Oncol. 2025, 16, 1172. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, H.; Wan, W.; Yang, H.; Zhao, J. Advances in nanotechnological approaches for the detection of early markers associated with severe cardiac ailments. Nanomedicine 2024, 19, 1487–1506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, Y.; Zhang, Y.; Fang, F.; Liu, J.; Xia, Y.; Liu, Y. Cardiac Biomarkers for the Detection and Management of Cancer Thera-py-Related Cardiovascular Toxicity. J. Cardiovasc. Dev. Dis. 2022, 9, 372. [Google Scholar] [CrossRef]
- Yu, A.F.; Ky, B. Roadmap for biomarkers of cancer therapy cardiotoxicity. Heart 2016, 102, 425–430. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
| Domain | Details |
|---|---|
| Definition | Cardiac dysfunction resulting from cancer therapy, particularly chemotherapeutic agents. Includes structural and functional damage such as LVEF decline and HF. |
| Diagnostic Criteria | - Symptomatic: ≥5% drop in LVEF to <55% - Asymptomatic: ≥10% drop in LVEF to <55% |
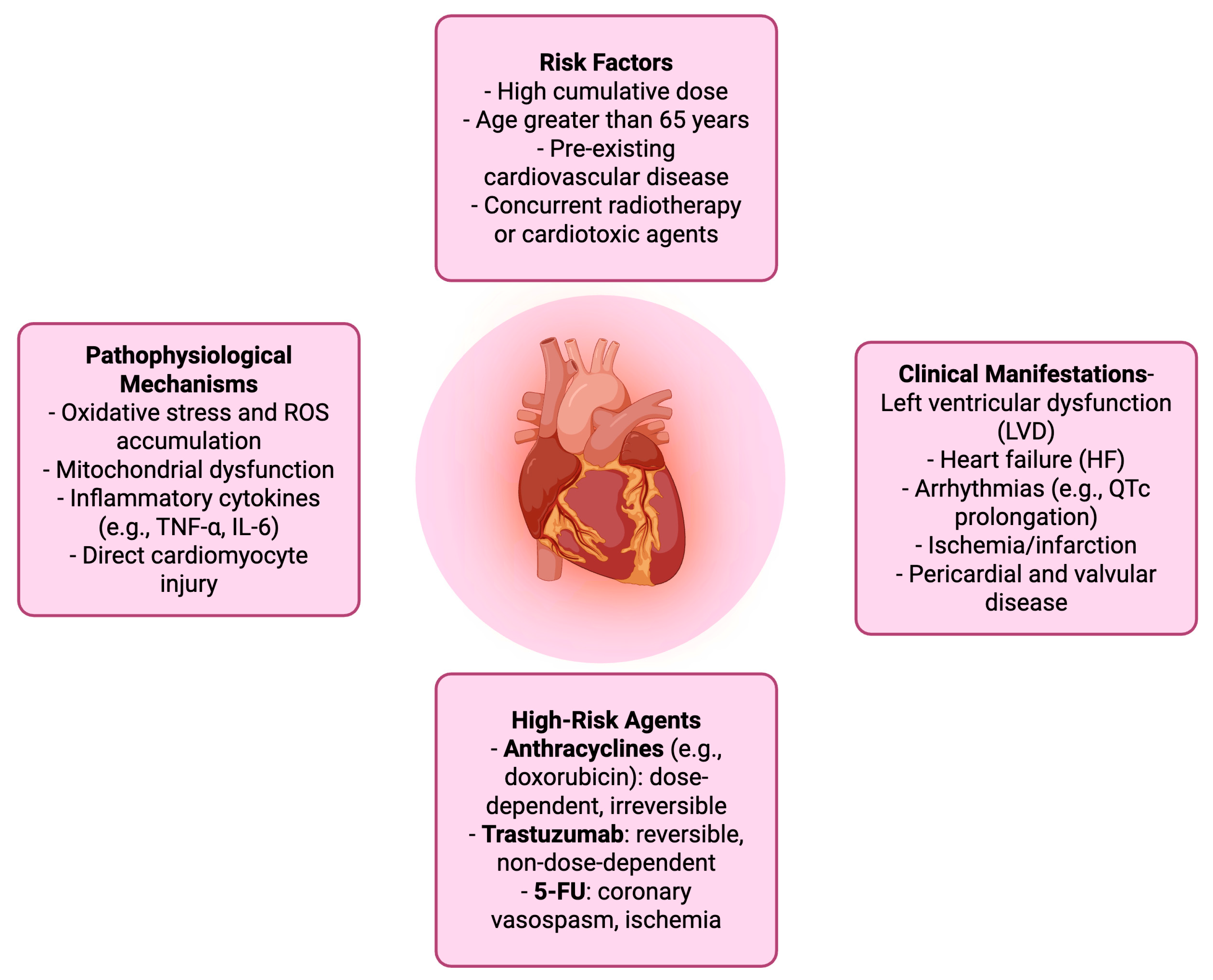
| Clinical Manifestations | - Left ventricular dysfunction (LVD) - Heart failure (HF) - Arrhythmias (e.g., QTc prolongation) - Ischemia/infarction - Pericardial and valvular disease |
| Pathophysiological Mechanisms | - Oxidative stress and ROS accumulation - Mitochondrial dysfunction - Inflammatory cytokines (e.g., TNF-α, IL-6) - Direct cardiomyocyte injury |
| High-Risk Agents | - Anthracyclines (e.g., doxorubicin): dose-dependent, irreversible - Trastuzumab: reversible, non-dose-dependent - 5-FU: coronary vasospasm, ischemia |
| Risk Factors | - High cumulative dose - Age >65 years - Pre-existing cardiovascular disease - Concurrent radiotherapy or cardiotoxic agents |
| Monitoring Modalities | - Echocardiography (LVEF, GLS) - Cardiac biomarkers (troponin, BNP) - ECG - Cardiac MRI |
| Preventive Strategies | - Dose limitation and scheduling adjustments - Use of cardioprotective agents (e.g., dexrazoxane) - Baseline and serial cardiac assessment |
| Management Approaches | - Temporary or permanent discontinuation of culprit agent - Initiation of HF therapy (e.g., ACE inhibitors, beta-blockers) - Multidisciplinary cardio-oncology care |
| Component | Description |
|---|---|
| P (Population) | Adult patients diagnosed with primary colorectal cancer undergoing chemotherapy treatment |
| I (Intervention) | Administration of chemotherapy regimens (FOLFOX, XELOX, 5-FU, capecitabine, oxaliplatin) |
| C (Comparison) | CRC patients not receiving chemotherapy, receiving non-cardiotoxic agents, or baseline cardiac function pre-chemotherapy |
| O (Outcome) | Incidence, mechanisms, and severity of cardiotoxicity (myocardial injury, arrhythmias, heart failure), as well as detection and monitoring strategies |
| Authors (Year) | Country | Study Design | Sample Size | Population Characteristics | Chemotherapy Regimen | Cardiotoxicity Outcome(s) |
|---|---|---|---|---|---|---|
| Wang et al. (2021) [23] | China | Prospective observational | 30 | Adults aged 37–64, diagnosed with primary CRC, no preexisting CVD | mFOLFOX6 | Reduced LVEF, GLS, LVtw; ECG changes; ↑ cTnT levels |
| Sonaglioni et al. (2020) [24] | Italy | Prospective observational | 25 | Adults (mean age 71.8 ± 7.5), mCRC, normotensive | Bevacizumab + FOLFOX | >15% decrease in GLS (36%); new-onset hypertension (20%) |
| Płońska-Gościniak et al. (2017) [25] | Poland | Prospective multicentre | 25 | Adults (12F), mean age 61.3 y, CRC (adenocarcinoma) | 5-FU or capecitabine regimens | ↓ S’IVS, S’lat, E’sept; transient QT prolongation |
| Dyhl-Polk et al. (2021) [26] | Denmark | Prospective observational | 108 | CRC and anal cancer patients receiving 5-FU for the first time | 5-FU ± cisplatin, FOLFOX, FOLFIRI | ST changes, ischemia, arrhythmias, ↑ copeptin, rare ↑ troponin |
| Visvikis et al. (2020) [27] | Greece | Prospective observational | 70 | Non-metastatic CRC (Stage II & III); no prior cardiac disease | FOLFOX or XELOX (Oxaliplatin + 5-FU/Capecitabine) | ↑ arterial stiffness: PWV, Aix75, SBP; no change in EF |
| Ben-Yakov et al. (2017) [28] | Canada/USA | Case report | 1 | 54-year-old male with rectal cancer, HTN, hyperlipidemia, smoker | 5-FU infusion | Chest pain, hyperacute T waves, reversible ST changes, vasospasm |
| Wong et al. (2025) [29] | Hong Kong | Retrospective cohort w/matching | 21, 216 (After propensity score matching) | Adults with CRC, balanced for CVD risk factors | 5-FU and capecitabine | 1.06% experienced major adverse cardiovascular event (MACE); no ↑ CV risk vs. controls; no difference between drugs |
| Liu et al. (2024) [30] | China | Retrospective cohort | 754 | CRC pts on 5-FU-based regimens, normal cardiac baseline, stratified by monocytes | 5-FU-based regimens | Chest pain, arrhythmias, ECG/ST-T changes, ↑ cardiac enzymes |
| Wang et al. (2023) [31] | China | Retrospective cohort | 916 | CRC patients, 5-FU or capecitabine-based chemo, no severe CVD at baseline | 5-FU or capecitabine | Chest pain, arrhythmia, dyspnea, ST-T changes, myocardial infarction |
| Huang et al. (2022) [32] | Taiwan | Nationwide retrospective cohort | 32, 35 | Stage II–III CRC post-resection, treated with UFT, non-UFT, or mixed 5-FU regimens | Tegafur-uracil (UFT), non-UFT, mixed | AMI, LTA, CHF, ischemic stroke, CV death |
| Lee et al. (2022) [33] | Hong Kong | Population-based cohort | 1037 CRC + 5078 controls | Stage II–III CRC survivors post-radical surgery, receiving adjuvant fluoropyrimidine-based chemo | Fluoropyrimidines (5-FU/capecitabine ± oxaliplatin) | Ischemic heart disease, heart failure, cardiomyopathy, stroke |
| McAndrew et al. (2021) [34] | Canada | Case series | 2 | CRC patients on CAPOX with no prior cardiac history; acute HF within days of first cycle | CAPOX (Capecitabine + Oxaliplatin) | Cardiogenic shock, reversible heart failure, ↓ LVEF < 20%, elevated lactate |
| Sami et al. (2025) [35] | USA | Case report | 1 | 74-year-old woman with sigmoid adenocarcinoma and severe anxiety | FOLFOX (5-FU + leucovorin + oxaliplatin) | Takotsubo cardiomyopathy (TCM); LV dysfunction; elevated troponin and BNP |
| Vargo et al. (2015) [36] | USA | Case report | 1 | 48-year-old woman with stage IIIc colon cancer, no cardiac risk factors | FOLFOX (5-FU + oxaliplatin) | Vasospastic angina during 5-FU infusion; recurrent chest pain |
| Authors (Year) | Measurement Tools | Timing of Outcome Assessment | Key Findings |
|---|---|---|---|
| Wang et al. (2021) [23] | 3D speckle-tracking echocardiography, serum cTnT | Before chemo; after cycles 1, 6, and 12 | MCI showed highest sensitivity for early cardiotoxicity; significant decline in strain measures & ↑ cTnT |
| Sonaglioni et al. (2020) [24] | 2D STE, BP, ECG, HS-cTnI | Baseline, 3 months, 6 months | GLS impaired over 6 months; no LVEF changes; 2D-STE effective for early cardiotoxicity detection |
| Płońska-Gościniak et al. (2017) [25] | Tissue Doppler Echo, ECG | Baseline, 3, 6, and 12 months | Chemotherapy utilizing 5-FU or capecitabine in CRC patients did not influence the conduction system, left ventricular structural features, or systolic function as assessed by left ventricular ejection fraction, nor is it linked to cardiovascular events in the following 12 months. Chemotherapy with 5-FU or capecitabine in CRC patients may induce minor alterations in cardiac function, detected exclusively through tissue analysis. Doppler echocardiography conducted after a duration of 12 months. Transient QT prolongation occurs during CTX and resolves upon discontinuation of CTX. |
| Dyhl-Polk et al. (2021) [26] | Holter ECG, 12-lead ECG, copeptin, troponin I | Before chemo and during 1st & 3rd/4th cycles | 14.1% had silent ischemia during first 5-FU infusion; 5.6% developed ACS; copeptin ↑ during treatment, troponin rarely elevated |
| Visvikis et al. (2020) [27] | ECG, Echo, SphygmoCor tonometry, Complior PWV, BP monitoring | Before and after full chemo regimen (6–12 cycles) | Significant post-chemo ↑ in Aix75, PWVc-f, PWVc-r (p < 0.001); changes independent of regimen; ↑ vascular burden post-adjuvant chemo requires follow-up |
| Ben-Yakov et al. (2017) [28] | ECG, coronary angiography, troponin, LV angiogram | During 2nd cycle of 5-FU infusion | Coronary vasospasm mimicking STEMI; no obstructive CAD; LV dilation with global hypokinesis; resolved with NTG and CCB; first EM case report of 5-FU vasospasm |
| Wong et al. (2025) [29] | Clinical records, troponin, survival analysis | 1-year follow-up | Fluoropyrimidines did not ↑ MACE risk; no significant difference between 5-FU and capecitabine |
| Liu et al. (2024) [30] | Clinical symptoms, ECG, echo, serum markers, SII | During treatment and up to 4 weeks post-completion | SII positively correlated with cardiotoxicity in low-monocyte subgroup; threshold effect observed in mid-tertile |
| Wang et al. (2023) [31] | Clinical records, ECG, echo, labs, CTCAE grading | During chemo and up to 4 weeks post-treatment | Age > 60, BMI ≥ 22.97, ≤3 cycles of chemo, and use of bevacizumab ↑ risk of 5-FU–induced cardiotoxicity; nomogram developed |
| Huang et al. (2022) [32] | TCR, NHIRD, TDR linkage; SIPTW adjusted modeling | Up to 14 years post-diagnosis | UFT group had ↑ heart failure, stroke, AMI risk vs. other groups; strongest in stage III and age ≥ 70; caution advised for this subset |
| Lee et al. (2022) [33] | Electronic medical records, ICD codes, competing risk modeling | 1–10 years post-treatment | Adjusted HR for CVD in chemo-treated CRC vs. controls = 2.11; strongest risk factors: age, male sex, diabetes, HTN, dyslipidemia; stroke risk notably elevated |
| McAndrew et al. (2021) [34] | ECG, TTE, Cardiac MRI, CT, coronary angiography, lab biomarkers | Day 3–7 post treatment initiation | Severe reversible cardiomyopathy; full cardiac recovery without infarction, vasospasm or myocarditis; Capecitabine implicated in acute HF without ST changes |
| Sami et al. (2025) [35] | ECG, TTE, coronary angiography, ventriculography, BNP, troponin | 3 days post-cycle 1; 1 month post-rechallenge | TCM resolved with conservative therapy; successful rechallenge with telemetry monitoring and no recurrence; highlights need for cardiac surveillance |
| Vargo et al. (2015) [36] | ECG, echo, cardiac catheterization, troponin | During all 10 cycles of 5-FU infusion | Rechallenge successful with inpatient cardiac monitoring, nitrates, and calcium channel blockers; no troponin leak or ST changes; no delayed toxicity at 10-month follow-up |
| Chemotherapy Regimen 1 | Studies Reporting, n | Total Sample Size 2 | Reported Cardiotoxicity Incidence/Risk 3 | Commonly Reported Cardiac Events | Representative References 4 |
|---|---|---|---|---|---|
| FOLFOX (5-FU + leucovorin + oxaliplatin) | 6 | ~1200 | 5–36% (higher for subclinical strain changes) | ↓ GLS, LVtw, vasospastic angina, arrhythmias, hypertension | [23,27,36] |
| CAPOX/XELOX (capecitabine + oxaliplatin) | 2 | 72 | 2.8–100% in case series | Acute HF, cardiogenic shock, ↓ LVEF | [27,34] |
| 5-FU monotherapy/infusional | 5 | >22,000 | 1.06% MACE in large cohorts; up to 14% silent ischemia | Chest pain, silent ischemia, vasospasm, arrhythmias | [26,28,29] |
| Capecitabine monotherapy | 3 | ~1000 | Similar to 5-FU in large cohorts; rare acute HF in case reports | Acute HF, arrhythmias, ischemia | [29,34] |
| Tegafur–uracil (UFT) | 1 | 32,351 | Increased risk of HF, stroke, AMI vs. non-UFT regimens (adjusted HRs significant in elderly, stage III) | HF, stroke, AMI | [32] |
| 5-FU + bevacizumab | 2 | ~150 | 20% new-onset hypertension; increased cardiotoxicity risk in predictive models | Hypertension, ischemia | [24,31] |
| 5-FU + cisplatin | 1 | 108 | 14.1% silent ischemia; 5.6% ACS | Silent ischemia, ACS | [26] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsokkou, S.; Konstantinidis, I.; Chatzikomnitsa, P.; Papakonstantinou, M.; Toutziari, E.; Giakoustidis, D.; Papamitsou, T.; Papadopoulos, V.; Giakoustidis, A. Unveiling the Mechanisms for the Development of Cardiotoxicity Following Chemotherapy Regimens Administration for Primary Colorectal Cancer: A Systematic Review. Cancers 2025, 17, 3129. https://doi.org/10.3390/cancers17193129
Tsokkou S, Konstantinidis I, Chatzikomnitsa P, Papakonstantinou M, Toutziari E, Giakoustidis D, Papamitsou T, Papadopoulos V, Giakoustidis A. Unveiling the Mechanisms for the Development of Cardiotoxicity Following Chemotherapy Regimens Administration for Primary Colorectal Cancer: A Systematic Review. Cancers. 2025; 17(19):3129. https://doi.org/10.3390/cancers17193129
Chicago/Turabian StyleTsokkou, Sophia, Ioannis Konstantinidis, Paraskevi Chatzikomnitsa, Menelaos Papakonstantinou, Evdokia Toutziari, Dimitrios Giakoustidis, Theodora Papamitsou, Vasileios Papadopoulos, and Alexandros Giakoustidis. 2025. "Unveiling the Mechanisms for the Development of Cardiotoxicity Following Chemotherapy Regimens Administration for Primary Colorectal Cancer: A Systematic Review" Cancers 17, no. 19: 3129. https://doi.org/10.3390/cancers17193129
APA StyleTsokkou, S., Konstantinidis, I., Chatzikomnitsa, P., Papakonstantinou, M., Toutziari, E., Giakoustidis, D., Papamitsou, T., Papadopoulos, V., & Giakoustidis, A. (2025). Unveiling the Mechanisms for the Development of Cardiotoxicity Following Chemotherapy Regimens Administration for Primary Colorectal Cancer: A Systematic Review. Cancers, 17(19), 3129. https://doi.org/10.3390/cancers17193129












