Determining Risk Factors Associated with Cardiovascular Complications in Patients with Acute Leukemia: A Systematic Review
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Information Sources and Search Strategy
2.2. Eligibility Criteria
2.2.1. Inclusion Criteria
- Studies published from the beginning of 2020 to the end of 2024.
- Theses, original studies, review articles, and conference papers with full text available.
- Studies that investigated risk factors associated with the development of cardiovascular complications in patients with AL.
- Studies that included different AL types, such as acute lymphoblastic leukemia (ALL) with B and T subtypes, acute myeloid leukemia (AML) with genetic variations, AML with dysplastic changes, treatment-related AML, and unspecified AML (such as minimally differentiated AML, AML without mutations, or with mutations), as well as specific types (acute myelomonocytic leukemia, monoblastic/monocytic leukemia, erythroleukemia, megakaryoblastic leukemia, and other rare cases); mixed-phenotype acute leukemia (MPAL) and acute leukemias with undefined lineage.
- Studies that included quantitative or qualitative analyses regarding the risk complications leading to cardiovascular issues in patients with AL.
- Studies that addressed cardiovascular complications related to bone marrow transplantation and treatments for AL, including complications arising from bone marrow transplants such as side effects of immunosuppressive drugs (e.g., cyclosporine, tacrolimus), direct complications related to the transplant process (e.g., cardiotoxicity from AL treatments or high doses prior to transplant), and late complications after transplantation (e.g., heart failure, arrhythmias, or coronary artery disease (CAD)). Additionally, complications from AL treatments such as chemotherapy with drugs like anthracyclines (e.g., doxorubicin, daunorubicin, idarubicin, mitoxantrone), alkylating agents (e.g., cyclophosphamide, busulfan, melphalan), antimetabolites (e.g., cytarabine, fludarabine, cladribine, clofarabine, azacitidine, decitabine), TKIs (e.g., sorafenib, quizartinib, imatinib, dasatinib, nilotinib, ponatinib), FLT3 inhibitors (e.g., midostaurin, gilteritinib), and isocitrate dehydrogenase (IDH) inhibitors (e.g., ivosidenib, enasidenib), monoclonal antibodies (e.g., rituximab, blinatumomab, gemtuzumab, ozogamicin, tisagenlecleucel), and drugs regulating apoptosis and cellular metabolism (e.g., venetoclax, etoposide, asparaginase) were considered. Other related treatments like radiation therapy and supportive treatments (e.g., colony-stimulating factors, blood transfusions, or infection prevention medications) were also included in this study.
- The cardiac complications included direct heart problems such as heart failure, arrhythmias, CAD, myocarditis, pericarditis, cardiomyopathy, valvular heart disease, hypertension, cardiotoxicity, endocarditis, cardiogenic shock, and Takotsubo cardiomyopathy.
- The vascular complications included issues with both large and small vessels such as deep vein thrombosis (DVT), pulmonary embolism (PE), stroke, peripheral artery disease (PAD), renal artery stenosis, aneurysm, vasculitis, pulmonary hypertension, electrolyte imbalances, and coagulopathies.
2.2.2. Exclusion Criteria
- Studies such as letters to the editor, protocols, preclinical studies, case reports, editorials, or opinions were excluded.
- Articles that only had an abstract available and did not provide full-text access were excluded.
- Articles that focused on other cancers such as solid cancers (e.g., breast cancer, lung cancer, prostate cancer, or colorectal cancer) or hematologic cancers unrelated to AL (e.g., lymphoma or multiple myeloma) were excluded.
- Articles that focused on complications and diseases caused by AL other than cardiovascular complications (e.g., neurological, renal, hepatic complications, or treatment-related infections) were excluded.
- Articles that discussed general chemotherapy, radiotherapy, or bone marrow transplant complications without a specific focus on AL or its related cardiovascular complications were excluded.
- Studies that investigated complications or diseases related to AL, such as gastrointestinal, pulmonary, or ocular complications, but with no focus on cardiovascular complications were excluded.
- Studies that explored general risk factors for cardiovascular complications but did not clearly analyze the impact of these factors in patients with AL were excluded.
- Articles focused on topics unrelated to the risk factors for cardiovascular complications (e.g., alternative treatments or prevention) were excluded.
- Studies that focused on clonal hematopoiesis and myeloproliferative neoplasms (MPNs), including chronic myeloid leukemia (CML), polycythemia vera (PV), essential thrombocythemia (ET), primary myelofibrosis (PMF), chronic neutrophilic leukemia (CNL), chronic eosinophilic leukemia/hypereosinophilic syndrome (CEL/HES), mast cell disease (MCD), myelodysplastic syndromes (MDS), myelodysplastic/myeloproliferative neoplasms (MDS/MPN), chronic myelomonocytic leukemia (CMML), juvenile myelomonocytic leukemia (JMML), and neoplasms related to gene rearrangements (PDGFRA, PDGFRB, FGFR1, and PCM1-JAK2), without addressing AL and its associated complications were excluded.
2.3. Selection Process
2.4. Data Collection Process and Data Items
2.5. Study Risk of Bias Assessment
2.6. Synthesis Methods
3. Results
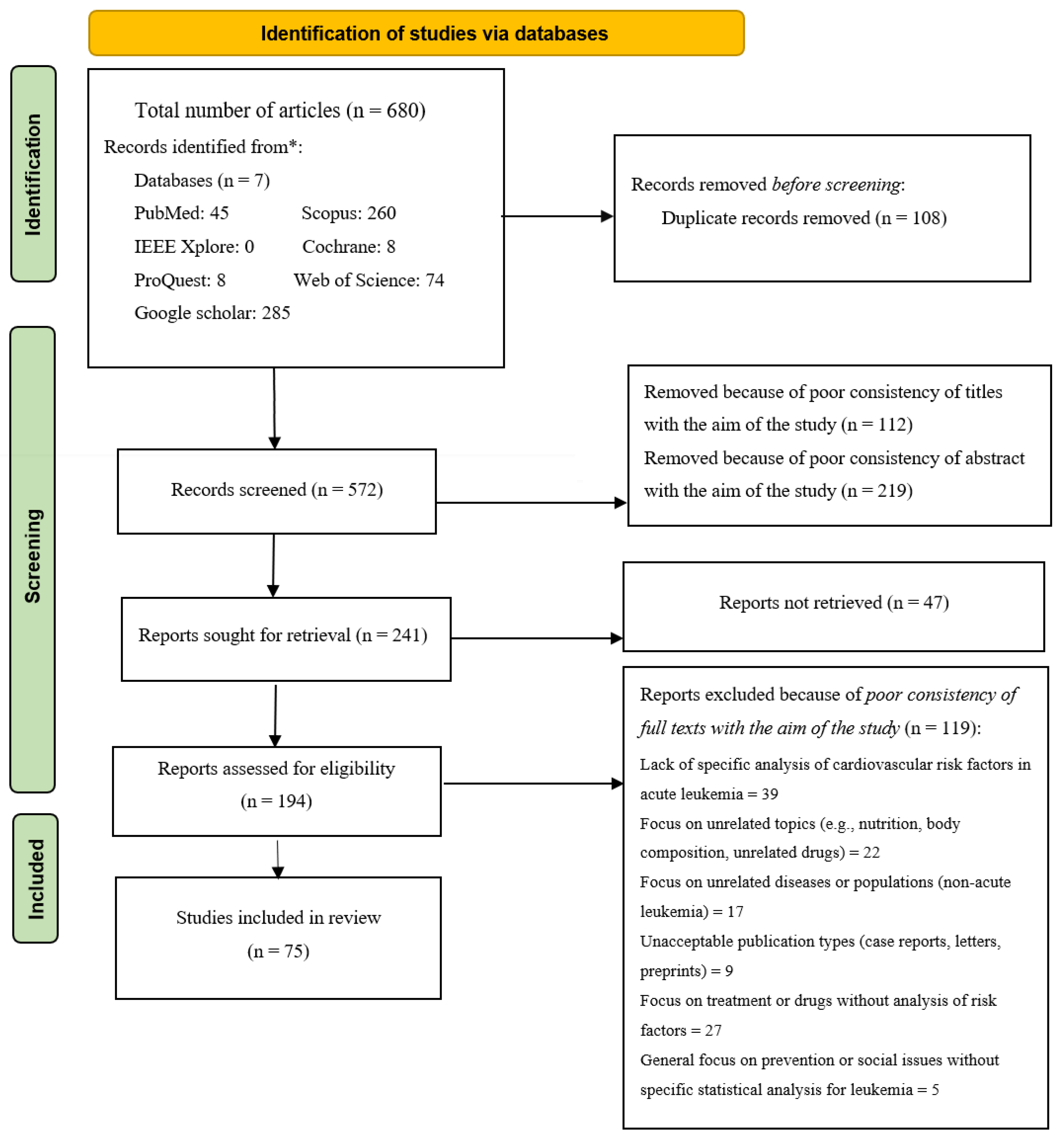
3.1. Study Selection
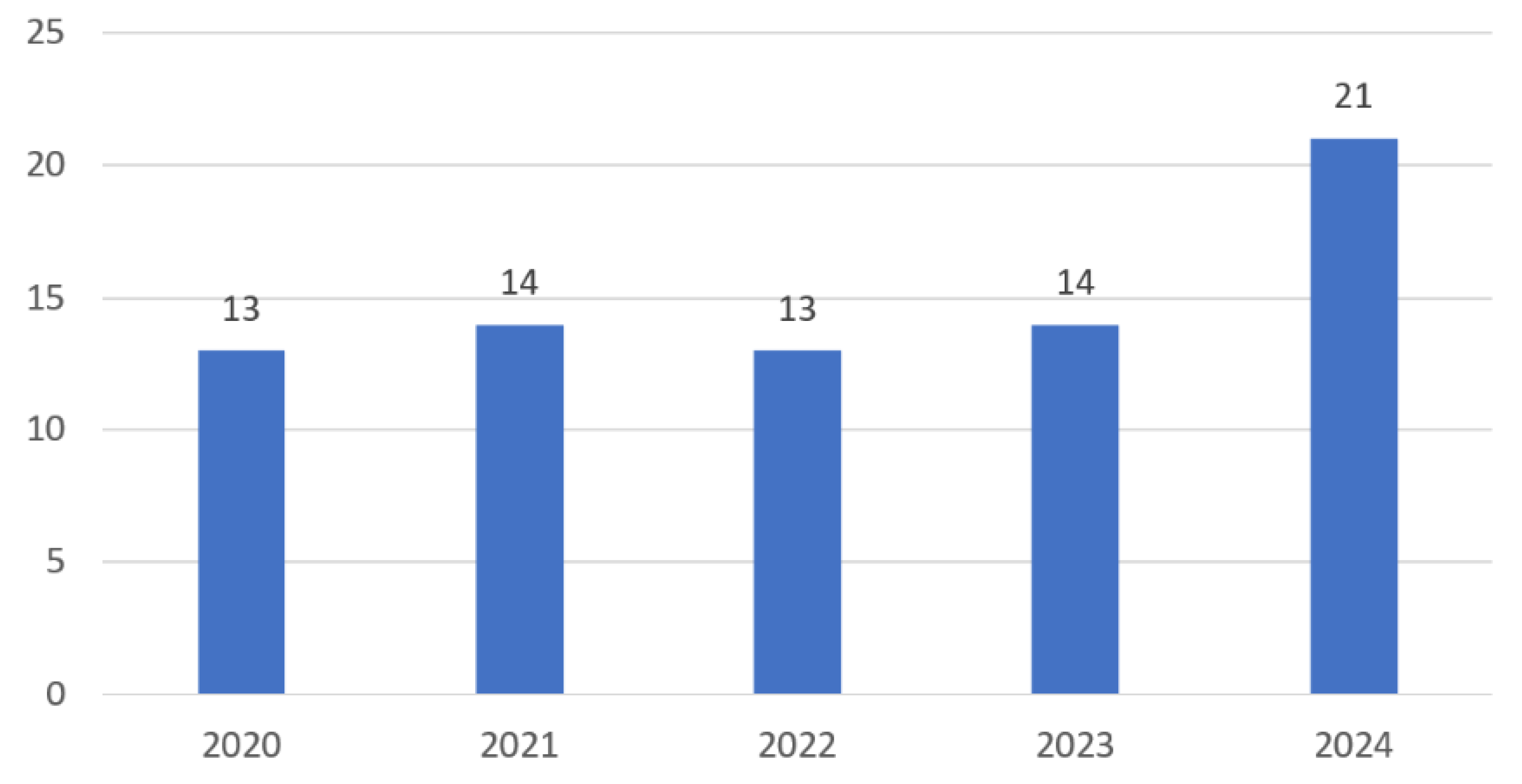
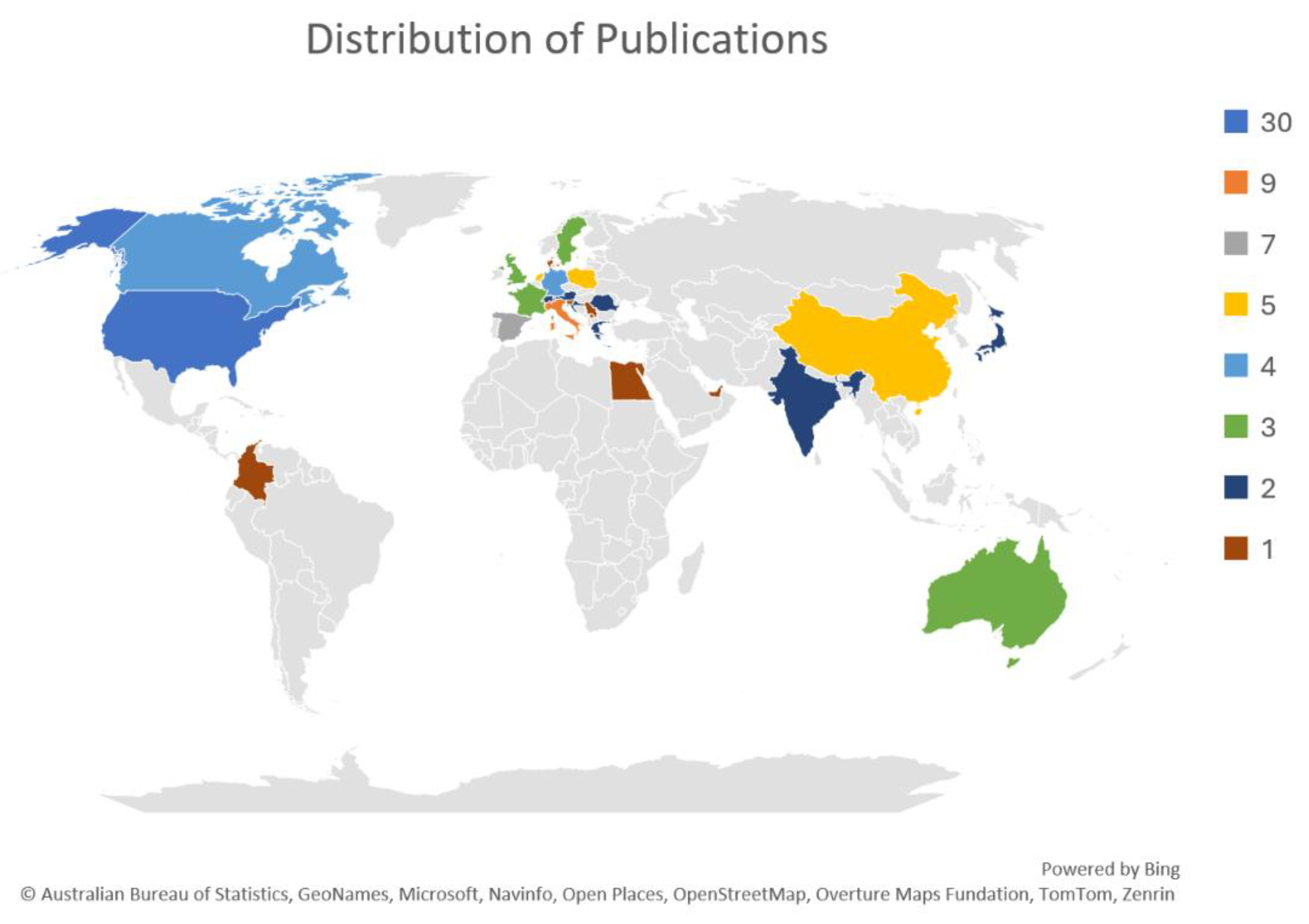
3.2. Study Characteristics
3.3. Risk of Bias in Studies
3.4. Results of Individual Studies
3.4.1. Research Methodology
3.4.2. Research Objectives
3.4.3. Type of Acute Leukemia
3.4.4. Risk Factors
3.4.5. Cardiovascular Complications
3.4.6. Time of Cardiovascular Complication Onset
3.4.7. Summary of Key Findings
3.5. Synthesis of the Results
4. Discussion
Research Implications
5. Limitations and Future Works
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACS | Acute Coronary Syndrome |
| AF | Atrial Fibrillation |
| AL | Acute Leukemia |
| ALL | Acute Lymphoblastic Leukemia |
| Allo-HCT/allo-HSCT/allo-SCT | Allogeneic Hematopoietic (Stem) Cell Transplantation |
| AML | Acute Myeloid Leukemia |
| AMSTAR 2 | A Measurement Tool to Assess Systematic Reviews (Version 2) |
| AOEs | Acute Onset Events |
| APL | Acute Promyelocytic Leukemia |
| ASCVD | Atherosclerotic Cardiovascular Disease |
| ATE | Arterial Thromboembolism |
| BMI | Body Mass Index |
| BNP | B-type Natriuretic Peptide |
| CAD | Coronary Artery Disease |
| CEL/HES | Chronic Eosinophilic Leukemia/Hypereosinophilic Syndrome |
| CHIP | Clonal Hematopoiesis of Indeterminate Potential |
| CML | Chronic Myeloid Leukemia |
| CMML | Chronic Myelomonocytic Leukemia |
| CNL | Chronic Neutrophilic Leukemia |
| CRS | Cytokine Release Syndrome |
| CTCAE/CTCAE 5.0 | Common Terminology Criteria for Adverse Events (version 5.0) |
| cTnT/hs-cTnT | (High-sensitivity) Cardiac Troponin T |
| CVD | Cardiovascular Disease |
| DD | Diastolic Dysfunction |
| DVT | Deep Vein Thrombosis |
| ECG | Electrocardiogram |
| ET | Essential Thrombocythemia |
| GLS | Global Longitudinal Strain |
| GVHD | Graft-versus-Host Disease |
| HSCT | Hematopoietic Stem Cell Transplantation |
| Hs-cTnI | High-Sensitivity Cardiac Troponin |
| IDH | Isocitrate Dehydrogenase |
| JBI | Joanna Briggs Institute |
| JMML | Juvenile Myelomonocytic Leukemia |
| LVD | Left Ventricular Dysfunction |
| LVEF | Left Ventricular Ejection Fraction |
| MCD | Mast Cell Disease |
| MDS/MPN | Myelodysplastic/Myeloproliferative Neoplasms |
| MDS | Myelodysplastic Syndromes |
| MPAL | Mixed-Phenotype Acute Leukemia |
| MPNs | Myeloproliferative Neoplasms |
| NIH | National Institutes of Health (Quality Assessment Tool) |
| NT-proBNP | N-terminal pro-B-type Natriuretic Peptide |
| PAD | Peripheral Artery Disease |
| PE | Pulmonary Embolism |
| Ph+ ALL | Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia |
| PMF | Primary Myelofibrosis |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PV | Polycythemia Vera |
| RoB 2 | Cochrane Risk-of-Bias Tool for Randomized Trials (Version 2) |
| ROBINS-I | Risk Of Bias In Non-randomized Studies-of Interventions |
| ROBIS | Risk Of Bias In Systematic Reviews |
| SANRA | Scale for the Assessment of Narrative Review Articles |
| t-AML | Therapy-Related Acute Myeloid Leukemia |
| TAPSE | Tricuspid Annular Plane Systolic Excursion |
| TBI | Total Body Irradiation |
| TIA | Transient Ischemic Attack |
| TKI | Tyrosine Kinase Inhibitors |
| TMI | Total Marrow Irradiation |
| VAs | Ventricular Arrhythmias |
| VTE | Venous Thromboembolism |
References
- Chennamadhavuni, A.; Lyengar, V.; Mukkamalla, S.K.R.; Shimanovsky, A. Leukemia. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Xiao, W.; Ma, L.; Shang, Y.; Yang, F.; Tan, Y.; Chen, G.; Wu, J.; Liang, Y.; Rouzi, T.; Wang, Q.; et al. Cardiac-Related Lesions in Newly Diagnosed Patients with Acute Leukemia: A Chinese Population-Based Real-World Study. Front. Med. 2022, 9, 844350. [Google Scholar] [CrossRef]
- Siaravas, K.C.; Moula, A.I.; Tzourtzos, I.S.; Ballas, C.E.; Katsouras, C.S. Acute and Chronic Cardiovascular Adverse Events in Patients with Acute Myeloid Leukemia: A Systematic Review. Cancers 2025, 17, 541. [Google Scholar] [CrossRef]
- Boluda, B.; Solana-Altabella, A.; Cano, I.; Martínez-Cuadrón, D.; Acuña-Cruz, E.; Torres-Miñana, L.; Rodríguez-Veiga, R.; Navarro-Vicente, I.; Martínez-Campuzano, D.; García-Ruiz, R.; et al. Incidence and Risk Factors for Development of Cardiac Toxicity in Adult Patients with Newly Diagnosed Acute Myeloid Leukemia. Cancers 2023, 15, 2267. [Google Scholar] [CrossRef]
- Araji, G.; Mustafa, A.; Niazi, M.; Wei, C.; Sharma, R.; Abu-Baker, S.; Khattar, G.; El-Sayegh, S.; Odaimi, M. Acute cardiovascular complications of disseminated intravascular coagulation in acute myeloid leukemia. Thromb. Res. 2024, 239, 109042. [Google Scholar] [CrossRef] [PubMed]
- Larsson, K.; Mattsson, M.; Ebrahim, F.; Glimelius, I.; Höglund, M. High prevalence and incidence of cardiovascular disease in chronic lymphocytic leukaemia: A nationwide population-based study. Br. J. Haematol. 2020, 190, e245–e248. [Google Scholar] [CrossRef]
- Calvillo-Argüelles, O.; Schoffel, A.; Capo-Chichi, J.M.; Abdel-Qadir, H.; Schuh, A.; Carrillo-Estrada, M.; Liu, S.; Gupta, V.; Schimmer, A.D.; Yee, K.; et al. Cardiovascular Disease Among Patients with AML and CHIP-Related Mutations. JACC CardioOncol. 2022, 4, 38–49. [Google Scholar] [CrossRef]
- Yong, J.H.; Mai, A.S.; Matetić, A.; Elbadawi, A.; Elgendy, I.Y.; Lopez-Fernandez, T.; Mamas, M.A. Cardiovascular Risk in Patients with Hematological Malignancies: A Systematic Review and Meta-Analysis. Am. J. Cardiol. 2024, 212, 80–102. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Colombo, A.; Lamantia, G.; Colombo, N.; Civelli, M.; De Giacomi, G.; Rubino, M.; Veglia, F.; Fiorentini, C.; Cipolla, C.M. Anthracycline-induced cardiomyopathy: Clinical relevance and response to pharmacologic therapy. J. Am. Coll. Cardiol. 2010, 55, 213–220. [Google Scholar] [CrossRef]
- Manouchehri, A.; Kanu, E.; Mauro, M.J.; Aday, A.W.; Lindner, J.R.; Moslehi, J. Tyrosine kinase inhibitors in leukemia and cardiovascular events: From mechanism to patient care. ATVB 2020, 40, 301–308. [Google Scholar] [CrossRef]
- Gaya, A.; Ashford, R. Cardiac complications of radiation therapy. Clin. Oncol. 2005, 17, 153–159. [Google Scholar] [CrossRef]
- Militaru, A.G.; Lighezan, D.F.; Cimpean, A.M.; Amaricai, E.; Militaru, M. Predicting Cardiovascular Risk Factors for Acute Leukemia Patients by Assessing Subclinical Atherosclerosis and Left Ventricular Function Before Chemotherapy. Life 2025, 15, 704. [Google Scholar] [CrossRef]
- Cornelissen, L.L.; Kreuger, A.L.; Caram-Deelder, C.; Huisman, M.V.; Middelburg, R.A.; Kerkhoffs, J.L.H.; von dem Borne, P.A.; Beckers, E.A.M.; de Vooght, K.M.K.; Kuball, J.; et al. Association between cardiovascular risk factors and intracranial hemorrhage in patients with acute leukemia. Eur. J. Haematol. 2022, 108, 310–318. [Google Scholar] [CrossRef]
- Alexandre, J.; Cautela, J.; Ederhy, S.; Damaj, G.L.; Salem, J.E.; Barlesi, F.; Farnault, L.; Charbonnier, A.; Mirabel, M.; Champiat, S. Cardiovascular toxicity related to cancer treatment: A pragmatic approach to the American and European cardio-oncology guidelines. J. Am. Heart Assoc. 2020, 9, e018403. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.R.; Kanneganti, R.; Abbasi, M.; Akhtari, M. Monitoring for Chemotherapy-Related Cardiotoxicity in the Form of Left Ventricular Systolic Dysfunction: A Review of Current Recommendations. JCO Oncol. Pract. 2021, 17, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ Syst. Rev. 2021, 10, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Barker, T.H.; Stone, J.C.; Sears, K.; Klugar, M.; Tufanaru, C.; Leonardi-Bee, J.; Aromataris, E.; Munn, Z. The revised JBI critical appraisal tool for the assessment of risk of bias for randomized controlled trials. JBI Evid. Synth. 2023, 21, 494–506. [Google Scholar] [CrossRef]
- Sterne, J.A.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef]
- Whiting, P.; Savović, J.; Higgins, J.P.; Caldwell, D.M.; Reeves, B.C.; Shea, B.; Davies, P.; Kleijnen, J.; Churchill, R. ROBIS: A new tool to assess risk of bias in systematic reviews was developed. J. Clin. Epidemiol. 2016, 69, 225–234. [Google Scholar] [CrossRef]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef]
- Thurmond, V.A. The point of triangulation. J. Nurs. Scholarsh. 2001, 33, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Sohani, Z.N.; Meyre, D.; de Souza, R.J.; Joseph, P.G.; Gandhi, M.; Dennis, B.B.; Norman, G.; Anand, S.S. Assessing the quality of published genetic association studies in meta-analyses: The quality of genetic studies (Q-Genie) tool. BMC Genet. 2015, 16, 50. [Google Scholar] [CrossRef] [PubMed]
- Barker, T.H.; Hasanoff, S.; Aromataris, E.; Stone, J.C.; Leonardi-Bee, J.; Sears, K.; Habibi, N.; Klugar, M.; Tufanaru, C.; Moola, S. The revised JBI critical appraisal tool for the assessment of risk of bias for cohort studies. JBI Evid. Synth. 2025, 23, 441–453. [Google Scholar] [CrossRef]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Qureshi, R.; Mattis, P.; Lisy, K. Chapter 7: Systematic reviews of etiology and risk. In Joanna Briggs Institute Reviewer’s Manual; JBI: Milwaukee, WI, USA, 2017; Volume 5, pp. 217–269. [Google Scholar]
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological quality of case series studies: An introduction to the JBI critical appraisal tool. JBI Evid. Synth. 2020, 18, 2127–2133. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- National Heart, L.; Institute, B. Quality Assessment Tool for Before-After (Pre-Post) Studies with no Control Group; National Heart, Lung, and Blood Institute: Bethesda, MD, USA, 2014. [Google Scholar]
- Udin, M.H.; Sunder, S.S.; Nepali, S.; Kattel, S.; Abdelradi, A.; Doyle, S.T.; Ionita, C.N.; Liu, Q.; Sharma, U.C.; Pokharel, S. Differential cardiac impacts of hematological malignancies and solid tumors: A histopathological and biomarker study. Cardiooncology 2024, 10, 90. [Google Scholar] [CrossRef] [PubMed]
- Desai, P.; Lonial, S.; Cashen, A.; Kamdar, M.; Flinn, I.; O’Brien, S.; Garcia, J.S.; Korde, N.; Moslehi, J.; Wey, M.; et al. A Phase 1 First-in-Human Study of the MCL-1 Inhibitor AZD5991 in Patients with Relapsed/Refractory Hematologic Malignancies. Clin. Cancer Res. 2024, 30, 4844–4855. [Google Scholar] [CrossRef]
- Poudel, S.; Shrestha, H.; Pan, Y.; Li, Q.; Li, K.; Im, C.; Dixon, S.B.; Ehrhardt, M.J.; Mulrooney, D.A.; Zhou, S.; et al. Serum Proteins Predict Treatment-Related Cardiomyopathy Among Survivors of Childhood Cancer. JACC CardioOncol. 2025, 7, 56–67. [Google Scholar] [CrossRef]
- Spannbauer, A.; Bergler-Klein, J. Cardio-Oncology: A New Discipline in Medicine and Its Relevance to Hematology. Hamostaseologie 2024, 44, 255–267. [Google Scholar] [CrossRef]
- Sameer, S.; N, P.; Kuppusamy, S.; Adole, P.S.; Kayal, S. Cardiac Autonomic and Endothelial Function in Acute Lymphoblastic Leukaemia Patients Immediately After Chemotherapy and at the Three-Month Follow-up. Cureus 2024, 16, e55108. [Google Scholar] [CrossRef]
- Salas, M.Q.; Cascos, E.; López-García, A.; Pérez, E.; Baile-González, M.; Martín Rodríguez, C.; Pascual Cascón, M.J.; Luque, M.; Esquirol, A.; Heras Fernando, I.; et al. Cardiac events after allo-HCT in patients with acute myeloid leukemia. Blood Adv. 2024, 8, 5497–5509. [Google Scholar] [CrossRef]
- Roganovic, J.; Haupt, R.; Bárdi, E.; Hjorth, L.; Michel, G.; Pavasovic, V.; Scheinemann, K.; van der Pal, H.J.; Zadravec Zaletel, L.; Amariutei, A.E.; et al. Late Adverse Effects after Treatment for Childhood Acute Leukemia. Acta Med. Acad. 2024, 53, 59–80. [Google Scholar] [CrossRef] [PubMed]
- Puła, B.; Kępski, J.; Misiewicz-Krzemińska, I.; Szmit, S. Left and right ventricular global longitudinal strain assessment together with biomarker evaluation may have a predictive and prognostic role in patients qualified for hematopoietic stem cell transplantation due to hematopoietic and lymphoid malignancies—A pilot study description. Cardiooncology 2024, 10, 9. [Google Scholar] [CrossRef]
- Onoue, T.; Matthews, A.H.; Vakilpour, A.; Kang, Y.; Lefebvre, B.; Smith, A.M.; McCurdy, S.R.; Fradley, M.G.; Carver, J.; Chittams, J.; et al. Cardiotoxicity of venetoclax in patients with acute myeloid leukemia: Comparison with anthracyclines. Cardio-Oncol. 2024, 10, 75. [Google Scholar] [CrossRef]
- Ma, L.; Wang, Q.; Li, X.; Shang, Y.; Zhang, N.; Wu, J.; Liang, Y.; Chen, G.; Tan, Y.; Liu, X.; et al. Development of a risk assessment model for cardiac injury in patients newly diagnosed with acute myeloid leukemia based on a multicenter, real-world analysis in China. BMC Cancer 2024, 24, 132. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ge, S.; Zhang, A. Pediatric Cardio-Oncology: Screening, Risk Stratification, and Prevention of Cardiotoxicity Associated with Anthracyclines. Children 2024, 11, 884. [Google Scholar] [CrossRef] [PubMed]
- Kępski, J.; Szmit, S.; Lech-Marańda, E. Time Relationship between the Occurrence of a Thromboembolic Event and the Diagnosis of Hematological Malignancies. Cancers 2024, 16, 3196. [Google Scholar] [CrossRef]
- Hellman, J.; Chaireti, R. Incidence and Risk Factors for Arterial Thrombosis in Patients with Acute Leukemia and Lymphoid Malignancies: A Retrospective Single-Center Study. Cancers 2024, 16, 2511. [Google Scholar] [CrossRef]
- Hammoud, R.A.; Liu, Q.; Dixon, S.B.; Onerup, A.; Mulrooney, D.A.; Huang, I.C.; Jefferies, J.L.; Rhea, I.B.; Ness, K.K.; Ehrhardt, M.J.; et al. The burden of cardiovascular disease and risk for subsequent major adverse cardiovascular events in survivors of childhood cancer: A prospective, longitudinal analysis from the St Jude Lifetime Cohort Study. Lancet Oncol. 2024, 25, 811–822. [Google Scholar] [CrossRef]
- Hammoud, R.A.; Mulrooney, D.A.; Rhea, I.B.; Yu, C.; Johnson, J.N.; Chow, E.J.; Ehrhardt, M.J.; Hudson, M.M.; Ness, K.K.; Armstrong, G.T.; et al. Modifiable Cardiometabolic Risk Factors in Survivors of Childhood Cancer: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2024, 6, 16–32. [Google Scholar] [CrossRef]
- Fernández-Avilés, C.; González-Manzanares, R.; Ojeda, S.; Molina, J.R.; Heredia, G.; Resúa, A.; Hidalgo, F.; López-Aguilera, J.; Mesa, D.; Anguita, M.; et al. Diastolic function assessment with left atrial strain in long-term survivors of childhood acute lymphoblastic leukemia. Rev. Esp. Cardiol. 2024, 77, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Kundavaram, R.; Kumar, A.; Konnepati, S.; Yadav, Y.S.; Chaudhary, N.K.; Malik, S.; Gogia, P. Acute Ventricular Dysfunction After Doxorubicin-Based Induction Therapy for Pediatric Acute Lymphoblastic Leukemia. Cureus 2024, 16, e75720. [Google Scholar] [CrossRef] [PubMed]
- Dogliotti, I.; Levis, M.; Martin, A.; Bartoncini, S.; Felicetti, F.; Cavallin, C.; Maffini, E.; Cerrano, M.; Bruno, B.; Ricardi, U.; et al. Maintain Efficacy and Spare Toxicity: Traditional and New Radiation-Based Conditioning Regimens in Hematopoietic Stem Cell Transplantation. Cancers 2024, 16, 865. [Google Scholar] [CrossRef]
- Diaz, A.N.R.; Hurtado, G.P.; Manzano, A.A.A.; Keyes, M.J.; Turissini, C.; Choudhary, A.; Curtin, C.; Dommaraju, S.; Warack, S.; Strom, J.B.; et al. Sex Differences in the Development of Anthracycline-Associated Heart Failure. J. Card. Fail. 2024, 30, 907–914. [Google Scholar] [CrossRef]
- Rique, A.; Cautela, J.; Thuny, F.; Michel, G.; Ovaert, C.; El Louali, F. Left Ventricular Longitudinal Strain Abnormalities in Childhood Exposure to Anthracycline Chemotherapy. Children 2024, 11, 378. [Google Scholar] [CrossRef] [PubMed]
- Barachini, S.; Buda, G.; Petrini, I. Cardiovascular Toxicity of Antineoplastic Treatments in Hematological Diseases: Focus on Molecular Mechanisms to Improve Therapeutic Management. J. Clin. Med. 2024, 13, 1574. [Google Scholar] [CrossRef]
- Ketterl, T.G.; Chow, E.J.; Koves, I.H.; Goodman, P.; Leisenring, W.M.; Ballard, S.; Dengel, D.R.; Moran, A.; Sinaiko, A.R.; Steinberger, J.; et al. Impact of Hematopoietic Cell Transplantation on Cardiovascular Risk Factors and Insulin Sensitivity. Transpl. Cell Ther. 2024, 30, e241–e243. [Google Scholar] [CrossRef]
- Wang, X.; Singh, P.; Zhou, L.; Sharafeldin, N.; Landier, W.; Hageman, L.; Burridge, P.; Yasui, Y.; Sapkota, Y.; Blanco, J.G.; et al. Genome-Wide Association Study Identifies ROBO2 as a Novel Susceptibility Gene for Anthracycline-Related Cardiomyopathy in Childhood Cancer Survivors. J. Clin. Oncol. 2023, 41, 1758–1769. [Google Scholar] [CrossRef]
- Zhou, X.; Weng, Y.; Jiang, T.; Ou, W.; Zhang, N.; Dong, Q.; Tang, X. Influencing factors of anthracycline-induced subclinical cardiotoxicity in acute leukemia patients. BMC Cancer 2023, 23, 976. [Google Scholar] [CrossRef]
- Mitrovic, M.; Pantic, N.; Sabljic, N.; Bukumiric, Z.; Virijevic, M.; Pravdic, Z.; Cvetkovic, M.; Rajic, J.; Bodrozic, J.; Milosevic, V.; et al. Arterial Thrombosis in Patients with Acute Myeloid Leukemia: Incidence and Risk Factors. Cancers 2023, 15, 3060. [Google Scholar] [CrossRef]
- Kantarjian, H.; Short, N.J.; Jain, N.; Sasaki, K.; Huang, X.; Haddad, F.G.; Khouri, I.; DiNardo, C.D.; Pemmaraju, N.; Wierda, W.; et al. Frontline combination of ponatinib and hyper-CVAD in Philadelphia chromosome-positive acute lymphoblastic leukemia: 80-months follow-up results. Am. J. Hematol. 2023, 98, 493–501. [Google Scholar] [CrossRef]
- Heredia, G.; Gonzalez-Manzanares, R.; Ojeda, S.; Molina, J.R.; Fernandez-Aviles, C.; Hidalgo, F.; Lopez-Aguilera, J.; Crespin, M.; Mesa, D.; Anguita, M.; et al. Right Ventricular Function in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia: From the CTOXALL Study. Cancers 2023, 15, 5158. [Google Scholar] [CrossRef]
- Gawlik, M.; Zimodro, J.M.; Gąsecka, A.; Filipiak, K.J.; Szmit, S. Cardiac Arrhythmias in Oncological Patients-Epidemiology, Risk Factors, and Management within the Context of the New ESC 2022 Guidelines. Curr. Oncol. Rep. 2023, 25, 1107–1115. [Google Scholar] [CrossRef]
- Fazal, M.; Wei, C.; Chuy, K.L.; Hussain, K.; Gomez, S.E.; Ba, S.S.; Pietrasik, G.; Yadav, N.; Ghazizadeh, Z.; Kapoor, R.; et al. Tyrosine kinase inhibitor-associated ventricular arrhythmias: A case series and review of literature. J. Interv. Card. Electrophysiol. 2023, 66, 1165–1175. [Google Scholar] [CrossRef]
- Bertrand, É.; Caru, M.; Harvey, A.; Dodin, P.; Jacquemet, V.; Curnier, D. Cardiac electrical abnormalities in childhood acute lymphoblastic leukemia survivors: A systematic review. Cardiooncology 2023, 9, 40. [Google Scholar] [CrossRef]
- Baum, J.; Lax, H.; Lehmann, N.; Merkel-Jens, A.; Beelen, D.W.; Jöckel, K.H.; Dührsen, U. Preventive health care in blood cancer survivors: Results from the ABC study. J. Cancer Res. Clin. Oncol. 2023, 149, 11531–11540. [Google Scholar] [CrossRef]
- Auberle, C.; Lenihan, D.; Gao, F.; Cashen, A. Late cardiac events after allogeneic stem cell transplant: Incidence, risk factors, and impact on overall survival. Cardiooncology 2023, 9, 1. [Google Scholar] [CrossRef]
- Alpman, M.S.; Toth, I.; Langebäck, A.; Broberg, A.M.; Herold, N. Incidence and surveillance of acute cardiovascular toxicities in paediatric acute lymphoblastic leukaemia: A retrospective population-based single-centre cohort study. EJC Paediatr. Oncol. 2023, 2, 100020. [Google Scholar] [CrossRef]
- Berisha, A.; Placci, A.; Piccaluga, P.P. Cardiotoxicity of Tyrosine Kinase Inhibitors in Philadelphia-Positive Leukemia Patients. Hemato 2023, 4, 68–75. [Google Scholar] [CrossRef]
- Januzzi, J.L.; Garasic, J.M.; Kasner, S.E.; McDonald, V.; Petrie, M.C.; Seltzer, J.; Mauro, M.; Croce, K.; Berman, E.; Deininger, M.; et al. Retrospective analysis of arterial occlusive events in the PACE trial by an independent adjudication committee. J. Hematol. Oncol. 2022, 15, 1. [Google Scholar] [CrossRef]
- Terada, C.I.; Onoue, K.; Fujii, T.; Itami, H.; Morita, K.; Uchiyama, T.; Takeda, M.; Nakagawa, H.; Nakano, T.; Baba, Y.; et al. Histopathological and epigenetic changes in myocardium associated with cancer therapy-related cardiac dysfunction. ESC Heart Fail. 2022, 9, 3031–3043. [Google Scholar] [CrossRef]
- Perpinia, A.S.; Kadoglou, N.; Vardaka, M.; Gkortzolidis, G.; Karavidas, A.; Marinakis, T.; Papachrysostomou, C.; Makaronis, P.; Vlachou, C.; Mantzourani, M.; et al. Pharmaceutical Prevention and Management of Cardiotoxicity in Hematological Malignancies. Pharmaceuticals 2022, 15, 1007. [Google Scholar] [CrossRef]
- Muggeo, P.; Scicchitano, P.; Muggeo, V.M.R.; Novielli, C.; Giordano, P.; Ciccone, M.M.; Faienza, M.F.; Santoro, N. Assessment of Cardiovascular Function in Childhood Leukemia Survivors: The Role of the Right Heart. Children 2022, 9, 1731. [Google Scholar] [CrossRef]
- Luo, Z.; Cheng, J.; Wang, Y. Cardiac Infiltration as the First Manifestation of Acute Lymphoblastic Leukemia: A Systematic Review. Front. Oncol. 2022, 12, 805981. [Google Scholar] [CrossRef]
- Lipshultz, E.R.; Chow, E.J.; Doody, D.R.; Armenian, S.H.; Asselin, B.L.; Baker, K.S.; Bhatia, S.; Constine, L.S.; Freyer, D.R.; Kopp, L.M.; et al. Cardiometabolic Risk in Childhood Cancer Survivors: A Report from the Children’s Oncology Group. Cancer Epidemiol. Biomark. Prev. 2022, 31, 536–542. [Google Scholar] [CrossRef]
- Gonzalez-Manzanares, R.; Castillo, J.C.; Molina, J.R.; Ruiz-Ortiz, M.; Mesa, D.; Ojeda, S.; Anguita, M.; Pan, M. Automated Global Longitudinal Strain Assessment in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia. Cancers 2022, 14, 1513. [Google Scholar] [CrossRef]
- Chianca, M.; Panichella, G.; Fabiani, I.; Giannoni, A.; L’Abbate, S.; Aimo, A.; Del Franco, A.; Vergaro, G.; Grigoratos, C.; Castiglione, V.; et al. Bidirectional Relationship Between Cancer and Heart Failure: Insights on Circulating Biomarkers. Front. Cardiovasc. Med. 2022, 9, 936654. [Google Scholar] [CrossRef]
- Bottinor, W.; Chow, E.J. Mitigating, monitoring, and managing long-term chemotherapy- and radiation-induced cardiac toxicity. Hematol. Am. Soc. Hematol. Educ. Program 2022, 2022, 251–258. [Google Scholar] [CrossRef]
- Arnán Sangerman, M.; Fernández Moreno, A.; García Quintana, A.; García-Vidal, C.; Olave Rubio, M.T.; Del Mar Tormo Díaz, M.; Vendranas, M.; Rodriguez Macias, G. Practical tips for managing FLT3 mutated acute myeloid leukemia with midostaurin. Expert Rev. Hematol. 2022, 15, 203–214. [Google Scholar] [CrossRef]
- Petrykey, K.; Rezgui, A.M.; Guern, M.L.; Beaulieu, P.; St-Onge, P.; Drouin, S.; Bertout, L.; Wang, F.; Baedke, J.L.; Yasui, Y.; et al. Genetic factors in treatment-related cardiovascular complications in survivors of childhood acute lymphoblastic leukemia. Pharmacogenomics 2021, 22, 885–901. [Google Scholar] [CrossRef]
- Oka, T.; Tada, Y.; Oboshi, M.; Kamada, R.; Yasui, T.; Shioyama, W.; Nishikawa, T.; Hino, A.; Ishikawa, J.; Fujita, M. Serial Changes in Cardiac Strain and Contractility After Hematopoietic Stem Cell Transplantation in Patients with Hematologic Malignancies. Int. Heart J. 2021, 62, 575–583. [Google Scholar] [CrossRef]
- Lubas, M.M.; Wang, M.; Jefferies, J.L.; Ness, K.K.; Ehrhardt, M.J.; Krull, K.R.; Mulrooney, D.A.; Srivastava, D.K.; Howell, R.M.; Robison, L.L.; et al. The Contribution of Stress and Distress to Cardiovascular Health in Adult Survivors of Childhood Cancer. Cancer Epidemiol. Biomark. Prev. 2021, 30, 286–294. [Google Scholar] [CrossRef]
- Lazăr, D.R.; Farcaş, A.D.; Blag, C.; Neaga, A.; Zdrenghea, M.T.; Căinap, C.; Lazăr, F.L.; Stef, A.; Căinap, S.S. Cardiotoxicity: A Major Setback in Childhood Leukemia Treatment. Dis. Markers 2021, 2021, 8828410. [Google Scholar] [CrossRef]
- El Amrousy, D.; El-Afify, D.; Khedr, R.; Ibrahim, A.M. Omega 3 fatty acids can reduce early doxorubicin-induced cardiotoxicity in children with acute lymphoblastic leukemia. Pediatr. Blood Cancer 2022, 69, e29496. [Google Scholar] [CrossRef]
- Kamaraju, S.; Mohan, M.; Zaharova, S.; Wallace, B.; McGraw, J.; Lokken, J.; Tierney, J.; Weil, E.; Fatunde, O.; Brown, S.A. Interactions between cardiology and oncology drugs in precision cardio-oncology. Clin. Sci. 2021, 135, 1333–1351. [Google Scholar] [CrossRef]
- Hoeben, B.A.W.; Wong, J.Y.C.; Fog, L.S.; Losert, C.; Filippi, A.R.; Bentzen, S.M.; Balduzzi, A.; Specht, L. Total Body Irradiation in Haematopoietic Stem Cell Transplantation for Paediatric Acute Lymphoblastic Leukaemia: Review of the Literature and Future Directions. Front. Pediatr. 2021, 9, 774348. [Google Scholar] [CrossRef]
- Gangaraju, R. Risk of Coronary Heart Disease in Blood or Marrow Transplant Survivors. Master’s Thesis, The University of Alabama at Birmingham, Birmingham, AL, USA, 2021. [Google Scholar]
- Duléry, R.; Mohty, R.; Labopin, M.; Sestili, S.; Malard, F.; Brissot, E.; Battipaglia, G.; Médiavilla, C.; Banet, A.; Van de Wyngaert, Z.; et al. Early Cardiac Toxicity Associated with Post-Transplant Cyclophosphamide in Allogeneic Stem Cell Transplantation. JACC CardioOncol. 2021, 3, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Diesch-Furlanetto, T.; Gabriel, M.; Zajac-Spychala, O.; Cattoni, A.; Hoeben, B.A.W.; Balduzzi, A. Late Effects After Haematopoietic Stem Cell Transplantation in ALL, Long-Term Follow-Up and Transition: A Step Into Adult Life. Front. Pediatr. 2021, 9, 773895. [Google Scholar] [CrossRef]
- Chow, E.J.; Doody, D.R.; Di, C.; Armenian, S.H.; Baker, K.S.; Bricker, J.B.; Gopal, A.K.; Hagen, A.M.; Ketterl, T.G.; Lee, S.J.; et al. Feasibility of a behavioral intervention using mobile health applications to reduce cardiovascular risk factors in cancer survivors: A pilot randomized controlled trial. J. Cancer Surviv. 2021, 15, 554–563. [Google Scholar] [CrossRef]
- Chen, D.H.; Tyebally, S.; Mallouppas, M.; Ghosh, A.K. CAR T Cell and BiTE Therapy—New Therapies, New Risks? Curr. Cardiovasc. Risk Rep. 2020, 15, 1. [Google Scholar] [CrossRef]
- Burns, E.A.; Gentille, C.; Trachtenberg, B.; Pingali, S.R.; Anand, K. Cardiotoxicity Associated with Anti-CD19 Chimeric Antigen Receptor T-Cell (CAR-T) Therapy: Recognition, Risk Factors, and Management. Diseases 2021, 9, 20. [Google Scholar] [CrossRef]
- Linares Ballesteros, A.; Sanguino Lobo, R.; Villada Valencia, J.C.; Arévalo Leal, O.; Plazas Hernández, D.C.; Aponte Barrios, N.; Perdomo Ramírez, I. Early-onset Cardiotoxicity assessment related to anthracycline in children with leukemia. A Prospect. Study. Colomb. Med. 2021, 52, e2034542. [Google Scholar] [CrossRef]
- Abrahão, R.; Huynh, J.C.; Benjamin, D.J.; Li, Q.W.; Winestone, L.E.; Muffly, L.; Keegan, T.H.M. Chronic medical conditions and late effects after acute myeloid leukaemia in adolescents and young adults: A population-based study. Int. J. Epidemiol. 2021, 50, 663–674. [Google Scholar] [CrossRef]
- Saussele, S.; Haverkamp, W.; Lang, F.; Koschmieder, S.; Kiani, A.; Jentsch-Ullrich, K.; Stegelmann, F.; Pfeifer, H.; La Rosée, P.; Goekbuget, N.; et al. Ponatinib in the Treatment of Chronic Myeloid Leukemia and Philadelphia Chromosome-Positive Acute Leukemia: Recommendations of a German Expert Consensus Panel with Focus on Cardiovascular Management. Acta Haematol. 2020, 143, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Orvain, C.; Balsat, M.; Tavernier, E.; Marolleau, J.P.; Pabst, T.; Chevallier, P.; de Gunzburg, N.; Cacheux, V.; Huguet, F.; Chantepie, S.; et al. Thromboembolism prophylaxis in adult patients with acute lymphoblastic leukemia treated in the GRAALL-2005 study. Blood 2020, 136, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Ociepa, T.; Posio, W.; Sawicki, M.; Urasiński, T. CIMT does not identify early vascular changes in childhood acute lymphoblastic leukemia survivors. Adv. Clin. Exp. Med. 2020, 29, 243–249. [Google Scholar] [CrossRef]
- Neuendorff, N.R.; Loh, K.P.; Mims, A.S.; Christofyllakis, K.; Soo, W.-K.; Bölükbasi, B.; Oñoro-Algar, C.; Hundley, W.G.; Klepin, H.D. Anthracycline-related cardiotoxicity in older patients with acute myeloid leukemia: A Young SIOG review paper. Blood Adv. 2020, 4, 762–775. [Google Scholar] [CrossRef]
- Leerink, J.M.; de Baat, E.C.; Feijen, E.A.M.; Bellersen, L.; van Dalen, E.C.; Grotenhuis, H.B.; Kapusta, L.; Kok, W.E.M.; Loonen, J.; van der Pal, H.J.H.; et al. Cardiac Disease in Childhood Cancer Survivors: Risk Prediction, Prevention, and Surveillance: JACC CardioOncology State-of-the-Art Review. JACC CardioOncol. 2020, 2, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Jamal, F.A.; Khaled, S.K. The Cardiovascular Complications of Chimeric Antigen Receptor T Cell Therapy. Curr. Hematol. Malig. Rep. 2020, 15, 130–132. [Google Scholar] [CrossRef]
- Herrmann, J. Adverse cardiac effects of cancer therapies: Cardiotoxicity and arrhythmia. Nat. Rev. Cardiol. 2020, 17, 474–502. [Google Scholar] [CrossRef]
- Giudice, V.; Vecchione, C.; Selleri, C. Cardiotoxicity of Novel Targeted Hematological Therapies. Life 2020, 10, 344. [Google Scholar] [CrossRef]
- Gavriilaki, E.; Sakellari, I.; Anyfanti, P.; Batsis, I.; Vardi, A.; Bousiou, Z.; Lazaridis, A.; Nikolaidou, B.; Zarifis, I.; Masmanidou, M.; et al. Assessment of Endothelial Injury and Pro-Coagulant Activity Using Circulating Microvesicles in Survivors of Allogeneic Hematopoietic Cell Transplantation. Int. J. Mol. Sci. 2020, 21, 9768. [Google Scholar] [CrossRef]
- Cook, J.; Litzow, M. Advances in Supportive Care for Acute Lymphoblastic Leukemia. Curr. Hematol. Malig. Rep. 2020, 15, 276–293. [Google Scholar] [CrossRef]
- Bhatia, S. Genetics of Anthracycline Cardiomyopathy in Cancer Survivors: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2020, 2, 539–552. [Google Scholar] [CrossRef]
- Mohamed, M.O.; Lopez-Mattei, J.C.; Parwani, P.; Iliescu, C.A.; Bharadwaj, A.; Kim, P.Y.; Palaskas, N.L.; Rashid, M.; Potts, J.; Kwok, C.S.; et al. Management strategies and clinical outcomes of acute myocardial infarction in leukaemia patients: Nationwide insights from United States hospitalisations. Int. J. Clin. Pract. 2020, 74, e13476. [Google Scholar] [CrossRef]
- Caro-Codón, J.; López-Fernández, T.; Álvarez-Ortega, C.; Zamora Auñón, P.; Rodríguez, I.R.; Gómez Prieto, P.; Buño Soto, A.; Canales Albendea, M.; Albaladejo, A.; Mediavilla, G.; et al. Cardiovascular risk factors during cancer treatment. Prevalence and prognostic relevance: Insights from the CARDIOTOX registry. Eur. J. Prev. Cardiol. 2022, 29, 859–868. [Google Scholar] [CrossRef]
- Koene, R.J.; Prizment, A.E.; Blaes, A.; Konety, S.H. Shared Risk Factors in Cardiovascular Disease and Cancer. Circulation 2016, 133, 1104–1114. [Google Scholar] [CrossRef]
- Hossain, M.J.; Xie, L. Sex disparity in childhood and young adult acute myeloid leukemia (AML) survival: Evidence from US population data. Cancer Epidemiol. 2015, 39, 892–900. [Google Scholar] [CrossRef]
- Li, Z.; Yu, L.; Hu, L.-J.; Zhao, T.; Duan, W.; Wang, J.; Jia, J.; Liu, J.; Qin, Y.-Z.; Jiang, H. Sex Differences in Disease Characteristics and Outcome in Adults with Acute Myeloid Leukemia from China. Blood 2024, 144, 6121. [Google Scholar] [CrossRef]
- Singh, S.K.; Lupo, P.J.; Scheurer, M.E.; Saxena, A.; Kennedy, A.E.; Ibrahimou, B.; Barbieri, M.A.; Mills, K.I.; McCauley, J.L.; Okcu, M.F.; et al. A childhood acute lymphoblastic leukemia genome-wide association study identifies novel sex-specific risk variants. Medicine 2016, 95, e5300. [Google Scholar] [CrossRef]
- Camilli, M.; Cipolla, C.M.; Dent, S.; Minotti, G.; Cardinale, D.M. Anthracycline Cardiotoxicity in Adult Cancer Patients: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2024, 6, 655–677. [Google Scholar] [CrossRef]
- Domercant, J.; Polin, N.; Jahangir, E. Cardio-Oncology: A Focused Review of Anthracycline-, Human Epidermal Growth Factor Receptor 2 Inhibitor-, and Radiation-Induced Cardiotoxicity and Management. Ochsner J. 2016, 16, 250–256. [Google Scholar]
- McGowan, J.V.; Chung, R.; Maulik, A.; Piotrowska, I.; Walker, J.M.; Yellon, D.M. Anthracycline chemotherapy and cardiotoxicity. Cardiovasc. Drug Ther. J. 2017, 31, 63–75. [Google Scholar] [CrossRef]
- Balough, E.; Ariza, A.; Asnani, A.; Hoeger, C.W. Cardiotoxicity of Anthracyclines. Cardiol. Clin. 2025, 43, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Hayek, S.S.; Zaha, V.G.; Bogle, C.; Deswal, A.; Langston, A.; Rotz, S.; Vasbinder, A.; Yang, E.; Okwuosa, T. Cardiovascular Management of Patients Undergoing Hematopoietic Stem Cell Transplantation: From Pretransplantation to Survivorship: A Scientific Statement from the American Heart Association. Circulation 2024, 149, e1113–e1127. [Google Scholar] [CrossRef] [PubMed]
- Salvaris, R.; Fedele, P.L. Targeted Therapy in Acute Lymphoblastic Leukaemia. J. Pers. Med. 2021, 11, e1113–e1127. [Google Scholar] [CrossRef]
- Singh, A.P.; Umbarkar, P.; Tousif, S.; Lal, H. Cardiotoxicity of the BCR-ABL1 tyrosine kinase inhibitors: Emphasis on ponatinib. Int. J. Cardiol. 2020, 316, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Shyam Sunder, S.; Sharma, U.C.; Pokharel, S. Adverse effects of tyrosine kinase inhibitors in cancer therapy: Pathophysiology, mechanisms and clinical management. Signal Transduct. Target. Ther. 2023, 8, 262. [Google Scholar] [CrossRef]
- Lee Chuy, K.; Nahhas, O.; Dominic, P.; Lopez, C.; Tonorezos, E.; Sidlow, R.; Straus, D.; Gupta, D. Cardiovascular Complications Associated with Mediastinal Radiation. Curr. Treat. Options Cardiovasc. Med. 2019, 21, 31. [Google Scholar] [CrossRef]
- Nishikawa, T.; Miyahara, E.; Kurauchi, K.; Watanabe, E.; Ikawa, K.; Asaba, K.; Tanabe, T.; Okamoto, Y.; Kawano, Y. Mechanisms of fatal cardiotoxicity following high-dose cyclophosphamide therapy and a method for its prevention. PLoS ONE 2015, 10, e0131394. [Google Scholar] [CrossRef]
- Zhao, Y.; He, R.; Oerther, S.; Zhou, W.; Vosough, M.; Hassan, M. Cardiovascular Complications in Hematopoietic Stem Cell Transplanted Patients. J. Pers. Med. 2022, 12, 1797. [Google Scholar] [CrossRef]
- Ohmoto, A.; Fuji, S. Cardiac complications associated with hematopoietic stem-cell transplantation. Bone Marrow Transplant. 2021, 56, 2637–2643. [Google Scholar] [CrossRef] [PubMed]
- Hermans, S.J.F.; Versluis, J.; Labopin, M.; Giebel, S.; van Norden, Y.; Moiseev, I.; Blaise, D.; Díez Martín, J.L.; Meijer, E.; Rovira, M.; et al. Prediction of Nonrelapse Mortality in Patients with Acute Myeloid Leukemia and Acute Lymphoblastic Leukemia Receiving Allogeneic Stem Cell Transplantation with Posttransplantation Cyclophosphamide-based Graft Versus Host Disease Prophylaxis. Hemasphere 2023, 7, e846. [Google Scholar] [CrossRef]
- Kayser, S.; Döhner, K.; Krauter, J.; Köhne, C.-H.; Horst, H.; Held, G.; Lilienfeld-Toal, M.; Wilhelm, S.; Kuendgen, A.; Götze, K.; et al. The impact of therapy-related acute myeloid leukemia (AML) on outcome in 2853 adult patients with newly diagnosed AML. Blood 2011, 117, 2137–2145. [Google Scholar] [CrossRef]
- Lica, J.J.; Pradhan, B.; Safi, K.; Jakóbkiewicz-Banecka, J.; Hellmann, A. Promising Therapeutic Strategies for Hematologic Malignancies: Innovations and Potential. Molecules 2024, 29, 4280. [Google Scholar] [CrossRef]
- Sanchez-Petitto, G.; Goloubeva, O.G.; Masur, J.; Childress, J.; Iqbal, T.; An, M.; Muhammad, S.; Lawson, J.; Li, G.; Barr, B.; et al. Clinical outcomes of patients with acute myeloid leukemia and cardiovascular disease. Leuk. Res. 2024, 138, 107456. [Google Scholar] [CrossRef]
- Yazdanparast, A.; Fathpour, G.; Saberianpour, S. Cardiovascular involvement in blood cancers: ALL, AML, CLL, and CML. Iran. J. Pediatr. Hematol. Oncol. 2021, 11, 270–279. [Google Scholar] [CrossRef]
- Sulicka-Grodzicka, J.; Chyrchel, B.; Totoń-Żurańska, J.; Nowak, E.; Wołkow, P.P.; Surdacki, A.; Grodzicki, T. Cranial Irradiation in Childhood Acute Lymphoblastic Leukemia Is Related to Subclinical Left Ventricular Dysfunction and Reduced Large Artery Compliance in Cancer Survivors. J. Clin. Med. 2019, 8, 1952. [Google Scholar] [CrossRef]
- Getz, K.D.; Sung, L.; Ky, B.; Gerbing, R.B.; Leger, K.J.; Leahy, A.B.; Sack, L.; Woods, W.G.; Alonzo, T.; Gamis, A.; et al. Occurrence of Treatment-Related Cardiotoxicity and Its Impact on Outcomes Among Children Treated in the AAML0531 Clinical Trial: A Report from the Children’s Oncology Group. J. Clin. Oncol. 2019, 37, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Sawaya, H.; Sebag, I.A.; Plana, J.C.; Januzzi, J.L.; Ky, B.; Tan, T.C.; Cohen, V.; Banchs, J.; Carver, J.R.; Wiegers, S.E.; et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ. Cardiovasc. Imaging 2012, 5, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, Y.; Zhang, Y.; Fang, F.; Liu, J.; Xia, Y.; Liu, Y. Cardiac Biomarkers for the Detection and Management of Cancer Therapy-Related Cardiovascular Toxicity. J. Cardiovasc. Dev. Dis. 2022, 9, 372. [Google Scholar] [CrossRef] [PubMed]
- Murtagh, G.; Januzzi, J.L.; Scherrer-Crosbie, M.; Neilan, T.G.; Dent, S.; Ho, J.E.; Appadurai, V.; McDermott, R.; Akhter, N. Circulating Cardiovascular Biomarkers in Cancer Therapeutics-Related Cardiotoxicity: Review of Critical Challenges, Solutions, and Future Directions. J. Am. Heart Assoc. 2023, 12, e029574. [Google Scholar] [CrossRef] [PubMed]
- Semeraro, G.C.; Cipolla, C.M.; Cardinale, D.M. Role of cardiac biomarkers in cancer patients. Cancers 2021, 13, 5426. [Google Scholar] [CrossRef] [PubMed]
- Pudil, R.; Mueller, C.; Čelutkienė, J.; Henriksen, P.A.; Lenihan, D.; Dent, S.; Barac, A.; Stanway, S.; Moslehi, J.; Suter, T.M.; et al. Role of serum biomarkers in cancer patients receiving cardiotoxic cancer therapies: A position statement from the Cardio-Oncology Study Group of the Heart Failure Association and the Cardio-Oncology Council of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 1966–1983. [Google Scholar] [CrossRef] [PubMed]
- Skitch, A.; Mital, S.; Mertens, L.; Liu, P.; Kantor, P.; Grosse-Wortmann, L.; Manlhiot, C.; Greenberg, M.; Nathan, P.C. Novel approaches to the prediction, diagnosis and treatment of cardiac late effects in survivors of childhood cancer: A multi-centre observational study. BMC Cancer 2017, 17, 519. [Google Scholar] [CrossRef] [PubMed]
- Kattih, B.; Shirvani, A.; Klement, P.; Garrido, A.; Gabdoulline, R.; Liebich, A.; Brandes, M.; Chaturvedi, A.; Seeger, T.; Thol, F.; et al. IDH Mutations Are Associated with an Increased Risk of Coronary Artery Disease and Cardiotoxicity in Patients with Established AML. Blood 2020, 136, 32–33. [Google Scholar] [CrossRef]
- Kemp, H.; Fenwarth, L.; Lebon, D.; Duployez, N.; Paubelle, E.; Joris, M.; Charbonnier, A.; Montes, L.; Preudhomme, C.; Tribouilloy, C.; et al. The Occurrence of Infection and TP53 Mutation Are Risk Factors for Cardiovascular Toxicity of Acute Myeloid Leukemia Induction Therapy. Blood 2023, 142, 3815. [Google Scholar] [CrossRef]
- Kang, Y.; Lefebvre, B.; Pamies, I.M.; Gill, S.I.; Doucette, A.G.; Denduluri, S.; Smith, A.M.; McCurdy, S.; Luger, S.; Carver, J.; et al. Symptomatic Heart Failure and Clonal Hematopoiesis-Related Mutations in Patients with Acute Myeloid Leukemia. Am. J. Cardiol. 2024, 226, 9–17. [Google Scholar] [CrossRef]
- Jaiswal, S.; Natarajan, P.; Silver, A.J.; Gibson, C.J.; Bick, A.G.; Shvartz, E.; McConkey, M.; Gupta, N.; Gabriel, S.; Ardissino, D.; et al. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 111–121. [Google Scholar] [CrossRef]
- Sikking, M.A.; Stroeks, S.L.; Waring, O.J.; Henkens, M.T.; Riksen, N.P.; Hoischen, A.; Heymans, S.R.; Verdonschot, J.A. Clonal hematopoiesis of indeterminate potential from a heart failure specialist’s point of view. J. Am. Heart Assoc. 2023, 12, e030603. [Google Scholar] [CrossRef]
- Blanco, J.G.; Sun, C.L.; Landier, W.; Chen, L.; Esparza-Duran, D.; Leisenring, W.; Mays, A.; Friedman, D.L.; Ginsberg, J.P.; Hudson, M.M.; et al. Anthracycline-related cardiomyopathy after childhood cancer: Role of polymorphisms in carbonyl reductase genes–a report from the Children’s Oncology Group. J. Clin. Oncol. 2012, 30, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Covarrubias, V.; Ghosh, D.; Lakhman, S.S.; Pendyala, L.; Blanco, J.G. A functional genetic polymorphism on human carbonyl reductase 1 (CBR1 V88I) impacts on catalytic activity and NADPH binding affinity. Drug Metab. Dispos. 2007, 35, 973–980. [Google Scholar] [CrossRef]
- Wojnowski, L.; Kulle, B.; Schirmer, M.; Schlüter, G.; Schmidt, A.; Rosenberger, A.; Vonhof, S.; Bickeböller, H.; Toliat, M.R.; Suk, E.K.; et al. NAD(P)H oxidase and multidrug resistance protein genetic polymorphisms are associated with doxorubicin-induced cardiotoxicity. Circulation 2005, 112, 3754–3762. [Google Scholar] [CrossRef]
- Cascales, A.; Pastor-Quirante, F.; Sánchez-Vega, B.; Luengo-Gil, G.; Corral, J.; Ortuño-Pacheco, G.; Vicente, V.; de la Peña, F.A. Association of anthracycline-related cardiac histological lesions with NADPH oxidase functional polymorphisms. Oncologist 2013, 18, 446–453. [Google Scholar] [CrossRef]
- Armenian, S.H.; Ding, Y.; Mills, G.; Sun, C.; Venkataraman, K.; Wong, F.L.; Neuhausen, S.L.; Senitzer, D.; Wang, S.; Forman, S.J.; et al. Genetic susceptibility to anthracycline-related congestive heart failure in survivors of haematopoietic cell transplantation. Br. J. Haematol. 2013, 163, 205–213. [Google Scholar] [CrossRef]
- Blaes, A.; Nohria, A.; Armenian, S.; Bergom, C.; Thavendiranathan, P.; Barac, A.; Sanchez-Petitto, G.; Desai, S.; Zullig, L.L.; Morgans, A.K.; et al. Cardiovascular Considerations After Cancer Therapy. JACC CardioOncol. 2025, 7, 1–19. [Google Scholar] [CrossRef] [PubMed]
- El-Harasis, M.A.; Hefazi, M.; Julakanti, R.; Hogan, W.J.; Litzow, M.R.; Patnaik, M.M.; Herrmann, J. Cardiovascular outcomes in patients receiving myeloablative vs. reduced intensity conditioning prior to allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia. Bone Marrow Transpl. 2021, 56, 508–510. [Google Scholar] [CrossRef]
- Schwinger, R.H. Pathophysiology of heart failure. Cardiovasc. Diagn. Ther. 2021, 11, 263. [Google Scholar] [CrossRef]
- Izquierdo-Condoy, J.S.; Arias-Intriago, M.; Becerra Cardona, D.A.; García-Cañarte, S.; Vinueza-Moreano, P. Anticancer Chemotherapy-Induced Atherosclerotic Cardiovascular Disease: A Comprehensive Review. Life 2025, 15, 245. [Google Scholar] [CrossRef]
- Xiong, Z.; Liao, Y.; Zhang, Z.; Wan, Z.; Liang, S.; Guo, J. Molecular Insights into Oxidative-Stress-Mediated Cardiomyopathy and Potential Therapeutic Strategies. Biomolecules 2025, 15, 670. [Google Scholar] [CrossRef]
- Ness, K.K.; Armenian, S.H.; Kadan-Lottick, N.; Gurney, J.G. Adverse effects of treatment in childhood acute lymphoblastic leukemia: General overview and implications for long-term cardiac health. Expert Rev. Hematol. 2011, 4, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidis, I.; Tsokkou, S.; Grigoriadis, S.; Chrysavgi, L.; Gavriilaki, E. Cardiotoxicity in Acute Myeloid Leukemia in Adults: A Scoping Study. Cancers 2024, 16, 2474. [Google Scholar] [CrossRef]
- Lazar, D.R.; Maniu, D.; Lazar, F.-L.; Blag, C.; Bota, M.; Zdrenghea, M.; Cainap, S. Exploring the baseline cardiac function and its correlation with risk stratification in children diagnosed with acute lymphoblastic leukemia. Ann. Hematol. 2025, 1–8. [Google Scholar] [CrossRef]
- Medeiros, B.C.; Possick, J.; Fradley, M. Cardiovascular, pulmonary, and metabolic toxicities complicating tyrosine kinase inhibitor therapy in chronic myeloid leukemia: Strategies for monitoring, detecting, and managing. Blood Rev. 2018, 32, 289–299. [Google Scholar] [CrossRef] [PubMed]

| No. | Authors/Year | Research Methodology | Research Objective | Type of Acute Leukemia | Risk Factors | Type of Cardiovascular Complications | Time of Cardiovascular Complication Onset | Key Findings |
|---|---|---|---|---|---|---|---|---|
| 1 | Udin et al. [29]/2024 | Quantitative study (retrospective, observational post-mortem analysis) | To examine histopathological features alongside cardiovascular biomarkers in patients with Hematological Malignancies and Solid Tumors who underwent post-mortem evaluation | AML, ALL |
| MI, CAD, arrhythmias, pericarditis, valvular heart disease, AF, cardiomyopathy, myocardial fibrosis, cardiac dysfunction, hypertension, electromechanical dysfunction, severe atherosclerosis | Early-Onset to Late-Onset | Patients with hematological malignancies, including acute leukemia, exhibited significant myocardial damage, marked by reduced cardiomyocyte nuclear density and disorganized collagen fibers, more so than in solid tumor cases. They also showed elevated BNP and low hemoglobin, indicating worsened cardiac dysfunction. Multivariate analysis identified increased right ventricular thickness, low diastolic blood pressure, and elevated cardiac troponin I as key predictors of cardiac death. |
| 2 | Desai et al. [30]/2024 | Quantitative study (Phase 1 clinical trial) | To assess the safety, tolerability, pharmacokinetics, and antitumor activity of AZD5991 (investigational MCL-1 inhibitor), both as a monotherapy and in combination with venetoclax, in patients with relapsed or refractory hematologic malignancies | AML |
| Cardiac arrest | Early-Onset | AZD5991 treatment, a human Myeloid Cell Leukemia 1 type inhibitor, was associated with cardiovascular complications, including elevated troponin in 10.3% of patients and increased troponin T in 54 of 65 cases. Importantly, no correlation was found between troponin elevation and pre-existing cardiovascular risk factors. |
| 3 | Poudel et al. [31]/2024 | Quantitative study (population-based case–control cohort study) | To determine if serum proteins and/or metabolites in asymptomatic childhood cancer survivors can discriminate symptomatic cardiomyopathy | ALL |
| Subclinical cardiomyopathy, severe cardiomyopathy | Late-Onset | The study identified 27 dysregulated serum proteins predicting severe cardiomyopathy with 83% accuracy. Key proteins (A-Kinase Anchoring Protein 4, splicing factor 3b subunit 1, Tubulin Alpha 4a) revealed mechanisms of anthracycline-induced damage, and the model outperformed traditional tools, supporting early detection and prevention in cancer survivors. |
| 4 | Spannbauer and Bergler-Klein [32]/2024 | Qualitative study (narrative review) | To evaluate the risk of ATE, MI, and ischemic stroke in cancer patients, particularly those with acute leukemia, explores the relationship between cancer treatments and cardiovascular toxicity, alongside the role of anticoagulation and antiplatelet therapies in preventing ATE | ALL, AML |
| ATE, MI, ischemic stroke, cardiomyopathy, AF, HF, cardiotoxicity | During Treatment to Late-Onset | Acute leukemia patients were at high risk for cardiovascular events, particularly in the first 6 months of diagnosis and treatment. Anthracyclines and radiation heighten cardiotoxicity, while AF increases ATE risk. The stroke risk score in AF underestimates this risk, and the efficacy of anticoagulation (Low Molecular Weight Heparin, Direct Oral Anticoagulants, warfarin) in prevention remained unclear. |
| 5 | Sameer et al. [33]/2024 | Quantitative study (longitudinal cohort study) | To assess cardiac autonomic function and levels of endothelial and inflammatory biomarkers in adult patients with ALL immediately after chemotherapy and at a three-month follow-up | ALL |
| Cardiovascular autonomic dysfunction, endothelial dysfunction | Early-Oonset | ALL patients showed heightened sympathetic activity, reduced parasympathetic modulation, and autonomic imbalance, indicating elevated cardiovascular risk. While autonomic function improved over time, persistent levels of Soluble Vascular Cell Adhesion Molecule-1, Soluble Intercellular Adhesion Molecule-1, and High-sensitivity C-Reactive Protein suggested ongoing endothelial dysfunction and inflammation. |
| 6 | Salas et al. [34]/2024 | Quantitative study (retrospective, multicenter, registry-based cohort study) | To investigate the incidence and predictors of early (first 100 days) and late cardiac events after allo-HCT in patients with AML treated with anthracyclines and explore the impact of post-transplant cyclophosphamide on cardiac complications and the effect of cardiac events on overall survival and nonrelapse mortality | AML |
| HF, MI, ischemia, arrhythmias, pericardiac effusion or pericarditis, moderate valvulopathy disease | Early-Onset | Early cardiac events (5.5% incidence) were linked to posttransplant cyclophosphamide use and preexisting cardiac risks before allo-HCT and were associated with higher nonrelapse mortality and lower overall survival. Late cardiac events (2.8% incidence) had no significant impact on nonrelapse mortality or overall survival. |
| 7 | Roganovic et al. [35]/2024 | Qualitative study (narrative review) | To raise awareness about the long-term adverse effects experienced by survivors of childhood acute leukemia, emphasizing the need for structured long-term surveillance and standardized follow-up care to manage these effects effectively | ALL, AML |
| Arrhythmias, cardiomyopathy, CAD, pericardial disease, valvular heart disease | Late-Onset | Leukemia treatments, especially in childhood cancers, caused serious long-term complications, including second malignancies, cardiovascular issues, and chronic health conditions. Cardiovascular complications were a significant concern after leukemia treatment, especially in patients who received high doses of anthracyclines or radiation. These complications occurred during treatment, early-onset or late-onset, with long-term risks of HF, hypertension, and CAD. |
| 8 | Puła et al. [36]/2024 | Quantitative study (pilot before–after study) | To determine the clinical utility of the new ST2 marker and to routinely assess cardiac parameters in patients undergoing HSCT | ALL, AML |
| Subclinical myocardial damage, Vascular disorders, Arrhythmias, HF, Cardiovascular death | During Treatment and Early-Onset | Echocardiographic GLS and biomarkers effectively predicted cardiovascular complications, detecting subclinical myocardial damage before symptoms appeared. Leukemia treatment history influences dysfunction severity, and patients with pre-existing cardiovascular risks were less likely to receive HSCT, indicating the need for early risk assessment. |
| 9 | Onoue et al. [37]/2024 | Quantitative study (retrospective cohort study) | To compare the incidence of MACE in patients with AML treated with venetoclax versus anthracyclines | AML |
| HF, MACE, AF, MI | During Treatment and Early-Onset | Venetoclax-treated patients showed a higher incidence of cardiovascular complications, including earlier-onset MACE and new AF, compared to those on anthracyclines. While HF rates were similar, venetoclax was linked to significantly higher mortality (78% vs. 41%). Echocardiogram use was more frequent in the anthracycline group, despite slightly lower LVEF. |
| 10 | Ma et al. [38]/2024 | Quantitative study (observational cohort study) | To develop and validate a personalized predictive nomogram and risk score to assess the risk of cardiac injury before chemotherapy in patients with newly diagnosed AML | AML |
| Cardiac injury, Cardiac enzyme abnormalities, Cardiac-related lesions, Cardiotoxicity, Other cardiac alterations | Before Treatment | The study identified four independent risk factors, including abnormal NT-proBNP, NPM1 mutations, and elevated WBC and RBC counts for cardiac injury in newly diagnosed AML patients. A predictive nomogram showed high accuracy (AUCs: 0.742, 0.750, 0.706), and higher risk scores were linked to worse overall survival in AML patients. |
| 11 | Liu et al. [39]/2024 | Qualitative study (narrative review) | To investigate cardiotoxicity prevention strategies in patients receiving anthracyclines, particularly in children with acute leukemia | ALL, AML |
| LVD, HF, Myocardial fibrosis, Hypertension, Arrhythmias, Endothelial dysfunction | Before Treatment to Late-Onset | Anthracyclines posed a dose-dependent cardiotoxicity risk. Dexrazoxane reduced early toxicity but raised concerns about secondary malignancies. Liposomal anthracyclines were less cardiotoxic, and long-term cardiac monitoring was essential. Combination therapies (beta-blockers, ACE inhibitors, statins) showed potential for cardio protection but needed further validation. |
| 12 | Kępski et al. [40]/2024 | Quantitative study (observational retrospective analysis cohort study) | To analyze the time-dependent relationship between the occurrence of venous thromboembolic (VTE) and arterial thromboembolic (ATE) events and the diagnosis of hematological malignancies, as well as the initiation of onco-hematological treatment | ALL, AML |
| VTE, ATE | During Treatment to Late-onset | AML and ALL raised VTE risk during treatment, with chemotherapy and disseminated intravascular coagulation contributing to AML, and higher VTE rates were seen in ALL. ATEs, often preceded cancer diagnosis in older patients, were linked to CAD but not general cardiovascular comorbidities. VTE and ATE impacted prognosis and treatment strategies in hematologic malignancies. |
| 13 | Hellman and Chaireti [41]/2024 | Quantitative study (retrospective cohort, single-center study) | To evaluate the incidence and risk factors of arterial thromboembolic events in a cohort of patients with acute leukemia and lymphoid malignancies during a 15-year period | ALL, AML |
| Thromboembolic Stroke, AMI, Arterial Thrombosis | Before Treatment to Early-Onset | The 15-year incidence of ATE was 1.4%, similar between AL and lymphoid malignancies. 68.8% of ATE cases were in male patients, which aligned with known higher cardiovascular risk in men. Many ATE cases in AL patients occurred at the time of diagnosis, before treatment initiation. A low platelet count was not protective against ATE, and some patients developed ATE despite severe thrombocytopenia. Almost 50% of patients did not restart antiplatelet agents after platelet recovery (>50 × 109/L), which may have increased ATE risk. |
| 14 | Hammoud et al. [42]/2024 | Quantitative study (prospective, longitudinal cohort study) | To characterize the prevalence of MACE and its association with the cumulative burden of non-MACE in survivors of childhood cancer | ALL, AML |
| MACE includes Cardiomyopathy, HF, LVSD, MI, and Stroke Non-MACE includes Arrhythmias, prolonged QT, Hypertension, Dyslipidemia, Structural defects, Ventricular dysfunction, vascular disease. | During Treatment to Late-Onset | The cumulative burden of non-MACE conditions significantly raised the risk of MACE, with one non-MACE condition increasing the risk 4.3-fold and four or more increasing it 11.1-fold. Major contributors included chest radiation ≥30 Gy and high-dose anthracyclines ≥250 mg/m2. Subclinical cardiovascular issues strongly predicted future MACE. Survivors of AML had the highest MACE burden by age 50. Black survivors and males carried a higher cumulative burden than their counterparts. |
| 15 | Hammoud et al. [43]/2024 | Qualitative study (state-of-the-art review) | To provide a comprehensive summary of the health consequences associated with cardiometabolic risk factors and frailty in survivors of childhood cancer and outline current recommendations for detection, prevention, and treatment while identifying knowledge gaps | ALL |
| Cardiomyopathy, CAD, Valvular heart disease, Arrhythmias, Hypertension, Pericardial disease | Late-Onset | Childhood cancer survivors were at a significantly higher risk of developing CVDs compared to the general population. Cardiometabolic risk factors (obesity, hypertension, diabetes, and dyslipidemia) contributed to increased morbidity and mortality. Survivors remained underdiagnosed and undertreated for these conditions. |
| 16 | Fernández-Avilés et al. [44]/2024 | Quantitative study (cross-sectional study) | To assess diastolic function in long-term survivors of childhood ALL using left atrial strain and conventional echocardiographic parameters | ALL |
| DD, Subclinical LVSD, Increased left atrial stiffness and reduced LAS values | Late-Onset | DD was present but subtle and was better detected with LAS than with conventional echocardiography. Patients exposed to higher doses of anthracyclines had more pronounced DD. Conventional echocardiographic parameters were not sensitive enough to detect early DD. LAS served as an early marker of cardiovascular dysfunction in childhood leukemia survivors. |
| 17 | Kundavaram et al. [45]/2024 | Quantitative study (cross-sectional study) | To evaluate the acute cardiotoxic effects of doxorubicin-based induction therapy in pediatric ALL patients using echocardiography | ALL including B-cell ALL, T-cell ALL |
| Systolic and diastolic dysfunction of both ventricles, Decreased LVEF and fractional shortening, right ventricular dysfunction, with a decrease in TAPSE and an increase in myocardial performance index, DD by reduced mitral and tricuspid E/A ratios and prolonged Isovolumic Relaxation Time | Early-Onset | Doxorubicin-induced cardiotoxicity can occur acutely, within 72 hours of administration. Both left and right ventricular dysfunctions were detected using echocardiography. The diastolic function was affected earlier than systolic function. Subclinical cardiac dysfunction was detected, emphasizing the need for early echocardiographic monitoring. The observed changes were not severe, but long-term follow-up was necessary to determine if these effects progressed. |
| 18 | Dogliotti et al. [46]/2024 | Qualitative study (narrative review) | To maintain efficacy while reducing toxicity in radiation-based conditioning regimens for HSCT, which includes evaluating total marrow and lymphoid irradiation as an alternative to TBI to reduce side effects while maintaining treatment effectiveness | ALL, AML |
| Hypertension, Cardiotoxicity | Early-Onset to Late-Onset | Total marrow irradiation and total marrow and lymphoid irradiation regimens may reduce cardiovascular toxicity compared to traditional TBI. There was a high prevalence of metabolic and cardiovascular risks in leukemia survivors who received HSCT with TBI. Alternative conditioning regimens with lower radiation exposure mitigate toxicity while preserving efficacy. |
| 19 | Diaz et al. [47]/2024 | Quantitative study (retrospective cohort study) | To assess the effect of female sex on the development of incident HF in adult patients treated with anthracyclines | ALL, AML |
| Cardiomyopathy, HF, Heart Failure with Reduced Ejection Fraction, Heart Failure with Mildly Reduced Ejection Fraction, Heart Failure with Preserved Ejection Fraction | Early-Onset | Female sex was not a risk factor for anthracycline-associated HF in adults, contrary to findings in pediatric populations. Older age, CAD, and HSCT were significant risk factors for developing HF. Leukemia patients had a higher HF risk due to a high rate of hematopoietic stem cell transplants, mostly presenting heart failure with preserved ejection fraction. Five-year HF incidence was 4.78%, consistent with prior data. |
| 20 | Rique et al. [48]/2024 | Quantitative study (monocentric longitudinal cross-sectional study) | To analyze the profile of left ventricular alterations in children treated with anthracyclines and to examine risk and protective factors, including physical activity | ALL, AML |
| LVD, Dilated cardiomyopathy, LVDD, right ventricular dysfunction | Late-Onset | 28.9% of patients had LV GLS abnormalities despite normal ejection fraction. Radiotherapy and high anthracycline doses (>240 mg/m2) increased cardiac dysfunction risk, while regular physical activity (>14 MET.h/week) was protective. LV GLS was more sensitive than LVEF for early cardiotoxicity detection. Dilated cardiomyopathy occurred in 3 patients. |
| 21 | Barachini et al. [49]/2024 | Qualitative study (narrative review) | To describe advancements in understanding the molecular physiopathology of treatment-related adverse events and to emphasize strategies for predicting, detecting, and managing chemotherapy-induced cardiotoxicity | ALL and mentions Blinatumomab, a Cluster of Differentiation 19/Cluster of Differentiation 3 bispecific antibody used for its treatment |
| HF, Myocarditis, Arrhythmias, AF, QT prolongation, hypertension, ischemic events, VTE, pulmonary hypertension, Pericardial disease | During Treatment to Late-Onset | Cardiotoxicity was a major concern in leukemia treatment, requiring ongoing monitoring. Early detection strategies (biomarkers, imaging) were crucial in preventing severe complications. Mitochondrial dysfunction played a central role in chemotherapy-induced cardiac damage. Newer targeted therapies (BTK inhibitors, CAR-T cells) had specific cardiac risks that needed tailored management. |
| 22 | Ketterl et al. [50]/2023 | Quantitative study (comparative cohort study) | To assess the insulin sensitivity and CVD risk factors in survivors of HCT and compare these factors with healthy sibling controls | ALL, AML |
| Dyslipidemia, Cardiometabolic risk | Late-Onset | HCT survivors had a higher prevalence of metabolic syndrome and cardiovascular risk factors than the general population and sibling controls. They showed insulin resistance, dyslipidemia (high total cholesterol, LDL, triglycerides, low HDL), and altered body composition with increased visceral fat and reduced lean body mass, even with normal BMI. TBI was strongly linked to these changes and elevated CVD risk. These factors contributed to early cardiovascular mortality, highlighting the need for early monitoring and intervention. |
| 23 | Wang et al. [51]/2023 | Quantitative study (genome-wide association study (GWAS)) | To identify genetic variants that modify the risk of anthracycline-related cardiomyopathy among childhood cancer survivors | ALL, AML |
| Cardiomyopathy, anthracycline-related cardiomyopathy, CHF, HF, heart transplantation | Late-Onset | The rs17736312 SNP in roundabout 2 gene was linked to increased risk of anthracycline-related cardiomyopathy, especially at high doses. AA genotype carriers showed significantly higher heart failure risk. The Slit-Robo pathway promoted fibrosis via Transforming Growth Factor β1/Smad, with key gene–dose interactions influencing late-onset cardiotoxicity. |
| 24 | Zhou et al. [52]/2023 | Quantitative study (observational prospective cohort study) | To explore biomarkers as early predictors of anthracycline-induced subclinical cardiotoxicity in acute leukemia patients | ALL, AML |
| Anthracycline-Induced Subclinical Cardiotoxicity, Cardiac Dysfunction, Potential Risk of Progression to CHF, LVD, and Arrhythmias | During Treatment | 17 of 51 patients developed anthracycline-induced subclinical cardiotoxicity after 3 chemotherapy cycles. Higher platelet count and blood glucose were linked to increased anthracycline-induced subclinical cardiotoxicity risk, while total and direct bilirubin might have been protective. Combining PLT and NT-proBNP had the best predictive value. Dynamic PLT changes after chemotherapy differed significantly between anthracycline-induced subclinical cardiotoxicity and non-anthracycline-induced subclinical cardiotoxicity groups. |
| 25 | Mitrovic et al. [53]/2023 | Quantitative study (retrospective cohort study) | To determine the incidence of ATEs in non-promyelocytic AML patients and identify potential risk factors for ATE development | AML excluding promyelocytic AML |
| Hypertension, HF, AF, Previous MI, Peripheral arterial disease, Stroke history ATEs which included Ischemic Stroke, Acute Lower Extremity Arterial Thrombosis, | During Treatment to Early-Onset | The incidence of ATE in AML patients was 2.9%, aligning with other cancer populations. Key risk factors included obesity (BMI > 30, 20× risk), prior thrombosis (4×), cardiovascular comorbidities (8×), and adverse cytogenetics (2×). ATE was associated with a high mortality rate (50%). The study indicated the need for primary thromboprophylaxis in high-risk AML patients, though bleeding risks from AML treatment posed challenges to implementation. |
| 26 | Kantarjian et al. [54]/2023 | Quantitative study (phase 2 clinical trial) | To assess the long-term efficacy and safety of the frontline combination of ponatinib (a third-generation BCR::ABL1 tyrosine kinase inhibitor) and Hyper fractionated Cyclophosphamide, Vincristine, Adriamycin, Dexamethasone chemotherapy in treating Philadelphia chromosome-positive ALL (Ph+ ALL) | Ph+ ALL |
| Hypertension, AOEs, MI, Unstable angina; Venous thromboembolic events including Pulmonary embolism, Renal vein thrombosis, DVT | During Treatment to Late-Onset | The combination of ponatinib and hyper fractionated Cyclophosphamide, Vincristine, Adriamycin, Dexamethasone achieved 100% complete response and 86% complete molecular remission, with strong long-term outcomes: 6-year event-free survival (65%) and overall survival (75%), largely avoiding the need for allo-SCT. However, cardiovascular toxicity was significant, with 52% developing hypertension (17% Grade 3), especially at 45 mg/day doses. Dose reductions (to 30 mg or 15 mg) improved safety without reducing efficacy. No fatal cardiovascular events occurred post-adjustment. Ponatinib also suppressed ABL1 T315I mutations effectively, with no relapses in these patients. |
| 27 | Heredia et al. [55]/2023 | Quantitative study (cross-sectional study) | To assess right ventricular function in long-term survivors of childhood ALL using echocardiographic conventional measurements and automated RV strain | ALL |
| Right Ventricular Systolic Dysfunction, Subclinical Right Ventricular Dysfunction, Reduced TAPSE, Decreased Right Ventricular Four-Chamber Strain, LVD | Late-Onset | Significant reduction in RV function happened among childhood leukemia survivors compared to healthy siblings, despite normal conventional echocardiographic parameters. Right ventricular free-wall strain identified subclinical dysfunction in 16.7% of survivors, a much higher prevalence than conventional measures. HSCT was linked to lower TAPSE values, suggesting it contributed to long-term RV impairment. Modifiable risk factors (obesity and smoking) were strongly associated with worsening RV function. |
| 28 | Gawlik et al. [56]/2023 | Qualitative study (narrative review) | To provide an update on epidemiology, risk factors, and management of cardiac arrhythmias in oncological patients within the context of the new European Society of Cardiology 2022 guidelines on cardio-oncology | APL |
| QT Prolongation, VAs | During Treatment to Late-Onset | Arsenic trioxide was highly effective for APL treatment but posed a significant risk of QT prolongation and VAs. 63% of patients experienced QT prolongation, including a case of asymptomatic torsades de pointes. These complications ranged from moderate to severe and could be life-threatening without proper management. Onset occurred during treatment, requiring close ECG monitoring and correction of modifiable risk factors such as electrolyte imbalances. Management followed ESC 2022 guidelines for arrhythmias. |
| 29 | Fazal et al. [57]/2023 | Qualitative study (retrospective case series and review of literature) | To describe and analyze cases of VAs associated with the use of TKIs in cancer patients | Ph+ ALL |
| VAs include non-sustained ventricular tachycardia, Sustained ventricular tachycardia, Ventricular fibrillation, Premature ventricular contractions | Early-Onset to Late-Onset | TKIs, including ibrutinib, zanubrutinib, dasatinib, and afatinib, were clearly associated with VAs. Arrhythmias often resolved after discontinuing TKIs, suggesting a drug-induced cause. However, some patients required long-term antiarrhythmic treatment or implantable cardioverter-defibrillators. Both those with and without prior cardiovascular disease were at risk, highlighting widespread susceptibility. |
| 30 | Boluda et al. [4]/2023 | Quantitative study (retrospective cohort study) | To evaluate the incidence, timing, and impact of cardiac events in patients with acute leukemia undergoing intensive and non-intensive chemotherapy | AML |
| HF, Arrhythmias, MI, Pericarditis, Hypertension | During Treatment to Early-Onset | Cardiac events were more common in older patients (≥65 years) and those with pre-existing cardiovascular conditions. Anthracyclines, FLT3 inhibitors, and intensive treatments increased cardiovascular risk. Infections (sepsis, pneumonia) and poor performance status further elevated complications. Cardiac issues negatively affected overall survival and treatment continuity. |
| 31 | Bertrand et al. [58]/2023 | Qualitative study (systematic review study) | To provide evidence on the prevalence, incidence, and risk factors of cardiac electrical abnormalities in childhood ALL survivors who have completed their treatment | ALL |
| Arrhythmias, Repolarization Disorders, Depolarization Disorders and Pathologic Q-waves, Conduction Disorders, Unclassified Abnormalities | Early-Onset to Late-Onset | Cardiac electrical abnormalities in childhood acute ALL survivors were generally infrequent but showed wide variability by type; heart rate abnormalities (0–68%) and repolarization disorders (0–30%) were more common, while depolarization disorders were rare. No consistent risk factors were identified, though anthracycline exposure and radiotherapy emerged as potential contributors. |
| 32 | Baum et al. [59]/2023 | Quantitative study (observational retrospective and prospective cohort study) | To analyze the utilization of preventive health care (cancer screening, cardiovascular screening, vaccination) among blood cancer survivors | ALL, AML |
| Arterial hypertension | Late-Onset | Blood cancer survivors, especially allo-HSCT recipients, showed higher rates of cardiovascular complications and greater use of preventive care (screenings for blood pressure, lipids, diabetes) than the general population. Coordinated care between oncologists and general practitioners led to the highest preventive care use, with GPs boosting cancer screening rates and oncologists improving vaccination coverage. |
| 33 | Auberle et al. [60]/2023 | Quantitative study (retrospective cohort study) | To describe the incidence of late cardiac events after allo-SCT, identify risk factors for their development, and evaluate their impact on overall survival | ALL, AML |
| HF, Decline in LVEF, Atrial Arrhythmias, VAs, CAD, Pericardial Effusion, Pericarditis | Late-Onset | Cardiovascular complications affected 22% of allo-SCT survivors within 5 years. Pre-existing conditions like HF, hypertension, diabetes, and CAD were strong predictors of late cardiac events, while transplant-related factors (TBI, conditioning regimen, TKI use) were not significantly associated. These late cardiac events severely impacted overall survival, with atrial arrhythmias carrying the highest mortality risk (High Risk 10.6). |
| 34 | Alpman et al. [61]/2023 | Quantitative study (retrospective population-based single-center cohort study) | To assess cardiovascular toxicity, including systemic hypertension, pericardial effusion, and thrombosis, in children undergoing anthracycline-containing treatment for ALL and to evaluate the effectiveness of standard cardiovascular surveillance methods | ALL |
| Systemic hypertension, Intracardiac thrombosis, Pericardial effusion, QTc prolongation and arrhythmia risk, Mild cardiac dysfunction, LVEF, Severe HF | During Treatment to Late-Onset | Standard echocardiographic parameters (LVEF, LV Shortening Fraction) failed to detect early cardiac dysfunction, highlighting the need for more sensitive methods like GLS. Hypertension occurred in 20% of patients, mainly due to corticosteroid therapy, but resolved in all cases. Intracardiac thrombosis was observed in 8.5% of patients, treated successfully with Low Molecular Weight Heparin. One case of early severe HF occurred despite standard echocardiographic surveillance, indicating the importance of individualized monitoring. |
| 35 | Berisha et al. [62]/2023 | Qualitative study (narrative review) | To examine the cardiovascular toxicity of TKIs used in treating Ph-positive leukemias and other cancers, with a particular focus on the effects of ponatinib, as well as other TKIs like sorafenib, sunitinib, lapatinib, gefitinib, and erlotinib | ALL, Ph-positive ALL |
| MI, Cardiomyopathy and CHF, Hypertension, AOEs, LVD | During Treatment to Late-Onset | Ponatinib carried the highest cardiovascular risk among TKIs, with notable rates of MI and AOEs. Nilotinib and dasatinib presented moderate cardiovascular risk, primarily associated with hypertension and LVD. Hypertension was common with all TKIs but differed in severity. Cardiovascular events occur even in patients without prior risk factors, pointing to TKI-induced cardiotoxicity rather than pre-existing conditions. |
| 36 | Januzzi et al. [63]/2022 | Quantitative study (retrospective cohort study) | To investigate the prevalence of baseline risk factors in patients with adjudicated AOEs both non-serious and serious, and to examine the associations between these risk factors, including cardiovascular conditions, and the occurrence of these events | ALL |
| Cardiac arrest, MI, Ischemic and hemorrhagic strokes, Superior mesenteric artery occlusion, Worsening of congestive HF, Peripheral ischemia, Mild to moderate tricuspid regurgitation, Intermittent ventricular tachycardia | During Treatment to Early-Onset | Leukemia therapies, particularly TKIs like nilotinib, were linked to increased cardiovascular events, including QTc prolongation and fatal AOEs. Many affected patients had pre-existing CVD or risk factors. Those with serious AOEs were more likely to be on antihypertensives, aspirin, and platelet inhibitors, underscoring the importance of baseline cardiovascular management. Despite these risks, long-term survival was comparable between patients with and without AOEs, indicating that early intervention and risk factor control were critical for improving outcomes. |
| 37 | Xiao et al. [2]/2022 | Quantitative study (single-center retrospective observational study) | To investigate baseline cardiac function and the risk of CVDs in patients with new-onset acute leukemia (AL), explore CVD risk factors present before treatment, and their association with prognostic survival | ALL. AML |
| Myocardial damage, Heart dysfunction, Elevated biomarkers of cardiac injury (highly sensitive troponin I, B-type natriuretic peptide, lactate dehydrogenase), Changes in echocardiography parameters (left ventricular internal diameter, ejection fraction) | Before Treatment | The leukemia blast ratio was positively correlated with cardiac damage, indicating that leukemia progression was linked to heart complications. Cardiac damage was evident in newly diagnosed acute leukemia patients even before chemotherapy, suggesting that leukemia itself contributed to cardiovascular issues. Hyperleukocytic leukemia was associated with more severe myocardial enzyme changes, likely due to leukemic cell accumulation and microvascular obstruction. The left ventricular internal diameter was the best predictor of heart damage, with age and EF identified as independent prognostic risk factors. |
| 38 | Terada et al. [64]/2022 | Quantitative study (observational histopathological cohort study) | To clarify the histopathology and mechanisms of cancer therapy-related cardiac dysfunction by evaluating myocardial tissue samples for fibrosis, cardiomyocyte abnormalities, epigenetic modifications (Tumor Protein 53 and H3K27ac histone modification) and seeks to identify risk factors for cancer therapy-related cardiac dysfunction | ALL, AML |
| Cardiac Dysfunction, HF Reduction in LVEF, Myocardial Fibrosis, Arrhythmias, Pericardial Effusion, Cardiomyocyte Damage | During Treatment to Late-Onset | Leukemia patients with therapy-related cardiac dysfunction showed fibrosis, cardiomyocyte damage, and elevated Tumor Protein 53/H3K27ac, indicating stress and epigenetic changes. Late-onset cases (>4.2 years) had worse outcomes (HR 7.61). Type 1 dysfunction (e.g., anthracyclines) caused irreversible, dose-dependent damage; Type 2 (e.g., trastuzumab) was potentially reversible. |
| 39 | Perpinia et al. [65]/2022 | Qualitative study (narrative review) | To examine the clinical manifestations, preventive strategies, and pharmaceutical management of cardiotoxicity in patients with hematologic malignancies undergoing anticancer drug therapy or HSCT | ALL, AML |
| HF, Arrhythmias, AF, Cardiomyopathy, Hypertension, Pericarditis and Pericardial Effusion, Pulmonary Arterial Hypertension, Arterial Thrombosis and MI, QT Interval Prolongation | During Treatment to Late-Onset | Cardiotoxicity was a major concern in leukemia treatment, especially for patients with pre-existing cardiovascular conditions, who faced significantly higher risk. Cardioprotective therapies like beta-blockers, ACE inhibitors, and statins offered promising protection, while dexrazoxane was FDA-approved specifically to prevent anthracycline-induced cardiotoxicity. Hematopoietic stem cell transplant survivors had a 4-fold increased risk of CVD compared to the general population. |
| 40 | Muggeo et al. [66]/2022 | Quantitative study (case–control study) | To evaluate left and right cardiac chamber performances and vascular endothelial function in childhood ALL survivors treated with anthracyclines | ALL |
| Left and right ventricular dysfunction, Systolic and diastolic dysfunction, Endothelial dysfunction, Increased left and right myocardial performance index, Reduced TAPSE, Higher arterial stiffness | Late-Onset | Childhood ALL survivors treated with anthracyclines showed subclinical cardiovascular impairment, affecting both left and right ventricular function, including reduced TAPSE indicating right heart dysfunction. Higher cumulative anthracycline doses correlated with worsening cardiac function. Additionally, survivors exhibited vascular Endothelial dysfunction, evidenced by reduced flow-mediated dilation. |
| 41 | Luo et al. [67]/2022 | Qualitative study (systematic review) | To document clinical features, diagnostic procedures, treatments, and outcomes of ALL cases that initially present with cardiac symptoms and provide management recommendations to help avoid misdiagnosis and improper treatment | ALL include T-ALL and B-ALL |
| Cardiac tamponade, Cardiac masses, Myocardial Hypertrophy, Leukemic infiltration of coronary arteries, Thrombotic occlusion, Pericardial Effusion, Arrhythmias and Conduction Abnormalities | Before Treatment to Late-Onset | Cardiac symptoms could be the first presentation of ALL, often leading to misdiagnosis by cardiologists. T-ALL commonly presented with pericardial effusion or tamponade, while B-ALL is linked to cardiac masses. Chemotherapy often resolved cardiac complications, but outcomes varied; some achieved complete remission, others faced fatal outcomes due to chemoresistance, delays, or severe cardiac dysfunction. Accurate diagnosis relied on cardiac imaging and biopsy. |
| 42 | Lipshultz et al. [68]/2022 | Quantitative study (cross-sectional study) | To assess the burden of potentially modifiable cardiometabolic risk factors among childhood cancer survivors and compare them with an age, sex, and race/ethnicity-matched general population to estimate future cardiovascular risk | ALL |
| Hypertension, Pre-hypertension, Dyslipidemia, Obesity, Metabolic Syndrome, Ischemic heart disease, HF | Early-Onset to Late-Onset | Survivors had higher rates of pre-hypertension and hypertension than the general population, with similar or better metabolic profiles (obesity, diabetes, dyslipidemia) compared to controls. Despite healthier lifestyle habits (less smoking, more physical activity), survivors faced a higher risk of ischemic heart disease and HF. Existing cardiovascular risk models underestimated risks in cancer survivors, emphasizing the need for specialized survivor-specific models. |
| 43 | Gonzalez-Manzanares et al. [69]/2022 | Quantitative study (retrospective single-center cross-sectional cohort study) | To assess the utility of automated GLS in LVSD in long-term childhood ALL | ALL |
| LVSD, LVEF, Subclinical LVSD with GLS < 18.5%, HF, Increased Hs-cTnI | Late-Onset | GLS was more sensitive than LVEF in detecting cardiotoxicity in long-term childhood leukemia survivors. 26.6% of survivors had subclinical LV dysfunction, while only 12.2% showed reduced LVEF. Higher anthracycline doses (>250 mg/m2), diabetes mellitus, and radiotherapy were significant predictors of LVEF reduction. Smoking and HSCT were independent predictors of reduced GLS. Biomarkers like NT-ProBNP and Hs-cTnI were not effective predictors of LV function. Automated GLS may have improved long-term cardiac monitoring for childhood leukemia survivors. |
| 44 | Cornelissen et al. [13]/2022 | Quantitative study (case–control study) | To explore whether cardiovascular risk factors can predict intracranial hemorrhage in patients with acute leukemia | ALL, AML, APL |
| Hypertension, Ischemic heart disease | Before Treatment to Early-Onset | Hypertension and ischemic heart disease were the strongest predictors of intracranial hemorrhage in acute leukemia, increasing risk by 12.9 and 12.1 times, respectively. Intracranial hemorrhage had a high mortality rate (47%) compared to non-intracranial hemorrhage patients (9%). Leukemia treatment itself (chemotherapy, SCT) contributed to vascular damage and bleeding risk, which may have worsened pre-existing cardiovascular conditions. Other cardiovascular risk factors showed a positive association with intracranial hemorrhage but were not statistically significant due to the small sample size. |
| 45 | Chianca et al. [70]/2022 | Qualitative study (narrative review) | To investigate the relationship between CHIP and the risk of CVDs in patients with AML, particularly in those treated with anthracycline chemotherapy | AML |
| HF, Cardiomyopathy (Dilated Cardiomyopathy), Arrhythmias, MI, Hypertension, Endothelial Dysfunction, Atherosclerosis | During Treatment to Late-Onset | CHIP-related mutations elevated cardiovascular risk in AML patients on anthracyclines. Elevated IL-6, TNF-α, C-reactive Protein, and Myeloperoxidase levels indicated inflammation and oxidative stress, predicting cardiotoxicity. Soluble Suppression of Tumorigenicity 2 was associated with poorer cardiovascular and leukemia outcomes, while pre-existing conditions like hypertension and atherosclerosis increased post-treatment complication risk. |
| 46 | Calvillo-Argüelles et al. [7]/2022 | Quantitative study (retrospective cohort study) | To examine the association between CHIP-related mutations and the risk of cardiovascular events in patients with AML, as well as the impact of these events on mortality | AML |
| HF, MI, Acute Coronary Syndrome, Arrhythmias, Transient Ischemic Attack, VTE, Hypertension and Hypotension, Pericardial Disease, Cardiomyopathy | During Treatment to Late-Onset | CHIP-related mutations were linked to a higher risk of cardiovascular events (HF, MI, arrhythmia) in AML patients. Anthracycline-based chemotherapy was strongly associated with both early and late-onset cardiotoxicity. Patients with cardiovascular events had higher mortality, and CHIP-related cardiovascular events independently predicted worse survival. Pre-existing CVD and traditional risk factors further increased the risk of complications. |
| 47 | Bottinor and Chow [71]/2022 | Qualitative study (narrative review with educational focus) | To discuss strategies for identifying childhood cancer survivors at increased risk for late cardiotoxicity, current monitoring approaches, and prevention strategies to reduce CVDs burden | AML |
| Cardiomyopathy, HF, Ischemic Heart Disease, Valvular Heart Disease, Stroke, LVD, Hypertension | Early-Onset to Late-Onset | Cumulative anthracycline exposure, especially mitoxantrone (10× cardiotoxicity of doxorubicin), greatly increased HF risk, often after years without symptoms. Studies suggested dexrazoxane was safe and effective in reducing cardiac toxicity in pediatric leukemia patients. Echocardiography, myocardial strain imaging, and cardiac MRI improved early detection of subclinical dysfunction. Pediatric cancer survivors required unique long-term cardiovascular management, as adult HF treatments did not fully apply. |
| 48 | Arnán Sangerman et al. [72]/2022 | Qualitative study (narrative review) | To provide practical recommendations for the use of midostaurin in the management of FLT3-mutated AML, optimize the use of midostaurin, minimize adverse effects, and provide a useful guide for routine clinical practice | FLT3-mutated AML |
| QTc interval prolongation, HF | During treatment to Early-Onset | Midostaurin may have prolonged QTc, especially with other QTc-prolonging drugs; dose adjustment was needed if QTc > 500 ms. Anthracycline regimens increased HF risk, particularly in patients with hypertension or diabetes. Regular ECGs, electrolyte monitoring, and baseline cardiac assessments (echo, NT-proBNP, troponin) were essential. ACE inhibitors or beta-blockers may have prevented cardiotoxicity. Resistance arose via FLT3 mutations or pathway shifts, requiring adaptive management. |
| 49 | Petrykey et al. [73]/2021 | Quantitative study (lifetime cohort study) | To identify genetic markers associated with treatment-related cardiovascular complications, particularly cardiotoxicity, in survivors of childhood ALL anthracycline-based chemotherapy | ALL |
| Cardiotoxicity, LVD, HF, Structural Changes, LV End-Diastolic Diameter | Late-Onset | Common Titin gene variants were linked to better cardiac function (higher LVEF and FS) and had a cardioprotective effect. Rare variants in several genes were associated with cardiovascular outcomes; some were protective, while others (like Carbonyl Reductase 1) increased cardiotoxicity risk. Rare ATP-binding Cassette C5 variants were protective in high-risk patients, while rare Carbonyl Reductase 1 variants increased risk in standard-risk patients. Rare Nucleotide-Oligomerization Domain 2 and Zinc Finger Protein 267 variants were significantly associated with FS and LVEF, suggesting a role in long-term cardiovascular risk. |
| 50 | Oka et al. [74]/2021 | Quantitative study (single-center observational cohort study) | To clarify the association between HSCT and GLS and to evaluate the serial changes in GLS and LVEF before and after HSCT | ALL, AML |
| GLS, LVEF, Diastolic dysfunction | Early-Onset | GLS decreased notably at 1-month post-HSCT and partially recovered by 6 months, indicating it as an early marker of subclinical dysfunction. LVEF also dropped at 1 month but normalized by 3 months, except in the low EF group where it continued to decline. DD (e’ and E/e’) persisted at 3 and 6 months. Lower baseline GLS predicted reduced LVEF at 6 months. BNP and troponin I levels rose at 1-month post-HSCT, signaling early myocardial stress, but returned to baseline by 6 months. |
| 51 | Lubas et al. [75]/2021 | Quantitative study (retrospective cohort study with prospective medical assessment) | To examine the contribution of emotional stress and distress to cardiac health in adult survivors of childhood cancer | ALL, AML |
| Arrhythmias, Cardiomyopathy, MI, Hypertension, Dyslipidemia, Metabolic syndrome | Late-Onset | Stress and emotional distress were associated with higher rates of hypertension, dyslipidemia, metabolic syndrome, and diabetes in survivors. Longitudinally, baseline stress predicted new cases of dysrhythmia, hypertension, and dyslipidemia over 3.9 years. About one-third of survivors had clinically significant distress, higher than community controls. Stress and distress were independent cardiovascular risk factors, emphasizing the need for psychological interventions to improve heart health in childhood cancer survivors. |
| 52 | Lazăr et al. [76]/2021 | Qualitative study (narrative review) | To investigate the challenges and complications associated with anthracycline induced cardiotoxicity in children treated for leukemia and to summarize the current understanding of the mechanisms of cardiotoxicity, identify risk factors, and discuss strategies for monitoring and managing cardiac dysfunction in pediatric cancer survivors | ALL |
| Cardiomyopathy, HF, Arrhythmias, DD, Myocarditis and Pericarditis | During Treatment to Late-Onset | Cardiotoxicity from anthracyclines occurred even at low doses due to genetic variability, particularly CBR3 and Solute Carrier 28 family member 3 polymorphisms. They caused irreversible cardiomyocyte loss, leading to chronic heart failure with <50% 5-year survival after symptom onset. Early, systematic cardiac evaluation was essential. Dexrazoxane, an iron-chelating agent, effectively reduced cardiotoxicity without affecting chemotherapy efficacy. |
| 53 | El Amrousy et al. [77]/2021 | Quantitative study (prospective randomized clinical trial) | To assess the possible protective effect of omega-3 fatty acids on early doxorubicin-induced cardiac toxicity in children with ALL | ALL |
| Doxorubicin-induced cardiotoxicity, LVD and Myocardial injury | Early-Onset | Omega-3 fatty acids reduced oxidative stress, preserved cardiac function, and lowered cardiac biomarkers in children with ALL receiving doxorubicin, compared to controls. They were a potential cardioprotective agent against doxorubicin-induced cardiotoxicity in pediatric ALL patients. |
| 54 | kamarajuet al. [78]/2021 | Qualitative study (narrative review) | To highlight the role of CYP450 and P-glycoprotein in drug interactions within the field of cardio-oncology and to provide an overview of cardiotoxicity related to cancer treatments and multidisciplinary treatment approach to reduce drug-related toxicities | AML |
| HF, Systolic and diastolic dysfunction; Arrhythmias include atrial and VAs, QT prolongation, Bradycardia, heart block; Pericarditis, myocarditis, Hypertension, Thrombosis | During Treatment to Late-Onset | Drug interactions mediated by CYP450 and P-glycoprotein transporters significantly impacted cardiovascular outcomes. Anthracyclines, TKIs, and alkylating agents were among the most cardiotoxic treatments. HSCT survivors had a 4.5-fold higher risk of CVD compared to the general population. |
| 55 | Hoeben et al. [79]/2021 | Qualitative study (narrative review) | To review the role of TBI in HSCT for pediatric ALL, including its immunosuppressive and anti-leukemic effects, clinical outcomes, toxicity, and potential future directions | ALL |
| Metabolic syndrome, Atherosclerosis, Hypertension, Increased risk of MI, stroke, and peripheral vascular disease, Subclinical systolic and diastolic dysfunction | Early-Onset to Late-Onset | TBI was linked to higher risks of metabolic and CVDs compared to chemotherapy-based conditioning. Higher doses of TBI (>10 Gy) correlated with increased cardiometabolic traits (higher fasting insulin, blood pressure, adverse lipid profiles). Late cardiovascular effects required lifelong monitoring, as cardiac disease risk increased over time in childhood cancer survivors. |
| 56 | Gangaraju et al. [80]/2021 | Quantitative study, thesis (retrospective cohort study) | To evaluate the long-term risk of coronary heart disease in blood or marrow transplant survivors and identify associated risk factors | ALL, AML |
| Coronary Heart Disease, Hypertension, Dyslipidemia, Congestive HF, Arrhythmias, Stroke, VTE | Early-Onset to Late-Onset | BMT survivors had a 7.2–11.7 times higher risk of coronary heart disease than siblings, with autologous BMT recipients at greater risk due to older age and pre-BMT chest radiation. Major risk factors included older age at BMT, male sex, pre-BMT chest radiation, and pre-existing cardiovascular risks. Coronary heart disease risk increased by 4% for every 100 cGy rise in radiation dose. |
| 57 | Duléry et al. [81]/2021 | Quantitative study (single-center retrospective cohort study) | To assess the incidence and clinical features of early cardiac events associated with post-transplant cyclophosphamide in allo-HSCT | ALL, AML |
| LVSD, Arrhythmias, Pericarditis, Acute Coronary Syndrome | Early-Onset | Post-transplant cyclophosphamide was linked to a higher incidence of early cardiac events (19% incidence in post-transplant cyclophosphamide group vs. 6% in non-post-transplant cyclophosphamide group), with early cardiac events patients showing significantly lower 2-year survival (31% vs. 64%). Pre-transplant cyclophosphamide exposure, sequential conditioning, older age, and prior cardiac events increased early cardiac events risk. Traditional cardiovascular risk factors were not strongly associated. Lowering post-transplant cyclophosphamide doses may have reduced cardiac toxicity, but more research is needed. |
| 58 | Diesch-Furlanetto et al. [82]/2021 | Qualitative study (narrative review) | To discuss the late effects of HSCT in ALL survivors, emphasizing the importance of long-term follow-up and transition to adult care to mitigate health risks and improve quality of life | ALL |
| Atherosclerosis CVD, Hypertension, CAD, Arterial Diseases, HF, Arrhythmias, Cardiac Dysfunction | During Treatment to Late-Onset | HSCT survivors had a significantly higher risk of CVD compared to the general population. Risk of premature CV-related death was 2.3 times higher in HSCT recipients. Metabolic syndrome was more prevalent in ALL survivors who underwent HSCT (39%) compared to those treated with chemotherapy alone (8%). Arterial diseases (atherosclerosis, stroke, and heart attack) were a major cause of long-term mortality in HSCT survivors. |
| 59 | Chow et al. [83]/2021 | Quantitative study (pilot randomized controlled trial) | To determine the feasibility of a remotely delivered mobile health intervention aimed at improving diet and physical activity in hematologic malignancy survivors to reduce cardiovascular risk factors | Acute Leukemia |
| Hypertension, Dyslipidemia | Late-Onset | Cancer survivors, particularly those treated for hematologic malignancies, were at an increased risk of CVD. Lifestyle factors such as hypertension, dyslipidemia, diabetes, smoking, poor diet, and physical inactivity further contributed to cardiovascular risk. A mobile health intervention using Fitbit trackers, diet tracking apps, and a Facebook peer support group was feasible and acceptable among survivors. While no statistically significant differences were found between intervention and control groups, the intervention favored improved diet quality, physical activity, and cardiovascular risk factors. |
| 60 | Chen et al. [84]/2021 | Qualitative study (narrative review) | To identify the current understanding of cardiovascular toxicity associated with CAR-T cell and bispecific T cell engager therapies in the treatment of relapsed and refractory hematologic malignancies | B cell ALL |
| Hypotension requiring vasopressors, Sinus tachycardia, Atrial arrythmia, VAs, LVSD, Decompensated cardiac failure, Cardiovascular death | Early-Onset | Cardiovascular toxicity was a significant but under-researched side effect of CAR-T cell and bispecific T cell engager therapies. Cytokine release syndrome was the strongest predictor of cardiovascular events. High-grade cytokine release syndrome (grades 3–4) increased the risk of cardiovascular events, including arrhythmias and cardiac failure. Early use of tocilizumab to treat cytokine release syndrome reduced the incidence of cardiovascular events. |
| 61 | Burns et al. [85]/2021 | Qualitative study (narrative review) | To review the recognition, risk factors, and management of cardiotoxicity associated with Anti-Cluster of Differentiation 19 Chimeric Antigen Receptor T-Cell therapy | B cell ALL |
| Tachycardia, hjypotension, pulmonary edema, depressed LVD, cardiac failure, myocardial injury, Arrhythmias, ST-segment changes, cardiac arrest | During Treatment to Early-Onset | Cardiotoxicity was closely linked to severe cytokine release syndrome. Troponin elevation was a predictive marker of cardiovascular risk. Older adults and patients with pre-existing cardiac risk factors (CAD, DD, etc.) were at higher risk. Expedited administration of tocilizumab (IL-6 inhibitor) may have reduced cardiac risk. Pediatric patients tended to recover cardiac function, whereas adults suffered long-term effects or fatal events. |
| 62 | Linares Ballesteros et al. [86]/2021 | Quantitative study (prospective descriptive cohort study) | To describe the incidence of early-onset cardiotoxicity in children with acute leukemia treated with chemotherapy | ALL, AML |
| Cardiac dysfunction related to cancer therapy, LVEF, electrocardiographic abnormalities, including impulse generation disturbances and repolarization disorders; pericardial effusion, valvular insufficiency, systemic arterial hypertension, pulmonary hypertension | Early-Onset | Anthracycline doses >150 mg/m2 were the main risk factor for early cardiotoxicity, affecting 17.9% of patients, especially in high-risk ALL and AML. Echocardiographic measures (LVEF, GLS) predicted cardiotoxicity, while biomarkers (troponin, BNP) did not. Pulmonary and systemic hypertension increased risk. Early GLS changes helped detect risk before symptoms, and ECG abnormalities did not correlate with cardiac dysfunction. |
| 63 | Abrahão et al. [87]/2020 | Quantitative study (population-based cohort study) | To estimate the cumulative incidence and investigate the main predictors of late effects among adolescent and young adult (AYA) survivors of AML | AML |
| Hypertension, ischemic heart disease, cardiomyopathy, HF | Late-Onset | Cardiovascular (18.6%), endocrine (26.1%), and respiratory (6.6%) diseases were common in AML survivors. HSCT doubled the risk of late effects, including heart disease. Non-favorable risk AML, minority ethnicity, and lower socioeconomic status were linked to higher rates of complications. |
| 64 | Saussele et al. [88]/2020 | Qualitative study (narrative review, expert consensus report) | To summarize current evidence regarding the efficacy and cardiovascular safety of Ponatinib in Philadelphia chromosome-positive Acute Leukemia and to provide recommendations for CV risk management | Ph+ ALL |
| AOEs, VTE, MI, Stroke, peripheral artery disease, hypertension | During Treatment to Early-Onset | Ponatinib was highly effective in treating Ph+ ALL, particularly in patients with the T315I mutation. Cardiovascular risk was dose-dependent, and dose reduction (from 45 mg to 30 mg or 15 mg) lowered CV risk while maintaining efficacy. Patients with pre-existing CV risk factors (hypertension, diabetes, hyperlipidemia) had a higher incidence of CV complications. Close collaboration between hematologists and cardiologists was recommended to manage CV risk while using Ponatinib. |
| 65 | Orvain et al. [89]/2020 | Quantitative study (multicenter prospective study-GRAALL-2005 controlled trial) | To assess the efficacy and safety of thrombotic prophylaxis in adult ALL patients receiving L-asparaginase therapy, with a focus on fibrinogen supplementation, antithrombin supplementation, fresh frozen plasma, and heparin | ALL |
| VTE, DVT, pulmonary embolism, cerebral venous thrombosis | During Treatment to Early-Onset | L-asparaginase significantly raised VTE risk, especially in patients with low antithrombin. Fibrinogen and Antithrombin supplementation did not reduce risk and may have increased thrombosis. Heparin prophylaxis, particularly unfractionated Heparin, was linked to higher VTE rates. Central venous lines, older age, high hemoglobin, and obesity were independent VTE risk factors. Cerebral venous thrombosis cases were severe, often requiring intensive care. |
| 66 | Ociepa et al. [90]/2020 | Quantitative study (cross-sectional Study) | To assess the cardiovascular risk in childhood survivors of ALL using carotid intima-media thickness as a potential early indicator of subclinical CVD | ALL |
| Arterial hypertension, endothelial dysfunction, possible atherosclerosis development, cardiovascular risk in adulthood | Late-Onset | Carotid Intima-Media Thickness was not a sensitive early marker of cardiovascular risk in childhood ALL survivors, showing no significant difference from controls or between hypertensive and normotensive survivors. Although hypertension was more common in this group, Carotid Intima-Media Thickness did not correlate strongly with BP, unlike in other pediatric conditions. Endothelial dysfunction and lipid profile changes may have been more relevant for early cardiovascular risk detection. |
| 67 | Neuendorff et al. [91]/2020 | Qualitative study (narrative review) | To review the prevalence of anthracycline-related LVD in patients with AML, particularly older adults, and discuss risk factors, assessment methods, management strategies, and alternative treatments | AML |
| Acute cardiotoxicity, chronic cardiotoxicity, acute HF, HF, arrhythmias, Myocarditis, subclinical LVEF decline, myocardial fibrosis | During Treatment to Late-Onset | Anthracyclines caused dose-dependent chronic cardiotoxicity in older AML patients, often leading to anthracycline-related LVD, HF, LVEF decline, and fibrosis. Risk was higher with preexisting heart conditions. Anthracycline-related LVD usually developed within a year and had poor recovery if not managed early. Alternatives like hypomethylating agents, venetoclax, and FLT3 inhibitors could be considered for high-risk patients. |
| 68 | Leerink et al. [92]/2020 | Qualitative study (state-of-the-art review) | To review the prevalence, risk factors, prevention strategies, and risk prediction models for cardiac disease in childhood cancer survivors, as well as the methods used for risk prediction, prevention, and surveillance. | Acute Leukemia |
| HF, CAD, valvular heart disease, pericardial disease, arrhythmias, myocardial fibrosis, hypertension, obesity, and metabolic syndrome | Late-Onset | Anthracyclines, chest radiotherapy, and mitoxantrone (10× more cardiotoxic) were key cardiac risk factors in childhood cancer survivors. Traditional CV risks (hypertension, obesity, dyslipidemia) worsened late-onset heart disease. HF and CAD were major non-cancer killers. Preventive strategies like dexrazoxane and liposomal anthracyclines were promising, though their long-term efficacy was still being studied. |
| 69 | Jamal and Khaled [93]/2020 | Qualitative study (narrative review) | To analyze the cardiovascular complications of CAR-T cell therapy, explore its mechanisms, and suggest approaches for clinical management | ALL |
| Cytokine release syndrome-induced cardiotoxicity, hypotension, tachycardia, cardiogenic shock, LV dysfunction, HF, arrhythmias, myocardial dysfunction, endothelial dysfunction, capillary leak syndrome | During Treatment to Early-Onset | Cardiotoxicity from cytokine release syndrome impaired vascular and heart function, often causing reversible LV dysfunction like stress cardiomyopathy. Pre-existing CVD increased risk, leading to more severe complications. Management strategies included fluid resuscitation, vasopressors, tocilizumab (IL-6 inhibitor), and cardio-oncology consultation. |
| 70 | Herrmann [94]/2020 | Qualitative study (narrative review) | To examine the cardiotoxic effects of cancer treatments, including chemotherapy, targeted therapies, immunotherapies, and radiation therapy; mechanisms of cardiotoxicity, risk factors, and preventive strategies | ALL |
| Cardiomyopathy, HF, arrhythmias, AF, ventricular tachycardia, hypertension, QT prolongation, sudden cardiac death, pericarditis, pericardial effusion, CAD, ischemia, myocarditis, thromboembolism, vascular toxicity | During Treatment to Late-Onset | Cancer therapies significantly impacted cardiovascular health, with newer treatments (targeted therapy, immunotherapy) presenting unique toxicity profiles. AF was an emerging major cardiac issue in cancer patients due to novel therapies. The combination of anthracyclines and targeted therapies (trastuzumab) increased the risk of irreversible cardiomyopathy. Early detection and monitoring (echocardiography, biomarkers like cardiac troponins) helped mitigate severe cardiac complications. |
| 71 | Giudice et al. [95]/2020 | Qualitative study (narrative review) | To analyze and summarize the cardiotoxic effects associated with targeted therapies used in hematology, particularly focusing on novel drugs like BTK inhibitors, PI3K inhibitors, IDH inhibitors, and monoclonal antibodies | AML |
| Hypertension, QT prolongation, arrhythmias, HF, CHF, LVD, acute coronary syndromes, MI, AOEs, VTE, pericardial and pleural effusion | During Treatment to Late-Onset | Pre-existing cardiovascular conditions and cumulative drug exposure significantly heightened the risk of cardiotoxicity in targeted leukemia treatments, affecting both survival and quality of life. Ponatinib and Nilotinib were linked to a high rate of AOEs, while BTK and PI3K inhibitors were strongly associated with QT prolongation and arrhythmias. FLT3 inhibitors and hypomethylating agents carried a moderate risk, causing QT prolongation and hypertension. Preventive strategies, such as ACE inhibitors, β-blockers, and statins, may have helped reduce cardiotoxicity severity. |
| 72 | Gavriilaki et al. [96]/2020 | Quantitative study (observational case–control study) | To assess vascular injury and pro-coagulant activity in allo-HCT survivors without existing allo-HCT complications or relapses, using circulating microvesicles as markers | ALL, AML |
| Endothelial dysfunction, increased thrombotic risk, hypertension, dyslipidemia, increased risk of CVD independent of traditional risk factors | Late-Onset | Endothelial dysfunction and thrombotic risk persisted long after allo-HCT, regardless of traditional cardiovascular risk factors. Myeloablative conditioning increased endothelial microvesicles, indicating ongoing vascular injury, while the history of thrombotic microangiopathy was associated with elevated erythrocyte microvesicles, reflecting a lasting thrombo-inflammatory effect. The correlation between platelet and endothelial microvesicles reinforced the link between endothelial injury and pro-coagulant activity. Since traditional risk factors (hypertension, dyslipidemia) did not fully explain the elevated cardiovascular risk, novel biomarkers like microvesicles were essential for improved risk stratification in allo-HCT survivors. |
| 73 | Cook and Litzow [97]/2020 | Qualitative study (narrative review) | To summarize emerging toxicities associated with modern immunotherapeutic and targeted treatment strategies for ALL and to provide insights into supportive care measures that can optimize patient outcomes | ALL |
| Vascular toxicity, vascular occlusive events, platelet dysfunction, QTc prolongation, arrhythmias, pulmonary artery hypertension | During Treatment to Late-Onset | Ponatinib and nilotinib carried a significant risk of vascular complications; comprehensive cardiovascular screening was recommended before initiating therapy. Dasatinib was associated with QTc prolongation, pleural effusion, and platelet dysfunction, and routine ECG and bleeding risk assessment were recommended. |
| 74 | Bhatia [98]/2020 | Qualitative study (narrative review) | To investigate the genetic factors contributing to anthracycline-related cardiomyopathy in cancer survivors, focusing on the role of genetic variants in modifying the risk | ALL, AML |
| Cardiomyopathy, HF, LVEF | Early-Onset to Late-Onset | A dose-dependent relationship existed between anthracycline exposure and cardiomyopathy risk. Genetic susceptibility played a role, with variants in genes e.g., SLC28A3, affecting cardiotoxicity risk. Concurrent risk factors (age at exposure, female sex, chest radiation, cardiovascular risk factors) exacerbated the risk. Potential preventive strategies included genetic screening and personalized monitoring. |
| 75 | Mohamed et al. [99]/2020 | Quantitative study (retrospective observational cohort study) | To evaluate the association between a leukemia diagnosis and clinical outcomes of AMI, focusing on clinical characteristics, management strategies, and outcomes in leukemia patients | ALL, AML |
| Cardiac tamponade, hemopericardium, coronary dissection, shock | Early-Onset to Late-Onset | Leukemia patients, particularly those with AML, experienced significantly worse cardiovascular outcomes after AMI, with a fourfold increase in mortality and a threefold increase in major Acute cardiovascular and cerebrovascular events risk. They were also less likely to undergo coronary angiography (48.5% vs. 64.5%) and Percutaneous Coronary Intervention (28.2% vs. 42.9%). These findings highlighted the need for a multidisciplinary approach involving both cardiologists and hematology-oncologists to optimize care and outcomes. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abasi, A.; Ayatollahi, H.; Rad, S.; Hajahmadipoor Rafsanjani, M. Determining Risk Factors Associated with Cardiovascular Complications in Patients with Acute Leukemia: A Systematic Review. Cancers 2025, 17, 2777. https://doi.org/10.3390/cancers17172777
Abasi A, Ayatollahi H, Rad S, Hajahmadipoor Rafsanjani M. Determining Risk Factors Associated with Cardiovascular Complications in Patients with Acute Leukemia: A Systematic Review. Cancers. 2025; 17(17):2777. https://doi.org/10.3390/cancers17172777
Chicago/Turabian StyleAbasi, Arezoo, Haleh Ayatollahi, Soroush Rad, and Marjan Hajahmadipoor Rafsanjani. 2025. "Determining Risk Factors Associated with Cardiovascular Complications in Patients with Acute Leukemia: A Systematic Review" Cancers 17, no. 17: 2777. https://doi.org/10.3390/cancers17172777
APA StyleAbasi, A., Ayatollahi, H., Rad, S., & Hajahmadipoor Rafsanjani, M. (2025). Determining Risk Factors Associated with Cardiovascular Complications in Patients with Acute Leukemia: A Systematic Review. Cancers, 17(17), 2777. https://doi.org/10.3390/cancers17172777





