Melanoma Clues Beyond Dermoscopic Patterns: Lesion Orientation to Langer’s Lines as a Predictor on the Trunk
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Number Needed to Excise (NNE)
3.2. Population Characteristics and Lesion Sites
3.3. Frequencies of Dermoscopic Features and Adherence to Langer’s Lines in Trunk Nevi and Melanomas
3.4. Predictors of Melanoma on the Trunk
3.5. Lesion Orientation Relative to Langer’s Lines in CM vs. Nevi
3.6. Subgroup Analysis by Anatomic Location: “Critical” vs. “Non-Critical” Sites
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CM | Cutaneous melanoma |
| SD | Standard deviation |
| OR | Odds ratio |
| NNE | Number needed to excise |
| ECM | Extracellular matrix |
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Schadendorf, D.; van Akkooi, A.C.J.; Berking, C.; Griewank, K.G.; Gutzmer, R.; Hauschild, A.; Stang, A.; Roesch, A.; Ugurel, S. Melanoma. Lancet 2018, 392, 971–984. [Google Scholar] [CrossRef] [PubMed]
- Rosendahl, C.; Tschandl, P.; Cameron, A.; Kittler, H. Diagnostic Accuracy of Dermatoscopy for Melanocytic and Nonmelanocytic Pigmented Lesions. J. Am. Acad. Dermatol. 2011, 64, 1068–1073. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, M.E.; Macaskill, P.; Holt, P.E.; Menzies, S.W. Dermoscopy Compared with Naked Eye Examination for the Diagnosis of Primary Melanoma: A Meta-Analysis of Studies Performed in a Clinical Setting. Br. J. Dermatol. 2008, 159, 669–676. [Google Scholar] [CrossRef]
- Argenziano, G.; Fabbrocini, G.; Carli, P.; De Giorgi, V.; Sammarco, E.; Delfino, M. Epiluminescence Microscopy for the Diagnosis of Doubtful Melanocytic Skin Lesions. Comparison of the ABCD Rule of Dermatoscopy and a New 7-Point Checklist Based on Pattern Analysis. Arch. Dermatol. 1998, 134, 1563–1570. [Google Scholar] [CrossRef]
- Nelson, K.C.; Swetter, S.M.; Saboda, K.; Chen, S.C.; Curiel-Lewandrowski, C. Evaluation of the Number-Needed-to-Biopsy Metric for the Diagnosis of Cutaneous Melanoma: A Systematic Review and Meta-Analysis. JAMA Dermatol. 2019, 155, 1167–1174. [Google Scholar] [CrossRef]
- Petty, A.J.; Ackerson, B.; Garza, R.; Peterson, M.; Liu, B.; Green, C.; Pavlis, M. Meta-Analysis of Number Needed to Treat for Diagnosis of Melanoma by Clinical Setting. J. Am. Acad. Dermatol. 2020, 82, 1158–1165. [Google Scholar] [CrossRef]
- Lallas, A.; Paschou, E.; Manoli, S.-M.; Papageorgiou, C.; Spyridis, I.; Liopyris, K.; Bobos, M.; Moutsoudis, A.; Lazaridou, E.; Apalla, Z. Dermatoscopy of Melanoma According to Type, Anatomic Site and Stage. Ital. J. Dermatol. Venerol. 2021, 156, 274–288. [Google Scholar] [CrossRef]
- Longo, C.; Pampena, R.; Moscarella, E.; Chester, J.; Starace, M.; Cinotti, E.; Piraccini, B.M.; Argenziano, G.; Peris, K.; Pellacani, G. Dermoscopy of Melanoma According to Different Body Sites: Head and Neck, Trunk, Limbs, Nail, Mucosal and Acral. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 1718–1730. [Google Scholar] [CrossRef]
- Whiteman, D.C.; Green, A.C.; Olsen, C.M. The Growing Burden of Invasive Melanoma: Projections of Incidence Rates and Numbers of New Cases in Six Susceptible Populations through 2031. J. Investig. Dermatol. 2016, 136, 1161–1171. [Google Scholar] [CrossRef]
- Cavallo, F.; Santaniello, U.; Bin, E.; Roccuzzo, G.; Giordano, S.; Agostini, A.; Merli, M.; Fava, P.; Quaglino, P.; Ribero, S.; et al. Dermoscopy of Melanoma According to Age Groups: A Retrospective Monocentric Study on 285 Patients. Cancers 2025, 17, 2597. [Google Scholar] [CrossRef]
- Sclerosing Melanocytic Lesions (Sclerosing Melanomas with Nevoid Features and Sclerosing Nevi with Pseudomelanomatous Features)—An Analysis of 90 Lesions—PMC. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC6043882/ (accessed on 14 August 2025).
- Hassanein, A.; Depick-Smith, N.; Magill, M.; Bandarchi, B. Focal Regression-Like Changes in Dysplastic Back Nevi: A Diagnostic Pitfall for Malignant Melanoma. Hassanein. J. Cutan. Pathol. 2005, 32, 91. [Google Scholar] [CrossRef]
- Borges, A.F. Relaxed Skin Tension Lines (RSTL) versus Other Skin Lines. Plast. Reconstr. Surg. 1984, 73, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Langer, K. On the Anatomy and Physiology of the Skin. I. The Cleavability of the Cutis. Br. J. Plast. Surg. 1978, 31, 3–8. [Google Scholar]
- McClenahan, P.; Blake, T.; Douglas, N.; Gilmore, S.; Soyer, H.P. Quantifying the Orientation of Acquired Melanocytic Nevi on the Back. Arch. Dermatol. 2012, 148, 857–859. [Google Scholar] [CrossRef]
- Argenziano, G.; Soyer, H.P.; Chimenti, S.; Talamini, R.; Corona, R.; Sera, F.; Binder, M.; Cerroni, L.; De Rosa, G.; Ferrara, G.; et al. Dermoscopy of Pigmented Skin Lesions: Results of a Consensus Meeting via the Internet. J. Am. Acad. Dermatol. 2003, 48, 679–693. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.; Puig, S. Dermoscopy, Digital Dermoscopy and Other Diagnostic Tools in the Early Detection of Melanoma and Follow-up of High-Risk Skin Cancer Patients. Acta Derm. Venereol. 2017, 97 (Suppl. S218), 14–21. [Google Scholar] [CrossRef]
- Marghoob, N.G.; Liopyris, K.; Jaimes, N. Dermoscopy: A Review of the Structures That Facilitate Melanoma Detection. J. Osteopath. Med. 2019, 119, 380–390. [Google Scholar] [CrossRef]
- Williams, N.M.; Rojas, K.D.; Reynolds, J.M.; Kwon, D.; Shum-Tien, J.; Jaimes, N. Assessment of Diagnostic Accuracy of Dermoscopic Structures and Patterns Used in Melanoma Detection. JAMA Dermatol. 2021, 157, 1–12. [Google Scholar] [CrossRef]
- Schweizer, A.; Fink, C.; Bertlich, I.; Toberer, F.; Mitteldorf, C.; Stolz, W.; Enk, A.; Kilian, S.; Haenssle, H.A. Differentiation of Combined Nevi and Melanomas: Case-Control Study with Comparative Analysis of Dermoscopic Features. JDDG J. Der Dtsch. Dermatol. Ges. 2020, 18, 111–118. [Google Scholar] [CrossRef]
- Carrera, C.; Marchetti, M.A.; Dusza, S.W.; Argenziano, G.; Braun, R.P.; Halpern, A.C.; Jaimes, N.; Kittler, H.J.; Malvehy, J.; Menzies, S.W.; et al. Validity and Reliability of Dermoscopic Criteria Used to Differentiate Nevi From Melanoma: A Web-Based International Dermoscopy Society Study. JAMA Dermatol. 2016, 152, 798–806. [Google Scholar] [CrossRef]
- Seidenari, S.; Ferrari, C.; Borsari, S.; Benati, E.; Ponti, G.; Bassoli, S.; Giusti, F.; Schianchi, S.; Pellacani, G. Reticular Grey-Blue Areas of Regression as a Dermoscopic Marker of Melanoma in Situ. Br. J. Dermatol. 2010, 163, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Osella-Abate, S.; Conti, L.; Annaratone, L.; Senetta, R.; Bertero, L.; Licciardello, M.; Caliendo, V.; Picciotto, F.; Quaglino, P.; Cassoni, P.; et al. Phenotypic Characterisation of Immune Cells Associated with Histological Regression in Cutaneous Melanoma. Pathology 2019, 51, 487–493. [Google Scholar] [CrossRef]
- Blessing, K.; McLaren, K.M. Histological Regression in Primary Cutaneous Melanoma: Recognition, Prevalence and Significance. Histopathology 1992, 20, 315–322. [Google Scholar] [CrossRef]
- Ribero, S.; Moscarella, E.; Ferrara, G.; Piana, S.; Argenziano, G.; Longo, C. Regression in Cutaneous Melanoma: A Comprehensive Review from Diagnosis to Prognosis. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 2030–2037. [Google Scholar] [CrossRef]
- Selim, M.A.; Vollmer, R.T.; Herman, C.M.; Pham, T.T.N.; Turner, J.W. Melanocytic Nevi with Nonsurgical Trauma: A Histopathologic Study. Am. J. Dermatopathol. 2007, 29, 134–136. [Google Scholar] [CrossRef]
- Verhaegen, P.D.H.M.; Res, E.M.; van Engelen, A.; Middelkoop, E.; van Zuijlen, P.P.M. A Reliable, Non-Invasive Measurement Tool for Anisotropy in Normal Skin and Scar Tissue. Skin Res. Technol. 2010, 16, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Elouneg, A.; Chambert, J.; Lejeune, A.; Lucot, Q.; Jacquet, E.; Bordas, S.P.A. Anisotropic Mechanical Characterization of Human Skin by in Vivo Multi-Axial Ring Suction Test. J. Mech. Behav. Biomed. Mater. 2023, 141, 105779. [Google Scholar] [CrossRef]
- Provenzano, P.P.; Inman, D.R.; Eliceiri, K.W.; Trier, S.M.; Keely, P.J. Contact Guidance Mediated Three-Dimensional Cell Migration Is Regulated by Rho/ROCK-Dependent Matrix Reorganization. Biophys. J. 2008, 95, 5374–5384. [Google Scholar] [CrossRef]
- Friedl, P.; Alexander, S. Cancer Invasion and the Microenvironment: Plasticity and Reciprocity. Cell 2011, 147, 992–1009. [Google Scholar] [CrossRef] [PubMed]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of Extracellular Matrix Remodelling in Tumour Progression and Metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar] [CrossRef] [PubMed]
- Taloni, A.; Alemi, A.A.; Ciusani, E.; Sethna, J.P.; Zapperi, S.; Porta, C.A.M.L. Mechanical Properties of Growing Melanocytic Nevi and the Progression to Melanoma. PLoS ONE 2014, 9, e94229. [Google Scholar] [CrossRef] [PubMed]
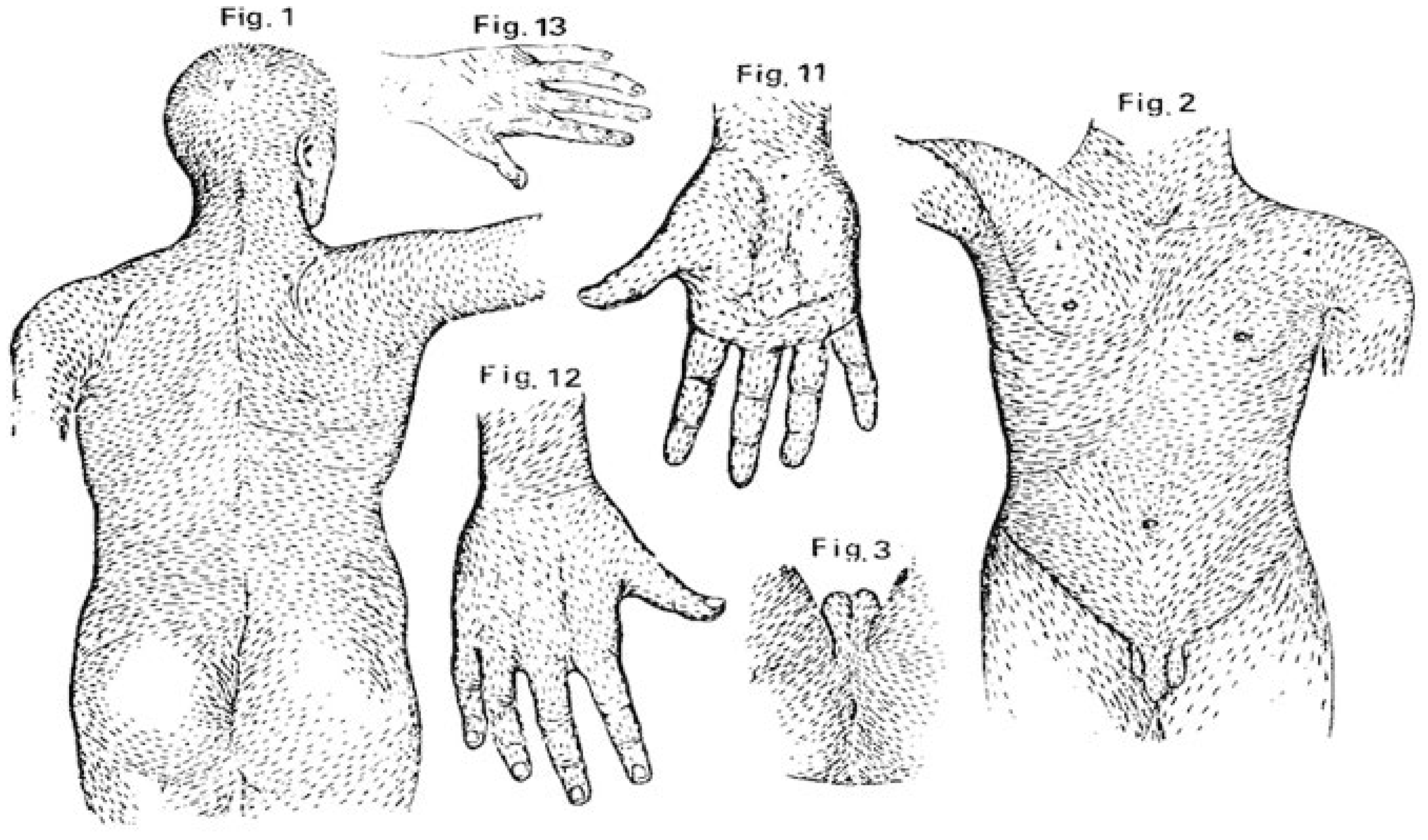
| Site/Area | Nevi, n (%) | CM, n (%) |
|---|---|---|
| Interscapular | 39 (17.2%) | 14 (14.9%) |
| Scapular | 43 (18.9%) | 16 (17.0%) |
| Pectoral | 22 (9.7%) | 10 (10.6%) |
| Sternal/Parasternal | 7 (3.1%) | 9 (9.6%) |
| Dorsal | 29 (12.8%) | 13 (13.8%) |
| Lumbar | 27 (11.9%) | 7 (7.4%) |
| Abdomen | 38 (16.7%) | 16 (17.0%) |
| Flank | 13 (5.7%) | 5 (5.3%) |
| Shoulder | 9 (4.0%) | 4 (4.3%) |
| Total | 227 (100%) | 94 (100%) |
| Parameter | Nevi, n (%) | CM, n (%) | p-Value |
|---|---|---|---|
| Asymmetry of pattern | 107 (47%) | 60 (64%) | 0.0052 |
| Asymmetry of color | 120 (53%) | 58 (62%) | 0.1911 |
| Atypical blotches | 23 (10%) | 21 (22%) | <0.01 |
| Blue-white veil | 11 (5%) | 21 (22%) | <0.01 |
| Hypopigmented structureless areas | 145 (64%) | 80 (85%) | <0.01 |
| Atypical network | 145 (64%) | 74 (79%) | 0.0114 |
| Regression (scar-like) | 34 (15%) | 21 (22%) | 0.1111 |
| Peppering | 48 (21%) | 33 (35%) | <0.01 |
| Irregular globules/dots | 130 (57%) | 32 (34%) | <0.01 |
| Pseudopods | 11 (5%) | 1 (1%) | 0.1041 |
| Radial streaks | 73 (32%) | 15 (16%) | <0.01 |
| Polymorphous vessels | 11 (5%) | 14 (15%) | <0.01 |
| Shiny white lines | 16 (7%) | 11 (12%) | 0.1279 |
| Angulated lines | 5 (2%) | 11 (12%) | <0.01 |
| Inverse network | 14 (6%) | 6 (6%) | 0.9420 |
| Prominent skin markings | 2 (1%) | 2 (2%) | 0.5956 |
| Gray color | 102 (45%) | 47 (50%) | 0.4497 |
| Globular pattern | 52 (23%) | 4 (4%) | <0.01 |
| Reticular pattern | 132 (58%) | 42 (45%) | 0.0228 |
| Homogeneous pattern | 20 (9%) | 4 (4%) | 0.1579 |
| Multicomponent pattern | 66 (29%) | 46 (49%) | <0.01 |
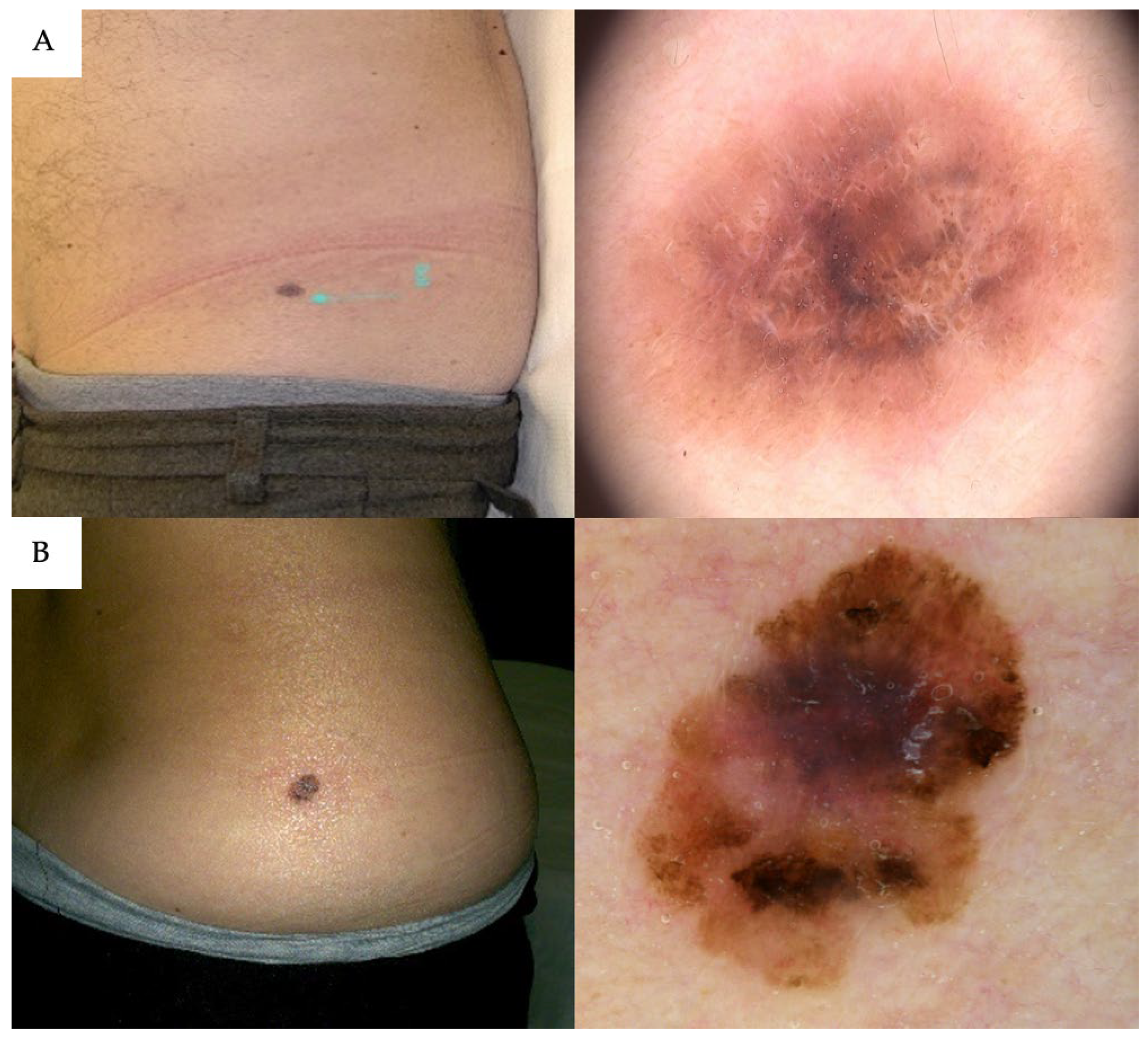
| Non-adherence to Langer’s lines | 64 (28%) | 66 (70%) | <0.01 |
| Parameter | Odds Ratio (OR) | 95% Confidence Interval (CI) | p-Value |
|---|---|---|---|
| Predictors of Melanoma (OR > 1) | |||
| Non-Adherence to Langer’s Lines | 5.55 | 3.22–9.81 | <0.001 |
| Angulated Lines | 5.24 | 1.56–20.97 | 0.07 |
| Blue-White Veil | 5.09 | 2.26–12.04 | <0.001 |
| Polymorphous Vessels | 4.06 | 1.67–10.19 | 0.02 |
| Hypopigmented Structureless Areas | 3.05 | 1.61–6.13 | <0.001 |
| Atypical Blotches | 2.35 | 1.16–4.76 | 0.18 |
| Multicomponent Pattern | 2.01 | 1.18–3.42 | 0.10 |
| Peppering | 2.30 | 1.07–3.37 | 0.29 |
| Atypical Network | 2.24 | 1.03–3.41 | 0.40 |
| Predictors of Nevi (OR < 1) | |||
| Globular Pattern | 0.24 | 0.07–0.63 | 0.04 |
| Radial Streaks | 0.41 | 0.21–0.77 | 0.06 |
| Irregular Globules/Dots | 0.46 | 0.27–0.78 | 0.04 |
| Parameter | Non-Critical Sites, OR (95%CI) | Critical Sites OR (95%CI) | p for Interaction |
|---|---|---|---|
| Non-Adherence to Langer’s Lines | 6.04 (2.72–14.14) | 5.29 (2.43–12.16) | 0.958 |
| Hypopigmented structureless areas | 2.19 (0.96–5.33) | 5.15 (1.76–19.16) | 0.461 |
| Blue-White Veil | 3.67 (1.00–14.72) | 6.44 (2.21–20.78) | 0.415 |
| Asymmetry of Color | 2.27 (1.06–5.02) | 0.63 (0.29–1.35) | 0.026 |
| Atypical Network | 2.81 (1.15–7.70) | 1.44 (0.65–3.35) | 0.240 |
| Multicomponent Pattern | 1.35 (0.60–2.97) | 2.60 (1.23–5.57) | 0.379 |
| Radial Streaks | 0.68 (0.29–1.55) | 0.16 (0.04–0.48) | 0.097 |
| Globular Pattern | 0.32 (0.05–1.27) | 0.20 (0.03–0.78) | 0.944 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santaniello, U.; Cavallo, F.; Diana, S.; Giordano, S.; Crespi, O.; Rosset, F.; Agostini, A.; Moirano, G.; Fava, P.; Quaglino, P.; et al. Melanoma Clues Beyond Dermoscopic Patterns: Lesion Orientation to Langer’s Lines as a Predictor on the Trunk. Cancers 2025, 17, 3064. https://doi.org/10.3390/cancers17183064
Santaniello U, Cavallo F, Diana S, Giordano S, Crespi O, Rosset F, Agostini A, Moirano G, Fava P, Quaglino P, et al. Melanoma Clues Beyond Dermoscopic Patterns: Lesion Orientation to Langer’s Lines as a Predictor on the Trunk. Cancers. 2025; 17(18):3064. https://doi.org/10.3390/cancers17183064
Chicago/Turabian StyleSantaniello, Umberto, Francesco Cavallo, Sara Diana, Silvia Giordano, Orsola Crespi, François Rosset, Andrea Agostini, Giovenale Moirano, Paolo Fava, Pietro Quaglino, and et al. 2025. "Melanoma Clues Beyond Dermoscopic Patterns: Lesion Orientation to Langer’s Lines as a Predictor on the Trunk" Cancers 17, no. 18: 3064. https://doi.org/10.3390/cancers17183064
APA StyleSantaniello, U., Cavallo, F., Diana, S., Giordano, S., Crespi, O., Rosset, F., Agostini, A., Moirano, G., Fava, P., Quaglino, P., Ribero, S., & Broganelli, P. (2025). Melanoma Clues Beyond Dermoscopic Patterns: Lesion Orientation to Langer’s Lines as a Predictor on the Trunk. Cancers, 17(18), 3064. https://doi.org/10.3390/cancers17183064







