Tumour-Infiltrating Lymphocytes, Tumour Cell Density, and Response to Neoadjuvant Short-Course Radiotherapy in Rectal Cancer: A Translational Sub-Study from the MRC CR07 Clinical Trial
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort
2.2. Sample Preparation
2.3. Tumour Cell Density Assessment
2.4. Tumour-Infiltrating Lymphocyte Density Assessment
2.5. Statistical Analysis
3. Results
3.1. Patient Clinicopathological Characteristics
3.2. Tumour Cell Density
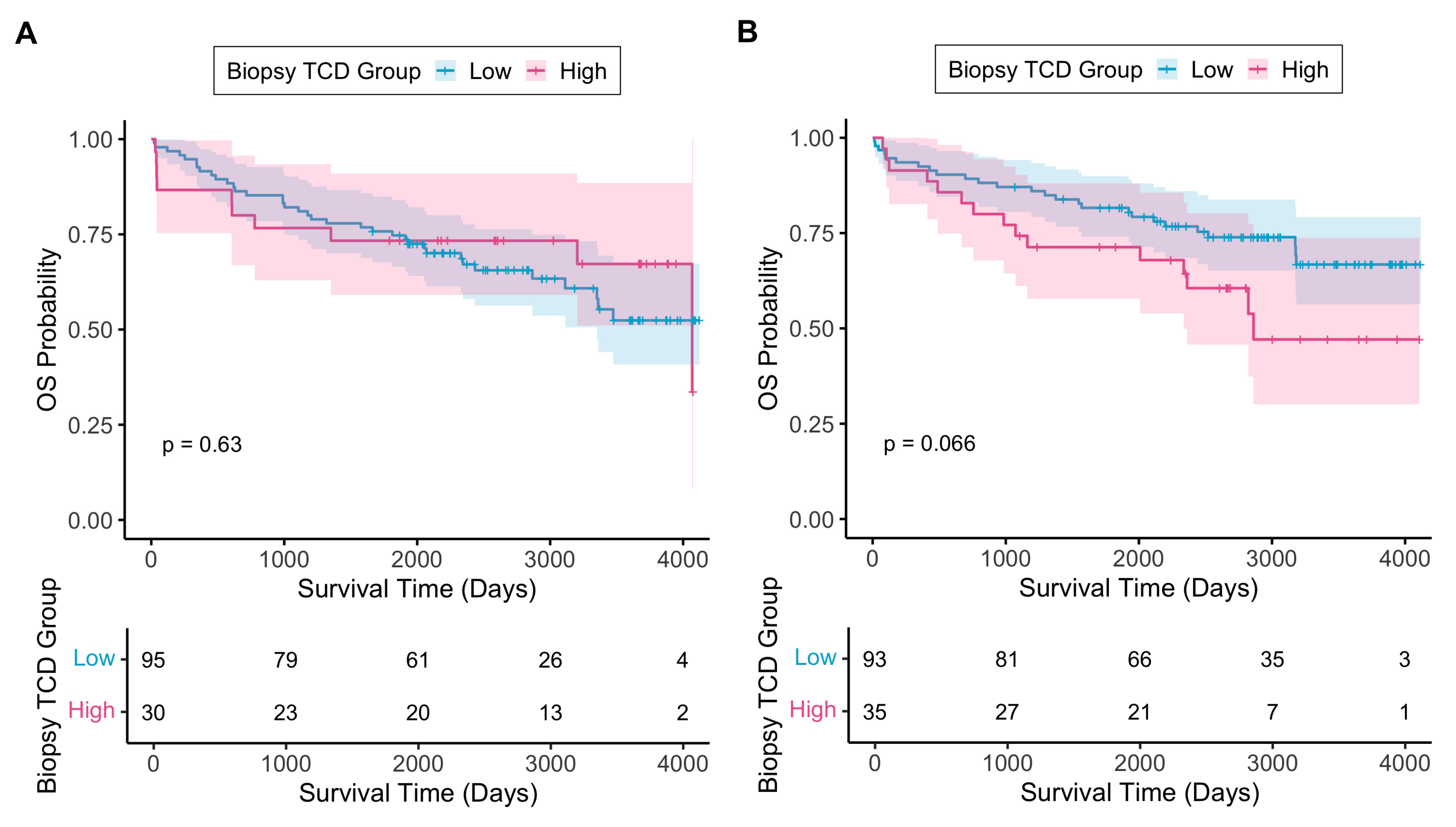
3.2.1. Biopsy Tumour Cell Density
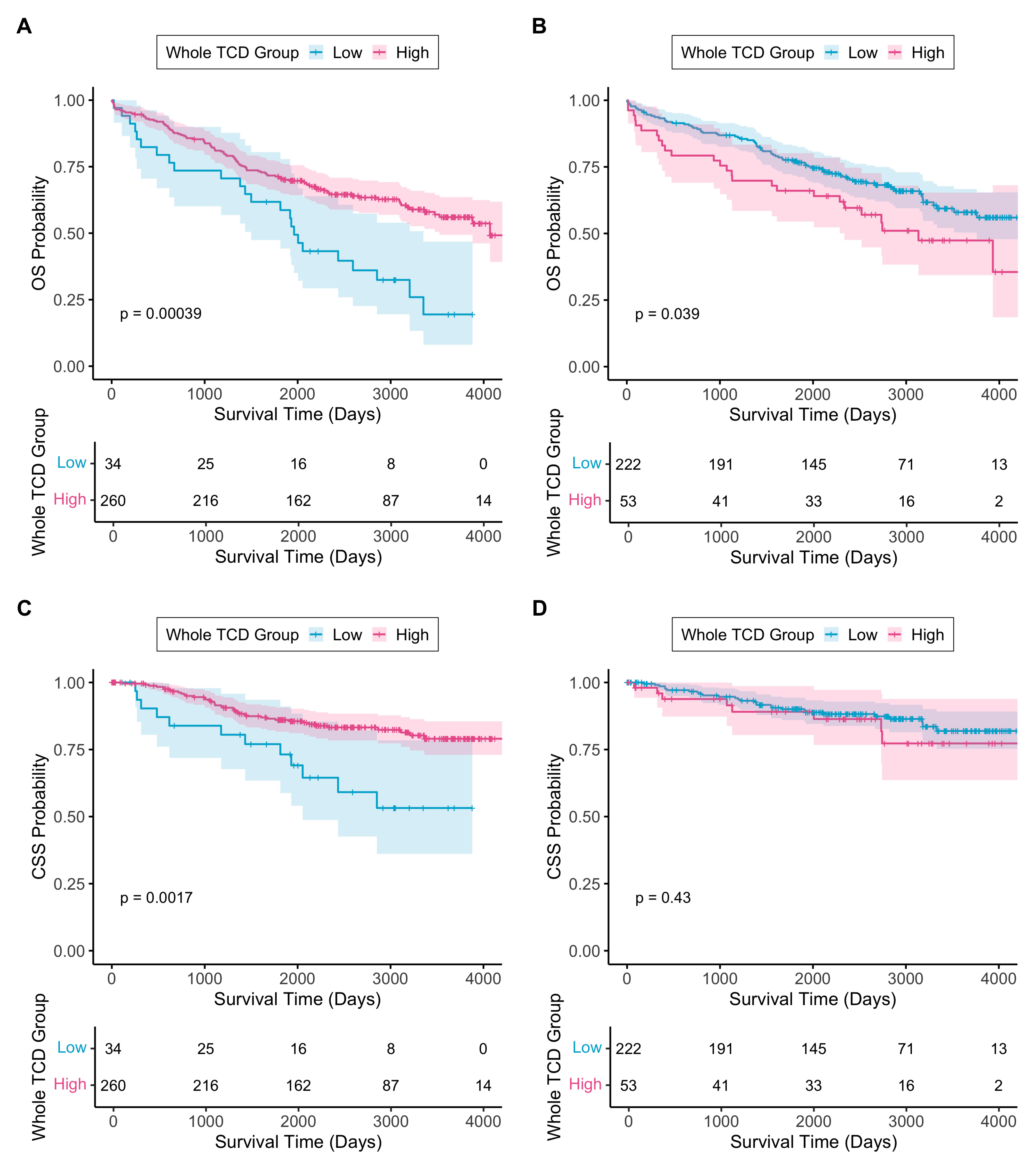
3.2.2. Resection Tumour Cell Density Across the Whole Tumour Area
3.2.3. Change in TCD Between Biopsy and Resection Specimens
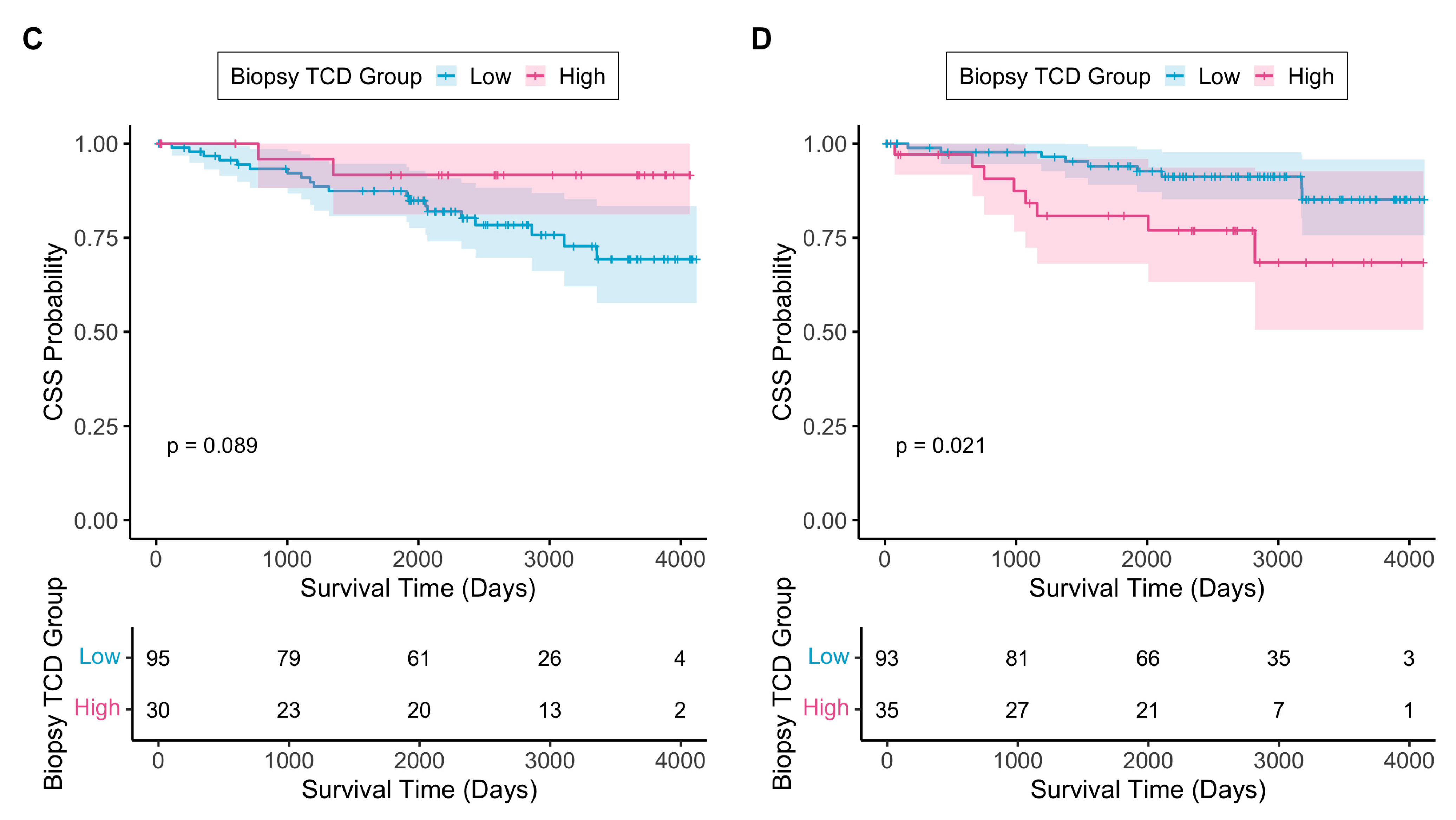
3.2.4. TCD as an Independent Prognostic Biomarker
3.3. Tumour-Infiltrating Lymphocytes
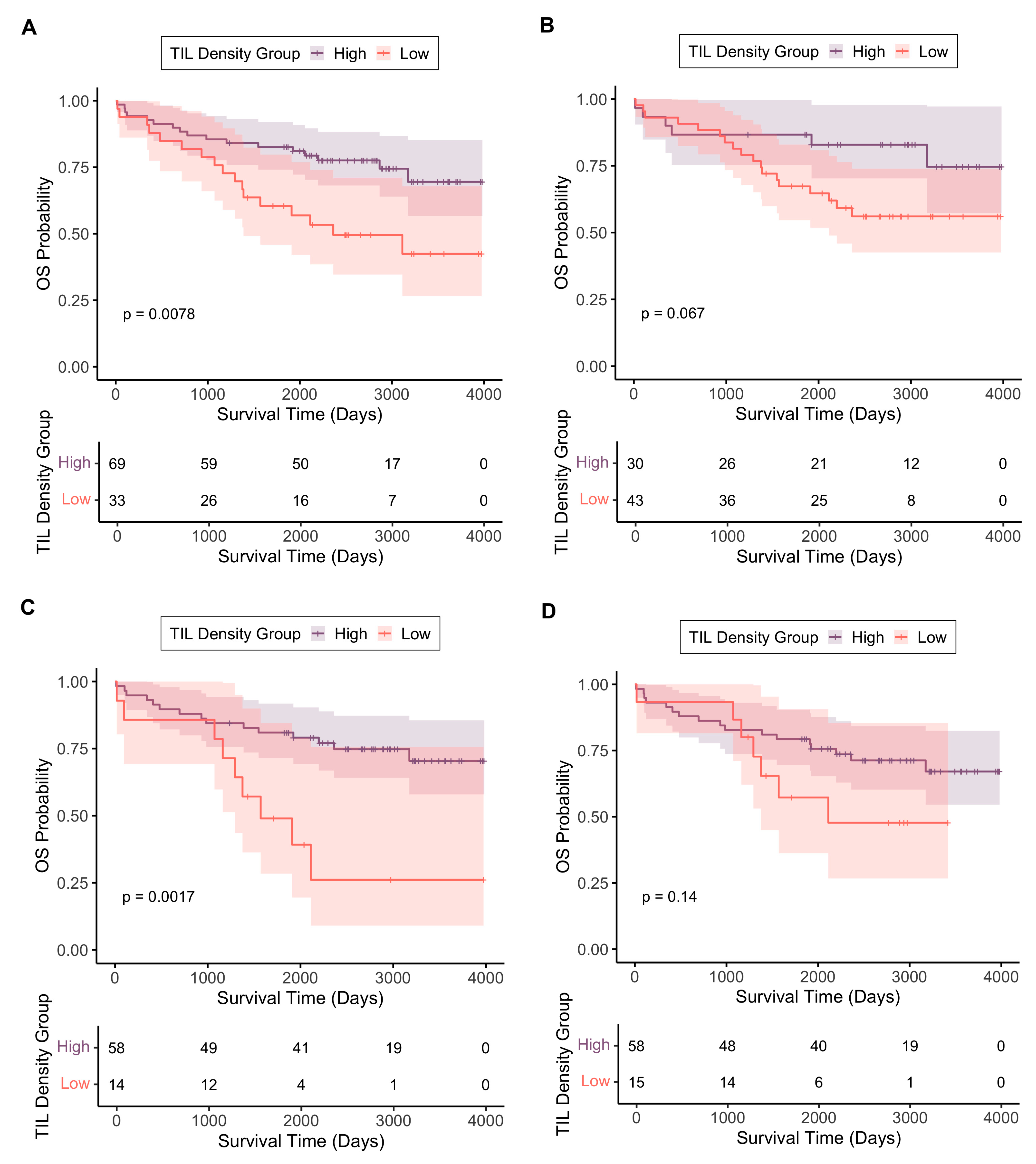
3.3.1. Tumour-Infiltrating Lymphocyte Density in Different Tumour Regions
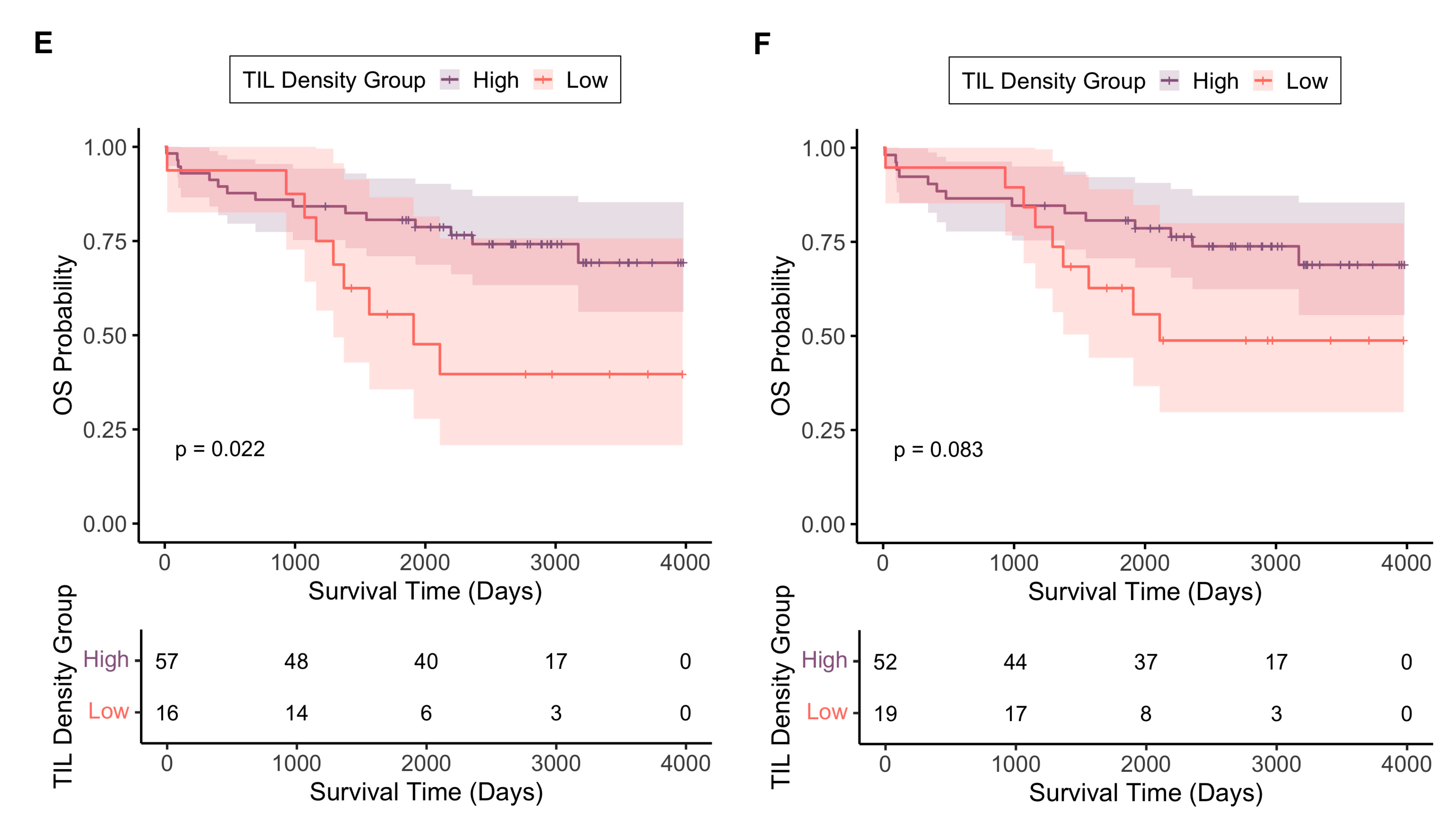
3.3.2. TIL Density as an Independent Prognostic Biomarker
3.4. TIL Density and TCD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Abraha, I.; Aristei, C.; Palumbo, I.; Lupattelli, M.; Trastulli, S.; Cirocchi, R.; De Florio, R.; Valentini, V. Preoperative radiotherapy and curative surgery for the management of localised rectal carcinoma. Cochrane Database Syst. Rev. 2018, 10, CD002102. [Google Scholar] [CrossRef]
- Feeney, G.; Sehgal, R.; Sheehan, M.; Hogan, A.; Regan, M.; Joyce, M.; Kerin, M. Neoadjuvant radiotherapy for rectal cancer management. World J. Gastroenterol. 2019, 25, 4850–4869. [Google Scholar] [CrossRef]
- Gaedcke, J.; Sahrhage, M.; Ebeling, M.; Azizian, A.; Rühlmann, F.; Bernhardt, M.; Grade, M.; Bechstein, W.O.; Germer, C.T.; Grützmann, R.; et al. Prognosis and quality of life in patients with locally advanced rectal cancer after abdominoperineal resection in the CAO/ARO/AIO-04 randomized phase 3 trial. Sci. Rep. 2025, 15, 5401. [Google Scholar] [CrossRef]
- Sebag-Montefiore, D.; Stephens, R.J.; Steele, R.; Monson, J.; Grieve, R.; Khanna, S.; Quirke, P.; Couture, J.; de Metz, C.; Myint, A.S.; et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): A multicentre, randomised trial. Lancet 2009, 373, 811–820. [Google Scholar] [CrossRef]
- West, N.P.; Dattani, M.; McShane, P.; Hutchins, G.; Grabsch, J.; Mueller, W.; Treanor, D.; Quirke, P.; Grabsch, H. The proportion of tumour cells is an independent predictor for survival in colorectal cancer patients. Br. J. Cancer 2010, 102, 1519–1523. [Google Scholar] [CrossRef]
- Idos, G.E.; Kwok, J.; Bonthala, N.; Kysh, L.; Gruber, S.B.; Qu, C. The Prognostic Implications of Tumor Infiltrating Lymphocytes in Colorectal Cancer: A Systematic Review and Meta-Analysis. Sci. Rep. 2020, 10, 3360. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liang, C.; Chen, M.; Su, W. Association between tumor-stroma ratio and prognosis in solid tumor patients: A systematic review and meta-analysis. Oncotarget 2016, 7, 68954–68965. [Google Scholar] [CrossRef]
- Smit, M.A.; van Pelt, G.W.; Terpstra, V.; Putter, H.; Tollenaar, R.; Mesker, W.E.; van Krieken, J. Tumour-stroma ratio outperforms tumour budding as biomarker in colon cancer: A cohort study. Int. J. Color. Dis. 2021, 36, 2729–2737. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Jin, Z.; Qian, Y.; Shen, Y.; Wang, Z. Prognostic Value of Tumor-Stroma Ratio in Rectal Cancer: A Systematic Review and Meta-analysis. Front. Oncol. 2021, 11, 685570. [Google Scholar] [CrossRef]
- Lopez de Rodas, M.; Villalba-Esparza, M.; Sanmamed, M.F.; Chen, L.; Rimm, D.L.; Schalper, K.A. Biological and clinical significance of tumour-infiltrating lymphocytes in the era of immunotherapy: A multidimensional approach. Nat. Rev. Clin. Oncol. 2025, 22, 163–181. [Google Scholar] [CrossRef]
- Teng, F.; Mu, D.; Meng, X.; Kong, L.; Zhu, H.; Liu, S.; Zhang, J.; Yu, J. Tumor infiltrating lymphocytes (TILs) before and after neoadjuvant chemoradiotherapy and its clinical utility for rectal cancer. Am. J. Cancer Res. 2015, 5, 2064–2074. [Google Scholar] [PubMed]
- Mlecnik, B.; Tosolini, M.; Kirilovsky, A.; Berger, A.; Bindea, G.; Meatchi, T.; Bruneval, P.; Trajanoski, Z.; Fridman, W.H.; Pagès, F.; et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J. Clin. Oncol. 2011, 29, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Domingo, E.; Kelly, C.; Hay, J.; Sansom, O.; Maka, N.; Oien, K.; Iveson, T.; Saunders, M.; Kerr, R.; Tomlinson, I.; et al. Prognostic and Predictive Value of Immunoscore in Stage III Colorectal Cancer: Pooled Analysis of Cases From the SCOT and IDEA-HORG Studies. J. Clin. Oncol. 2024, 42, 2207–2218. [Google Scholar] [CrossRef]
- Wankhede, D.; Halama, N.; Kloor, M.; Edelmann, D.; Brenner, H.; Hoffmeister, M. Prognostic Value of CD8+ T Cells at the Invasive Margin Is Comparable to the Immune Score in Nonmetastatic Colorectal Cancer: A Prospective Multicentric Cohort Study. Clin. Cancer Res. 2025, 31, 1711–1718. [Google Scholar] [CrossRef]
- Ugolini, F.; De Logu, F.; Iannone, L.F.; Brutti, F.; Simi, S.; Maio, V.; de Giorgi, V.; Maria di Giacomo, A.; Miracco, C.; Federico, F.; et al. Tumor-Infiltrating Lymphocyte Recognition in Primary Melanoma by Deep Learning Convolutional Neural Network. Am. J. Pathol. 2023, 193, 2099–2110. [Google Scholar] [CrossRef]
- Choi, S.; Cho, S.I.; Jung, W.; Lee, T.; Choi, S.J.; Song, S.; Park, G.; Park, S.; Ma, M.; Pereira, S.; et al. Deep learning model improves tumor-infiltrating lymphocyte evaluation and therapeutic response prediction in breast cancer. NPJ Breast Cancer 2023, 9, 71. [Google Scholar] [CrossRef]
- Liu, D.H.W.; Kim, Y.-W.; Sefcovicova, N.; Laye, J.P.; Hewitt, L.C.; Irvine, A.F.; Vromen, V.; Janssen, Y.; Davarzani, N.; Fazzi, G.E.; et al. Tumour infiltrating lymphocytes and survival after adjuvant chemotherapy in patients with gastric cancer: Post-hoc analysis of the CLASSIC trial. Br. J. Cancer 2023, 128, 2318–2325. [Google Scholar] [CrossRef]
- Westwood, A.C.; Wilson, B.I.; Laye, J.; Grabsch, H.I.; Mueller, W.; Magee, D.R.; Quirke, P.; West, N.P. Deep-learning enabled combined measurement of tumour cell density and tumour infiltrating lymphocyte density as a prognostic biomarker in colorectal cancer. BJC Rep. 2025, 3, 12. [Google Scholar] [CrossRef]
- Falk, T.; Mai, D.; Bensch, R.; Çiçek, Ö.; Abdulkadir, A.; Marrakchi, Y.; Böhm, A.; Deubner, J.; Jäckel, Z.; Seiwald, K.; et al. U-Net: Deep learning for cell counting, detection, and morphometry. Nat. Methods 2019, 16, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Kassambara, A.K.M.; Biecek, P. Survminer: Drawing Survival Curves Using ‘ggplot2’. R Package Version 0502024. Available online: https://cran.r-project.org/web/packages/survminer/index.html (accessed on 9 June 2025).
- Sobin, L.H.; Fleming, I.D. TNM Classification of Malignant Tumors, fifth edition (1997). Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer 1997, 80, 1803–1804. [Google Scholar] [CrossRef]
- Bouzourene, H.; Bosman, F.T.; Seelentag, W.; Matter, M.; Coucke, P. Importance of tumor regression assessment in predicting the outcome in patients with locally advanced rectal carcinoma who are treated with preoperative radiotherapy. Cancer 2002, 94, 1121–1130. [Google Scholar] [CrossRef]
- Gao, J.; Shen, Z.; Deng, Z.; Mei, L. Impact of Tumor-Stroma Ratio on the Prognosis of Colorectal Cancer: A Systematic Review. Front. Oncol. 2021, 11, 738080. [Google Scholar] [CrossRef] [PubMed]
- Gollins, S.; West, N.; Sebag-Montefiore, D.; Susnerwala, S.; Falk, S.; Brown, N.; Saunders, M.; Quirke, P.; Ray, R.; Parsons, P.; et al. A prospective phase II study of pre-operative chemotherapy then short-course radiotherapy for high risk rectal cancer: COPERNICUS. Br. J. Cancer 2018, 119, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, A.; Fontana, E.; Nyamundanda, G.; Ragulan, C.; Patil, Y.; Mansfield, D.; Kingston, J.; Errington-Mais, F.; Bottomley, D.; von Loga, K.; et al. Differential and longitudinal immune gene patterns associated with reprogrammed microenvironment and viral mimicry in response to neoadjuvant radiotherapy in rectal cancer. J. Immunother. Cancer 2021, 9, e001717. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Cheadle, E.J.; Illidge, T.M. Understanding the Effects of Radiotherapy on the Tumour Immune Microenvironment to Identify Potential Prognostic and Predictive Biomarkers of Radiotherapy Response. Cancers 2020, 12, 2835. [Google Scholar] [CrossRef]
- Galon, J.; Costes, A.; Sanchez-Cabo, F.; Kirilovsky, A.; Mlecnik, B.; Lagorce-Pagès, C.; Tosolini, M.; Camus, M.; Berger, A.; Wind, P.; et al. Type, Density, and Location of Immune Cells Within Human Colorectal Tumors Predict Clinical Outcome. Science 2006, 313, 1960–1964. [Google Scholar] [CrossRef]
- Pagès, F.; Mlecnik, B.; Marliot, F.; Bindea, G.; Ou, F.-S.; Bifulco, C.; Lugli, A.; Zlobec, I.; Rau, T.T.; Berger, M.D.; et al. International validation of the consensus Immunoscore for the classification of colon cancer: A prognostic and accuracy study. Lancet 2018, 391, 2128–2139. [Google Scholar] [CrossRef]
- Alexander, P.G.; McMillan, D.C.; Park, J.H. The local inflammatory response in colorectal cancer—Type, location or density? A systematic review and meta-analysis. Cancer Treat. Rev. 2020, 83, 101949. [Google Scholar] [CrossRef]
- Orhan, A.; Khesrawi, F.; Tvilling Madsen, M.; Peuliche Vogelsang, R.; Dohrn, N.; Kanstrup Fiehn, A.-M.; Gögenur, I. Tumor-Infiltrating Lymphocytes as Biomarkers of Treatment Response and Long-Term Survival in Patients with Rectal Cancer: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 636. [Google Scholar] [CrossRef]
- Fridman, W.H.; Zitvogel, L.; Sautès–Fridman, C.; Kroemer, G. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 2017, 14, 717–734. [Google Scholar] [CrossRef]
- Gilbert, A.; Homer, V.; Brock, K.; Korsgen, S.; Geh, I.; Hill, J.; Gill, T.; Hainsworth, P.; Tutton, M.; Khan, J.; et al. Quality-of-life outcomes in older patients with early-stage rectal cancer receiving organ-preserving treatment with hypofractionated short-course radiotherapy followed by transanal endoscopic microsurgery (TREC): Non-randomised registry of patients unsuitable for total mesorectal excision. Lancet Healthy Longev. 2022, 3, e825–e838. [Google Scholar]
- Boustani, J.; Derangère, V.; Bertaut, A.; Adotevi, O.; Morgand, V.; Charon-Barra, C.; Ghiringhelli, F.; Mirjolet, C. Radiotherapy Scheme Effect on PD-L1 Expression for Locally Advanced Rectal Cancer. Cells 2020, 9, 2071. [Google Scholar] [CrossRef] [PubMed]
- Graham Martínez, C.; Barella, Y.; Kus Öztürk, S.; Ansems, M.; Gorris, M.A.J.; van Vliet, S.; Marijnen, C.A.M.; Nagtegaal, I.D. The immune microenvironment landscape shows treatment-specific differences in rectal cancer patients. Front. Immunol. 2022, 13, 1011498. [Google Scholar] [CrossRef] [PubMed]
- Raje, P.; Kunitake, H.; Cauley, C.E.; Goldstone, R.N.; Lee, G.C.; Ricciardi, R. Delayed Surgery after Neoadjuvant Short-course Radiation for Rectal Cancer Improves Pathologic Outcomes without Impacting Survival: A National Cancer Database Analysis. J. Gastrointest. Cancer 2024, 56, 30. [Google Scholar] [CrossRef]
- Alexander, P.G.; Roseweir, A.K.; Pennel, K.A.F.; van Wyk, H.C.; Powell, A.G.M.T.; McMillan, D.C.; Horgan, P.G.; Kelly, C.; Hay, J.; Sansom, O.; et al. The Glasgow Microenvironment Score associates with prognosis and adjuvant chemotherapy response in colorectal cancer. Br. J. Cancer 2021, 124, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Firmbach, D.; Benz, M.; Kuritcyn, P.; Bruns, V.; Lang-Schwarz, C.; Stuebs, F.A.; Merkel, S.; Leikauf, L.-S.; Braunschweig, A.-L.; Oldenburger, A.; et al. Tumor–Stroma Ratio in Colorectal Cancer—Comparison between Human Estimation and Automated Assessment. Cancers 2023, 15, 2675. [Google Scholar] [CrossRef]


| Patient Clinicopathological Characteristics | Tumour Cell Density Cohort | Median TCD (%) Across Resection Whole Tumour Area (IQR) | p-Value | TIL Density Cohort | Median TIL Density (/mm2) Across Resection Whole Tumour Area (IQR) | p-Value | |||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | ||||||
| All patients | 569 | 100 | 27.3 | n/a | 102 | 100 | 1304 | n/a | |
| Sex | Male | 402 | 70.7 | 26.7 (17.7–35.4) | 0.10 | 67 | 65.7 | 1377 (835–1703) | 0.75 |
| Female | 167 | 28.0 | 28.9 (18.9–38.7) | 35 | 34.3 | 1262 (900–1628) | |||
| Age | <65 years | 265 | 46.6 | 25.8 (18.1–35.7) | 0.17 | 54 | 52.9 | 1297 (896–1607) | 0.64 |
| ≥65 years | 304 | 53.4 | 28.6 (17.9–36.6) | 48 | 47.1 | 1323 (819–1770) | |||
| (y)pT | 1 | 32 | 5.6 | 32.0 (15.4–38.3) | 0.27 | 6 | 5.9 | 1389 (1217–1981) | 0.64 |
| 2 | 159 | 27.9 | 24.5 (16.0–34.8) | 32 | 31.4 | 1407 (1017–1733) | |||
| 3 | 335 | 59.2 | 28.0 (19.7–36.3) | 56 | 54.9 | 1203 (826–1614) | |||
| 4 | 38 | 6.7 | 26.6 (16.4–35.5) | 8 | 7.8 | 1135 (711–1671) | |||
| Unknown | 5 | 0.9 | 28.0 (18.3–31.9) | 0 | 0 | n/a | |||
| (y)pN | 0 | 310 | 54.5 | 27.3 (18.0–36.1) | 0.50 | 59 | 57.8 | 1299 (979–1700) | 0.40 |
| 1 | 159 | 27.9 | 28.0 (18.3–35.6) | 28 | 27.5 | 1364 (567–1626) | |||
| 2 | 95 | 16.7 | 27.8 (17.8–36.9) | 15 | 14.7 | 1287 (760–1632) | |||
| Unknown | 5 | 0.9 | 28.0 (18.3–31.9) | 0 | 0 | n/a | |||
| TNM stage | I | 140 | 24.6 | 25.5 (15.4–35.5) | 0.18 | 30 | 29.4 | 1514 (1144–2027) | 0.11 |
| II | 151 | 26.5 | 28.7 (20.8–36.2) | 28 | 27.5 | 1314 (950–1621) | |||
| III | 238 | 41.8 | 28.2 (18.6–36.2) | 41 | 40.2 | 1168 (598–1615) | |||
| IV | 4 | 0.7 | 21.7 (16.2–27.1) | 0 | 0 | n/a | |||
| Unknown | 36 | 6.3 | 23.1 (18.1–31.4) | 3 | 2.9 | 1685 (1298–1999) | |||
| Tumour grade | Other | 483 | 84.9 | 28.2 (18.8–36.6) | 0.036 | 93 | 91.2 | 1349 (944–1659) | 0.37 |
| Poorly differentiated | 82 | 14.4 | 24.5 (16.2–34.2) | 8 | 7.8 | 972 (708–1449) | |||
| Unknown | 4 | 0.7 | 10.3 (4.5–15.7) | 1 | 1.0 | 579 | |||
| Trial arm | SCRT | 275 | 48.3 | 19.9 (12.9–26.7) | <0.001 | 73 | 71.6 | 1210 (677–1539) | 0.0013 |
| Control | 294 | 51.7 | 34.3 (27.7–40.5) | 29 | 28.4 | 1615 (1110–2109) | |||
| Patient Clinicopathological Characteristics | Univariate Hazard Ratio (95% Confidence Interval) | p-Value | Multivariate Hazard Ratio (95% Confidence Interval) | p-Value | |
|---|---|---|---|---|---|
| Sex | Female | 1.00 | 1.00 | ||
| Male | 1.50 (1.09–2.07) | 0.01 | 1.53 (1.11–2.11) | 0.009 | |
| Age | <65 years | 1.00 | 1.00 | ||
| ≥65 years | 2.24 (1.68–2.98) | <0.001 | 2.33 (1.74–3.11) | <0.001 | |
| (y)pT | 1 | 1.00 | n/a | n/a | |
| 2 | 1.31 (0.59–2.92) | 0.514 | |||
| 3 | 2.44 (1.14–5.22) | 0.021 | |||
| 4 | 5.63 (2.43–13.05) | <0.001 | |||
| (y)pN | 0 | 1.00 | n/a | n/a | |
| 1 | 1.32 (0.97–1.81) | 0.082 | |||
| 2 | 2.10 (1.50–2.94) | <0.001 | |||
| TNM stage | I | 1.00 | 1.00 | ||
| II | 1.33 (0.89–1.99) | 0.165 | 1.31 (0.87–1.97) | 0.196 | |
| III | 1.77 (1.23–2.54) | 0.002 | 1.84 (1.28–2.65) | <0.001 | |
| IV | 5.92 (2.11–16.58) | <0.001 | 7.13 (2.48–20.47) | <0.001 | |
| Tumour grade | Other | 1.00 | 1.00 | ||
| Poorly differentiated | 1.28 (0.90–1.83) | 0.176 | 1.14 (0.79–1.65) | 0.489 | |
| Trial arm | Control | 1.00 | 1.00 | ||
| SCRT | 0.93 (0.71–1.22) | 0.595 | 0.96 (0.73–1.26) | 0.747 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Callaghan, J.P.; Jarrett, R.; Westwood, A.C.; Laye, J.; Quirke, P.; Magee, D.R.; Bottomley, D.; Sebag-Montefiore, D.; Thompson, L.; Meade, A.; et al. Tumour-Infiltrating Lymphocytes, Tumour Cell Density, and Response to Neoadjuvant Short-Course Radiotherapy in Rectal Cancer: A Translational Sub-Study from the MRC CR07 Clinical Trial. Cancers 2025, 17, 3040. https://doi.org/10.3390/cancers17183040
Callaghan JP, Jarrett R, Westwood AC, Laye J, Quirke P, Magee DR, Bottomley D, Sebag-Montefiore D, Thompson L, Meade A, et al. Tumour-Infiltrating Lymphocytes, Tumour Cell Density, and Response to Neoadjuvant Short-Course Radiotherapy in Rectal Cancer: A Translational Sub-Study from the MRC CR07 Clinical Trial. Cancers. 2025; 17(18):3040. https://doi.org/10.3390/cancers17183040
Chicago/Turabian StyleCallaghan, Jonathan P., Ross Jarrett, Alice C. Westwood, Jon Laye, Philip Quirke, Derek R. Magee, Daniel Bottomley, David Sebag-Montefiore, Lindsay Thompson, Angela Meade, and et al. 2025. "Tumour-Infiltrating Lymphocytes, Tumour Cell Density, and Response to Neoadjuvant Short-Course Radiotherapy in Rectal Cancer: A Translational Sub-Study from the MRC CR07 Clinical Trial" Cancers 17, no. 18: 3040. https://doi.org/10.3390/cancers17183040
APA StyleCallaghan, J. P., Jarrett, R., Westwood, A. C., Laye, J., Quirke, P., Magee, D. R., Bottomley, D., Sebag-Montefiore, D., Thompson, L., Meade, A., Grabsch, H. I., & West, N. P. (2025). Tumour-Infiltrating Lymphocytes, Tumour Cell Density, and Response to Neoadjuvant Short-Course Radiotherapy in Rectal Cancer: A Translational Sub-Study from the MRC CR07 Clinical Trial. Cancers, 17(18), 3040. https://doi.org/10.3390/cancers17183040






