Tolerability and Safety Assessment of Adjuvant Chemoradiotherapy with S-1 after Limited Surgery for T1 or T2 Lower Rectal Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Methods
2.2. Study Cohort and Eligibility
2.3. Surgical and Postoperative Treatment Protocol
2.4. Follow-Up and Evaluation
2.5. Sample Size Calculation
2.6. Statistical Analysis
3. Results
3.1. Patient Background
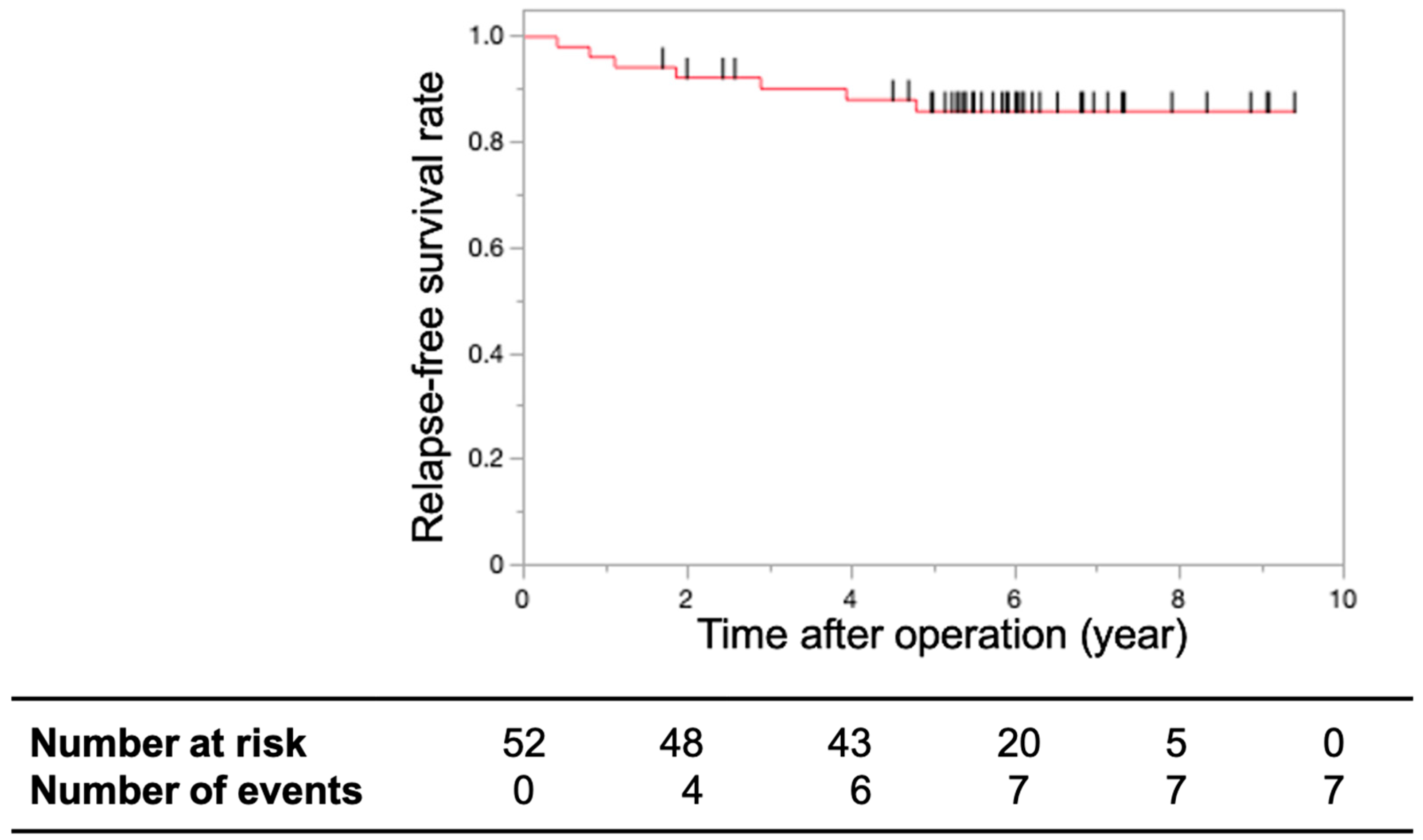
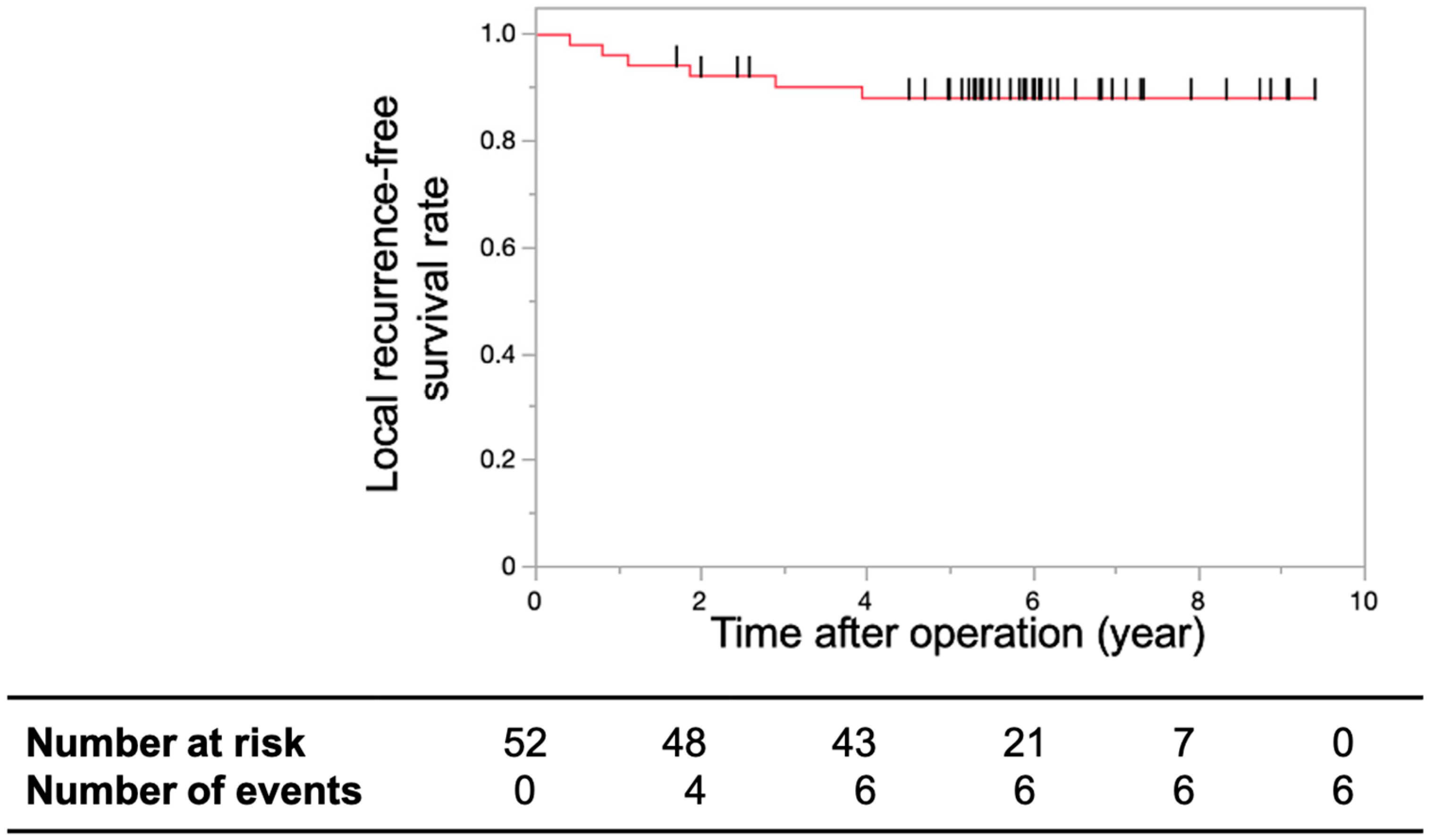
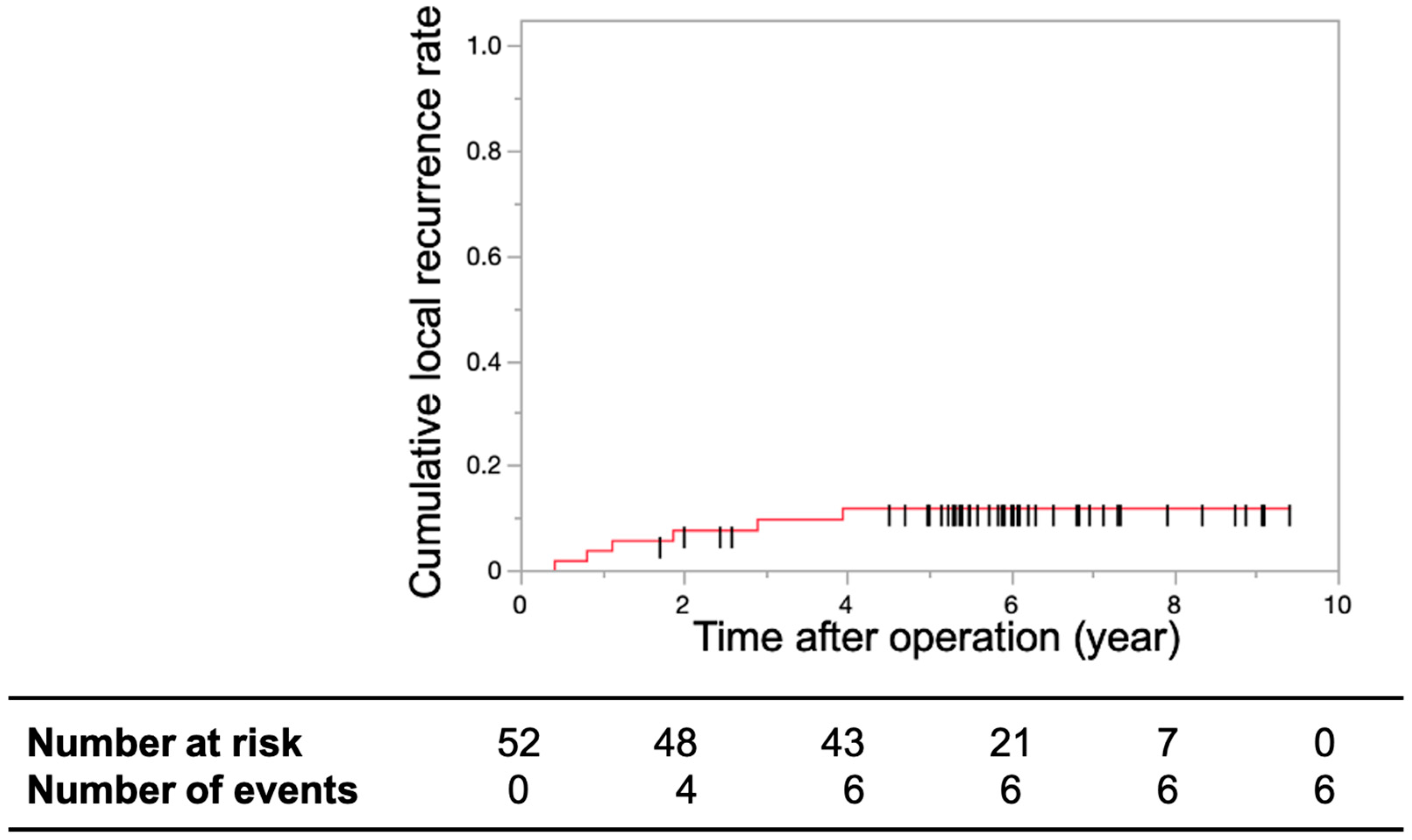
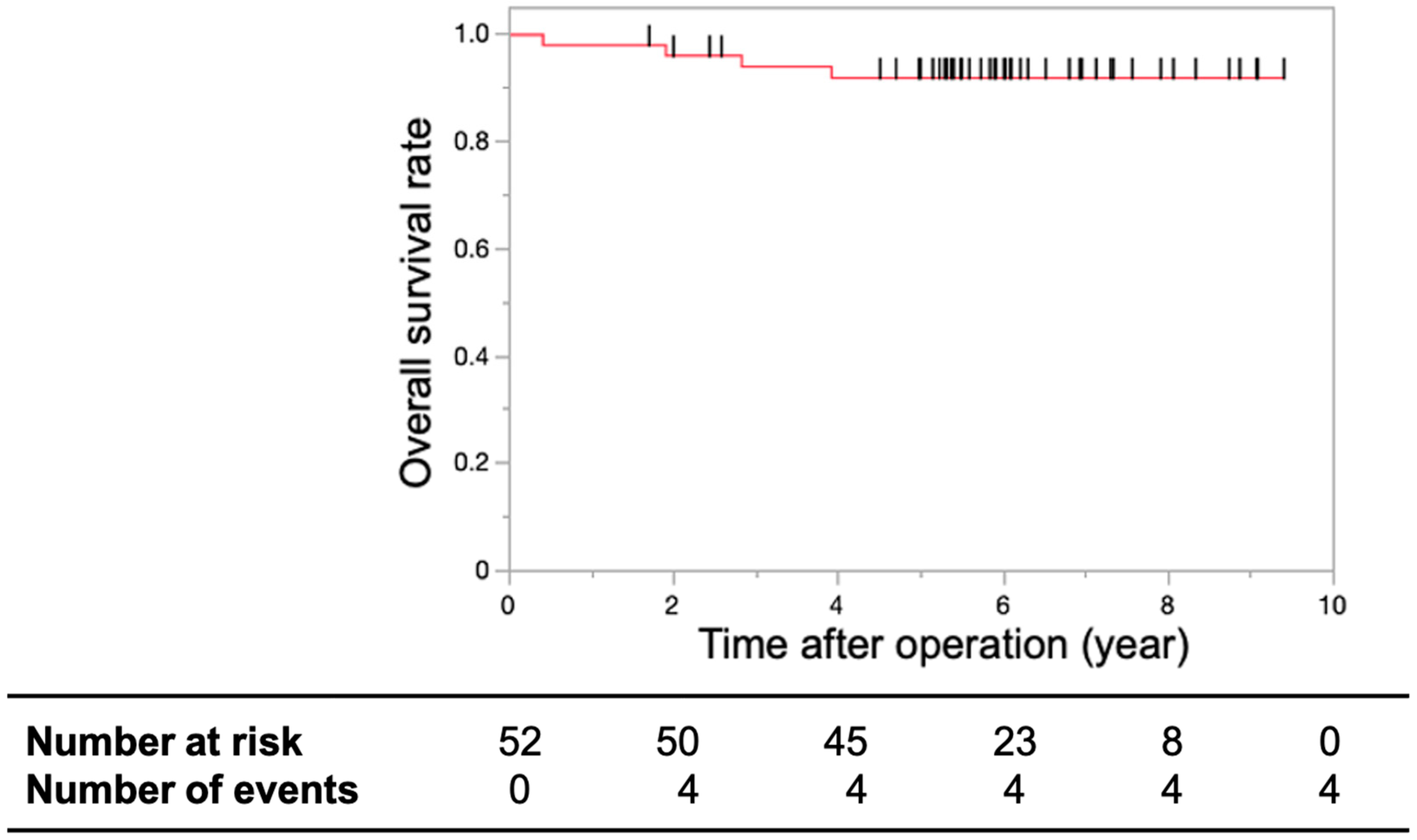
3.2. Primary and Secondary Endpoints
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heald, R.J.; Ryall, R.D. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986, 1, 1479–1482. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology-Rectal Cancer, version 3; National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2024. [Google Scholar]
- Penna, M.; Hompes, R.; Arnold, S.; Wynn, G.; Austin, R.; Warusavitarne, J.; Moran, B.; Hanna, G.B.; Mortensen, N.J.; Tekkis, P.P.; et al. Transanal total mesorectal excision: International registry results of the first 720 cases. Ann. Surg. 2017, 266, 111–117. [Google Scholar] [CrossRef]
- de Lacy, F.B.; van Laarhoven, J.J.E.M.; Pena, R.; Arroyave, M.C.; Bravo, R.; Cuatrecasas, M.; Lacy, A.M. Transanal total mesorectal excision: Pathological results of 186 patients with mid and low rectal cancer. Surg. Endosc. 2018, 32, 2442–2447. [Google Scholar] [CrossRef]
- Peeters, K.C.; van de Velde, C.J.; Leer, J.W.; Martijn, H.; Junggeburt, J.M.; Kranenbarg, E.K.; Steup, W.H.; Wiggers, T.; Rutten, H.J.; Marijnen, C.A. Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: Increased bowel dysfunction in irradiated patients—A Dutch colorectal cancer group study. J. Clin. Oncol. 2005, 23, 6199–6206. [Google Scholar] [CrossRef]
- Rutten, H.J.T.; den Dulk, M.; Lemmens, V.E.P.P.; van de Velde, C.J.H.; Marijnen, C.A.M. Controversies of total mesorectal excision for rectal cancer in elderly patients. Lancet Oncol. 2008, 9, 494–501. [Google Scholar] [CrossRef]
- Swellengrebel, H.A.; Marijnen, C.A.; Verwaal, V.J.; Vincent, A.; Heuff, G.; Gerhards, M.F.; van Geloven, A.A.; van Tets, W.F.; Verheij, M.; Cats, A. Toxicity and complications of preoperative chemoradiotherapy for locally advanced rectal cancer. Br. J. Surg. 2011, 98, 418–426. [Google Scholar] [CrossRef]
- Hager, T.; Gall, F.P.; Hermanek, P. Local excision of cancer of the rectum. Dis. Colon Rectum 1983, 26, 149–151. [Google Scholar] [CrossRef]
- Whiteway, J.; Nicholls, R.J.; Morson, B.C. The role of surgical local excision in the treatment of rectal cancer. Br. J. Surg. 1985, 72, 694–697. [Google Scholar] [CrossRef]
- Biggers, O.R.; Beart, R.W., Jr.; Ilstrup, D.M. Local excision of rectal cancer. Dis. Colon Rectum 1986, 29, 374–377. [Google Scholar] [CrossRef]
- Coco, C.; Magistrelli, P.; Granone, P.; Roncolini, G.; Picciocchi, A. Conservative surgery for early cancer of the distal rectum. Dis. Colon Rectum 1992, 35, 131–136. [Google Scholar] [CrossRef]
- Heimann, T.M.; Oh, C.; Steinhagen, R.M.; Greenstein, A.J.; Perez, C.; Aufses, A.H. Surgical treatment of tumors of the distal rectum with sphincter preservation. Ann. Surg. 1992, 216, 432–436, discussion 436. [Google Scholar] [CrossRef]
- Graham, R.A.; Atkins, M.B.; Karp, D.D.; Wazer, D.E.; Hackford, A.W. Local excision of rectal carcinoma. Early results with combined chemoradiation therapy using 5-fluorouracil and leucovorin. Dis. Colon Rectum 1994, 37, 308–312. [Google Scholar] [CrossRef]
- Taylor, R.H.; Hay, J.H.; Larsson, S.N. Transanal local excision of selected low rectal cancers. Am. J. Surg. 1998, 175, 360–363. [Google Scholar] [CrossRef]
- Jessup, J.M.; Bothe, A., Jr.; Stone, M.D.; Gray, C.; Bleday, R.; Busse, P.M.; Huberman, M.; Mayer, R.J.; Steele, G. Preservation of Sphincter Function in Rectal Carcinoma by a Multimodality Treatment Approach. Surg. Oncol. Clin. N. Am. 1992, 1, 137–145. [Google Scholar] [CrossRef]
- Frazee, R.C.; Patel, R.; Belew, M.; Roberts, J.W.; Hendricks, J.C. Transanal excision of rectal carcinoma. Am. Surg. 1995, 61, 714–717. [Google Scholar]
- Paty, P.B.; Nash, G.M.; Baron, P.; Zakowski, M.; Minsky, B.D.; Blumberg, D.; Nathanson, D.R.; Guillem, J.G.; Enker, W.E.; Cohen, A.M.; et al. Long-term results of local excision for rectal cancer. Ann. Surg. 2002, 236, 522–529, discussion 529. [Google Scholar] [CrossRef]
- Nash, G.M.; Weiser, M.R.; Guillem, J.G.; Temple, L.K.; Shia, J.; Gonen, M.; Wong, W.D.; Paty, P.B. Long-term survival after transanal excision of T1 rectal cancer. Dis. Colon Rectum 2009, 52, 577–582. [Google Scholar] [CrossRef]
- SenGupta, S.; Tjandra, J.J. Local excision of rectal cancer: What is the evidence? Dis. Colon Rectum 2001, 44, 1345–1361. [Google Scholar] [CrossRef]
- Sasaki, T.; Ito, Y.; Ohue, M.; Kanemitsu, Y.; Kobatake, T.; Ito, M.; Moriya, Y.; Saito, N. Postoperative chemoradiotherapy after local resection for high-risk T1 to T2 low rectal cancer: Results of a single-arm, multi-institutional, phase II clinical trial. Dis. Colon Rectum 2017, 60, 914–921. [Google Scholar] [CrossRef]
- Hashiguchi, Y.; Muro, K.; Saito, Y.; Ito, Y.; Ajioka, Y.; Hamaguchi, T.; Hasegawa, K.; Hotta, K.; Ishida, H.; Ishiguro, M.; et al. Japanese society for cancer of the colon and rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int. J. Clin. Oncol. 2020, 25, 1–42. [Google Scholar] [CrossRef]
- Tei, M.; Noura, S.; Ohue, M.; Kitakaze, M.; Takahashi, H.; Miyoshi, N.; Uemura, M.; Mizushima, T.; Murata, K.; Doki, Y.; et al. Tolerability and safety of adjuvant chemoradiotherapy with S-1 after limited surgery for T1 or T2 lower rectal cancer. Int. J. Clin. Oncol. 2021, 26, 2046–2052. [Google Scholar] [CrossRef] [PubMed]
- Japanese Society for Cancer of the Colon and Rectum. Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma, 3rd ed.; Kanehara & Comp., Ltd.: Tokyo, Japan, 2019. [Google Scholar]
- Watanabe, T.; Muro, K.; Ajioka, Y.; Hashiguchi, Y.; Ito, Y.; Saito, Y.; Hamaguchi, T.; Ishida, H.; Ishiguro, M.; Ishihara, S.; et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int. J. Clin. Oncol. 2018, 23, 1–34. [Google Scholar] [CrossRef]
- Kirwan, W.O.; Turnbull, R.B., Jr.; Fazio, V.W.; Weakley, F.L. Pullthrough operation with delayed anastomosis for rectal cancer. Br. J. Surg. 1978, 65, 695–698. [Google Scholar] [CrossRef]
- Jorge, J.M.; Wexner, S.D. Etiology and management of fecal incontinence. Dis. Colon Rectum 1993, 36, 77–97. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.H.; Harris, J.; Rosenberg, P.J.; Sause, W.T.; Fisher, B.J.; Hoffman, J.P.; Kraybill, W.G.; Byhardt, R.W. Anal sphincter conservation for patients with adenocarcinoma of the distal rectum: Long-term results of Radiation Therapy Oncology Group protocol 89-02. Int. J. Radiat. Oncol. Biol. Phys. 2000, 46, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Léonard, D.; Remue, C.; Kartheuser, A. The transanal endoscopic microsurgery procedure: Standards and extended indications. Dig. Dis. 2012, 30 (Suppl. 2), 85–90. [Google Scholar] [CrossRef]
- Devane, L.A.; Burke, J.P.; Kelly, J.J.; Albert, M.R. Transanal minimally invasive surgery for rectal cancer. Ann. Gastroenterol. Surg. 2021, 5, 39–45. [Google Scholar] [CrossRef]
- Bignell, M.B.; Ramwell, A.; Evans, J.R.; Dastur, N.; Simson, J.N. Complications of transanal endoscopic microsurgery (TEMS): A prospective audit. Colorectal Dis. 2010, 12, e99–e103. [Google Scholar] [CrossRef]
- Maglio, R.; Muzi, G.M.; Massimo, M.M.; Masoni, L. Transanal minimally invasive surgery (TAMIS): New treatment for early rectal cancer and large rectal polyps—Experience of an Italian center. Am. Med. Surg. 2015, 81, 273–277. [Google Scholar] [CrossRef]
- Noguchi, M.; Shitara, K.; Kawazoe, A.; Yamamoto, D.; Takii, Y.; Saito, Y.; Sato, T.; Horimatsu, T.; Ishikawa, H.; Ito, Y.; et al. Short-term safety of adjuvant chemoradiotherapy after local resection for patients with high-risk submucosal invasive rectal cancer: A single-arm, multicenter phase II trial. Jpn. J. Clin. Oncol. 2021, 51, 707–712. [Google Scholar] [CrossRef]
- Hiraki, M.; Tanaka, T.; Hiraki, Y.; Watanabe, T.; Sato, H.; Aibe, H.; Kitahara, K. Short-term outcomes of preoperative chemoradiotherapy with S-1 for locally advanced rectal cancer. Mol. Clin. Oncol. 2021, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Inomata, M.; Akagi, T.; Nakajima, K.; Etoh, T.; Tahara, K.; Matsumoto, T.; Ogawa, T.; Fujii, K.; Shiromizu, A.; Kitano, S. A prospective feasibility study to evaluate neoadjuvant-synchronous S-1 with radiotherapy for locally advanced rectal cancer: A multicentre phase II trial. Mol. Clin. Oncol. 2016, 4, 510–514. [Google Scholar] [CrossRef]
- Biviano, I.; Balla, A.; Badiali, D.; Quaresima, S.; D’Ambrosio, G.; Lezoche, E.; Corazziari, E.; Paganini, A.M. Anal function after endoluminal locoregional resection by transanal endoscopic microsurgery and radiotherapy for rectal cancer. Color. Dis. 2017, 19, O177–O185. [Google Scholar] [CrossRef] [PubMed]
- Allaix, M.E.; Rebecchi, F.; Giaccone, C.; Mistrangelo, M.; Morino, M. Long-term functional results and quality of life after transanal endoscopic microsurgery. Br. J. Surg. 2011, 98, 1635–1643. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Saito, N.; Sugito, M.; Kobayashi, A.; Nishizawa, Y.; Tsunoda, Y. Analysis of clinical factors associated with anal function after intersphincteric resection for very low rectal cancer. Dis. Colon Rectum 2009, 52, 64–70. [Google Scholar] [CrossRef]
- Kakodkar, R.; Gupta, S.; Nundy, S. Low anterior resection with total mesorectal excision for rectal cancer: Functional assessment and factors affecting outcome. Color. Dis. 2006, 8, 650–656. [Google Scholar] [CrossRef]
- Hermanek, P. Polypectomy in the colorectum: Histological and oncological aspects. Endoscopy 1983, 15, 158–161. [Google Scholar] [CrossRef]
- Kikuchi, R.; Takano, M.; Takagi, K.; Fujimoto, N.; Nozaki, R.; Fujiyoshi, T.; Uchidaet, Y. Management of early invasive colorectal cancer. Risk of recurrence and clinical guidelines. Dis. Colon Rectum 1995, 38, 1286–1295. [Google Scholar] [CrossRef]
- Kadota, T.; Ikematsu, H.; Sasaki, T.; Saito, Y.; Ito, M.; Mizutani, T.; Ogawa, G.; Shitara, K.; Ito, Y.; Kushima, R.; et al. Protocol for a single-arm confirmatory trial of adjuvant chemoradiation for patients with high-risk rectal submucosal invasive cancer after local resection: Japan Clinical Oncology Group Study JCOG1612 (RESCUE study). BMJ Open 2020, 10, e034947. [Google Scholar] [CrossRef]
- Borstlap, W.A.A.; Tanis, P.J.; Koedam, T.W.A.; Marijnen, C.A.; Cunningham, C.; Dekker, E.; van Leerdam, M.E.; Meijer, G.; van Grieken, N.; Nagtegaal, I.D.; et al. A multi-centred randomised trial of radical surgery versus adjuvant chemoradiotherapy after local excision for early rectal cancer. BMC Cancer 2016, 16, 513. [Google Scholar] [CrossRef]

| SM, N = 36 (%) | MP, N = 16 (%) | p Value | |
|---|---|---|---|
| Age, median (IQR) [<65/≥65] | 64 (54–68) 21 (58.3%)/15 (41.7%) | 70.5 (65–75) 4 (25.0%)/12 (75.0%) | 0.004 * 0.037 ** |
| Sex [Male / Female] | 20 (55.6%)/16 (44.4%) | 7 (43.7%)/8 (56.3%) | 0.551 |
| ECOG-PS [0/1] | 33 (100%)/0 (0%) | 14 (87.5%)/2 (12.5%) | 0.102 |
| Location [Rb/P] | 35 (97.1%)/1 (2.9%) | 15 (93.7%)/1 (6.3%) | 1.000 |
| Histological type [tub1/tub2] | 18 (51.4%)/17 (48.6%) | 6 (40.0%)/9 (60.0%) | 0.545 |
| Greatest diameter (median/IQR) [<22/≥22] | 20 (18–25) 17 (50.0%)/17 (50.0%) | 23.5 (20–27) 6 (37.5%)/10 (62.5%) | 0.321 * 0.546 ** |
| Lymphatic invasion [+/−] | 11 (30.6%)/25 (69.4%) | 8 (50.0%)/8 (50.0%) | 0.220 |
| Venous invasion [+/−] | 13 (36.1%)/23 (63.9%) | 11 (68.8%)/5 (31.2%) | 0.038 |
| Budding grade [1/2–3] | 23 (79.3%)/6 (20.7%) | 1 (25.0%)/3 (75.0%) | 0.052 |
| Preoperative CEA, median (IQR) (ng/mL) [<2.0/≥2.0] | 1.95 (1.4–2.78) 18 (50.0%)/18 (50.0%) | 2.1 (1.55–3.23) 6 (37.5%)/10 (62.5%) | 0.579 0.549 ** |
| Preoperative CA19-9, median (IQR) (ng/mL) [<6.5/≥6.5] | 6.5 (3.78–12.75) 18 (50.0%)/18 (50.0%) | 6.5 (3.4–9.75) 9 (56.3%)/7 (43.7%) | 0.960 * 1.000 ** |
| Operation approach (Transanal excision/EMR or ESD) | 21 (58.3%)/15 (41.7%) | 16 (100%)/0 (0%) | 0.002 |
| Operation time median (IQR) [<68/≥68] | 82 (45.3–99.5) 13 (43.3%)/17 (56.7%) | 59.5 (35.3–74.5) 10 (62.5%)/6 (37.5%) | 0.134 * 0.353 ** |
| SM, N = 36 (%) | MP, N = 16 (%) | p Value | |
|---|---|---|---|
| Adverse events (Grade 3–4) [+/−] | 0 (0%)/36 (100%) | 1 (6.2%)/15 (93.8%) | 0.3077 |
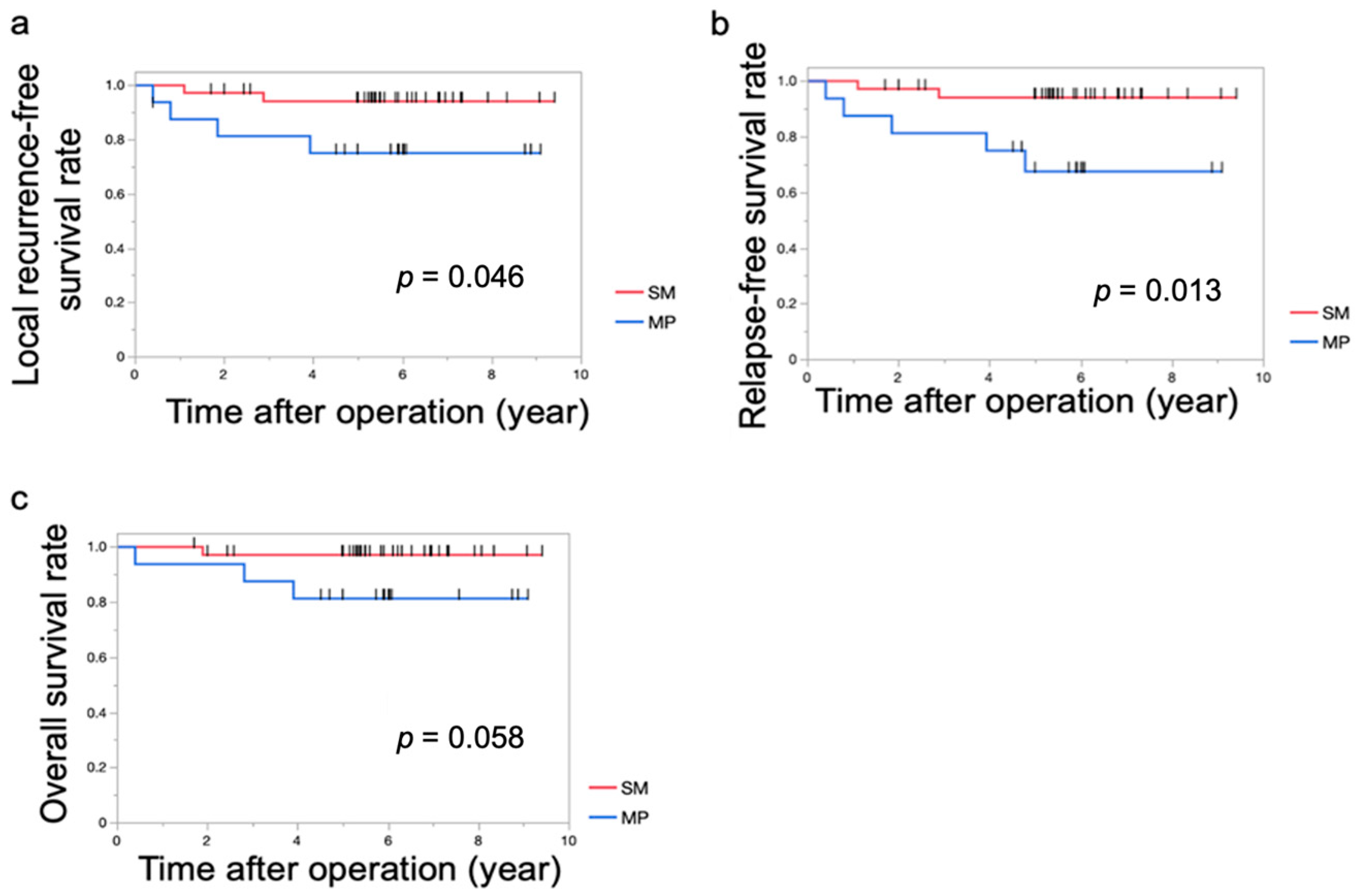
| Locoregional recurrence [+/−] | 2 (6.1%)/31 (93.9%) | 4 (26.7%)/11 (73.3%) | 0.0672 |
| Regional lymph node recurrence | 0 (0%) | 1 (6.2%) | NA |
| Case No. | Age | Sex | PS | Medical History | CEA (ng/mL) | CA19-9 (U/mL) | Surgery | Op time (min) | Blood Loss (mL) | Location | Tumor Size (mm) | Histology | Tumor Invasion | Ly | V | BD | Genetic Information |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 62 | F | 0 | None | 2.2 | 7 | TAE | 69 | 0 | Rb | 27 × 15 | tub2 | MP | 0 | 2 | NA | RAS-mt (KRAS G13D)/BRAF-wt/MSS |
| 2 | 70 | F | 0 | Varicose veins | 0.9 | <2 | TAE | 35 | 40 | Rb | 25 × 20 | tub2 | MP | 0 | 2 | NA | NA |
| 3 | 60 | F | 0 | Hypertension, appendicitis | 1.5 | 8 | TAE | 52 | 0 | Rb | 23 × 22 | tub2 | MP | 0 | 3 | 2 | RAS-mt |
| 4 | 49 | M | 0 | Anal fistula | 3.9 | 15 | TAE | 97 | 0 | Rb | 20 × 20 | tub2 | SM | 1 | 3 | 1 | RAS-wt/BRAF-wt/MSS |
| 5 | 73 | M | 0 | Asthma, AF | 2.4 | <2 | TAE | 67 | 0 | Rb | 13 × 13 | tub1 | MP | 0 | 1 | NA | MSS |
| 6 | 54 | F | 0 | None | 1.1 | 14 | ESD | 107 | 5 | Rb | 55 × 29 | tub2 | SM. | 1 | 0 | 1 | RAS-mt |
| Factors | HR | 95% CI | p Value |
|---|---|---|---|
| Age [≥65/<65] | 0.697 | 0.156–3.116 | 0.637 |
| Sex [Male/Female] | 0.706 | 0.317–6.330 | 0.649 |
| Tumor invasion [MP/M.S.] | 6.178 | 1.197–31.890 | 0.030 |
| Lymphatic invasion [+/−] | 4.722 | 0.568–39.240 | 0.151 |
| Venous invasion [+/−] | 2.863 | 0.555–14.761 | 0.209 |
| CEA [≥2.0/<2.0] | 1.152 | 0.258–5.149 | 0.853 |
| CA19-9 [≥6.5/<6.5] | 1.39 | 0.311–6.217 | 0.666 |
| Case No. | TS-1 Dosage (mg) | Dose Reduction or Discontinuation of Treatment | Complication (CD III or IV) | Recurrence Site | RFS (Month) | Survival Outcome | OS (Month) |
|---|---|---|---|---|---|---|---|
| 1 | 80 | Discontinuation of TS-1 due to leukopenia (G2) * | None | Bilateral iliac lymph node | 57 | Survival | 69 |
| 2 | 120 | None | None | Local | 47 | Survival | 57 |
| 3 | 120 | None | None | Local, lung, pelvic lymph node | 9 | Death | 33 |
| 4 | 120 | None | None | Local | 34 | Death | 46 |
| 5 | 120 | None | None | Local | 22 | Death | 45 |
| 6 | 100 | None | None | Local | 13 | Death | 22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyoshi, N.; Uemura, M.; Noura, S.; Yasui, M.; Nishimura, J.; Tei, M.; Matsuda, C.; Morita, S.; Inoue, A.; Tamagawa, H.; et al. Tolerability and Safety Assessment of Adjuvant Chemoradiotherapy with S-1 after Limited Surgery for T1 or T2 Lower Rectal Cancer. Cancers 2024, 16, 3360. https://doi.org/10.3390/cancers16193360
Miyoshi N, Uemura M, Noura S, Yasui M, Nishimura J, Tei M, Matsuda C, Morita S, Inoue A, Tamagawa H, et al. Tolerability and Safety Assessment of Adjuvant Chemoradiotherapy with S-1 after Limited Surgery for T1 or T2 Lower Rectal Cancer. Cancers. 2024; 16(19):3360. https://doi.org/10.3390/cancers16193360
Chicago/Turabian StyleMiyoshi, Norikatsu, Mamoru Uemura, Shingo Noura, Masayoshi Yasui, Junichi Nishimura, Mitsuyoshi Tei, Chu Matsuda, Shunji Morita, Akira Inoue, Hiroki Tamagawa, and et al. 2024. "Tolerability and Safety Assessment of Adjuvant Chemoradiotherapy with S-1 after Limited Surgery for T1 or T2 Lower Rectal Cancer" Cancers 16, no. 19: 3360. https://doi.org/10.3390/cancers16193360
APA StyleMiyoshi, N., Uemura, M., Noura, S., Yasui, M., Nishimura, J., Tei, M., Matsuda, C., Morita, S., Inoue, A., Tamagawa, H., Mokutani, Y., Yoshioka, S., Fujii, M., Kato, S., Sekido, Y., Ogino, T., Yamamoto, H., Murata, K., Doki, Y., & Eguchi, H., on behalf of Multicenter Clinical Study Group of Osaka University, Colorectal Group (CSGO-CG). (2024). Tolerability and Safety Assessment of Adjuvant Chemoradiotherapy with S-1 after Limited Surgery for T1 or T2 Lower Rectal Cancer. Cancers, 16(19), 3360. https://doi.org/10.3390/cancers16193360






