Carbon-Ion Radiotherapy for Prostate Cancer in Patients with a History of Surgery for Benign Prostatic Hyperplasia
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. CIRT
2.3. ADT
2.4. Follow-Up and Endpoint Assessment
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Adverse Events (AEs)
3.2.1. Acute AEs
3.2.2. Late AEs
3.2.3. Cystoscopic Findings in Patients with Hematuria
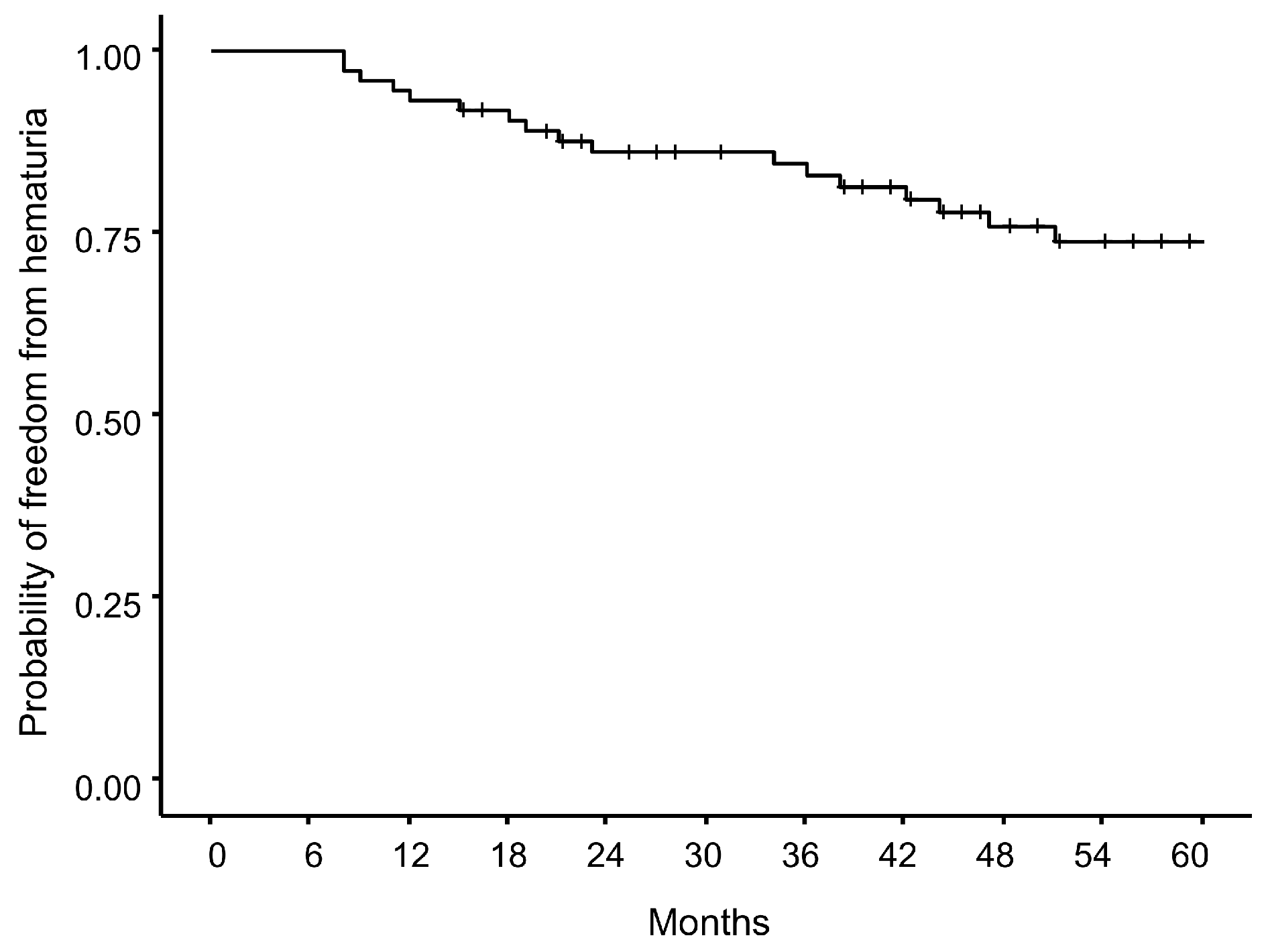
3.3. Hematuria Risk and Time-to-Event Analyses
3.3.1. Risk-Factor Analysis for Hematuria
3.3.2. Time-to-Event Analysis of Hematuria
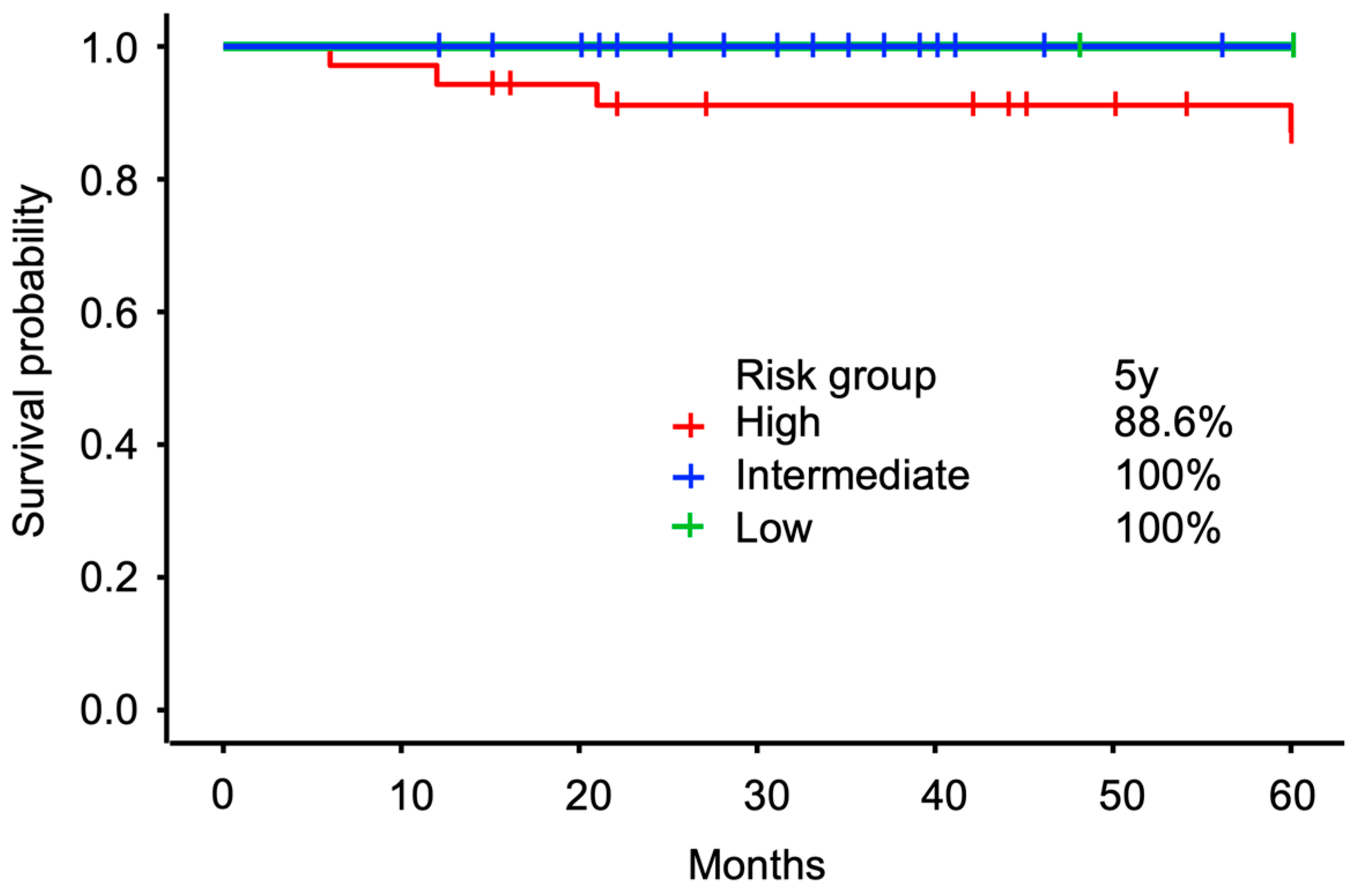
3.4. Cumulative Incidence of Grade ≥ 2 GU AEs
3.5. Oncological Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CIRT | Carbon-ion radiation therapy |
| RBE | Relative biological effectiveness |
| BPH | Benign prostatic hyperplasia |
| TURP | Transurethral resection of the prostate |
| HoLEP | Holmium laser enucleation of the prostate |
| GU | Genitourinary |
| GI | Gastrointestinal |
| PSA | Prostate-specific antigen |
| bRFS | Biochemical recurrence-free survival |
| J-CROS | Japan Carbon-Ion Radiation Oncology Study |
| AE | Adverse events |
| IQR | Interquartile range |
| PVP | Photoselective vaporization of the prostate |
| SPP | Simple suprapubic prostatectomy |
| TUMT | Transurethral microwave therapy |
| NCCN | National Comprehensive Cancer Network |
| CT | Computed tomography |
| PROs | Patient-reported outcomes |
| PVR | Post-void residual urine volume |
| EBRT | External beam radiation therapy |
| SBRT | Stereotactic body radiation therapy |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Dearnaley, D.; Syndikus, I.; Mossop, H.; Khoo, V.; Birtle, A.; Bloomfield, D.; Graham, J.; Kirkbride, P.; Logue, J.; Malik, Z.; et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016, 17, 1047–1060. [Google Scholar] [CrossRef]
- Kamada, T.; Tsujii, H.; Blakely, E.A.; Debus, J.; De Neve, W.; Durante, M.; Jakel, O.; Mayer, R.; Orecchia, R.; Potter, R.; et al. Carbon ion radiotherapy in Japan: An assessment of 20 years of clinical experience. Lancet Oncol. 2015, 16, e93–e100. [Google Scholar] [CrossRef]
- Tsujii, H.; Kamada, T. A review of update clinical results of carbon ion radiotherapy. Jpn. J. Clin. Oncol. 2012, 42, 670–685. [Google Scholar] [CrossRef]
- Ishikawa, H.; Hiroshima, Y.; Kanematsu, N.; Inaniwa, T.; Shirai, T.; Imai, R.; Suzuki, H.; Akakura, K.; Wakatsuki, M.; Ichikawa, T.; et al. Carbon-ion radiotherapy for urological cancers. Int. J. Urol. 2022, 29, 1109–1119. [Google Scholar] [CrossRef]
- Porto, J.G.; Bhatia, A.M.; Bhat, A.; Suarez Arbelaez, M.C.; Blachman-Braun, R.; Shah, K.; Malpani, A.; Lopategui, D.; Herrmann, T.R.W.; Marcovich, R.; et al. Evaluating transurethral resection of the prostate over twenty years: A systematic review and meta-analysis of randomized clinical trials. World J. Urol. 2024, 42, 639. [Google Scholar] [CrossRef] [PubMed]
- Mebust, W.K.; Holtgrewe, H.L.; Cockett, A.T.; Peters, P.C. Transurethral prostatectomy: Immediate and postoperative complications. a cooperative study of 13 participating institutions evaluating 3,885 patients. J. Urol. 1989, 141, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Michalak, J.; Tzou, D.; Funk, J. HoLEP: The gold standard for the surgical management of BPH in the 21(st) Century. Am. J. Clin. Exp. Urol. 2015, 3, 36–42. [Google Scholar]
- Huck, C.; Achard, V.; Zilli, T. Surgical Treatments of Benign Prostatic Hyperplasia and Prostate Cancer Stereotactic Radiotherapy: Impact on Long-Term Genitourinary Toxicity. Clin. Oncol. 2022, 34, e392–e399. [Google Scholar] [CrossRef]
- Carbin, D.D.; Abou Chedid, W.; Hindley, R.; Eden, C. Outcomes of robot-assisted radical prostatectomy in men after trans-urethral resection of the prostate: A matched-pair analysis. J. Robot. Surg. 2024, 18, 158. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Duan, X.; Du, Y.; Mou, X.; Hu, T.; Cai, T.; Liu, J.; Cui, S.; Wu, T. Radical prostatectomy after previous transurethral resection of the prostate: Oncological, surgical and functional outcomes-a meta-analysis. World J. Urol. 2020, 38, 1919–1932. [Google Scholar] [CrossRef] [PubMed]
- Menard, J.; de la Taille, A.; Hoznek, A.; Allory, Y.; Vordos, D.; Yiou, R.; Abbou, C.C.; Salomon, L. Laparoscopic radical prostatectomy after transurethral resection of the prostate: Surgical and functional outcomes. Urology 2008, 72, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Leyh-Bannurah, S.R.; Liakos, N.; Oelke, M.; Wagner, C.; Schuette, A.; Fangmeyer, B.; Zinke, J.; Wasiri, D.; Mendrek, M.; Witt, J.H. Perioperative and Postoperative Outcomes of Robot-Assisted Radical Prostatectomy in Prostate Cancer Patients with Prior Transurethral Subvesical Deobstruction: Results of a High-Volume Center. J. Urol. 2021, 206, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Huck, C.; Achard, V.; Maitre, P.; Murthy, V.; Zilli, T. Stereotactic body radiation therapy for prostate cancer after surgical treatment of prostatic obstruction: Impact on urinary morbidity and mitigation strategies. Clin. Transl. Radiat. Oncol. 2024, 45, 100709. [Google Scholar] [CrossRef]
- Onal, C.; Guler, O.C.; Elmali, A.; Demirhan, B.; Yavuz, M. The impact of age on clinicopathological features and treatment results in patients with localised prostate cancer receiving definitive radiotherapy. Acta Oncol. 2024, 63, 858–866. [Google Scholar] [CrossRef]
- Zelefsky, M.J.; Levin, E.J.; Hunt, M.; Yamada, Y.; Shippy, A.M.; Jackson, A.; Amols, H.I. Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 1124–1129. [Google Scholar] [CrossRef]
- Lee, C.T.; Dong, L.; Ahamad, A.W.; Choi, H.; Cheung, R.; Lee, A.K.; Horne, D.F., Jr.; Breaux, A.J.; Kuban, D.A. Comparison of treatment volumes and techniques in prostate cancer radiation therapy. Am. J. Clin. Oncol. 2005, 28, 618–625. [Google Scholar] [CrossRef]
- Inaniwa, T.; Furukawa, T.; Kase, Y.; Matsufuji, N.; Toshito, T.; Matsumoto, Y.; Furusawa, Y.; Noda, K. Treatment planning for a scanned carbon beam with a modified microdosimetric kinetic model. Phys. Med. Biol. 2010, 55, 6721–6737. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Rassweiler, J.; Teber, D.; Kuntz, R.; Hofmann, R. Complications of transurethral resection of the prostate (TURP)--incidence, management, and prevention. Eur. Urol. 2006, 50, 969–979, discussion 980. [Google Scholar] [CrossRef]
- Salmivalli, A.; Ettala, O.; Nurminen, P.; Kinnala, P.; Bostrom, P.J.; Kyto, V. Short- and long-term risks of photoselective laser vaporization of the prostate: A population-based comparison with transurethral resection of the prostate. Ann. Med. 2023, 55, 1287–1294. [Google Scholar] [CrossRef]
- Li, S.; Zeng, X.T.; Ruan, X.L.; Weng, H.; Liu, T.Z.; Wang, X.; Zhang, C.; Meng, Z.; Wang, X.H. Holmium laser enucleation versus transurethral resection in patients with benign prostate hyperplasia: An updated systematic review with meta-analysis and trial sequential analysis. PLoS ONE 2014, 9, e101615. [Google Scholar] [CrossRef]
- Elmansy, H.M.; Kotb, A.; Elhilali, M.M. Holmium laser enucleation of the prostate: Long-term durability of clinical outcomes and complication rates during 10 years of followup. J. Urol. 2011, 186, 1972–1976. [Google Scholar] [CrossRef]
- Hilscher, M.; Roder, A.; Helgstrand, J.T.; Klemann, N.; Brasso, K.; Vickers, A.J.; Stroomberg, H.V. Risk of prostate cancer and death after benign transurethral resection of the prostate-A 20-year population-based analysis. Cancer 2022, 128, 3674–3680. [Google Scholar] [CrossRef]
- Rosenhammer, B.; Lausenmeyer, E.M.; Mayr, R.; Burger, M.; Eichelberg, C. HoLEP provides a higher prostate cancer detection rate compared to bipolar TURP: A matched-pair analysis. World J. Urol. 2018, 36, 2035–2041. [Google Scholar] [CrossRef]
- Neerhut, T.; Grills, R.; Lynch, R.; Preece, P.D.; McLeod, K. Genitourinary toxicity in patients receiving TURP prior to hypofractionated radiotherapy for clinically localized prostate cancer: A scoping review. Urol. Oncol. 2024, 42, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Odrazka, K.; Dolezel, M.; Vanasek, J.; Vaculikova, M.; Zouhar, M.; Sefrova, J.; Paluska, P.; Vosmik, M.; Kohlova, T.; Kolarova, I.; et al. Late toxicity after conformal and intensity-modulated radiation therapy for prostate cancer: Impact of previous surgery for benign prostatic hyperplasia. Int. J. Urol. 2010, 17, 784–790. [Google Scholar] [CrossRef]
- Nomiya, T.; Tsuji, H.; Kawamura, H.; Ohno, T.; Toyama, S.; Shioyama, Y.; Nakayama, Y.; Nemoto, K.; Tsujii, H.; Kamada, T. A multi-institutional analysis of prospective studies of carbon ion radiotherapy for prostate cancer: A report from the Japan Carbon ion Radiation Oncology Study Group (J-CROS). Radiother. Oncol. 2016, 121, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.H.; Gilling, P.J.; Kennett, K.M.; Frampton, C.; Westenberg, A.M.; Fraundorfer, M.R. A randomized trial comparing holmium laser enucleation of the prostate with transurethral resection of the prostate for the treatment of bladder outlet obstruction secondary to benign prostatic hyperplasia in large glands (40 to 200 grams). J. Urol. 2003, 170, 1270–1274. [Google Scholar] [CrossRef] [PubMed]
- Reich, O.; Gratzke, C.; Stief, C.G. Techniques and long-term results of surgical procedures for BPH. Eur. Urol. 2006, 49, 970–978, discussion 978. [Google Scholar] [CrossRef]
- Utsumi, T.; Suzuki, H.; Wakatsuki, M.; Kobayashi, K.; Okato, A.; Nakajima, M.; Aoki, S.; Sumiya, T.; Ichikawa, T.; Akakura, K.; et al. Development of Novel Nomograms to Predict 5- and 7-Year Biochemical-Recurrence-Free Survival in High-Risk Prostate Cancer Patients After Carbon-Ion Radiotherapy and Androgen Deprivation Therapy. Appl. Sci. 2025, 15, 804. [Google Scholar] [CrossRef]
- Okonogi, N.; Tsuji, H.; Kobayashi, K.; Nakajima, M.; Aoki, S.; Utsumi, T.; Suzuki, H.; Akakura, K.; Ichikawa, T.; Ishikawa, H. A Phase I/II Study of Ultra-Hypofractionated Carbon-ion Radiation therapy for Low- and Intermediate-Risk Localized Prostate Cancer. Adv. Radiat. Oncol. 2025, 10, 101705. [Google Scholar] [CrossRef]
- Cousin, W.; Ho, M.L.; Desai, R.; Tham, A.; Chen, R.Y.; Kung, S.; Elabd, C.; Conboy, I.M. Regenerative capacity of old muscle stem cells declines without significant accumulation of DNA damage. PLoS ONE 2013, 8, e63528. [Google Scholar] [CrossRef]
- Paulino, A.C.; Constine, L.S.; Rubin, P.; Williams, J.P. Normal tissue development, homeostasis, senescence, and the sensitivity to radiation injury across the age spectrum. Semin. Radiat. Oncol. 2010, 20, 12–20. [Google Scholar] [CrossRef]
- Spampinato, S.F.; Caruso, G.I.; De Pasquale, R.; Sortino, M.A.; Merlo, S. The Treatment of Impaired Wound Healing in Diabetes: Looking among Old Drugs. Pharmaceuticals 2020, 13, 60. [Google Scholar] [CrossRef]
- Folkert, M.R.; Sato, R.; Yu, J.B.; Vannan, D.; Bhattacharyya, S.; Noriega, C.; Hamstra, D.A. Bowel Disorder Incidence and Rectal Spacer Use in Patients with Prostate Cancer Undergoing Radiotherapy. JAMA Netw. Open 2025, 8, e250491. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.H.; Ko, I.C.; Leung, D.K.; Yuen, S.K.; Siu, B.; Yuan, C.; Teoh, J.Y. Does biodegradable peri-rectal spacer mitigate treatment toxicities in radiation therapy for localised prostate cancer-a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2025. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Guercio, A.; Lombardo, R.; Turchi, B.; Romagnoli, M.; Franco, A.; D’Annunzio, S.; Fusco, F.; Pastore, A.L.; Al Salhi, Y.; Fuschi, A.; et al. Patient satisfaction and decision regret in patients undergoing radical prostatectomy: A multicenter analysis. Int. Urol. Nephrol. 2025. online ahead of print. [Google Scholar] [CrossRef]
- Choo, R.; Do, V.; Herschorn, S.; DeBoer, G.; Danjoux, C.; Morton, G.; Cheng, C.H.; Barak, I.; Preiner, J. Urodynamic changes at 18 months post-therapy in patients treated with external beam radiotherapy for prostate carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 290–296. [Google Scholar] [CrossRef]
- Miyazawa, Y.; Nakayama, H.; Kawamura, H.; Miyasaka, Y.; Onishi, M.; Kaminuma, T.; Sekine, Y.; Matsui, H.; Ohno, T.; Suzuki, K. Analysis of urinary function and prostate volume changes in localized prostate cancer patients treated with carbon-ion radiotherapy; a prospective study. Radiat. Oncol. 2024, 19, 165. [Google Scholar] [CrossRef]
- Hamdy, F.C.; Donovan, J.L.; Lane, J.A.; Mason, M.; Metcalfe, C.; Holding, P.; Davis, M.; Peters, T.J.; Turner, E.L.; Martin, R.M.; et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N. Engl. J. Med. 2016, 375, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Bill-Axelson, A.; Holmberg, L.; Garmo, H.; Rider, J.R.; Taari, K.; Busch, C.; Nordling, S.; Haggman, M.; Andersson, S.O.; Spangberg, A.; et al. Radical prostatectomy or watchful waiting in early prostate cancer. N. Engl. J. Med. 2014, 370, 932–942. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. SEER Cancer Stat Facts: Prostate Cancer. Available online: https://seer.cancer.gov/statfacts/html/prost.html (accessed on 5 September 2025).
- Schaeffer, E.M.; Srinivas, S.; Adra, N.; An, Y.; Barocas, D.; Bitting, R.; Bryce, A.; Chapin, B.; Cheng, H.H.; D’Amico, A.V.; et al. Prostate Cancer, Version 4.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2023, 21, 1067–1096. [Google Scholar] [CrossRef]
- Slevin, F.; Zattoni, F.; Checcucci, E.; Cumberbatch, M.G.K.; Nacchia, A.; Cornford, P.; Briers, E.; De Meerleer, G.; De Santis, M.; Eberli, D.; et al. A Systematic Review of the Efficacy and Toxicity of Brachytherapy Boost Combined with External Beam Radiotherapy for Nonmetastatic Prostate Cancer. Eur. Urol. Oncol. 2024, 7, 677–696. [Google Scholar] [CrossRef]
- Meng, Y.; Liu, J.; Shen, B.; Xu, H.; Wu, D.; Ying, Y. Evaluation of the safety and efficacy of stereotactic body radiotherapy for radio-recurrent prostate cancer: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2025, 28, 718–726. [Google Scholar] [CrossRef] [PubMed]



| Factors | n (%), Median [IQR] |
|---|---|
| Follow-up period (months) | 60 [48.0–70.75] |
| Age at CIRT (years) | 75.5 [73.0–79.0] |
| Age at BPH surgery (years) | 67 [63.0–72.0] |
| Interval from BPH surgery to CIRT | 8 [3.0–14.0] |
| Previous BPH surgical treatment | |
| TURP | 49 (66.2%) |
| HoLEP | 15 (20.3%) |
| TUEB | 3 (4.1%) |
| PVP | 3 (4.1%) |
| SPP | 3 (4.1%) |
| TUMT | 1 (1.4%) |
| (SPP + TURP) | 1 (1.4%) |
| (TURP + HoLEP) | 2 (2.7%) |
| T stage | |
| T1c | 5 (6.8%) |
| T2a | 31 (41.9%) |
| T2b | 10 (14.0%) |
| T2c | 10 (14.0%) |
| T3a | 13 (17.6%) |
| T3b | 3 (4.1%) |
| Tx | 2 (2.7%) |
| Pretreatment PSA (ng/mL) | 7.7 [5.7–13.1] |
| <10 | 48 (64.9%) |
| ≥10 and ≤20 | 14 (18.9%) |
| >20 | 12 (16.2%) |
| Gleason Score | |
| 6 | 6 (8.1%) |
| 7 | 43 (58.1%) |
| 8 | 10 (13.5%) |
| 9 | 15 (20.3%) |
| Risk group (NCCN) | |
| Low | 3 (4.1%) |
| Intermediate | 36 (48.6%) |
| High | 35 (47.3%) |
| Dose fractionation | |
| 57.6 Gy in 16 fractions | 15 (20.3%) |
| 51.6 Gy in 12 fractions | 49 (66.2%) |
| 54.0 Gy in 12 fractions | 10 (13.5%) |
| Comorbidities | |
| Diabetes mellitus | 9 (12.2%) |
| Anticoagulant therapy | 13 (17.6%) |
| Grade 0–1 | Grade 2 | Grade 3 | |
|---|---|---|---|
| Acute AEs | |||
| GU | 94.6% | 5.4% | 0% |
| GI | 100% | 0% | 0% |
| Late AEs | |||
| GU | 91.8% | 6.8% | 1.4% |
| GI | 98.6% | 1.4% | 0% |
| Grade 2 | Grade 3 | |
|---|---|---|
| Acute GU AEs | ||
| Urinary frequency | 4.1% | 0% |
| Urinary tract pain | 1.4% | 0% |
| Late GU AEs | ||
| Hematuria | 4.1% | 1.4% |
| Urinary frequency | 1.4% | 0% |
| Urethral stricture | 1.4% | 0% |
| Late GI AEs | ||
| Rectal hemorrhage | 1.4% | 0% |
| G1 ≥ Hematuria | G2 ≥ Hematuria | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | |||||
| Variable | OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value |
| Age at CIRT (years) | 0.99 (0.89–1.1) | 0.887 | 1.10 (0.96–1.12) | 0.185 | 1.01 (0.83–1.23) | 0.888 | 1.03 (0.81–1.30) | 0.804 |
| Interval from BPH surgery to CIRT | 0.93 (0.85–1.02) | 0.126 | 0.87 (0.75–0.98) | * 0.041 | 0.99 (0.85–0.92) | 0.918 | 0.95 (0.75–1.16) | 0.651 |
| Diabetes mellitus | 1.67 (0.37–7.48) | 0.505 | 3.47 (0.75–27.2) | 0.219 | 9.0 (1.09–74.2) | * 0.041 | 9.64 (1.46–14.7) | 0.068 |
| Anticoagulant therapy | 1.49 (0.40–5.59) | 0.553 | 2.26 (0.44–11.6) | 0.318 | 5.36 (0.68–42.2) | 0.111 | 4.63 (3.12–90.1) | 0.252 |
| Risk group: intermediate | 1.76 (0.60–5.45) | 0.310 | 2.57 (0.66–11.3) | 0.187 | 0.97 (0.01–8.48) | 0.977 | 1.51 (1.20–2.50) | 0.749 |
| Risk group: low | 0.00 (NA) | 0.991 | 0.00 (NA) | 0.994 | 0.00 (NA) | 0.995 | 0.00 (NA) | 0.997 |
| Surgery: TURP | 2.6 (0.61–18.0) | 0.246 | 10.3 (1.41–127) | * 0.037 | 0.913 (0.11–19.3) | 0.939 | 2.95 (9.30–242) | 0.571 |
| Total dose | 1.15 (0.94–1.42) | 0.184 | 1.20 (0.92–1.57) | 0.185 | 0.992 (0.65–1.52) | 0.972 | 1.03 (0.56–1.65) | 0.917 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okato, A.; Miura, K.; Yamaguchi, T.; Nakajima, M.; Makishima, H.; Utsumi, T.; Akakura, K.; Suzuki, H.; Wakatsuki, M.; Tsuji, H.; et al. Carbon-Ion Radiotherapy for Prostate Cancer in Patients with a History of Surgery for Benign Prostatic Hyperplasia. Cancers 2025, 17, 3039. https://doi.org/10.3390/cancers17183039
Okato A, Miura K, Yamaguchi T, Nakajima M, Makishima H, Utsumi T, Akakura K, Suzuki H, Wakatsuki M, Tsuji H, et al. Carbon-Ion Radiotherapy for Prostate Cancer in Patients with a History of Surgery for Benign Prostatic Hyperplasia. Cancers. 2025; 17(18):3039. https://doi.org/10.3390/cancers17183039
Chicago/Turabian StyleOkato, Atsushi, Kosei Miura, Tomoki Yamaguchi, Mio Nakajima, Hirokazu Makishima, Takanobu Utsumi, Koichiro Akakura, Hiroyoshi Suzuki, Masaru Wakatsuki, Hiroshi Tsuji, and et al. 2025. "Carbon-Ion Radiotherapy for Prostate Cancer in Patients with a History of Surgery for Benign Prostatic Hyperplasia" Cancers 17, no. 18: 3039. https://doi.org/10.3390/cancers17183039
APA StyleOkato, A., Miura, K., Yamaguchi, T., Nakajima, M., Makishima, H., Utsumi, T., Akakura, K., Suzuki, H., Wakatsuki, M., Tsuji, H., Ichikawa, T., & Ishikawa, H. (2025). Carbon-Ion Radiotherapy for Prostate Cancer in Patients with a History of Surgery for Benign Prostatic Hyperplasia. Cancers, 17(18), 3039. https://doi.org/10.3390/cancers17183039








