Lipid Storage and Therapy Resistance in Chronic Myeloid Leukaemia: A Novel Perspective on Targeting Metabolic Vulnerabilities
Simple Summary
Abstract
1. Introduction
2. Reprogramming of Lipid Metabolism in Cancer: Emerging Insights in CML
3. Lipid Droplets as a Potential Therapeutic Target in CML
4. Targeting Lipid Storage in CML as an Adjuvant Therapy
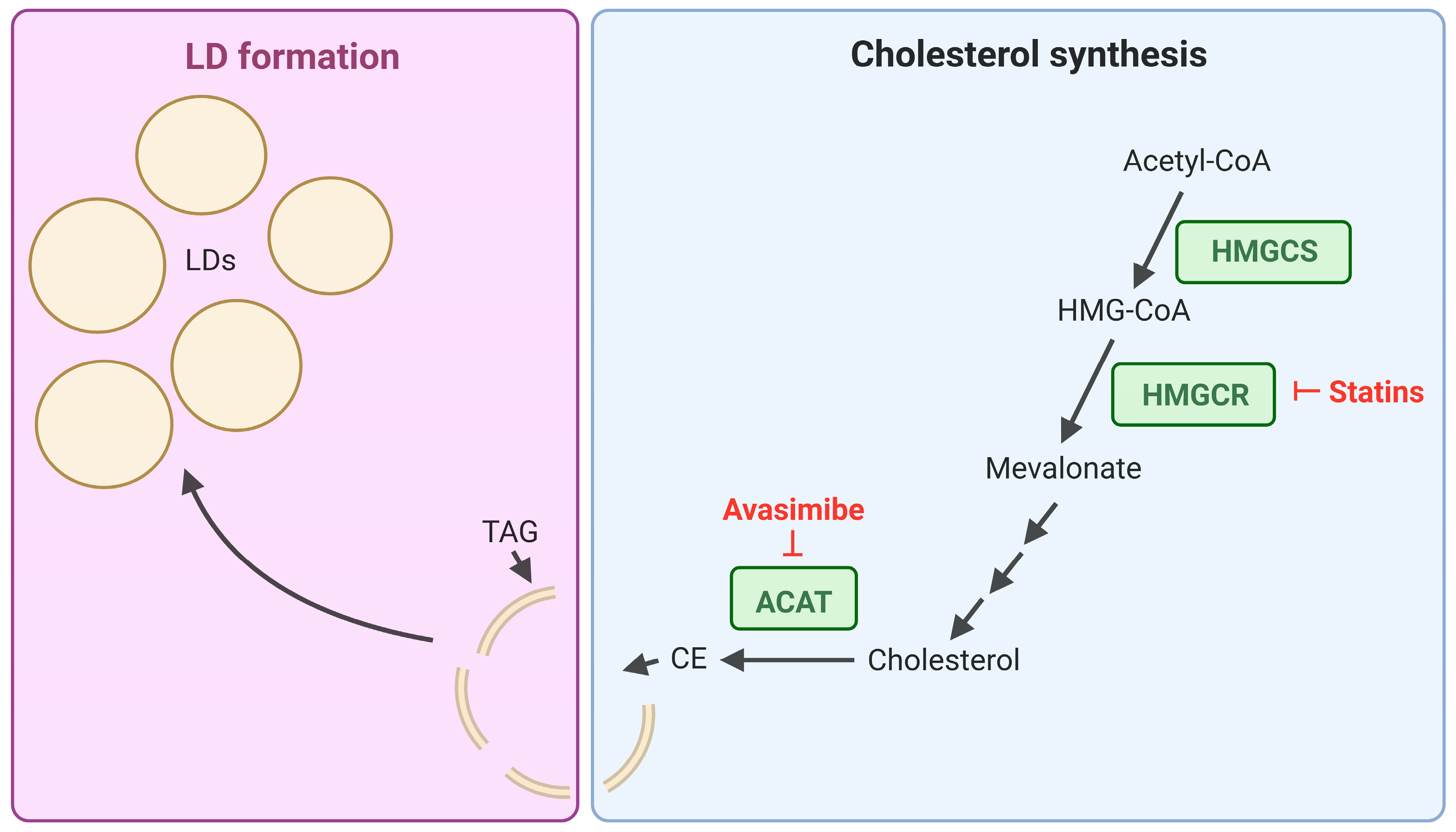
4.1. Inhibition of Lipid Biosynthesis Pathways: Targeting Cholesterol Storage in CML
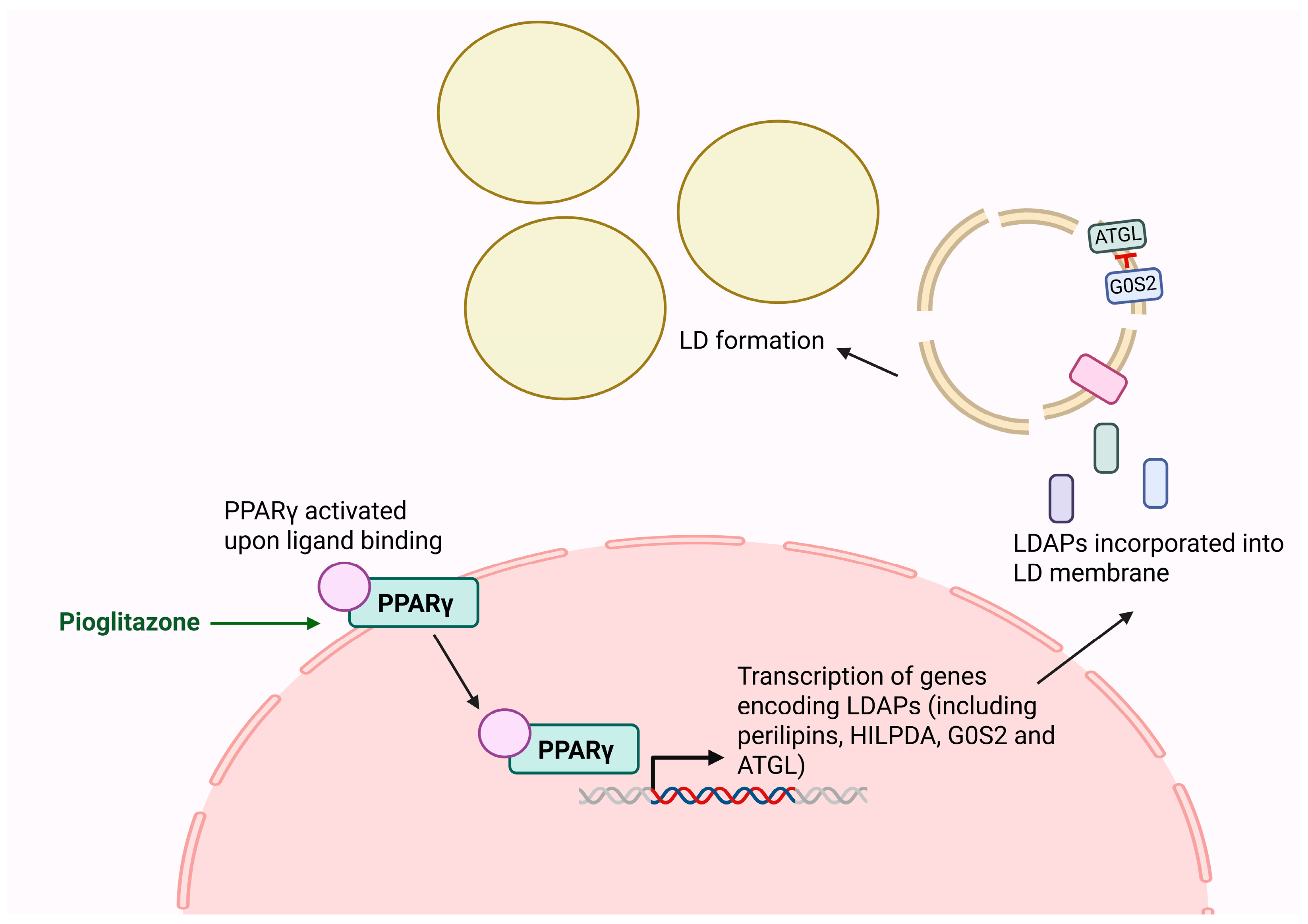
4.2. Targeting LD-Associated Proteins via PPARγ
4.3. Targeting Autophagic Regulation of LDs
5. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Rowley, J.D. A New Consistent Chromosomal Abnormality in Chronic Myelogenous Leukaemia identified by Quinacrine Fluorescence and Giemsa Staining. Nature 1973, 243, 290–293. [Google Scholar] [CrossRef]
- WHO Classification of Tumours, 5th ed.; WHO Press: Geneva, Switzerland, 2024.
- Shtivelman, E.; Lifshitz, B.; Gale, R.P.; Canaani, E. Fused transcript of abl and bcr genes in chronic myelogenous leukaemia. Nature 1985, 315, 550–554. [Google Scholar] [CrossRef]
- Daley, G.Q.; Baltimore, D. Transformation of an interleukin 3-dependent hematopoietic cell line by the chronic myelogenous leukemia-specific P210bcr/abl protein. Proc. Natl. Acad. Sci. USA 1988, 85, 9312–9316. [Google Scholar] [CrossRef] [PubMed]
- Mughal, T.I.; Radich, J.P.; Deininger, M.W.; Apperley, J.F.; Hughes, T.P.; Harrison, C.J.; Gambacorti-Passerini, C.; Saglio, G.; Cortes, J.; Daley, G.Q. Chronic myeloid leukemia: Reminiscences and dreams. Haematologica 2016, 101, 541–558. [Google Scholar] [CrossRef] [PubMed]
- Apperley, J.F.; Milojkovic, D.; Cross, N.C.P.; Hjorth-Hansen, H.; Hochhaus, A.; Kantarjian, H.; Lipton, J.H.; Malhotra, H.; Niederwieser, D.; Radich, J.; et al. 2025 European LeukemiaNet recommendations for the management of chronic myeloid leukemia. Leukemia 2025, 39, 1797–1813. [Google Scholar] [CrossRef] [PubMed]
- Branford, S.; Seymour, J.F.; Grigg, A.; Arthur, C.; Rudzki, Z.; Lynch, K.; Hughes, T. BCR-ABL Messenger RNA Levels Continue to Decline in Patients with Chronic Phase Chronic Myeloid Leukemia Treated with Imatinib for More Than 5 Years and Approximately Half of All First-Line Treated Patients Have Stable Undetectable BCR-ABL Using Strict Sensitivity Criteria. Clin. Cancer Res. 2007, 13, 7080–7085. [Google Scholar] [CrossRef]
- Mahon, F.X.; Réa, D.; Guilhot, J.; Guilhot, F.; Huguet, F.; Nicolini, F.; Legros, L.; Charbonnier, A.; Guerci, A.; Varet, B.; et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: The prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010, 11, 1029–1035. [Google Scholar] [CrossRef]
- Ross, D.M.; Branford, S.; Seymour, J.F.; Schwarer, A.P.; Arthur, C.; Yeung, D.T.; Dang, P.; Goyne, J.M.; Slader, C.; Filshie, R.J.; et al. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: Results from the TWISTER study. Blood 2013, 122, 515–522. [Google Scholar] [CrossRef]
- Hughes, T.P.; Hochhaus, A.; Kantarjian, H.M.; Cervantes, F.; Guilhot, F.; Niederwieser, D.; le Coutre, P.D.; Rosti, G.; Ossenkoppele, G.; Lobo, C.; et al. Safety and efficacy of switching to nilotinib 400 mg twice daily for patients with chronic myeloid leukemia in chronic phase with suboptimal response or failure on front-line imatinib or nilotinib 300 mg twice daily. Haematologica 2014, 99, 1204–1211. [Google Scholar] [CrossRef][Green Version]
- Boddu, P.; Shah, A.R.; Borthakur, G.; Verstovsek, S.; Garcia-Manero, G.; Daver, N.; Kadia, T.; Ravandi, F.; Jain, N.; Alhuraiji, A.; et al. Life after ponatinib failure: Outcomes of chronic and accelerated phase CML patients who discontinued ponatinib in the salvage setting. Leuk. Lymphoma 2018, 59, 1312–1322. [Google Scholar] [CrossRef]
- Jabbour, E.; Kantarjian, H. Chronic myeloid leukemia: 2020 update on diagnosis, therapy and monitoring. Am. J. Hematol. 2020, 95, 691–709. [Google Scholar] [CrossRef] [PubMed]
- Brioli, A.; Lomaia, E.; Fabisch, C.; Sacha, T.; Klamova, H.; Morozova, E.; Golos, A.; Ernst, P.; Olsson-Stromberg, U.; Zackova, D.; et al. Management and outcome of patients with chronic myeloid leukemia in blast phase in the tyrosine kinase inhibitor era—Analysis of the European LeukemiaNet Blast Phase Registry. Leukemia 2024, 38, 1072–1080. [Google Scholar] [CrossRef]
- Corbin, A.S.; Agarwal, A.; Loriaux, M.; Cortes, J.; Deininger, M.W.; Druker, B.J. Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity. J. Clin. Investig. 2011, 121, 396–409. [Google Scholar] [CrossRef]
- Flynn, K.E.; Atallah, E. Quality of Life and Long-Term Therapy in Patients with Chronic Myeloid Leukemia. Curr. Hematol. Malig. Rep. 2016, 11, 80–85. [Google Scholar] [CrossRef]
- Cortes, J.E.; Kim, D.W.; Pinilla-Ibarz, J.; le Coutre, P.; Paquette, R.; Chuah, C.; Nicolini, F.E.; Apperley, J.F.; Khoury, H.J.; Talpaz, M.; et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N. Engl. J. Med. 2013, 369, 1783–1796. [Google Scholar] [CrossRef] [PubMed]
- Schoenbeck, K.L.; Atallah, E.; Lin, L.; Weinfurt, K.P.; Cortes, J.; Deininger, M.W.N.; Kota, V.; Larson, R.A.; Mauro, M.J.; Oehler, V.G.; et al. Patient-Reported Functional Outcomes in Patients With Chronic Myeloid Leukemia After Stopping Tyrosine Kinase Inhibitors. J. Natl. Cancer Inst. 2022, 114, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Poudel, G.; Tolland, M.G.; Hughes, T.P.; Pagani, I.S. Mechanisms of Resistance and Implications for Treatment Strategies in Chronic Myeloid Leukaemia. Cancers 2022, 14, 3300. [Google Scholar] [CrossRef]
- Ng, J.J.; Ong, S.T. Therapy Resistance and Disease Progression in CML: Mechanistic Links and Therapeutic Strategies. Curr. Hematol. Malig. Rep. 2022, 17, 181–197. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Broadfield, L.A.; Pane, A.A.; Talebi, A.; Swinnen, J.V.; Fendt, S.-M. Lipid metabolism in cancer: New perspectives and emerging mechanisms. Dev. Cell 2021, 56, 1363–1393. [Google Scholar] [CrossRef]
- Kuntz, E.M.; Baquero, P.; Michie, A.M.; Dunn, K.; Tardito, S.; Holyoake, T.L.; Helgason, G.V.; Gottlieb, E. Targeting mitochondrial oxidative phosphorylation eradicates therapy-resistant chronic myeloid leukemia stem cells. Nat. Med. 2017, 23, 1234–1240. [Google Scholar] [CrossRef]
- Cruz, A.L.S.; Barreto, E.d.A.; Fazolini, N.P.B.; Viola, J.P.B.; Bozza, P.T. Lipid droplets: Platforms with multiple functions in cancer hallmarks. Cell Death Dis. 2020, 11, 105. [Google Scholar] [CrossRef]
- Petan, T.; Jarc, E.; Jusović, M. Lipid Droplets in Cancer: Guardians of Fat in a Stressful World. Molecules 2018, 23, 1941. [Google Scholar] [CrossRef] [PubMed]
- Björntorp, P.; Sjöström, L. Carbohydrate storage in man: Speculations and some quantitative considerations. Metabolism 1978, 27, 1853–1865. [Google Scholar] [CrossRef]
- Lodhi, I.J.; Wei, X.; Semenkovich, C.F. Lipoexpediency: De novo lipogenesis as a metabolic signal transmitter. Trends Endocrinol. Metab. 2011, 22, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gandemer, G.; Durand, G.; Pascal, G. Relative contribution of the main tissues and organs to body fatty acid synthesis in the rat. Lipids 1983, 18, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Blanco, E.; Bruns, I.; Neumann, F.; Fischer, J.C.; Graef, T.; Rosskopf, M.; Brors, B.; Pechtel, S.; Bork, S.; Koch, A.; et al. Molecular signature of CD34+ hematopoietic stem and progenitor cells of patients with CML in chronic phase. Leukemia 2007, 21, 494–504. [Google Scholar] [CrossRef][Green Version]
- Dowhan, W.; Bogdanov, M. Chapter 1 Functional roles of lipids in membranes. In New Comprehensive Biochemistry; Elsevier: Amsterdam, The Netherlands, 2002; Volume 36, pp. 1–35. [Google Scholar] [CrossRef]
- Singer, S.J.; Nicolson, G.L. The Fluid Mosaic Model of the Structure of Cell Membranes. Science 1972, 175, 720–731. [Google Scholar] [CrossRef]
- Bonora, M.; Patergnani, S.; Rimessi, A.; De Marchi, E.; Suski, J.M.; Bononi, A.; Giorgi, C.; Marchi, S.; Missiroli, S.; Poletti, F.; et al. ATP synthesis and storage. Purinergic Signal. 2012, 8, 343–357. [Google Scholar] [CrossRef]
- Wymann, M.P.; Schneiter, R. Lipid signalling in disease. Nat. Rev. Mol. Cell Biol. 2008, 9, 162–176. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Kinder, M.; Wei, C.; Shelat, S.G.; Kundu, M.; Zhao, L.; Blair, I.A.; Puré, E. Hematopoietic stem cell function requires 12/15-lipoxygenase–dependent fatty acid metabolism. Blood 2010, 115, 5012–5022. [Google Scholar] [CrossRef]
- Liu, X.; Lv, M.; Zhang, W.; Zhan, Q. Dysregulation of cholesterol metabolism in cancer progression. Oncogene 2023, 42, 3289–3302. [Google Scholar] [CrossRef]
- Epstein, J.I.; Carmichael, M.; Partin, A.W. OA-519 (fatty acid synthase) as an independent predictor of pathologic stage in adenocarcinoma of the prostate. Urology 1995, 45, 81–86. [Google Scholar] [CrossRef]
- Liu, T.; Peng, F.; Yu, J.; Tan, Z.; Rao, T.; Chen, Y.; Wang, Y.; Liu, Z.; Zhou, H.; Peng, J. LC-MS-based lipid profile in colorectal cancer patients: TAGs are the main disturbed lipid markers of colorectal cancer progression. Anal. Bioanal. Chem. 2019, 411, 5079–5088. [Google Scholar] [CrossRef] [PubMed]
- Pinkham, K.; Park, D.J.; Hashemiaghdam, A.; Kirov, A.B.; Adam, I.; Rosiak, K.; da Hora, C.C.; Teng, J.; Cheah, P.S.; Carvalho, L.; et al. Stearoyl CoA Desaturase Is Essential for Regulation of Endoplasmic Reticulum Homeostasis and Tumor Growth in Glioblastoma Cancer Stem Cells. Stem Cell Rep. 2019, 12, 712–727. [Google Scholar] [CrossRef]
- Ventura, R.; Mordec, K.; Waszczuk, J.; Wang, Z.; Lai, J.; Fridlib, M.; Buckley, D.; Kemble, G.; Heuer, T.S. Inhibition of de novo Palmitate Synthesis by Fatty Acid Synthase Induces Apoptosis in Tumor Cells by Remodeling Cell Membranes, Inhibiting Signaling Pathways, and Reprogramming Gene Expression. eBioMedicine 2015, 2, 808–824. [Google Scholar] [CrossRef]
- Qiu, S.; Sheth, V.; Yan, C.; Liu, J.; Chacko, B.K.; Li, H.; Crossman, D.K.; Fortmann, S.D.; Aryal, S.; Rennhack, A.; et al. Metabolic adaptation to tyrosine kinase inhibition in leukemia stem cells. Blood 2023, 142, 574–588. [Google Scholar] [CrossRef] [PubMed]
- Kohlmann, A.; Kipps, T.J.; Rassenti, L.Z.; Downing, J.R.; Shurtleff, S.A.; Mills, K.I.; Gilkes, A.F.; Hofmann, W.K.; Basso, G.; Dell’orto, M.C.; et al. An international standardization programme towards the application of gene expression profiling in routine leukaemia diagnostics: The Microarray Innovations in LEukemia study prephase. Br. J. Haematol. 2008, 142, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Radich, J.P.; Dai, H.; Mao, M.; Oehler, V.; Schelter, J.; Druker, B.; Sawyers, C.; Shah, N.; Stock, W.; Willman, C.L.; et al. Gene expression changes associated with progression and response in chronic myeloid leukemia. Proc. Natl. Acad. Sci. USA 2006, 103, 2794–2799. [Google Scholar] [CrossRef]
- Zhong, F.; Zhang, X.; Wang, Z.; Li, X.; Huang, B.; Kong, G.; Wang, X. The therapeutic and biomarker significance of ferroptosis in chronic myeloid leukemia. Front. Immunol. 2024, 15, 1402669. [Google Scholar] [CrossRef]
- Giustacchini, A.; Thongjuea, S.; Barkas, N.; Woll, P.S.; Povinelli, B.J.; Booth, C.A.G.; Sopp, P.; Norfo, R.; Rodriguez-Meira, A.; Ashley, N.; et al. Single-cell transcriptomics uncovers distinct molecular signatures of stem cells in chronic myeloid leukemia. Nat. Med. 2017, 23, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Geng, F.; Pan, M.; Wu, X.; Zhong, Y.; Wang, C.; Tian, Z.; Cheng, C.; Zhang, R.; Puduvalli, V.; et al. Targeting DGAT1 Ameliorates Glioblastoma by Increasing Fat Catabolism and Oxidative Stress. Cell Metab. 2020, 32, 229–242.e8. [Google Scholar] [CrossRef]
- Grachan, J.J.; Kery, M.; Giaccia, A.J.; Denko, N.C.; Papandreou, I. Lipid droplet storage promotes murine pancreatic tumor growth. Oncol. Rep. 2021, 45, 21. [Google Scholar] [CrossRef]
- Olzmann, J.A.; Carvalho, P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 2019, 20, 137–155. [Google Scholar] [CrossRef]
- Ding, Y.; Wu, Y.; Zeng, R.; Liao, K. Proteomic profiling of lipid droplet-associated proteins in primary adipocytes of normal and obese mouse. Acta Biochim. Biophys. Sin. 2012, 44, 394–406. [Google Scholar] [CrossRef]
- Brasaemle, D.L.; Dolios, G.; Shapiro, L.; Wang, R. Proteomic Analysis of Proteins Associated with Lipid Droplets of Basal and Lipolytically Stimulated 3T3-L1 Adipocytes. J. Biol. Chem. 2004, 279, 46835–46842. [Google Scholar] [CrossRef]
- Kimmel, A.R.; Brasaemle, D.L.; McAndrews-Hill, M.; Sztalryd, C.; Londos, C. Adoption of PERILIPIN as a unifying nomenclature for the mammalian PAT-family of intracellular lipid storage droplet proteins. J. Lipid Res. 2010, 51, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, A.S.; Egan, J.J.; Wek, S.A.; Garty, N.B.; Blanchette-Mackie, E.J.; Londos, C. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J. Biol. Chem. 1991, 266, 11341–11346. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lu, X.; Lombès, M.; Rha, G.B.; Chi, Y.I.; Guerin, T.M.; Smart, E.J.; Liu, J. The G0/G1 switch gene 2 regulates adipose lipolysis through association with adipose triglyceride lipase. Cell Metab. 2010, 11, 194–205. [Google Scholar] [CrossRef]
- Smirnova, E.; Goldberg, E.B.; Makarova, K.S.; Lin, L.; Brown, W.J.; Jackson, C.L. ATGL has a key role in lipid droplet/adiposome degradation in mammalian cells. EMBO Rep. 2006, 7, 106–113. [Google Scholar] [CrossRef]
- Guo, Y.; Cordes, K.R.; Farese, R.V., Jr.; Walther, T.C. Lipid droplets at a glance. J. Cell Sci. 2009, 122, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Farese, R.V., Jr.; Walther, T.C. Lipid Droplets Finally Get a Little R-E-S-P-E-C-T. Cell 2009, 139, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Listenberger, L.L.; Brown, D.A. Lipid droplets. Curr. Biol. 2008, 18, R237–R238. [Google Scholar] [CrossRef]
- Wang, C.-W. Lipid droplets, lipophagy, and beyond. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2016, 1861, 793–805. [Google Scholar] [CrossRef]
- Bailey, A.P.; Koster, G.; Guillermier, C.; Hirst, E.M.A.; MacRae, J.I.; Lechene, C.P.; Postle, A.D.; Gould, A.P. Antioxidant Role for Lipid Droplets in a Stem Cell Niche of Drosophila. Cell 2015, 163, 340–353. [Google Scholar] [CrossRef] [PubMed]
- Zechner, R.; Madeo, F.; Kratky, D. Cytosolic lipolysis and lipophagy: Two sides of the same coin. Nat. Rev. Mol. Cell Biol. 2017, 18, 671–684. [Google Scholar] [CrossRef]
- Kimmel, A.R.; Sztalryd, C. The Perilipins: Major Cytosolic Lipid Droplet–Associated Proteins and Their Roles in Cellular Lipid Storage, Mobilization, and Systemic Homeostasis. Annu. Rev. Nutr. 2016, 36, 471–509. [Google Scholar] [CrossRef]
- Singh, R.; Kaushik, S.; Wang, Y.; Xiang, Y.; Novak, I.; Komatsu, M.; Tanaka, K.; Cuervo, A.M.; Czaja, M.J. Autophagy regulates lipid metabolism. Nature 2009, 458, 1131–1135. [Google Scholar] [CrossRef]
- Cui, W.; Sathyanarayan, A.; Lopresti, M.; Aghajan, M.; Chen, C.; Mashek, D.G. Lipophagy-derived fatty acids undergo extracellular efflux via lysosomal exocytosis. Autophagy 2021, 17, 690–705. [Google Scholar] [CrossRef] [PubMed]
- Dupont, N.; Chauhan, S.; Arko-Mensah, J.; Castillo, E.F.; Masedunskas, A.; Weigert, R.; Robenek, H.; Proikas-Cezanne, T.; Deretic, V. Neutral lipid stores and lipase PNPLA5 contribute to autophagosome biogenesis. Curr. Biol. 2014, 24, 609–620. [Google Scholar] [CrossRef]
- Accioly, M.T.; Pacheco, P.; Maya-Monteiro, C.M.; Carrossini, N.; Robbs, B.K.; Oliveira, S.S.; Kaufmann, C.; Morgado-Diaz, J.A.; Bozza, P.T.; Viola, J.P. Lipid bodies are reservoirs of cyclooxygenase-2 and sites of prostaglandin-E2 synthesis in colon cancer cells. Cancer Res. 2008, 68, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Jarc, E.; Kump, A.; Malavašič, P.; Eichmann, T.O.; Zimmermann, R.; Petan, T. Lipid droplets induced by secreted phospholipase A2 and unsaturated fatty acids protect breast cancer cells from nutrient and lipotoxic stress. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2018, 1863, 247–265. [Google Scholar] [CrossRef] [PubMed]
- Geng, F.; Cheng, X.; Wu, X.; Yoo, J.Y.; Cheng, C.; Guo, J.Y.; Mo, X.; Ru, P.; Hurwitz, B.; Kim, S.-H.; et al. Inhibition of SOAT1 Suppresses Glioblastoma Growth via Blocking SREBP-1–Mediated Lipogenesis. Clin. Cancer Res. 2016, 22, 5337–5348. [Google Scholar] [CrossRef]
- Mancini, C.; Menegazzi, G.; Peppicelli, S.; Versienti, G.; Guasti, D.; Pieraccini, G.; Rovida, E.; Lulli, M.; Papucci, L.; Dello Sbarba, P.; et al. BCR::ABL1 expression in chronic myeloid leukemia cells in low oxygen is regulated by glutamine via CD36-mediated fatty acid uptake. Cancer Cell Int. 2025, 25, 176. [Google Scholar] [CrossRef]
- Jang, H.-J.; Woo, Y.-M.; Naka, K.; Park, J.-H.; Han, H.-J.; Kim, H.-J.; Kim, S.-H.; Ahn, J.-S.; Kim, T.; Kimura, S.; et al. Statins Enhance the Molecular Response in Chronic Myeloid Leukemia when Combined with Tyrosine Kinase Inhibitors. Cancers 2021, 13, 5543. [Google Scholar] [CrossRef]
- Ellis, M.; Krashin, E.; Hamburger-Avnery, O.; Gan, S.; Elis, A.; Ashur-Fabian, O. The anti-leukemic and lipid lowering effects of imatinib are not hindered by statins in CML: A retrospective clinical study and in vitro assessment of lipid-genes transcription. Leuk. Lymphoma 2017, 58, 1172–1177. [Google Scholar] [CrossRef]
- Prost, S.; Relouzat, F.; Spentchian, M.; Ouzegdouh, Y.; Saliba, J.; Massonnet, G.; Beressi, J.-P.; Verhoeyen, E.; Raggueneau, V.; Maneglier, B.; et al. Erosion of the chronic myeloid leukaemia stem cell pool by PPARγ agonists. Nature 2015, 525, 380–383. [Google Scholar] [CrossRef]
- Rousselot, P.; Prost, S.; Guilhot, J.; Roy, L.; Etienne, G.; Legros, L.; Charbonnier, A.; Coiteux, V.; Cony-Makhoul, P.; Huguet, F.; et al. Pioglitazone together with imatinib in chronic myeloid leukemia: A proof of concept study. Cancer 2017, 123, 1791–1799. [Google Scholar] [CrossRef]
- Yanamandra, U.; Yadav, N.; Pramanik, S.; Kapoor, R.; Mishra, K.; Khurana, H.; Sharma, S.; Das, S. Supplemental Pioglitazone to Patients of CML with Suboptimal TKI Response: A Pragmatic Pilot Study. Indian J. Hematol. Blood Transfus. 2023, 39, 71–76. [Google Scholar] [CrossRef]
- Pagnano, K.B.B.; Lopes, A.B.P.; Miranda, E.C.; Delamain, M.T.; Duarte, G.O.; Rodrigues, B.R.V.; Povoa, V.M.O.; Furlin, G.C.P.; Vianna, J.C.; da Silva, M.A.S.; et al. Efficacy and safety of pioglitazone in a phase 1/2 imatinib discontinuation trial (EDI-PIO) in chronic myeloid leukemia with deep molecular response. Am. J. Hematol. 2020, 95, E321–E323. [Google Scholar] [CrossRef]
- Horne, G.A.; Stobo, J.; Kelly, C.; Mukhopadhyay, A.; Latif, A.L.; Dixon-Hughes, J.; McMahon, L.; Cony-Makhoul, P.; Byrne, J.; Smith, G.; et al. A randomised phase II trial of hydroxychloroquine and imatinib versus imatinib alone for patients with chronic myeloid leukaemia in major cytogenetic response with residual disease. Leukemia 2020, 34, 1775–1786. [Google Scholar] [CrossRef] [PubMed]
- Maxfield, F.R.; Tabas, I. Role of cholesterol and lipid organization in disease. Nature 2005, 438, 612–621. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Li, J.; Traer, E.; Tyner, J.W.; Zhou, A.; Oh, S.T.; Cheng, J.X. Cholesterol esterification inhibition and imatinib treatment synergistically inhibit growth of BCR-ABL mutation-independent resistant chronic myelogenous leukemia. PLoS ONE 2017, 12, e0179558. [Google Scholar] [CrossRef] [PubMed]
- Tardif, J.-C.; Grégoire, J.; L’Allier, P.L.; Anderson, T.J.; Bertrand, O.; Reeves, F.; Title, L.M.; Alfonso, F.; Schampaert, E.; Hassan, A.; et al. Effects of the Acyl Coenzyme A:Cholesterol Acyltransferase Inhibitor Avasimibe on Human Atherosclerotic Lesions. Circulation 2004, 110, 3372–3377. [Google Scholar] [CrossRef] [PubMed]
- Delphine, R.; Tristan, M.; Thomas, C.; Jean-François, G.; François, G.; Hervé, D.; Emmanuel, M. Early onset hypercholesterolemia induced by the 2nd-generation tyrosine kinase inhibitor nilotinib in patients with chronic phase-chronic myeloid leukemia. Haematologica 2014, 99, 1197–1203. [Google Scholar] [CrossRef]
- Asari, K.; Sun, W.T.; Kok, Z.H.; Lam, Y.H.; Ng, B.L.; Saunders, V.; White, D.L.; Chuah, C.; Xiang, W. Simvastatin enhances the efficacy of nilotinib in chronic myeloid leukaemia by post-translational modification and drug transporter modulation. Anti-Cancer Drugs 2021, 32, 526–536. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, T.; Dou, Y.; Zhang, S.; Liu, H.; Khishignyam, T.; Li, X.; Zuo, D.; Zhang, Z.; Jin, M.; et al. Atorvastatin Exerts Antileukemia Activity via Inhibiting Mevalonate-YAP Axis in K562 and HL60 Cells. Front. Oncol. 2019, 9, 1032. [Google Scholar] [CrossRef]
- Yang, Y.-C.; Huang, W.-F.; Chuan, L.-M.; Xiao, D.-W.; Zeng, Y.-L.; Zhou, D.-A.; Xu, G.-Q.; Liu, W.; Huang, B.; Hu, Q. In vitro and in vivo Study of Cell Growth Inhibition of Simvastatin on Chronic Myelogenous Leukemia Cells. Chemotherapy 2008, 54, 438–446. [Google Scholar] [CrossRef]
- Martin, P.D.; Dane, A.L.; Nwose, O.M.; Schneck, D.W.; Warwick, M.J. No Effect of Age or Gender on the Pharmacokinetics of Rosuvastatin: A New HMG-CoA Reductase Inhibitor. J. Clin. Pharmacol. 2002, 42, 1116–1121. [Google Scholar] [CrossRef] [PubMed]
- Horňák, T.; Semerád, L.; Žáčková, D.; Weinbergerová, B.; Šustková, Z.; Procházková, J.; Bělohlávková, P.; Stejskal, L.; Rohoň, P.; Faber, E.; et al. Analysis of serum lipids, cardiovascular risk, and indication for statin use during nilotinib and imatinib therapy in de novo CML patients—Results from real-life prospective study. Leuk. Lymphoma 2020, 61, 494–496. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Spiegelman, B.M. PPARγ: A Nuclear Regulator of Metabolism, Differentiation, and Cell Growth. J. Biol. Chem. 2001, 276, 37731–37734. [Google Scholar] [CrossRef]
- de la Rosa Rodriguez, M.A.; Kersten, S. Regulation of lipid droplet-associated proteins by peroxisome proliferator-activated receptors. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2017, 1862, 1212–1220. [Google Scholar] [CrossRef]
- Zandbergen, F.; Mandard, S.; Escher, P.; Tan, N.S.; Patsouris, D.; Jatkoe, T.; Rojas-Caro, S.; Madore, S.; Wahli, W.; Tafuri, S.; et al. The G0/G1 switch gene 2 is a novel PPAR target gene. Biochem. J. 2005, 392, 313–324. [Google Scholar] [CrossRef]
- Fajas, L.; Auboeuf, D.; Raspé, E.; Schoonjans, K.; Lefebvre, A.-M.; Saladin, R.; Najib, J.; Laville, M.; Fruchart, J.-C.; Deeb, S.; et al. The Organization, Promoter Analysis, and Expression of the Human PPARγ Gene. J. Biol. Chem. 1997, 272, 18779–18789. [Google Scholar] [CrossRef] [PubMed]
- Tontonoz, P.; Hu, E.; Graves, R.; Budavari, A.; Spiegelman, B. mPPAR gamma 2: Tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994, 8, 1224–1234. [Google Scholar] [CrossRef]
- Prost, S.; Le Dantec, M.; Augé, S.; Le Grand, R.; Derdouch, S.; Auregan, G.; Déglon, N.; Relouzat, F.; Aubertin, A.-M.; Maillere, B.; et al. Human and simian immunodeficiency viruses deregulate early hematopoiesis through a Nef/PPARγ/STAT5 signaling pathway in macaques. J. Clin. Investig. 2008, 118, 1765–1775. [Google Scholar] [CrossRef][Green Version]
- Schepers, H.; van Gosliga, D.; Wierenga, A.T.J.; Eggen, B.J.L.; Schuringa, J.J.; Vellenga, E. STAT5 is required for long-term maintenance of normal and leukemic human stem/progenitor cells. Blood 2007, 110, 2880–2888. [Google Scholar] [CrossRef]
- Gonzalez, M.A.; Olivas, I.M.; Bencomo-Alvarez, A.E.; Rubio, A.J.; Barreto-Vargas, C.; Lopez, J.L.; Dang, S.K.; Solecki, J.P.; McCall, E.; Astudillo, G.; et al. Loss of G0/G1 switch gene 2 (G0S2) promotes disease progression and drug resistance in chronic myeloid leukaemia (CML) by disrupting glycerophospholipid metabolism. Clin. Transl. Med. 2022, 12, e1146. [Google Scholar] [CrossRef]
- Waugh, J.; Keating, G.M.; Plosker, G.L.; Easthope, S.; Robinson, D.M. Pioglitazone. Drugs 2006, 66, 85–109. [Google Scholar] [CrossRef] [PubMed]
- Apsel Winger, B.; Shah Neil, P. PPARγ: Welcoming the New Kid on the CML Stem Cell Block. Cancer Cell 2015, 28, 409–411. [Google Scholar] [CrossRef]
- Hochhaus, A.; Saglio, G.; Hughes, T.P.; Larson, R.A.; Kim, D.W.; Issaragrisil, S.; le Coutre, P.D.; Etienne, G.; Dorlhiac-Llacer, P.E.; Clark, R.E.; et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia 2016, 30, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, L.; Kok, C.H.; Saunders, V.A.; Goyne, J.M.; Dang, P.; Leclercq, T.M.; Hughes, T.P.; White, D.L. Increased peroxisome proliferator-activated receptor γ activity reduces imatinib uptake and efficacy in chronic myeloid leukemia mononuclear cells. Haematologica 2017, 102, 843–853. [Google Scholar] [CrossRef]
- Póvoa, V.M.O.; Delafiori, J.; Dias-Audibert, F.L.; de Oliveira, A.N.; Lopes, A.B.P.; de Paula, E.V.; Pagnano, K.B.B.; Catharino, R.R. Metabolic shift of chronic myeloid leukemia patients under imatinib–pioglitazone regimen and discontinuation. Med. Oncol. 2021, 38, 100. [Google Scholar] [CrossRef]
- Levy, J.M.M.; Towers, C.G.; Thorburn, A. Targeting autophagy in cancer. Nat. Rev. Cancer 2017, 17, 528–542. [Google Scholar] [CrossRef]
- Li, J.; Kim, S.G.; Blenis, J. Rapamycin: One Drug, Many Effects. Cell Metab. 2014, 19, 373–379. [Google Scholar] [CrossRef]
- Wu, X.; Geng, F.; Cheng, X.; Guo, Q.; Zhong, Y.; Cloughesy, T.F.; Yong, W.H.; Chakravarti, A.; Guo, D. Lipid Droplets Maintain Energy Homeostasis and Glioblastoma Growth via Autophagic Release of Stored Fatty Acids. iScience 2020, 23, 101569. [Google Scholar] [CrossRef]
- Xu, G.; Jiang, Y.; Xiao, Y.; Liu, X.D.; Yue, F.; Li, W.; Li, X.; He, Y.; Jiang, X.; Huang, H.; et al. Fast clearance of lipid droplets through MAP1S-activated autophagy suppresses clear cell renal cell carcinomas and promotes patient survival. Oncotarget 2016, 7, 6255–6265. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Czaja, M.J. Regulation of lipid stores and metabolism by lipophagy. Cell Death Differ. 2013, 20, 3–11. [Google Scholar] [CrossRef]
- Wu, M.; Chen, G.; Li, X.; Ma, W.; Chen, Y.; Gong, Y.; Zheng, H.; Gu, G.; Ding, Y.; Dong, P.; et al. Free fatty acids derived from lipophagy enhanced resistance to anoikis by activating Src in high-invasive clear cell renal cell carcinoma cells. Cell. Signal. 2025, 127, 111622. [Google Scholar] [CrossRef] [PubMed]
- Bellodi, C.; Lidonnici, M.R.; Hamilton, A.; Helgason, G.V.; Soliera, A.R.; Ronchetti, M.; Galavotti, S.; Young, K.W.; Selmi, T.; Yacobi, R.; et al. Targeting autophagy potentiates tyrosine kinase inhibitor–induced cell death in Philadelphia chromosome–positive cells, including primary CML stem cells. J. Clin. Investig. 2009, 119, 1109–1123. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.M.; Hughes, T.P. Treatment-free remission in patients with chronic myeloid leukaemia. Nat. Rev. Clin. Oncol. 2020, 17, 493–503. [Google Scholar] [CrossRef] [PubMed]
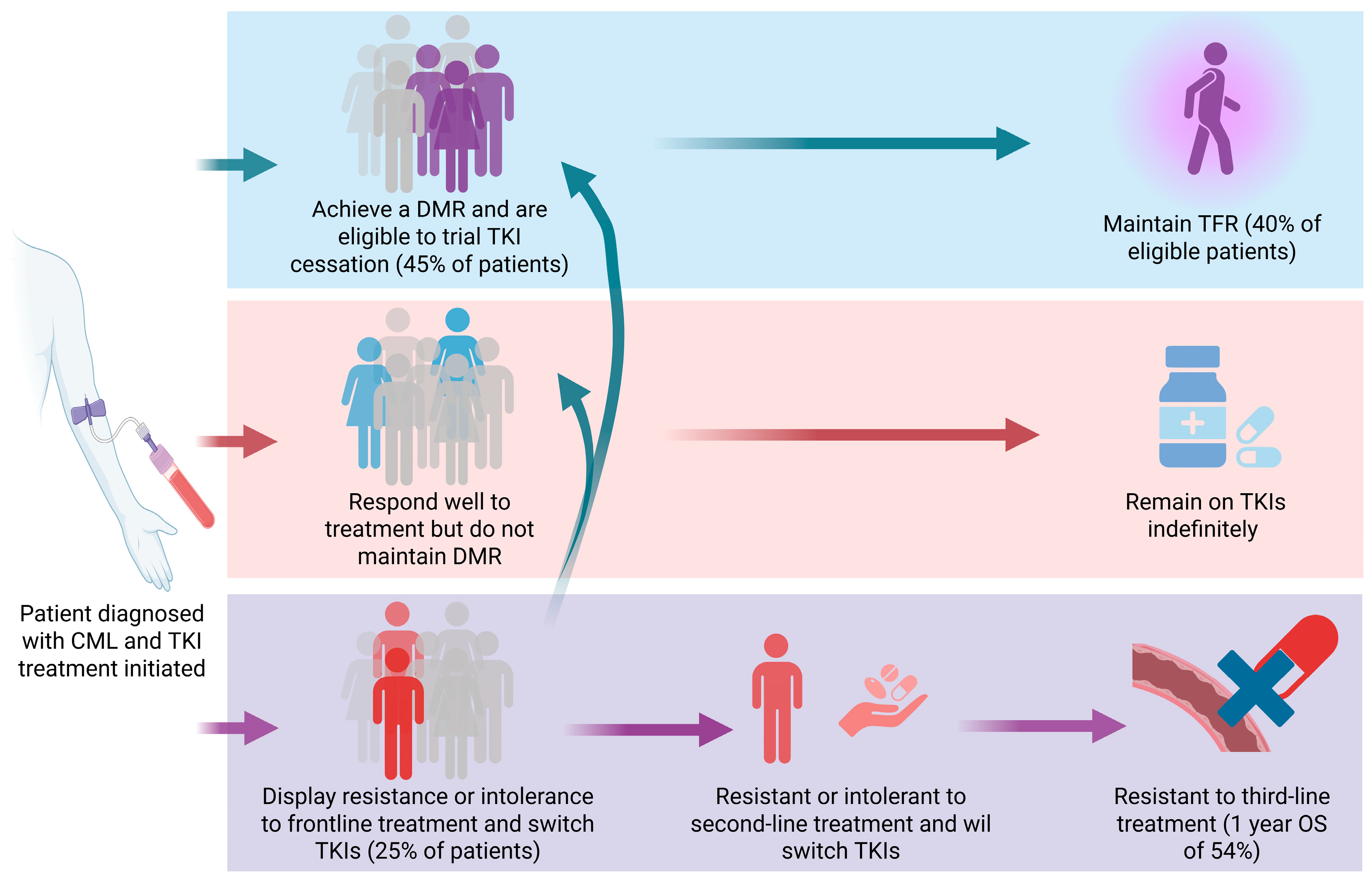


| Drug Class | Drugs | Associated LD Pathway | No. of Patients | Patient Characteristics | Trial/Study Type | Outcome | Reference |
|---|---|---|---|---|---|---|---|
| HMG-CoA Reductase inhibitor | Atorvastatin, simvastatin, pravastatin, fluvastatin | Mevalonate pathway; Cholesterol synthesis | 408; n = 88 imatinib + statin, n = 320 imatinib only | Chronic phase; median age 52 y; statin group undergoing statin therapy prior to initiation of imatinib and continued for at least 3 years alongside imatinib; 84 pairs selected for propensity score matching. | Retrospective study | Non-significant increase in MMR in the imatinib + statin group at 3 years (77.3% vs. 62.5%) compared to imatinib only group; Significant increase in DMR in the imatinib + statin at 5 years (55.8% vs. 41%) compared to imatinib only group. | [69] |
| HMG-CoA Reductase inhibitor; PPARα agonist | Atorvastatin, simvastatin, pravastatin, rosuvastatin; fenofibrate | Cholesterol synthesis: lipolysis | 40; n = 19 imatinib + statin, n = 21 imatinib only | Chronic phase; median age 66 y; statin group undergoing statin therapy prior to and throughout imatinib treatment; comparable baseline characteristics of patients in each group. | Retrospective study | No difference in time to BCR::ABL1 reduction to BCR::ABL1 < 1%, <0.1%, <0.01% and undetectable; no difference in time taken to WBC normalisation. | [70] |
| PPARγ agonist | Pioglitazone | Transcription of LDAPs | 3; n = 3 imatinib + pioglitazone | CMR not achieved after 4–6 years on imatinib; age range of 62–67 y; n = 2 patients with Type II diabetes. | Case study | CMR was achieved in each patient within 12 months following pioglitazone introduction. | [71] |
| PPARγ agonist | Pioglitazone | Transcription of LDAPs | 24; n = 24 imatinib + pioglitazone | Chronic phase; median age 61 y; MR4.5 not achieved after median 73 months on imatinib. | Clinical trial: 2009-011675-79 | Imatinib + pioglitazone increased cumulative incidence of MR4.5 to 56% over 12 months (compared to 23% in historical cohort treated with imatinib only). | [72] |
| PPARγ agonist | Pioglitazone | Transcription of LDAPs | 31; n = 31 TKI (imatinib, nilotinib, dasatinib) + pioglitazone | Failure to achieve MMR after 12–15 months on TKI but BCR::ABL1 mutation negative; median age 54 y. | Clinical trial | TKI + pioglitazone led to a significant reduction in BCR::ABL1 expression (1-log in 87% of patients for a median duration of 602 days); At time of censoring data 48.3% achieved MMR and 19.3% achieved DMR; during follow up, disease progressed in 38% of patients. | [73] |
| PPARγ agonist | Pioglitazone | Transcription of LDAPs | 32; n = 32 imatinib + pioglitazone (1 patient lost to centre transfer) | Chronic phase; median age 54 y, maintained MR4.5 for ≥3 y but have not achieved TFR. | Clinical trial: NCT02852486 | TFR incidence at 19 months was 60%, comparable to TFR incidence after imatinib alone. | [74] |
| Lysomotropic agent | Hydroxychloroquine (HCQ) | Autophagy | 62 (12 lost to follow up/consent withdrawal, physician intervention; n = 25 imatinib + HCQ, n = 25 imatinib only | Chronic phase; median age imatinib + HCQ group 50 y and imatinib only group 49.5 y; patients achieved MMR but still BCR::ABL1+ after 12 months on imatinib. | Clinical trial: NCT01227135 | No difference in percentage of patients achieving >0.5-log reduction in BCR::ABL1 at 12 months; 20.8% higher rate of achieving 0.5-log reduction in BCR::ABL1 in the imatinib + HCQ group compared to imatinib only group (did not reach statistical significance). | [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tolland, M.; Ross, D.M.; White, D.; Hughes, T.P.; Pagani, I.S. Lipid Storage and Therapy Resistance in Chronic Myeloid Leukaemia: A Novel Perspective on Targeting Metabolic Vulnerabilities. Cancers 2025, 17, 3033. https://doi.org/10.3390/cancers17183033
Tolland M, Ross DM, White D, Hughes TP, Pagani IS. Lipid Storage and Therapy Resistance in Chronic Myeloid Leukaemia: A Novel Perspective on Targeting Metabolic Vulnerabilities. Cancers. 2025; 17(18):3033. https://doi.org/10.3390/cancers17183033
Chicago/Turabian StyleTolland, Molly, David M. Ross, Deborah White, Timothy P. Hughes, and Ilaria S. Pagani. 2025. "Lipid Storage and Therapy Resistance in Chronic Myeloid Leukaemia: A Novel Perspective on Targeting Metabolic Vulnerabilities" Cancers 17, no. 18: 3033. https://doi.org/10.3390/cancers17183033
APA StyleTolland, M., Ross, D. M., White, D., Hughes, T. P., & Pagani, I. S. (2025). Lipid Storage and Therapy Resistance in Chronic Myeloid Leukaemia: A Novel Perspective on Targeting Metabolic Vulnerabilities. Cancers, 17(18), 3033. https://doi.org/10.3390/cancers17183033







