Presence of Adipophilin-Positive Cancer-Associated Fibroblasts Is an Independent Poor Prognostic Indicator and Is Correlated with Immature-Type Desmoplastic Reaction in Patients with Colorectal Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Histopathological Analysis
2.3. Immunohistochemical Analysis
2.4. Statistical Analyses
3. Results
3.1. Clinicopathological Characteristics of the Study Cohort
3.2. Histopathological Characteristics
3.3. Immunohistochemical Characteristics
3.4. Correlation Between Lipid-Laden CAFs and Clinicopathological Features
3.5. Prognostic Significance of the Type of DR and the Presence of Lipid-Laden CAFs
3.6. Cox Proportional Hazards Regression Analysis for OS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADP | Adipophilin |
| alpha-SMA | alpha-smooth muscle actin |
| CAF | Cancer-associated fibroblast |
| CRC | Colorectal cancer |
| DR | Desmoplastic reaction |
| FASN | Fatty acid synthase |
| IM type DR | Immature-type desmoplastic reaction |
| OS | Overall survival |
| TB | Tumor budding |
| TME | Tumor microenvironment |
References
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Kobayashi, T.; Ishida, M.; Miki, H.; Yamamoto, N.; Harino, T.; Yagyu, T.; Hori, S.; Hatta, M.; Hashimoto, Y.; Kotsuka, M.; et al. Prognostic scoring system based on indicators reflecting the tumor glandular differentiation and microenvironment for patients with colorectal cancer. Sci. Rep. 2024, 14, 14188. [Google Scholar] [CrossRef]
- Weiser, M.R.; Hsu, M.; Bauer, P.S.; Chapman, W.C., Jr.; González, I.A.; Chatterjee, D.; Lingam, D.; Mutch, M.G.; Keshinro, A.; Shia, J.; et al. Clinical calculator based on molecular and clinicopathologic characteristics predicts recurrence following resection of stage I-III colon cancer. J. Clin. Oncol. 2021, 39, 911–919. [Google Scholar] [CrossRef]
- Knijn, N.; van Exsel, U.E.M.; de Noo, M.E.; Nagtegaal, I.D. The value of intramural vascular invasion in colorectal cancer—A systematic review and meta-analysis. Histopathology 2018, 72, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Knijn, N.; Mogk, S.C.; Teerenstra, S.; Simmer, F.; Nagtegaal, I.D. Perineural invasion is a strong prognostic factor in colorectal cancer: A systematic review. Am. J. Surg. Pathol. 2016, 40, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Lugli, A.; Zlobec, I.; Berger, M.D.; Kirsch, R.; Nagtegaal, I.D. Tumour budding in solid cancers. Nat. Rev. Clin. Oncol. 2021, 18, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Kajiwara, Y.; Ajioka, Y.; Sugai, T.; Sekine, S.; Ishiguro, M.; Takashima, A.; Kanemitsu, Y. Histopathological atlas of desmoplastic reaction characterization in colorectal cancer. Jpn. J. Clin. Oncol. 2021, 51, 1004–1012. [Google Scholar] [CrossRef]
- Ueno, H.; Jones, A.M.; Wilkinson, K.H.; Jass, J.R.; Talbot, I.C. Histological categorisation of fibrotic cancer stroma in advanced rectal cancer. Gut 2004, 53, 581–586. [Google Scholar] [CrossRef]
- Li, Z.W.; He, L.; Zheng, Z.; Zhang, Q.; Xu, Y.T.; Chen, J.Y.; Shi, J.; Huang, W.B.; Fan, X.S. Combined assessment of tumour cell nest size and desmoplastic reaction as an excellent prognostic predictor in oesophageal squamous cell carcinoma. Histopathology 2022, 80, 1112–1120. [Google Scholar] [CrossRef]
- Noda, Y.; Ishida, M.; Ueno, Y.; Fujisawa, T.; Iwai, H.; Tsuta, K. Novel pathological predictive factors for extranodal extension in oral squamous cell carcinoma: A retrospective cohort study based on tumor budding, desmoplastic reaction, tumor-infiltrating lymphocytes, and depth of invasion. BMC Cancer 2022, 22, 402. [Google Scholar] [CrossRef]
- Farc, O.; Cristea, V. An overview of the tumor microenvironment, from cells to complex networks (Review). Exp. The. Med. 2021, 21, 96. [Google Scholar] [CrossRef]
- Ueno, H.; Shinto, E.; Shimazaki, H.; Kajiwara, Y.; Sueyama, T.; Yamamoto, J.; Hase, K. Histologic categorization of desmoplastic reaction: Its relevance to the colorectal cancer microenvironment and prognosis. Ann. Surg. Oncol. 2015, 22, 1504–1512. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Ishiguro, M.; Nakatani, E.; Ishikawa, T.; Uetake, H.; Murotani, K.; Matsui, S.; Teramukai, S.; Sugai, T.; Ajioka, Y.; et al. Prognostic value of desmoplastic reaction characterisation in stage II colon cancer: Prospective validation in a Phase 3 study (SACURA Trial). Br. J. Cancer 2021, 124, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Kanemitsu, Y.; Sekine, S.; Ishiguro, M.; Ito, E.; Hashiguchi, Y.; Kondo, F.; Shimazaki, H.; Mochizuki, S.; Kajiwara, Y.; et al. Desmoplastic pattern at the tumor front defines poor-prognosis subtypes of colorectal cancer. Am. J. Surg. Pathol. 2017, 41, 1506–1512. [Google Scholar] [CrossRef]
- Ueno, H.; Kanemitsu, Y.; Sekine, S.; Ishiguro, M.; Ito, E.; Hashiguchi, Y.; Kondo, F.; Shimazaki, H.; Kajiwara, Y.; Okamoto, K.; et al. A multicenter study of the prognostic value of desmoplastic reaction categorization in stage II colorectal cancer. Am. J. Surg. Pathol. 2019, 43, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Vogel, F.C.E.; Chaves-Filho, A.B.; Schulze, A. Lipids as mediators of cancer progression and metastasis. Nat. Cancer 2024, 5, 16–29. [Google Scholar] [CrossRef]
- Boroughs, L.K.; DeBerardinis, R.J. Metabolic pathways promoting cancer cell survival and growth. Nat. Cell Biol. 2015, 17, 351–359. [Google Scholar] [CrossRef]
- Xia, L.; Oyang, L.; Lin, J.; Tan, S.; Han, Y.; Wu, N.; Yi, P.; Tang, L.; Pan, Q.; Rao, S.; et al. The cancer metabolic reprogramming and immune response. Mol. Cancer 2021, 20, 28. [Google Scholar] [CrossRef]
- Snaebjornsson, M.T.; Janaki-Raman, S.; Schulze, A. Greasing the wheels of the cancer machine: The role of lipid metabolism in cancer. Cell Metab. 2020, 31, 62–76. [Google Scholar] [CrossRef]
- Ganguly, D.; Chandra, R.; Karalis, J.; Teke, M.; Aguilera, T.; Maddipati, R.; Wachsmann, M.B.; Ghersi, D.; Siravegna, G.; Zeh, H.J., 3rd; et al. Cancer-associated fibroblasts: Versatile players in the tumor microenvironment. Cancers 2020, 12, 2652. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, X.Y.; Zhang, P.; He, T.C.; Han, J.H.; Zhang, R.; Lin, J.; Fan, J.; Lu, L.; Zhu, W.W.; et al. Cancer-derived exosomal HSPC111 promotes colorectal cancer liver metastasis by reprogramming lipid metabolism in cancer-associated fibroblasts. Cell Death. Dis. 2022, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, Y.; Li, X.; Liu, Z.; Sun, Y.; Liu, H.; Shao, Z.; Jiang, E.; Zhou, X.; Shang, Z. Enhanced lipid biosynthesis in oral squamous cell carcinoma cancer-associated fibroblasts contributes to tumor progression: Role of IL8/AKT/p-ACLY axis. Cancer Sci. 2024, 115, 1433–1445. [Google Scholar] [CrossRef] [PubMed]
- Niu, N.; Shen, X.; Wang, Z.; Chen, Y.; Weng, Y.; Yu, F.; Tang, Y.; Lu, P.; Liu, M.; Wang, L.; et al. Tumor cell-intrinsic epigenetic dysregulation shapes cancer-associated fibroblasts heterogeneity to metabolically support pancreatic cancer. Cancer Cell 2024, 42, 869–884.e9. [Google Scholar] [CrossRef]
- Gong, J.; Lin, Y.; Zhang, H.; Liu, C.; Cheng, Z.; Yang, X.; Zhang, J.; Xiao, Y.; Sang, N.; Qian, X.; et al. Reprogramming of lipid metabolism in cancer-associated fibroblasts potentiates migration of colorectal cancer cells. Cell Death. Dis. 2020, 11, 267. [Google Scholar] [CrossRef]
- Wang, J.; Xi, M.J.; Lu, Q.; Xia, B.H.; Liu, Y.Z.; Yang, J.L. Single-cell and bulk transcriptomics identifies a tumor-specific CD36+ cancer-associated fibroblast subpopulation in colorectal cancer. Cancer Commun. 2024, 44, 495–498. [Google Scholar] [CrossRef]
- Li, F.Z.; Fang, S. Adipophilin: Roles in physiology and pathology. J. Clin. Pathol. 2023, 76, 98–102. [Google Scholar] [CrossRef]
- Hashiguchi, Y.; Muro, K.; Saito, Y.; Ito, Y.; Ajioka, Y.; Hamaguchi, T.; Hasegawa, K.; Hotta, K.; Ishida, H.; Ishiguro, M.; et al. Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int. J. Clin. Oncol. 2020, 25, 1–42. [Google Scholar] [CrossRef]
- Kobayashi, T.; Ishida, M.; Miki, H.; Hatta, M.; Hamada, M.; Hirose, Y.; Sekimoto, M. Significance of desmoplastic reactions on tumor deposits in patients with colorectal cancer. Oncol. Lett. 2022, 25, 1. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Lugli, A.; Kirsch, R.; Ajioka, Y.; Bosman, F.; Cathomas, G.; Dawson, H.; El Zimaity, H.; Fléjou, J.F.; Hansen, T.P.; Hartmann, A.; et al. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod. Pathol. 2017, 30, 1299–1311. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, M.; Yoshizawa, A.; Sumiyoshi, S.; Sonobe, M.; Menju, T.; Hirata, M.; Momose, M.; Date, H.; Haga, H. Adipophilin expression in lung adenocarcinoma is associated with apocrine-like features and poor clinical prognosis: An immunohistochemical study of 328 cases. Histopathology 2017, 70, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Ishida, M.; Ryota, H.; Yamamoto, T.; Kosaka, H.; Hirooka, S.; Yamaki, S.; Kotsuka, M.; Matsui, Y.; Yanagimoto, H.; et al. Adipophilin expression is an indicator of poor prognosis in patients with pancreatic ductal adenocarcinoma: An immunohistochemical analysis. Pancreatology 2019, 19, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, K.; Ishida, M.; Yanai, H.; Tsuta, K.; Sekimoto, M.; Sugie, T. Adipophilin expression is an independent marker for poor prognosis of patients with triple-negative breast cancer: An immunohistochemical study. PLoS ONE 2020, 15, e0242563. [Google Scholar] [CrossRef]
- Hirai, H.; Tada, Y.; Nakaguro, M.; Kawakita, D.; Sato, Y.; Shimura, T.; Tsukahara, K.; Kano, S.; Ozawa, H.; Okami, K.; et al. The clinicopathological significance of the adipophilin and fatty acid synthase expression in salivary duct carcinoma. Virchows. Arch. 2020, 477, 291–299. [Google Scholar] [CrossRef]
- Lai, T.T.; Ishida, M.; Kosaka, H.; Matsui, K.; Matsushima, H.; Yamamoto, H.; Kiguchi, G.; Van Nguyen, K.; Inoue, K.; Takada, M.; et al. The prognostic impact of adipophilin expression on long-term survival following liver resection in patients with colorectal liver metastases. Cancers 2024, 16, 3827. [Google Scholar] [CrossRef]
- Wu, D.; Zhuo, L.; Wang, X. Metabolic reprogramming of carcinoma-associated fibroblasts and its impact on metabolic heterogeneity of tumors. Semin. Cell Dev. Biol. 2017, 64, 125–131. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, Y.; Shi, Z.; Liu, J.; Sun, P.; Hou, X.; Zhang, J.; Zhao, S.; Zhou, B.P.; Mi, J. Metabolic reprogramming of cancer-associated fibroblasts by IDH3α downregulation. Cell Rep. 2015, 10, 1335–1348. [Google Scholar] [CrossRef]
- Ko, Y.H.; Lin, Z.; Flomenberg, N.; Pestell, R.G.; Howell, A.; Sotgia, F.; Lisanti, M.P.; Martinez-Outschoorn, U.E. Glutamine fuels a vicious cycle of autophagy in the tumor stroma and oxidative mitochondrial metabolism in epithelial cancer cells: Implications for preventing chemotherapy resistance. Cancer Biol. Ther. 2011, 12, 1085–1097. [Google Scholar] [CrossRef]
- Hsu, W.H.; LaBella, K.A.; Lin, Y.; Xu, P.; Lee, R.; Hsieh, C.E.; Yang, L.; Zhou, A.; Blecher, J.M.; Wu, C.J.; et al. Oncogenic KRAS drives lipofibrogenesis to promote angiogenesis and colon cancer progression. Cancer Discov. 2023, 13, 2652–2673. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Ishida, M.; Yanai, H.; Tsuta, K.; Sekimoto, M.; Sugie, T. Association between fatty acid synthase and adipophilin expression in triple-negative breast cancer. Mol. Clin. Oncol. 2022, 16, 80. [Google Scholar] [CrossRef]
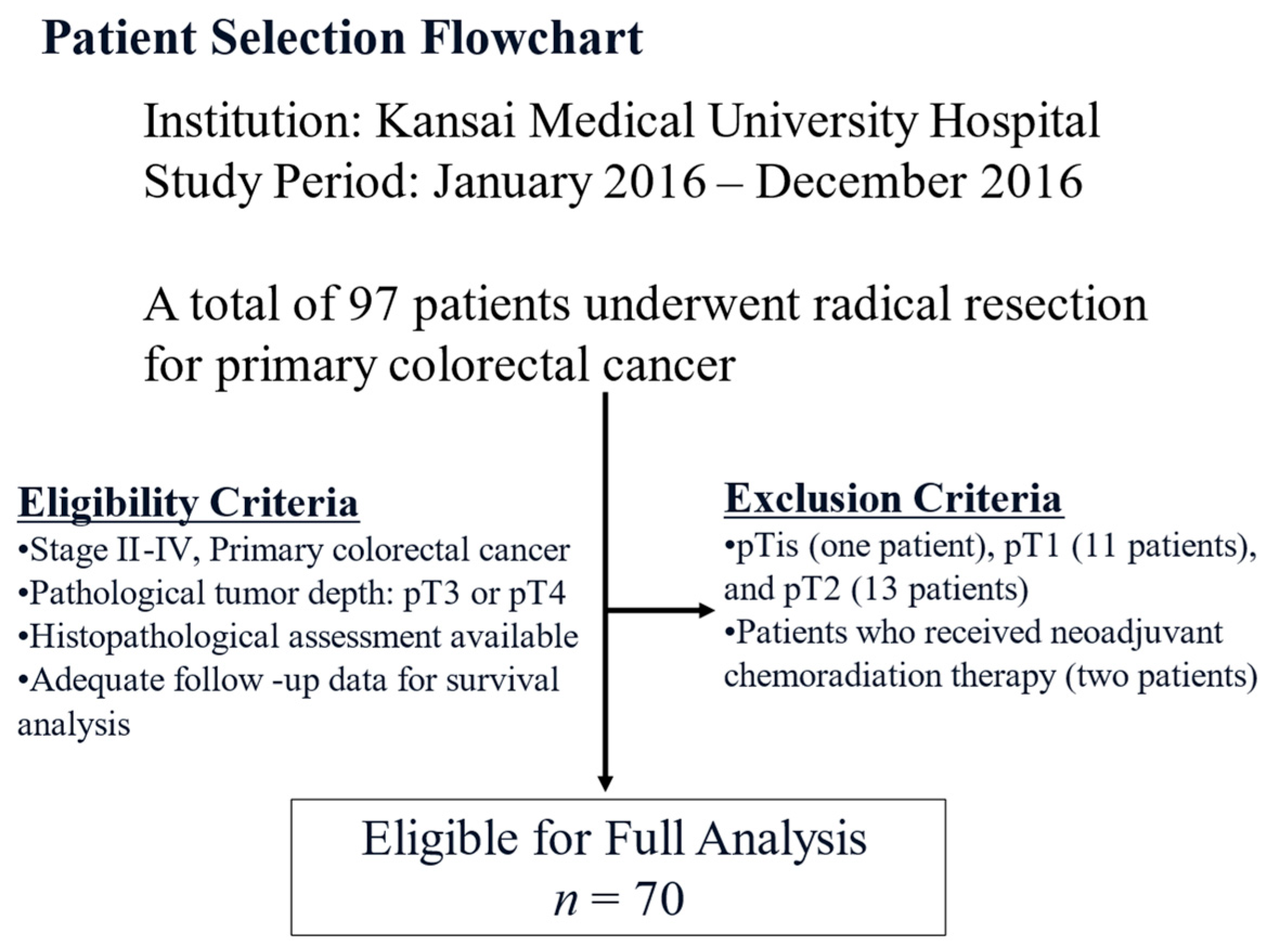
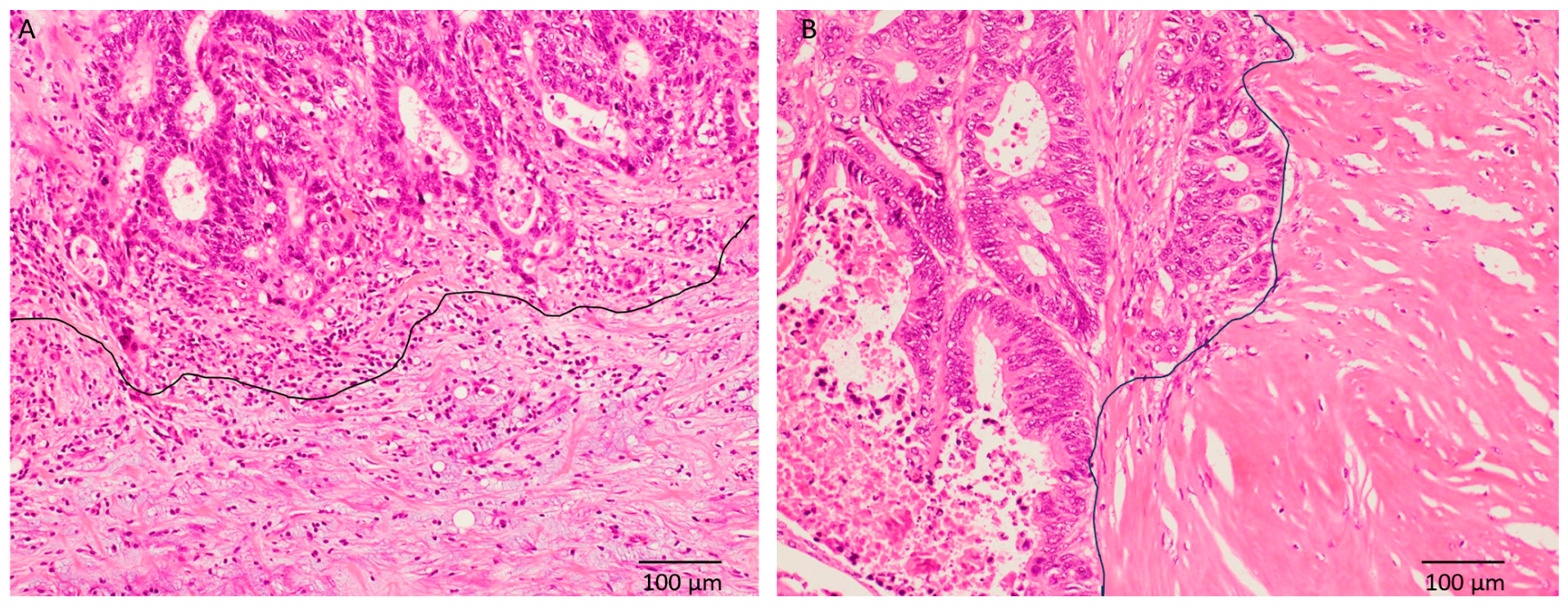
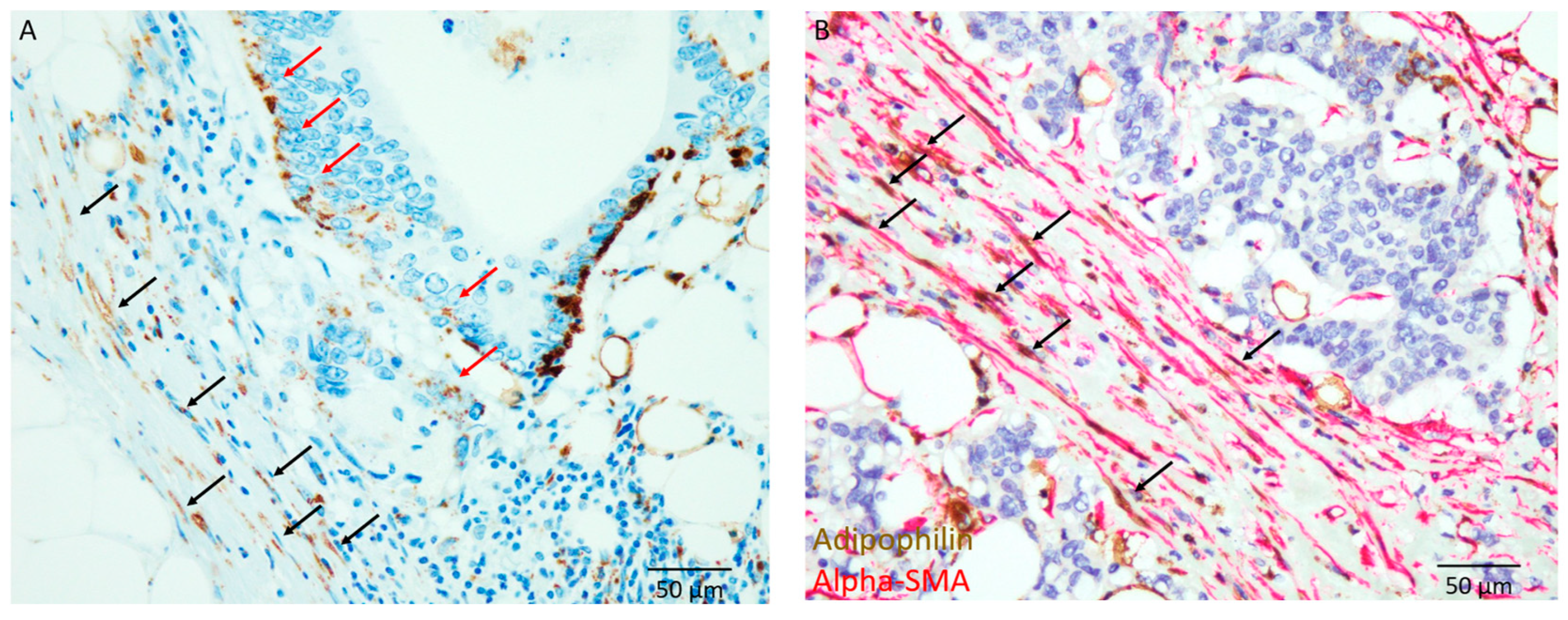
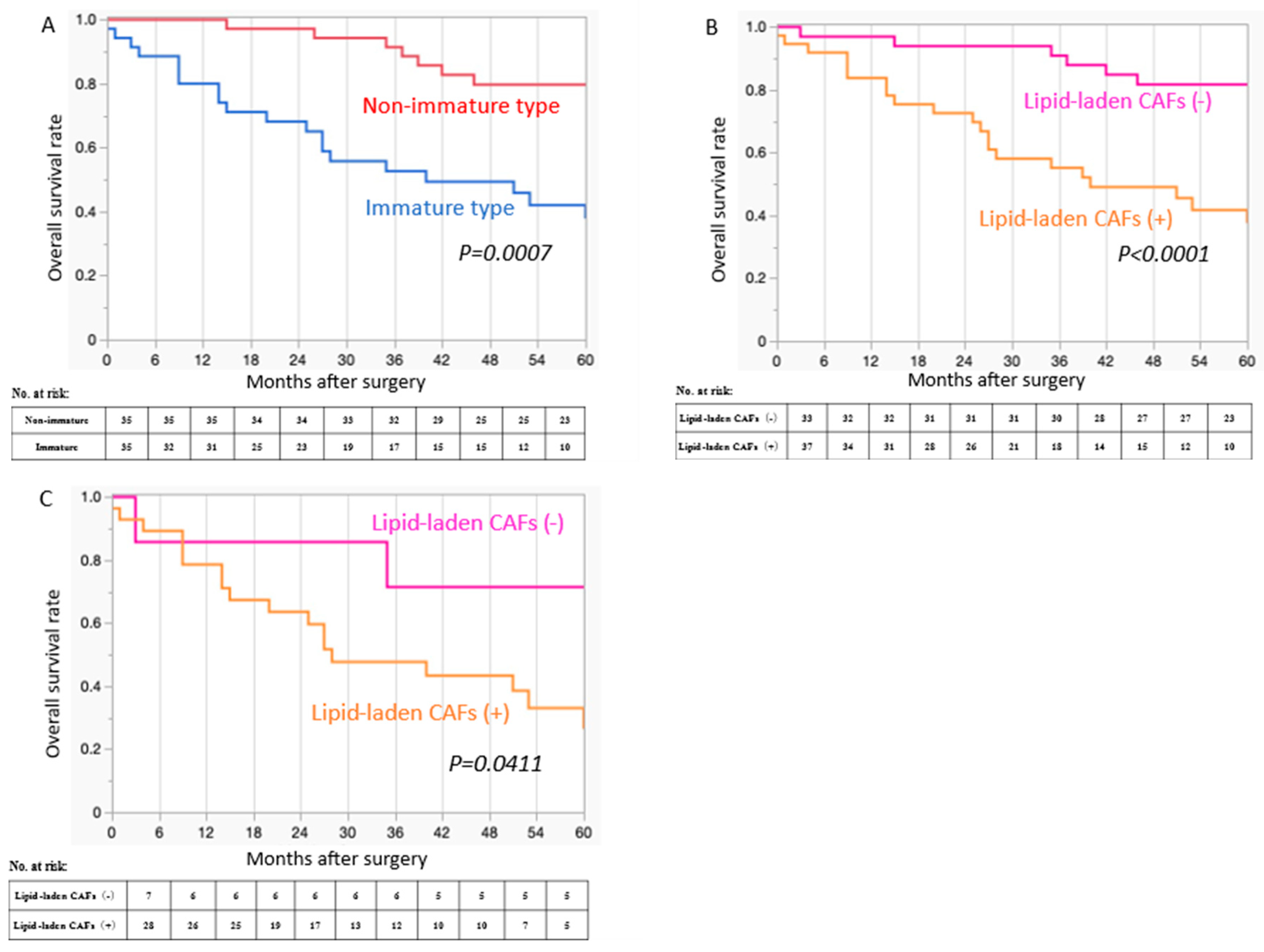
| Variables | Total Number (%) | Immature, n = 35 (%) | Non-Immature, n = 35 (%) | p-Value |
|---|---|---|---|---|
| Median age, years (range) | 74 (47–89) | 73 (58–86) | 74 (47–89) | 0.976 |
| Sex | ||||
| Male | 41 (59) | 20 (57) | 21 (60) | 0.808 |
| Female | 29 (41) | 15 (43) | 14 (40) | |
| Location | ||||
| Right-side | 40 (57) | 16 (46) | 24 (68) | 0.136 |
| Left-side | 26 (37) | 16 (46) | 10 (29) | |
| Rectum | 4 (6) | 3 (8) | 1 (3) | |
| pT | ||||
| pT3 | 46 (66) | 18 (51) | 28 (80) | 0.0118 |
| pT4 | 24 (34) | 17 (49) | 7 (20) | |
| pN | ||||
| Negative | 34 (49) | 9 (26) | 25 (71) | 0.0001 |
| Positive | 36 (51) | 26 (74) | 10 (29) | |
| pStage | ||||
| II | 32 (46) | 8 (23) | 24 (69) | 0.0006 |
| III | 25 (36) | 17 (48) | 8 (23) | |
| IV | 13 (18) | 10 (29) | 3 (8) | |
| Tumour differentiation | ||||
| Low-grade | 66 (94) | 33 (94) | 33 (94) | |
| High-grade | 4 (6) | 2 (6) | 2 (6) | 0.99 |
| Tumour budding | ||||
| TB1 | 21 (30) | 9 (25) | 12 (34) | |
| TB2+3 | 49 (70) | 26 (75) | 23 (66) | 0.433 |
| Lymphatic invasion | ||||
| Negative | 24 (34) | 12 (34) | 12 (34) | |
| Positive | 46 (66) | 23 (66) | 23 (66) | 0.99 |
| Venous invasion | ||||
| Negative | 7 (10) | 2 (5) | 5 (14) | 0.232 |
| Positive | 63 (90) | 33 (95) | 30 (86) | |
| Lipid-laden cancer-associated fibroblasts | ||||
| Absent | 33 (47) | 7 (20) | 26 (74) | <0.0001 |
| Present | 37 (53) | 28 (80) | 9 (26) | |
| Adipophilin expression in total carcinoma cells (%) median (range) | 10 (0–90) | 15 (0–90) | 5 (0–90) | 0.1207 |
| Adipophilin expression in carcinoma cells | ||||
| Low (<10%) | 41 (58) | 17 (49) | 24 (69) | 0.0894 |
| High (≧10%) | 29 (42) | 18 (51) | 11 (31) | |
| Adipophilin expression in carcinoma cells at the invasive front | ||||
| Absent | 19 (27) | 4 (11) | 15 (43) | 0.0063 |
| Present | 51 (73) | 31 (89) | 20 (57) |
| Lipid-Laden Cancer-Associated Fibroblasts | |||
|---|---|---|---|
| Variables | Present, n = 37 (%) | Absent, n = 33 (%) | p-Value |
| Median age, years (range) | 75 (58–89) | 71 (47–85) | 0.145 |
| Sex | |||
| Male | 21 (57) | 20 (60) | 0.744 |
| Female | 16 (43) | 13 (40) | |
| Location | |||
| Right-side | 22 (60) | 18 (55) | 0.514 |
| Left-side | 12 (32) | 14 (42) | |
| Rectum | 3 (8) | 1 (3) | |
| pT | |||
| pT3 | 21 (56) | 25 (76) | 0.0946 |
| pT4 | 16 (43) | 8 (24) | |
| pN | |||
| Negative | 11 (30) | 23 (70) | 0.0008 |
| Positive | 26 (70) | 10 (30) | |
| pStage | |||
| II | 10 (27) | 22 (66) | 0.0035 |
| III | 17 (46) | 8 (24) | |
| IV | 10 (27) | 3 (10) | |
| Tumor differentiation | |||
| Low-grade | 35 (96) | 31 (94) | 0.906 |
| High-grade | 2 (4) | 2 (6) | |
| Tumor budding | |||
| TB1 | 7 (19) | 14 (42) | |
| TB2+3 | 30 (81) | 19 (58) | 0.0322 |
| Lymphatic invasion | |||
| Negative | 12 (32) | 12 (36) | 0.7294 |
| Positive | 25 (68) | 21 (64) | |
| Venous invasion | |||
| Negative | 1 (3) | 6 (18) | 0.0321 |
| Positive | 36 (97) | 27 (82) | |
| Desmoplastic reaction | |||
| Immature type | 28 (76) | 7 (21) | <0.0001 |
| Non-immature type | 9 (24) | 26 (79) | |
| Adipophilin expression in carcinoma cells | |||
| Low (<10%) | 22 (59) | 19 (58) | 0.873 |
| High (≧10%) | 15 (41) | 14 (42) | |
| Adipophilin expression in carcinoma cells at the invasive front | |||
| Absent | 4 (11) | 15 (45) | 0.0014 |
| Present | 33 (89) | 18 (55) | |
| Variables | Dead Patients n = 30 (%) | Alive Patients n = 40 (%) | Univariate p-Value | Multivariate p-Value | HR | 95% CI |
|---|---|---|---|---|---|---|
| pT4 | 14 (47) | 10 (25) | 0.0588 | - | - | - |
| Immature-type desmoplastic reaction | 21 (70) | 14 (35) | 0.0038 | 0.387 | 1.744 | 0.482–6.094 |
| Presence of lymph node metastasis | 21 (70) | 15 (38) | 0.0071 | 0.172 | 2.057 | 0.631–6.671 |
| Presence of lymphatic invasion | 21 (70) | 25 (63) | 0.513 | - | - | - |
| Presence of vascular invasion | 29 (97) | 34 (85) | 0.107 | - | - | - |
| Tumor differentiation (high-grade) | 3 (10) | 1 (2.5) | 0.181 | - | - | - |
| Tumor budding (TB) 2+3 | 23 (77) | 26 (65) | 0.291 | - | - | - |
| Adipophilin expression in carcinoma cells (more than 10%) | 14 (47) | 15 (38) | 0.441 | - | - | - |
| Presence of adipophilin expression in carcinoma cells at the invasive front | 24 (80) | 27 (68) | 0.244 | - | - | - |
| Presence of lipid-laden cancer-associated fibroblasts | 23 (77) | 14 (35) | 0.0005 | 0.0368 | 3.65 | 1.082–13.038 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, T.; Ishida, M.; Uehara, H.; I, S.; Yamada, N.; Igarashi, Y.; Hagiwara, C.; Mori, Y.; Hirose, Y.; Watanabe, J. Presence of Adipophilin-Positive Cancer-Associated Fibroblasts Is an Independent Poor Prognostic Indicator and Is Correlated with Immature-Type Desmoplastic Reaction in Patients with Colorectal Cancer. Cancers 2025, 17, 3006. https://doi.org/10.3390/cancers17183006
Kobayashi T, Ishida M, Uehara H, I S, Yamada N, Igarashi Y, Hagiwara C, Mori Y, Hirose Y, Watanabe J. Presence of Adipophilin-Positive Cancer-Associated Fibroblasts Is an Independent Poor Prognostic Indicator and Is Correlated with Immature-Type Desmoplastic Reaction in Patients with Colorectal Cancer. Cancers. 2025; 17(18):3006. https://doi.org/10.3390/cancers17183006
Chicago/Turabian StyleKobayashi, Toshinori, Mitsuaki Ishida, Hiroki Uehara, Shoichiro I, Norikazu Yamada, Yuto Igarashi, Chie Hagiwara, Yoshihiro Mori, Yoshinobu Hirose, and Jun Watanabe. 2025. "Presence of Adipophilin-Positive Cancer-Associated Fibroblasts Is an Independent Poor Prognostic Indicator and Is Correlated with Immature-Type Desmoplastic Reaction in Patients with Colorectal Cancer" Cancers 17, no. 18: 3006. https://doi.org/10.3390/cancers17183006
APA StyleKobayashi, T., Ishida, M., Uehara, H., I, S., Yamada, N., Igarashi, Y., Hagiwara, C., Mori, Y., Hirose, Y., & Watanabe, J. (2025). Presence of Adipophilin-Positive Cancer-Associated Fibroblasts Is an Independent Poor Prognostic Indicator and Is Correlated with Immature-Type Desmoplastic Reaction in Patients with Colorectal Cancer. Cancers, 17(18), 3006. https://doi.org/10.3390/cancers17183006







