1. Introduction
In recent years, the diagnostic and therapeutic approach to prostate cancer (PCa) has undergone significant advancements [
1,
2]. The traditional reliance on systematic biopsies and Prostate Specific Antigen (PSA) alone has been progressively replaced by more precise and individualized strategies [
3,
4]. The widespread use of multiparametric magnetic resonance imaging (mpMRI), often combined with targeted fusion-guided biopsies, has significantly improved the detection and risk stratification of clinically significant PCa. In parallel, the integration of novel imaging modalities, such as PSMA PET/CT, has enhanced staging accuracy, particularly for high-risk and recurrent disease [
5]. Furthermore, the increasing availability of nomograms, risk calculators, and mobile health applications has facilitated shared decision-making and tailored treatment planning, empowering both patients and clinicians in choosing the most appropriate therapeutic pathway [
6,
7,
8,
9,
10,
11,
12].
Currently, radical prostatectomy (RP) remains the gold standard surgical treatment for localized PCa disease. Over the years, RP has evolved significantly in terms of surgical technique, perioperative outcomes, and oncological efficacy, especially with the adoption of minimally invasive and robotic-assisted approaches [
13,
14,
15]. This procedure, performed either via open, laparoscopic, or robot-assisted approaches, aims to achieve complete oncological control while minimizing functional side effects. One of the most common complications of RP is urinary incontinence (UI), which reduces quality of life (QoL) of patients treated [
16,
17,
18,
19]. In particular, UI can persist for weeks or months after surgery and may require the use of pads, behavioral therapy, or even surgical interventions, significantly impacting daily activities and psychological well-being [
20,
21].
Due to the high frequency of post-RP UI and the reduced QoL, possible predictors were recently studied in order to better stratify patients for the most appropriate treatment. In this context, identifying preoperative factors that may influence functional outcomes has become a crucial goal in urological research and clinical practice. Better risk stratification may help clinicians select patients who could benefit from specific counseling, early rehabilitation, or nerve-sparing techniques. Possible predictors of urinary continence (UC) recovery, evaluated from preoperative magnetic resonance imaging (MRI), might be prostatic apex shape according to Lee type (LT), median lobe (ML) presence and its intravesical protrusion (IPP), prostatic urethral length (PUL), or membranous urethral length (MUL) [
17,
18,
22,
23,
24,
25,
26]. These parameters are measurable with high reproducibility and may provide objective data to guide surgical planning and anticipate postoperative functional results.
Other possible predictors might be age, body mass index (BMI), International Prostate Symptoms Score (IPSS), Gleason Score (GS), disease stage, and use of a nerve sparing technique (NST) during RP [
23,
24,
27]. These variables, which are routinely collected in the preoperative work-up, may affect both oncological and functional outcomes, and their potential role in continence recovery is increasingly recognized in the literature. For instance, younger patients or those undergoing bilateral nerve sparing might have better chances of regaining continence earlier.
We aimed to find predictors of post-RP UC recovery and how much they could affect it, in order to better identify high UI risk patients who might benefit from specific treatments. The ultimate goal is to move toward a more personalized approach to prostate cancer surgery, where the choice of technique and the timing of rehabilitation can be tailored according to individual risk profiles. This could improve patient satisfaction, reduce anxiety, and promote a faster return to normal life.
2. Materials and Methods
A monocentric retrospective study was designed and the primary outcome was defined as the UC recovery at 0, 1, 3, and 6 months after robot-assisted radical prostatectomy (RARP) in PCa patients.
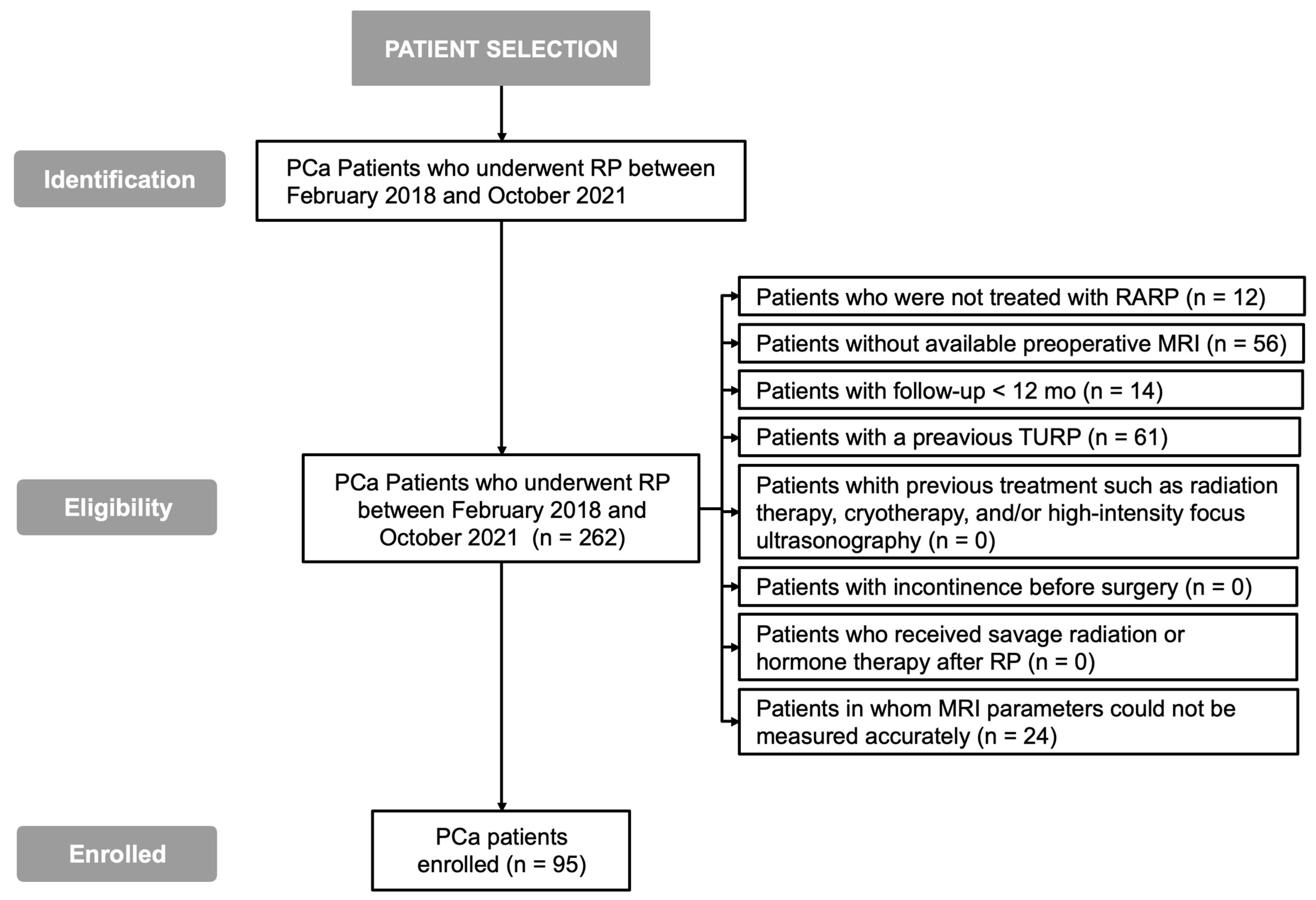
PCa patients who underwent RP between February 2018 and October 2021 were included. Patients who were not treated with RARP, with no available MRI, with follow-up shorter than 12 months, who underwent transurethral resection of the prostate (TURP) or other PCa treatment (such as radiotherapy, hormone therapy, cryotherapy, and/or focused high intensity ultrasound), or with a MRI where parameters could not be accurately measured were excluded.
UC recovery was defined as a daily pad usage less than or equal to one.
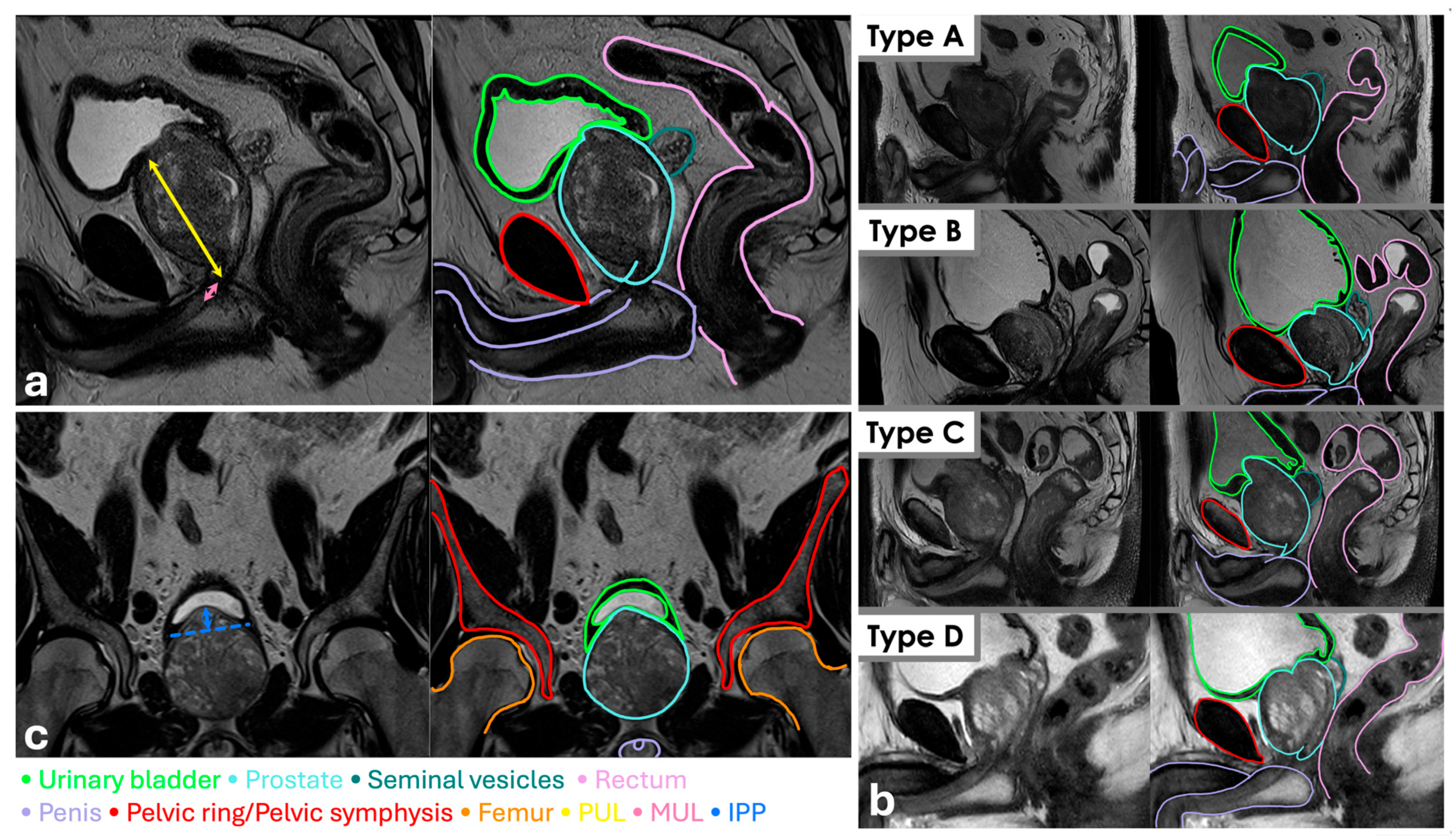
For every patient, at the diagnosis, age, weight, height, body mass index (calculated as quotient between weight and height squared), PSA, PSA Density (PSAD), GS at biopsy, ISUP, clinic stage, IPSS, and QoL were collected. An expert radiologist (V.G.), blinded to postoperative outcomes, evaluated every MRI, estimating prostatic volume (PV), PUL, MUL, prostatic apex shape, ML presence, and IPP. PV was calculated as the product among
and the three maximum diameters measured at T2 weighted images in sagittal and axial sections. PUL was measured from prostatic base to apex at T2 weighted images in sagittal section (
Figure 1a) and MUL from prostatic apex to urethral bulb at T2 weighted images in sagittal section (
Figure 1a). Apex shape was classified according to LT at T2 weighted images in sagittal section (
Figure 1b) and IPP was measured from intravesical protrusion apex and vesical base at T2 weighted images in coronal section (
Figure 1c).
During RARP, the execution of a NST or a bladder neck sparing technique was collected. During follow-up, every patient was encouraged to do Kegel’s exercises in order to strengthen their pelvic floor and was questioned about their daily pad usage.
Descriptive tables show clinical and radiological data with median and interquartile range (IQR), for continuous variable, or absolute number with percentage of the total, for categorical variables. Multivariate logistic regressions were performed to test predictors for UC recovery at vesical catheter (VC) removal, 1, 3, and 6 months from RARP. Covariates included age, BMI, PV, IPSS (all continuously coded), NST (yes vs. no), ISUP (1 vs. 2 vs. 3 vs. 4), PUL, MUL, ML, IPP (continuously coded), and LT (A vs. B vs. C vs. D) as independent variables. Statistical results were presented as odds ratios (ORs) with 95% confidence interval (95% CI) and p-value considered statistically significant if less than or equal to 0.05. Statistical analyses were conducted by Jamovi (version 2.4.12).
3. Results
A total of 262 PCa patients who underwent RP between February 2018 and October 2021 were identified (
Figure 2). A total of 12 patients who did not undergo RARP, 56 patients without an available preoperative MRI, 14 patients with follow-up <12 months, 61 patients who underwent TURP, and 24 patients in whom MRI parameters could not be accurately measured were excluded. Therefore, 95 patients were enrolled.
The demographic and clinical characteristics of the study population are summarized in
Table 1. The median age was 66 years (IQR 62–70), with a median height of 1.75 m (IQR 1.70–1.78), median weight of 80 kg (IQR 72–85), and a median BMI of 26.12 kg/m
2 (IQR 23.88–28.09). The median PSA level was 7.5 ng/mL (IQR 5.5–9.6), while the median PV was 45 cc (IQR 34–59), resulting in a PSAD of 0.16 ng/mL/cc (IQR 0.10–0.26). Regarding baseline urinary symptoms, the median IPSS was 9 (IQR 6–14). QoL, assessed through the IPSS-related question, ranged from 0 in 23 patients (26.1%) to 6 in 2 patients (2.1%). At diagnosis, the majority of patients were classified as ISUP grade 2 (
n = 59, 62.1%), followed by ISUP 3 (
n = 29, 30.5%), ISUP 5 (
n = 4, 4.2%), and ISUP 1 (
n = 3, 3.2%). In terms of clinical staging, most patients were staged as cT2c (
n = 63, 66.3%), while the remaining were distributed as follows: cT3c (
n = 12, 12.6%), cT3a (
n = 11, 11.6%), cT2a (
n = 6, 6.3%), and cT2b (
n = 3, 3.2%). Based on MRI findings, 26 patients (27.4%) were classified as LT A, 20 (21.1%) as LT B, 18 (18.9%) as LT C, and 31 (32.6%) as LT D. MRI also identified a median PUL of 36 mm (IQR 31–42), a median MUL of 15 mm (IQR 13–16), and a median IPP of 0 mm (IQR 0–0). Median ML was reported in 23 patients (24.2%). During follow-up, the proportion of patients regaining UC increased progressively over time. Immediately after VC removal, 32 patients (33.7%) had recovered UC. At 12 months postoperatively, UC recovery had occurred in 93 patients (97.8%).
Table 2,
Table 3,
Table 4,
Table 5 and
Table 6 show multivariate logistic regression results: only two models reported statistically significant results. LT C was a significant predictor of continence recovery at VC removal with respect to LT A in the model described in
Table 2 (OR 0.1; 95% CI 0.04–0.71;
p-value 0.01) (
Table 2).
The value of IPSS was found to be a statistically significant predictor on continence at 1 month after RARP in the model in
Table 6 (OR 1.08 (1.00–1.17; 0.04)).
No further statistical predictors were found for PUL, MUL, or ML presence, and IPP did not show any statistically significant impact on UC recovery in the models considered.
4. Discussion
This study aimed to evaluate the potential role of preoperative MRI-derived anatomic parameters in predicting UC recovery after RARP. Our findings suggest that only two variables were statistically associated with UC recovery at specific time points. First, at the time of VC removal, patients classified as having LT C on preoperative MRI showed a significantly higher likelihood of being continent compared to those with LT A. Second, an increase in preoperative IPSS was significantly associated with a reduced probability of UC recovery one month after RARP. However, no other variables, such as MUL, PUL, IPP, or the presence of ML, demonstrated a statistically significant correlation with UC recovery at any of the follow-up intervals considered.
These findings, although partially significant, may suggest that a clear and consistent association between UC recovery and preoperative MRI parameters is still elusive. One possible interpretation is that the anatomical characteristics evaluated may have a limited predictive role when considered in isolation, or that they primarily influence early UC recovery rather than long-term outcomes. Alternatively, the lack of broader statistical significance could reflect limitations in sample size, inter-observer variability in MRI interpretation, or the multifactorial nature of continence recovery after prostatectomy.
Our results are partially in line with previous studies in the literature, which show that urethral length, urethral sphincter dimensions, preoperative IPSS, and prostatic shape may be correlated to postoperative incontinence [
28,
29,
30,
31,
32,
33,
34,
35,
36]. Sauer et al. evaluated the relationship between LT classification and UC recovery, reporting no significant differences in continence outcomes between different LT groups at 3 months (
p = 0.24) and 6 months (
p = 0.61) following RP [
18]. Their findings support the hypothesis that LT may not have a durable or consistent impact on long-term continence outcomes. Similarly, Hikita et al. conducted a multivariate logistic regression analysis and found no statistically significant correlation between MUL and UC recovery, either at 1 month (OR 0.91; 95% CI 0.82–1.00;
p = 0.06) or 12 months (OR 0.95; 95% CI 0.85–1.10;
p = 0.39) postoperatively [
23]. Interestingly, in their study, IPSS again emerged as a predictor of early continence: higher IPSS values were significantly associated with reduced UC recovery after one month (OR 1.14; 95% CI 1.02–1.28;
p = 0.02), although not after 12 months (OR 0.99; 95% CI 0.87–1.14;
p = 0.39) [
4]. They also observed that patients with IPP greater than 5 mm experienced higher IPSS values postoperatively and had a lower rate of pad-free status at 1 month, suggesting that IPP might be used as a potential cutoff for identifying patients at high risk for early urinary incontinence.
On the other hand, some studies have yielded contrasting results, particularly regarding the role of MUL [
17,
24,
26]. A recent meta-analysis highlighted a significant correlation between longer MUL and improved UC recovery at various time points [
26]. Specifically, the analysis reported a hazard ratio (HR) of 1.05 (95% CI 1.02–1.08;
p < 0.001) for early recovery, and higher odds ratios (ORs) for recovery at 1 month (OR 1.16; 95% CI 1.09–1.23;
p < 0.001), 3 months (OR 1.08; 95% CI 1.03–1.14;
p = 0.004), 6 months (OR 1.12; 95% CI 1.03–1.15;
p < 0.001), and 12 months (OR 1.12; 95% CI 1.03–1.22;
p = 0.006) following surgery. These findings suggest that MUL may serve as a robust and consistent predictor of both early and late UC recovery. Similar observations have been reported by other authors as well, reinforcing the importance of urethral length as a relevant anatomical factor [
37]. Both the existing literature and our current findings indicate that patients undergoing nerve-sparing surgery achieve significantly better urinary continence outcomes compared to those without nerve preservation [
38,
39,
40]. This advantage is likely related to the preservation of neurovascular structures that contribute to sphincter function and pelvic floor innervation, thereby facilitating earlier and more complete recovery of continence.
The present study must be interpreted in light of several limitations. Firstly, its retrospective design inherently introduces the risk of selection bias and limits the strength of causal inferences. Secondly, although all patients underwent preoperative MRI, image acquisition was not centralized, and inter-reader variability in LT classification or measurement of parameters such as MUL and IPP could have influenced the accuracy of data. Moreover, the relatively small sample size and the strict inclusion/exclusion criteria reduced the number of eligible patients, potentially impacting the statistical power of our analysis. It is also possible that the relatively low event rate in some follow-up intervals contributed to the lack of statistical significance in some variables.
Despite these limitations, our findings contribute to the ongoing discussion about the predictive role of anatomical features observed on preoperative MRI in determining postoperative continence outcomes. The heterogeneity of results reported in the literature underscores the complexity of UC recovery, which likely depends on a combination of anatomical, functional, surgical, and rehabilitative factors. Future prospective, multicentric studies with larger patient cohorts and standardized MRI protocols will be essential to clarify the true impact of specific MRI parameters—such as LT classification, MUL, and IPP—on continence recovery.
In this context, identifying patients at higher risk of persistent incontinence [
31,
41,
42] is not only relevant for clinical prediction but also crucial for shared decision-making. Providing patients with accurate, personalized information about their individual risk of postoperative incontinence—based on their preoperative anatomical characteristics and functional status is essential to foster informed consent. A well-structured and transparent informed consent process [
43,
44,
45,
46] should include a discussion of these tailored risks, aligning surgical expectations and promoting early engagement in postoperative rehabilitation strategies when appropriate.