The Effect of Lurbinectedin as a Monotherapy and in Combination with Ionizing Radiation on Sarcoma Cell Lines
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Cell Culture
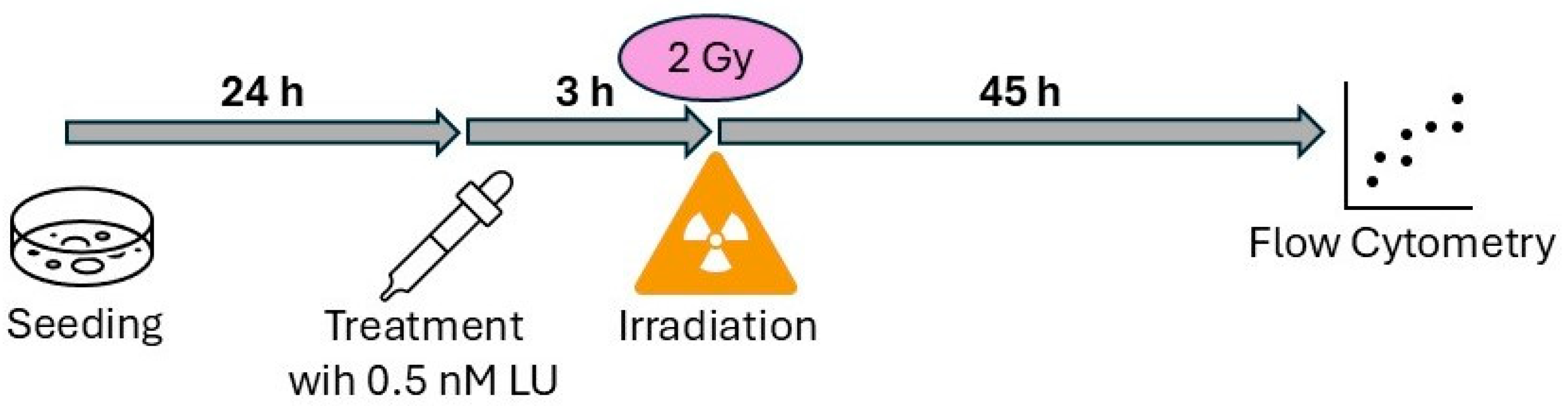
2.2. Treatment with Lurbinectedin and Radiotherapy
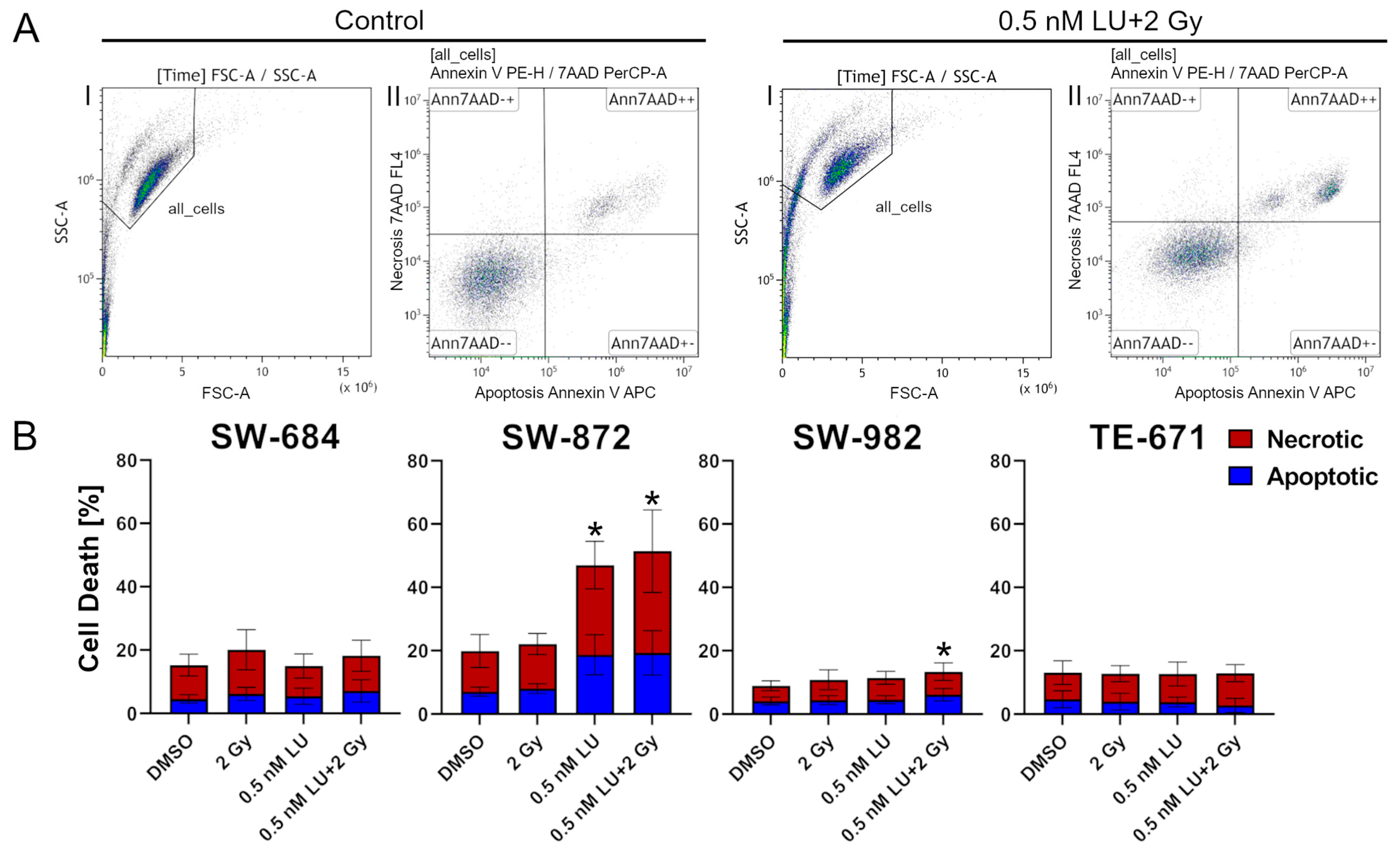
2.3. Assay for Apoptosis/Necrosis and Cell Cycle Distribution Following Single-Dose Ionizing Radiation (4-Day Protocol)
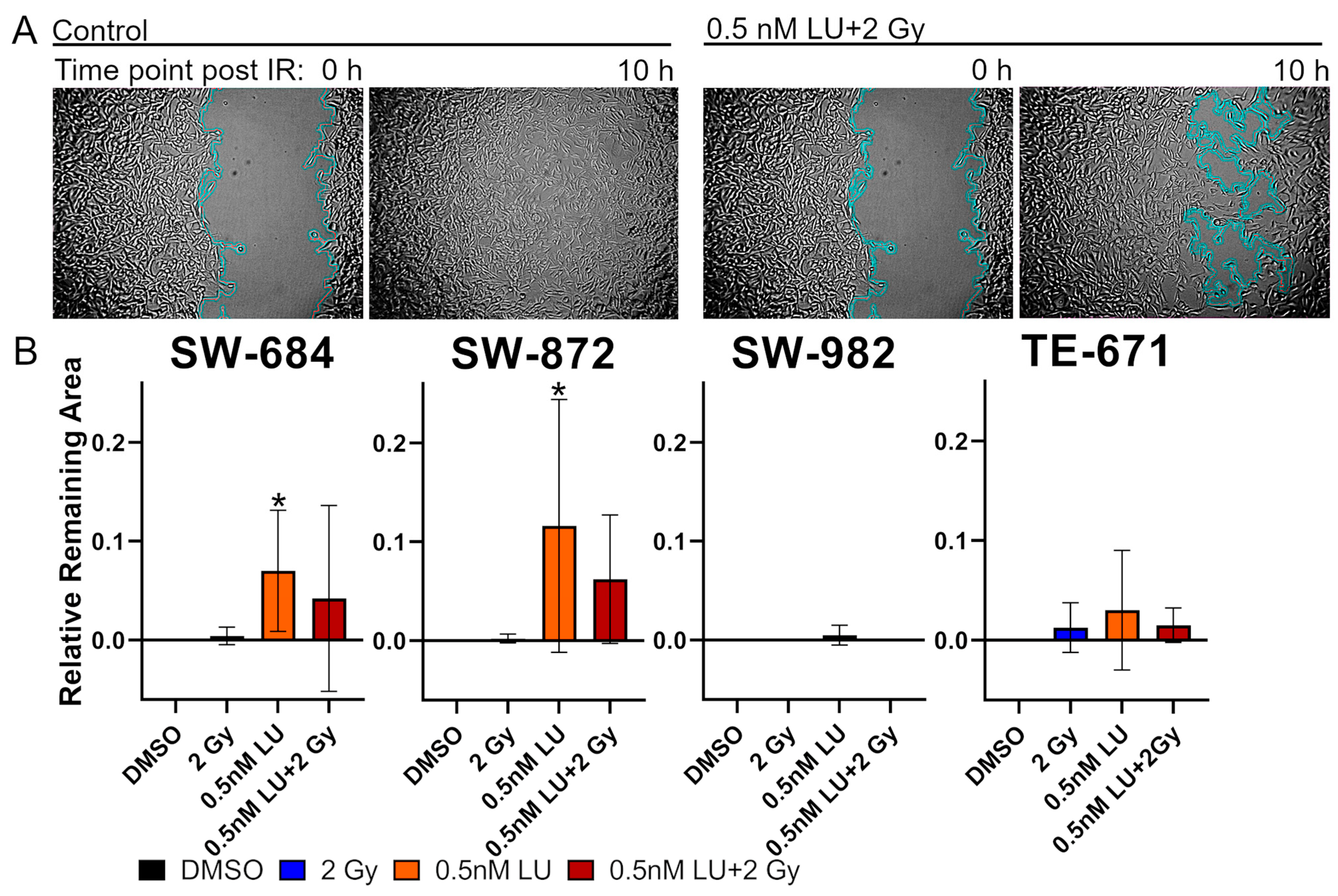
2.4. Scratch Assay
2.5. Assay for Clonogenic Survival
2.6. Assay for Apoptosis/Necrosis and Cell Cycle Distribution Combined with Clonogenic Survival Following Fractionated Ionizing Radiation (6-Day Protocol)
2.7. Statistics
3. Results
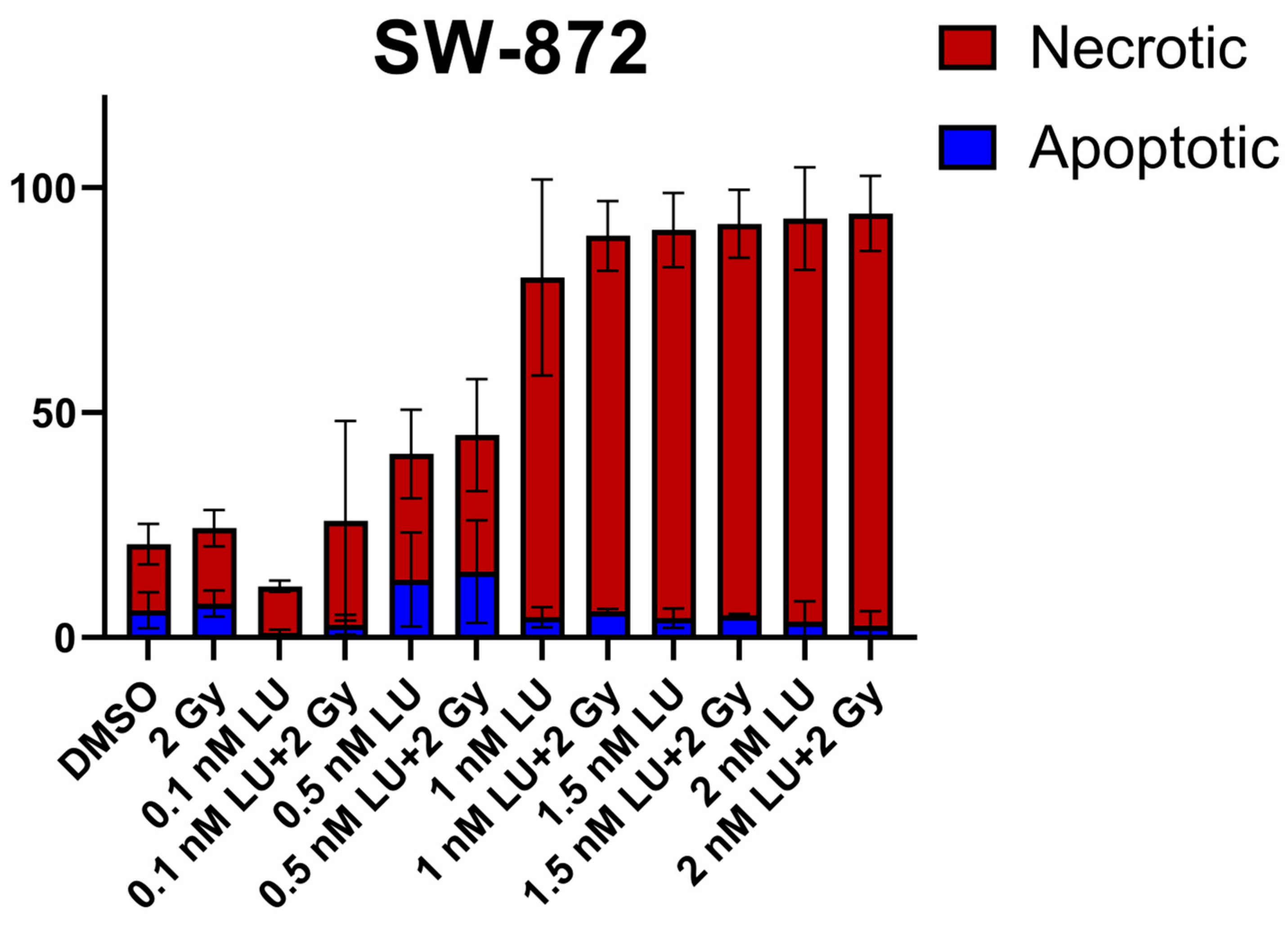
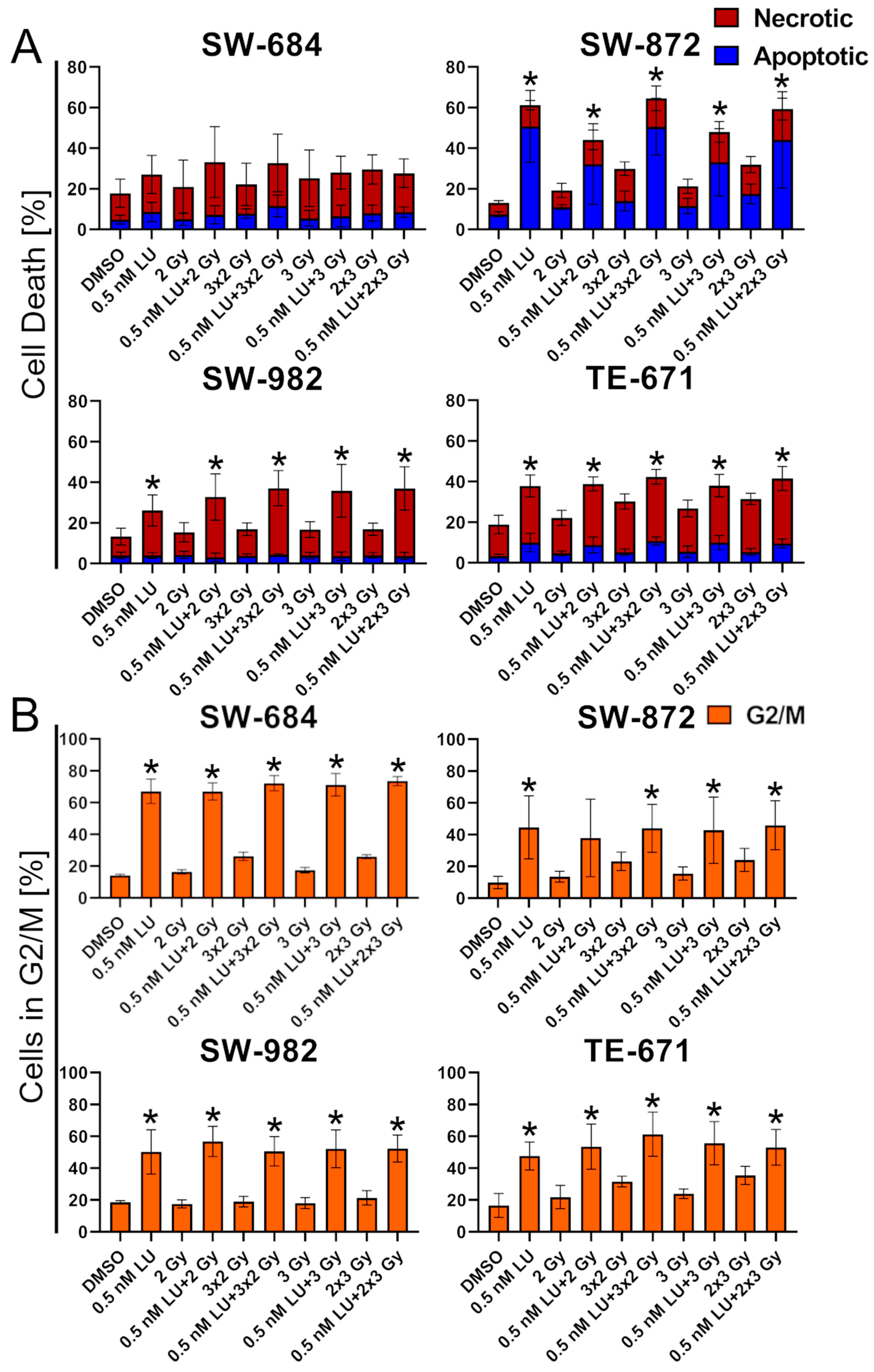
3.1. Apoptosis/Necrosis Induction by Lurbinectedin and Lurbinectedin + 2Gy Following the 4-Day Protocol
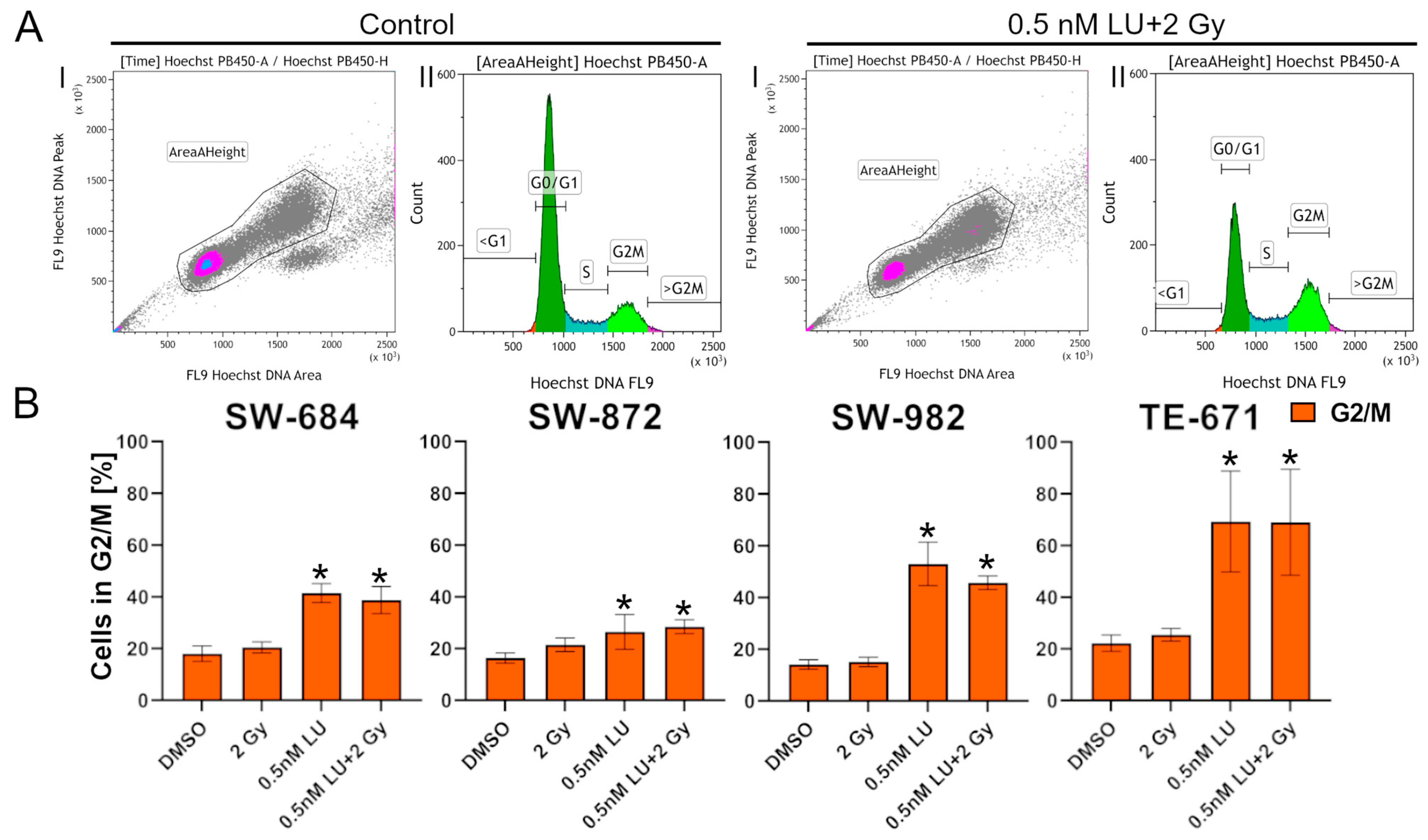
3.2. Effects of Lurbinectedin and Lurbinectedin + 2Gy on the Cell Cycle Following the 4-Day Protocol
3.3. Scratch Assay
3.4. G2/M Cell Cycle Distribution
3.5. Effects of Lurbinectedin and the Combination of Lurbinectedin and Fractionated Ionizing Radiation on Apoptosis/Necrosis and Cell Cycle Arrest
3.6. Effects of Lurbinectedin and the Combination of Lurbinectedin with Ionizing Radiation on the Clonogenic Survival
4. Discussion
4.1. The Effects of LU as a Monotherapy
4.2. The Effects of LU in Combination with RT
4.3. The Heterogenic Reaction of the Different STS Cell Lines
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| STS | Soft Tissue Sarcoma |
| LU | Lurbinectedin |
| IR | Ionizing radiation |
| RT | Radiotherapy |
| CT | Chemotherapy |
Appendix A
References
- Vodanovich, D.A.; Choong, P.F.M. Soft-Tissue Sarcomas. Indian J. Orthop. 2018, 52, 35–44. [Google Scholar] [CrossRef]
- Miwa, S.; Yamamoto, N.; Tsuchiya, H. Sarcoma: Molecular Pathology, Diagnostics, and Therapeutics. Int. J. Mol. Sci. 2023, 24, 5833. [Google Scholar] [CrossRef]
- Burningham, Z.; Hashibe, M.; Spector, L.; Schiffman, J.D. The Epidemiology of Sarcoma. Clin. Sarcoma Res. 2012, 2, 14. [Google Scholar] [CrossRef]
- Sbaraglia, M.; Bellan, E.; Dei Tos, A.P. The 2020 Who Classification of Soft Tissue Tumours: News and Perspectives. Pathologica 2021, 113, 70–84. [Google Scholar] [CrossRef]
- Kunisada, T.; Nakata, E.; Fujiwara, T.; Hosono, A.; Takihira, S.; Kondo, H.; Ozaki, T. Soft-Tissue Sarcoma in Adolescents and Young Adults. Int. J. Clin. Oncol. 2023, 28, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nagar, S.P.; Mytelka, D.S.; Candrilli, S.D.; D’Yachkova, Y.; Lorenzo, M.; Kasper, B.; Lopez-Martin, J.A.; Kaye, J.A. Treatment Patterns and Survival among Adult Patients with Advanced Soft Tissue Sarcoma: A Retrospective Medical Record Review in the United Kingdom, Spain, Germany, and France. Sarcoma 2018, 2018, 5467057. [Google Scholar] [CrossRef] [PubMed]
- Spalato-Ceruso, M.; Ghazzi, N.E.; Italiano, A. New Strategies in Soft Tissue Sarcoma Treatment. J. Hematol. Oncol. 2024, 17, 76. [Google Scholar] [CrossRef]
- Nakamura, T.; Matsumine, A.; Matsubara, T.; Asanuma, K.; Uchida, A.; Sudo, A. The Symptom-to-Diagnosis Delay in Soft Tissue Sarcoma Influence the Overall Survival and the Development of Distant Metastasis. J. Surg. Oncol. 2011, 104, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Lochner, J.; Menge, F.; Vassos, N.; Hohenberger, P.; Kasper, B. Prognosis of Patients with Metastatic Soft Tissue Sarcoma: Advances in Recent Years. Oncol. Res. Treat. 2020, 43, 613–619. [Google Scholar] [CrossRef]
- Kobus, M.; Roohani, S.; Ehret, F.; Flörcken, A.; Striefler, J.K.; Brandes, F.; Märdian, S.; Rau, D.; Wittenberg, S.; Öllinger, R.; et al. The Role of Neoadjuvant Radiochemotherapy in the Management of Localized high-Grade Soft Tissue Sarcoma. Radiat. Oncol. 2022, 17, 139. [Google Scholar] [CrossRef]
- Matsui, J.K.; Jackson, S.; Fang, J.; Mohler, D.G.; Steffner, R.J.; Avedian, R.S.; Charville, G.W.; Rijn, M.V.; Million, L.; Chin, A.L.; et al. Association of Histologic Subtype with Radiation Response and Survival Outcomes in Synovial Sarcoma. Adv. Radiat. Oncol. 2025, 10, 101718. [Google Scholar] [CrossRef]
- O’Sullivan, B.; Davis, A.M.; Turcotte, R.; Bell, R.; Catton, C.; Chabot, P.; Wunder, J.; Kandel, R.; Goddard, K.; Sadura, A.; et al. Preoperative Versus Postoperative Radiotherapy in Soft-Tissue Sarcoma of the Limbs: A Randomised Trial. The Lancet 2002, 359, 2235–2241. [Google Scholar] [CrossRef]
- Soyfer, V.; Corn, B.W.; Kollender, Y.; Tempelhoff, H.; Meller, I.; Merimsky, O. Radiation Therapy for Palliation of Sarcoma Metastases: A Unique and Uniform Hypofractionation Experience. Sarcoma 2010, 2010, 927972. [Google Scholar] [CrossRef]
- Tween, H.; Peake, D.; Spooner, D.; Sherriff, J. Radiotherapy for the Palliation of Advanced Sarcomas-the Effectiveness of Radiotherapy in Providing Symptomatic Improvement for Advanced Sarcomas in a Single Centre Cohort. Healthcare 2019, 7, 120. [Google Scholar] [CrossRef]
- Zwanenburg, A.; Price, G.; Löck, S. Artificial Intelligence for Response Prediction and Personalisation in Radiation Oncology. Strahlenther. Und Onkol. 2025, 201, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Cammelli, S.; Cortesi, A.; Buwenge, M.; Zamagni, A.; Ferioli, M.; Ghigi, G.; Romeo, A.; Morganti, A.G. The Role of Radiotherapy in Adult Soft Tissues Sarcoma of the Extremities. Eur. J. Orthop. Surg. Traumatol. 2021, 31, 1583–1596. [Google Scholar] [CrossRef] [PubMed]
- Roeder, F. Radiation Therapy in Adult Soft Tissue Sarcoma-Current Knowledge and Future Directions: A Review and Expert Opinion. Cancers 2020, 12, 3242. [Google Scholar] [CrossRef]
- Italiano, A.; Mathoulin-Pelissier, S.; Cesne, A.L.; Terrier, P.; Bonvalot, S.; Collin, F.; Michels, J.J.; Blay, J.Y.; Coindre, J.M.; Bui, B. Trends in Survival for Patients with Metastatic Soft-Tissue Sarcoma. Cancer 2011, 117, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Arbor, K.; Dubey, R. Doxorubicin. In Statpearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Mulder, R.L.; Paulides, M.; Langer, T.; Kremer, L.C.; van Dalen, E.C. Cyclophosphamide Versus Ifosfamide for Paediatric and Young Adult Bone and Soft Tissue Sarcoma Patients. Cochrane Database Syst. Rev. 2015, 2015, Cd006300. [Google Scholar] [CrossRef]
- Gamboa, A.C.; Gronchi, A.; Cardona, K. Soft-Tissue Sarcoma in Adults: An Update on the Current State of Histiotype-Specific Management in an Era of Personalized Medicine. CA Cancer J. Clin. 2020, 70, 200–229. [Google Scholar] [CrossRef]
- Miwa, S.; Hayashi, K.; Demura, S. Pathology, Diagnosis, and Management of Sarcoma. Int. J Mol. Sci. 2024, 25, 6609. [Google Scholar] [CrossRef]
- Eichler, M.; Hentschel, L.; Singer, S.; Hornemann, B.; Richter, S.; Hofbauer, C.; Hohenberger, P.; Kasper, B.; Andreou, D.; Pink, D.; et al. Health Related Quality of Life over Time in German Sarcoma Patients. An Analysis of Associated Factors—Results of the Prosa Study. Front. Endocrinol. 2023, 29, 1166838. [Google Scholar] [CrossRef]
- Hoofnagle, J.; Navarro, V.; Kleiner, D.; Knoben, J.; Tontchev, G.; Harris, S., Jr.; Serrano, J.; Seeff, L.; Bethea, G. Lurbinectedin. In Livertox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012. [Google Scholar]
- Belgiovine, C.; Bello, E.; Liguori, M.; Craparotta, I.; Mannarino, L.; Paracchini, L.; Beltrame, L.; Marchini, S.; Galmarini, C.M.; Mantovani, A.; et al. Lurbinectedin Reduces Tumour-Associated Macrophages and the Inflammatory Tumour Microenvironment in Preclinical Models. Br. J. Cancer 2017, 117, 628–638. [Google Scholar] [CrossRef]
- Gadducci, A.; Cosio, S. Trabectedin and Lurbinectedin: Mechanisms of Action, Clinical Impact, and Future Perspectives in Uterine and Soft Tissue Sarcoma, Ovarian Carcinoma, and Endometrial Carcinoma. Front. Oncol. 2022, 12, 914342. [Google Scholar] [CrossRef] [PubMed]
- Santamaría Nuñez, G.; Robles, C.M.; Giraudon, C.; Martínez-Leal, J.F.; Compe, E.; Coin, F.; Aviles, P.; Galmarini, C.M.; Egly, J.M. Lurbinectedin Specifically Triggers the Degradation of Phosphorylated Rna Polymerase Ii and the Formation of DNA Breaks in Cancer Cells. Mol. Cancer Ther. 2016, 15, 2399–2412. [Google Scholar] [CrossRef]
- Allavena, P.; Belgiovine, C.; Digifico, E.; Frapolli, R.; D’Incalci, M. Effects of the Anti-Tumor Agents Trabectedin and Lurbinectedin on Immune Cells of the Tumor Microenvironment. Front. Oncol. 2022, 12, 851790. [Google Scholar] [CrossRef] [PubMed]
- Miolo, G.; Viel, A.; Canzonieri, V.; Baresic, T.; Buonadonna, A.; Santeufemia, D.A.; Lara, D.P.; Corona, G. Association of the Germline Brca2 Missense Variation Glu2663lys with High Sensitivity to Trabectedin-Based Treatment in Soft Tissue Sarcoma. Cancer Biol. Ther. 2016, 17, 1017–1021. [Google Scholar] [CrossRef][Green Version]
- Povo-Retana, A.; Landauro-Vera, R.; Alvarez-Lucena, C.; Cascante, M.; Boscá, L. Trabectedin and Lurbinectedin Modulate the Interplay between Cells in the Tumour Microenvironment-Progresses in Their Use in Combined Cancer Therapy. Molecules 2024, 29, 331. [Google Scholar] [CrossRef]
- Grabenbauer, F.; Sabine, S. Im Labyrinth Dem Ziel Entgegen: Doxorubicin/Trabectedin Bei Leiomyosarkomen Im Metastasierten Und Initial Nichtresektablen Stadium. Strahlenther. Und Onkol. 2025, 201, 82–84. [Google Scholar] [CrossRef]
- Grosso, F.; D’Ambrosio, L.; Zucchetti, M.; Ibrahim, T.; Tamberi, S.; Matteo, C.; Rulli, E.; Comandini, D.; Palmerini, E.; Baldi, G.G.; et al. Pharmacokinetics, Safety, and Activity of Trabectedin as First-Line Treatment in Elderly Patients Who Are Affected by Advanced Sarcoma and Are Unfit to Receive Standard Chemotherapy: A Phase 2 Study (Tr1us Study) from the Italian Sarcoma Group. Cancer 2020, 126, 4726–4734. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Teruel, C.; Gonzalez, I.; Trocóniz, I.F.; Lubomirov, R.; Soto, A.; Fudio, S. Population-Pharmacokinetic and Covariate Analysis of Lurbinectedin (Pm01183), a New Rna Polymerase Ii Inhibitor, in Pooled Phase I/Ii Trials in Patients with Cancer. Clin. Pharmacokinet. 2019, 58, 363–374. [Google Scholar] [CrossRef]
- Wang, F.; Shen, Y.; Xu, X.; Lv, L.; Li, Y.; Liu, J.; Li, M.; Guo, A.; Guo, S.; Jin, F. Selective Tissue Distribution and Long Circulation Endowed by Paclitaxel Loaded Pegylated Poly(Ε-Caprolactone-Co-L-Lactide) Micelles Leading to Improved Anti-Tumor Effects and Low Systematic Toxicity. Int. J. Pharm. 2013, 456, 101–112. [Google Scholar] [CrossRef]
- Patel, S.; Petty, W.J.; Sands, J.M. An Overview of Lurbinectedin as a New Second-Line Treatment Option for Small Cell Lung Cancer. Ther. Adv. Med. Oncol. 2021, 13, 17588359211020529. [Google Scholar] [CrossRef] [PubMed]
- Grignani, G.; Le Cesne, A.; Martín-Broto, J. Trabectedin as Second-Line Treatment in Advanced Soft Tissue Sarcoma: Quality of Life and Safety Outcomes. Future Oncol. 2022, 18, 13–22. [Google Scholar] [CrossRef]
- Grigorian, A.; O’Brien, C.B. Hepatotoxicity Secondary to Chemotherapy. J. Clin. Transl. Hepatol. 2014, 2, 95–102. [Google Scholar] [CrossRef]
- Elez, M.E.; Tabernero, J.; Geary, D.; Macarulla, T.; Kang, S.P.; Kahatt, C.; Pita, A.S.; Teruel, C.F.; Siguero, M.; Cullell-Young, M.; et al. First-in-Human Phase I Study of Lurbinectedin (Pm01183) in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2014, 20, 2205–2214. [Google Scholar] [CrossRef]
- Leary, A.; Oaknin, A.; Trigo, J.M.; Moreno, V.; Delord, J.P.; Boni, V.; Braña, I.; Fernández, C.; Kahatt, C.; Nieto, A.; et al. Pooled Safety Analysis of Single-Agent Lurbinectedin in Patients with Advanced Solid Tumours. Eur. J. Cancer 2023, 192, 113259. [Google Scholar] [CrossRef] [PubMed]
- Manzo, A.; Sforza, V.; Carillio, G.; Palumbo, G.; Montanino, A.; Sandomenico, C.; Costanzo, R.; Esposito, G.; Laudato, F.; Mercadante, E.; et al. Lurbinectedin in Small Cell Lung Cancer. Front. Oncol. 2022, 12, 932105. [Google Scholar] [CrossRef] [PubMed]
- Cote, G.M.; Haddox, C.L.; Choy, E.; Merriam, P.A.; Mazzola, E.; Venkataraman, V.; Alcindor, T.; Wagner, A.J.; Demetri, G.D.; George, S. Safety and Efficacy of the Combination Lurbinectedin Plus Doxorubicin from a Phase 1b Trial in Patients with Advanced/Metastatic Soft-Tissue Sarcoma. Clin. Cancer Res. 2024, 30, 2702–2708. [Google Scholar] [CrossRef]
- Subbiah, V.; Braña, I.; Longhi, A.; Boni, V.; Delord, J.P.; Awada, A.; Boudou-Rouquette, P.; Sarantopoulos, J.; Shapiro, G.I.; Elias, A.; et al. Antitumor Activity of Lurbinectedin, a Selective Inhibitor of Oncogene Transcription, in Patients with Relapsed Ewing Sarcoma: Results of a Basket Phase Ii Study. Clin. Cancer Res. 2022, 28, 2762–2770. [Google Scholar] [CrossRef]
- Augsburger, D.; Nelson, P.J.; Kalinski, T.; Udelnow, A.; Knösel, T.; Hofstetter, M.; Qin, J.W.; Wang, Y.; Gupta, A.S.; Bonifatius, S.; et al. Current Diagnostics and Treatment of Fibrosarcoma -Perspectives for Future Therapeutic Targets and Strategies. Oncotarget 2017, 8, 104638–104653. [Google Scholar] [CrossRef] [PubMed]
- GmbH, Cytion Biosciences. Sw-684-Zellen. Available online: https://www.cytion.com/de/SW-684-Zellen/300422 (accessed on 27 August 2025).
- Dorand, R.D.; Donna, D.; Davis, E.J.; Park, B.H. Abstract 3974: Investigating Doxorubicin Resistance in Fibrosarcoma. Cancer Res. 2022, 82, 3974. [Google Scholar] [CrossRef]
- Lee, C.Y.; The, M.; Meng, C.; Bayer, F.P.; Putzker, K.; Müller, J.; Streubel, J.; Woortman, J.; Sakhteman, A.; Resch, M.; et al. Illuminating Phenotypic Drug Responses of Sarcoma Cells to Kinase Inhibitors by Phosphoproteomics. Mol. Syst. Biol. 2024, 20, 28–55. [Google Scholar] [CrossRef]
- Grethlein, S.J. Histology Driven Systemic Therapy of Liposarcoma-Ready for Prime Time? Transl. Gastroenterol. Hepatol. 2018, 3, 96. [Google Scholar] [CrossRef]
- Vos, M.; Koseła-Paterczyk, H.; Rutkowski, P.; van Leenders, G.J.L.H.; Normantowicz, M.; Lecyk, A.; Sleijfer, S.; Verhoef, C.; Grünhagen, D.J. Differences in Recurrence and Survival of Extremity Liposarcoma Subtypes. Eur. J. Surg. Oncol. 2018, 44, 1391–1397. [Google Scholar] [CrossRef]
- GmbH, Cytion Biosciences. Sw-872 Cells. Available online: https://www.cytion.com/SW-872-Cells/300405 (accessed on 28 July 2025).
- LaPensee, E.W.; Reddy, S.P.; Hugo, E.R.; Schwemberger, S.J.; Ben-Jonathan, N. Ls14 Cells: A Model for Chemoresistance in Liposarcoma. Cancer Biol. Ther. 2007, 6, 519–524. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Luk, S.J.; IJsselsteijn, M.E.; Somarakis, A.; Acem, I.; de Bruijn, I.B.; Szuhai, K.; Bovee, J.V.M.G.; de Miranda, N.F.C.C.; Falkenburg, J.H.F.; Heemskerk, M.H.M. Immunological Differences between Monophasic and Biphasic Synovial Sarcoma with Implications for Immunotherapy. Cancer Immunol. Immunother. 2024, 74, 31. [Google Scholar] [CrossRef] [PubMed]
- Sultan, I.; Rodriguez-Galindo, C.; Saab, R.; Yasir, S.; Casanova, M.; Ferrari, A. Comparing Children and Adults with Synovial Sarcoma in the Surveillance, Epidemiology, and End Results Program, 1983 to 2005: An Analysis of 1268 Patients. Cancer 2009, 115, 3537–3547. [Google Scholar] [CrossRef]
- Qi, Y.; Dong, S.S.; He, Y.L.; Liu, Z.H.; Huang, Y.L.; Wang, N.; Zhang, Z.; Li, Z.; Shi, M.E.T.H.T.M.; Feng, X.; et al. Syt-Ssx1 Enhances the Invasiveness and Maintains Stem-Like Cell Properties in Synovial Sarcoma Via Induction of Tgf-Β1/Smad Signaling. BMC Cancer 2022, 22, 166. [Google Scholar] [CrossRef]
- Joyner, D.E.; Albritton, K.H.; Bastar, J.D.; Randall, R.L. G3139 Antisense Oligonucleotide Directed against Antiapoptotic Bcl-2 Enhances Doxorubicin Cytotoxicity in the Fu-Sy-1 Synovial Sarcoma Cell Line. J. Orthop. Res. 2006, 24, 474–480. [Google Scholar] [CrossRef]
- Liu, A.; Feng, B.; Gu, W.; Cheng, X.; Tong, T.; Zhang, H.; Hu, Y. The Cd133+ Subpopulation of the Sw982 Human Synovial Sarcoma Cell Line Exhibits Cancer Stem-Like Characteristics. Int. J. Oncol. 2013, 42, 1399–1407. [Google Scholar] [CrossRef]
- Lohberger, B.; Rinner, B.; Stuendl, N.; Absenger, M.; Liegl-Atzwanger, B.; Walzer, S.M.; Windhager, R.; Leithner, A. Aldehyde Dehydrogenase 1, a Potential Marker for Cancer Stem Cells in Human Sarcoma. PLoS ONE 2012, 7, e43664. [Google Scholar] [CrossRef]
- Skapek, S.X.; Ferrari, A.; Gupta, A.A.; Lupo, P.J.; Butler, E.; Shipley, J.; Barr, F.G.; Hawkins, D.S. Rhabdomyosarcoma. Nat. Rev. Dis. Primers 2019, 5, 1. [Google Scholar] [CrossRef]
- Mandeville, H.C. Radiotherapy in the Management of Childhood Rhabdomyosarcoma. Clin. Onco. 2019, 31, 462–670. [Google Scholar] [CrossRef] [PubMed]
- Stratton, M.R.; Darling, J.; Pilkington, G.J.; Lantos, P.L.; Reeves, B.R.; Cooper, C.S. Characterization of the Human Cell Line Te671. Carcinogenesis 1989, 10, 899–905. [Google Scholar] [CrossRef]
- Friedman, H.S.; Schold, S.C., Jr.; Varia, M.; Bigner, D.D. Chemotherapy and Radiation Therapy of Human Medulloblastoma in Athymic Nude Mice. Cancer Res. 1983, 43, 3088–3093. [Google Scholar]
- Dodd, R.D.; Scherer, A.; Huang, W.; McGivney, G.R.; Gutierrez, W.R.; Laverty, E.A.; Ashcraft, K.A.; Stephens, V.R.; Yousefpour, P.; Saha, S.; et al. Tumor Subtype Determines Therapeutic Response to Chimeric Polypeptide Nanoparticle-Based Chemotherapy in Pten-Deleted Mouse Models of Sarcoma. Clin. Cancer Res. 2020, 26, 5036–5047. [Google Scholar] [CrossRef]
- Takahashi, R.; Mabuchi, S.; Kawano, M.; Sasano, T.; Matsumoto, Y.; Kuroda, H.; Kozasa, K.; Hashimoto, K.; Sawada, K.; Kimura, T. Preclinical Investigations of Pm01183 (Lurbinectedin) as a Single Agent or in Combination with Other Anticancer Agents for Clear Cell Carcinoma of the Ovary. PLoS ONE 2016, 11, e0151050. [Google Scholar] [CrossRef] [PubMed]
- Tummala, T.; Sevilla Uruchurtu, A.S.; Cruz, A.; Huntington, K.E.; George, A.; Liguori, N.R.; Zhang, L.; Zhou, L.; Abbas, A.E.; Azzoli, C.G.; et al. Preclinical Synergistic Combination Therapy of Lurbinectedin with Irinotecan and 5-Fluorouracil in Pancreatic Cancer. Curr. Oncol. 2023, 30, 9611–9626. [Google Scholar] [CrossRef] [PubMed]
- Herault, O.; Colombat, P.; Domenech, J.; Degenne, M.; Bremond, J.L.; Sensebe, L.; Bernard, M.C.; Binet, C. A Rapid Single-Laser Flow Cytometric Method for Discrimination of Early Apoptotic Cells in a Heterogenous Cell Population. Br. J. Haematol. 1999, 104, 530–537. [Google Scholar] [CrossRef]
- Belloc, F.; Dumain, P.; Boisseau, M.R.; Jalloustre, C.; Reiffers, J.; Bernard, P.; Lacombe, F. A Flow Cytometric Method Using Hoechst 33342 and Propidium Iodide for Simultaneous Cell Cycle Analysis and Apoptosis Determination in Unfixed Cells. Cytometry 1994, 17, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Cory, G. Scratch-Wound Assay. Methods Mol. Biol. 2011, 769, 25–30. [Google Scholar]
- Munshi, A.; Hobbs, M.; Meyn, R.E. Clonogenic Cell Survival Assay. Methods Mol. Med. 2005, 110, 21–28. [Google Scholar]
- Nuth, M.; Benakanakere, M.R.; Ricciardi, R.P. Discovery of a Potent Cytotoxic Agent That Promotes G(2)/M Phase Cell Cycle Arrest and Apoptosis in a Malignant Human Pharyngeal Squamous Carcinoma Cell Line. Int. J. Oncol. 2022, 60, 41. [Google Scholar] [CrossRef] [PubMed]
- Halloran, P.J.; Fenton, R.G. Irreversible G2-M Arrest and Cytoskeletal Reorganization Induced by Cytotoxic Nucleoside Analogues. Cancer Res. 1998, 58, 3855–3865. [Google Scholar] [PubMed]
- Evans, D.M.; Fang, J.; Silvers, T.; Delosh, R.; Laudeman, J.; Ogle, C.; Reinhart, R.; Selby, M.; Bowles, L.; Connelly, J.; et al. Exposure Time Versus Cytotoxicity for Anticancer Agents. Cancer Chemother. Pharmacol. 2019, 84, 359–371. [Google Scholar] [CrossRef]
- Schultz, C.W.; Zhang, Y.; Elmeskini, R.; Zimmermann, A.; Fu, H.; Murai, Y.; Wangsa, D.; Kumar, S.; Takahashi, N.; Atkinson, D.; et al. Atr Inhibition Augments the Efficacy of Lurbinectedin in Small-Cell Lung Cancer. EMBO Mol. Med. 2023, 15, e17313. [Google Scholar] [CrossRef]
- Moreno, I.; Hernández, T.; Calvo, E.; Fudio, S.; Kahatt, C.; Martínez, S.; Iglesias, J.L.; Calafati, R.O.; Pérez-Ramos, L.; Montilla, L.; et al. Pharmacokinetics and Safety of Lurbinectedin Administrated with Itraconazole in Cancer Patients: A Drug-Drug Interaction Study. Mar. Drugs. 2024, 22, 178. [Google Scholar] [CrossRef]
- Hallare, J.; Gerriets, V. Elimination Half-Life of Drugs; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Shin, S.Y.; Yong, Y.; Kim, C.G.; Lee, Y.H.; Lim, Y. Deoxypodophyllotoxin Induces G2/M Cell Cycle Arrest and Apoptosis in Hela Cells. Cancer Lett. 2010, 287, 231–239. [Google Scholar] [CrossRef]
- Marshall, S.; Nakano, K.; Sugiura, Y.; Taira, S.; Ono, M.; Tomomatsu, J.; Takahashi, S. Outcome for Advanced or Metastatic Soft Tissue Sarcoma of Nonextremities Treated with Doxorubicin-Based Chemotherapy: A Retrospective Study from a Single Cancer Institution. Sarcoma 2018, 2018, 8926598. [Google Scholar] [CrossRef]
- Landry, M.; Nelson, D.; Choi, E.; DuRoss, A.; Sun, C. Development of a G2/M Arrest High-Throughput Screening Method Identifies Potent Radiosensitizers. Transl. Oncol. 2022, 16, 101336. [Google Scholar] [CrossRef] [PubMed]
- Sui, M.; Zhang, H.; Di, X.; Chang, J.; Shen, Y.; Fan, W. G2 Checkpoint Abrogator Abates the Antagonistic Interaction between Antimicrotubule Drugs and Radiation Therapy. Radiother. Oncol. 2012, 104, 243–248. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Biade, S.; Stobbe, C.C.; Chapman, J.D. The Intrinsic Radiosensitivity of Some Human Tumor Cells Throughout Their Cell Cycles. Radiat. Res. 1997, 147, 416–421. [Google Scholar] [CrossRef]
- Eriksson, D.; Stigbrand, T. Radiation-Induced Cell Death Mechanisms. Tumour. Biol. 2010, 31, 363–372. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsheimer, H.; Schiller, P.; Semrau, S.; Grabenbauer, F.; Fietkau, R.; Distel, L.V.; Hildebrand, L.S. The Effect of Lurbinectedin as a Monotherapy and in Combination with Ionizing Radiation on Sarcoma Cell Lines. Cancers 2025, 17, 2930. https://doi.org/10.3390/cancers17172930
Alsheimer H, Schiller P, Semrau S, Grabenbauer F, Fietkau R, Distel LV, Hildebrand LS. The Effect of Lurbinectedin as a Monotherapy and in Combination with Ionizing Radiation on Sarcoma Cell Lines. Cancers. 2025; 17(17):2930. https://doi.org/10.3390/cancers17172930
Chicago/Turabian StyleAlsheimer, Hannah, Paula Schiller, Sabine Semrau, Felix Grabenbauer, Rainer Fietkau, Luitpold V. Distel, and Laura S. Hildebrand. 2025. "The Effect of Lurbinectedin as a Monotherapy and in Combination with Ionizing Radiation on Sarcoma Cell Lines" Cancers 17, no. 17: 2930. https://doi.org/10.3390/cancers17172930
APA StyleAlsheimer, H., Schiller, P., Semrau, S., Grabenbauer, F., Fietkau, R., Distel, L. V., & Hildebrand, L. S. (2025). The Effect of Lurbinectedin as a Monotherapy and in Combination with Ionizing Radiation on Sarcoma Cell Lines. Cancers, 17(17), 2930. https://doi.org/10.3390/cancers17172930







