Dynamic Tumor Tracking (DTT) for Hepatocellular Carcinoma Using the Vero4DRT Gimbaled Linac Stereotactic Body Radiation Therapy (SBRT) System
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Radiation Therapy
2.3. Outcomes of Interest, Follow-Up, and Censoring
2.4. Statistics
3. Results
3.1. Patients and Treatment
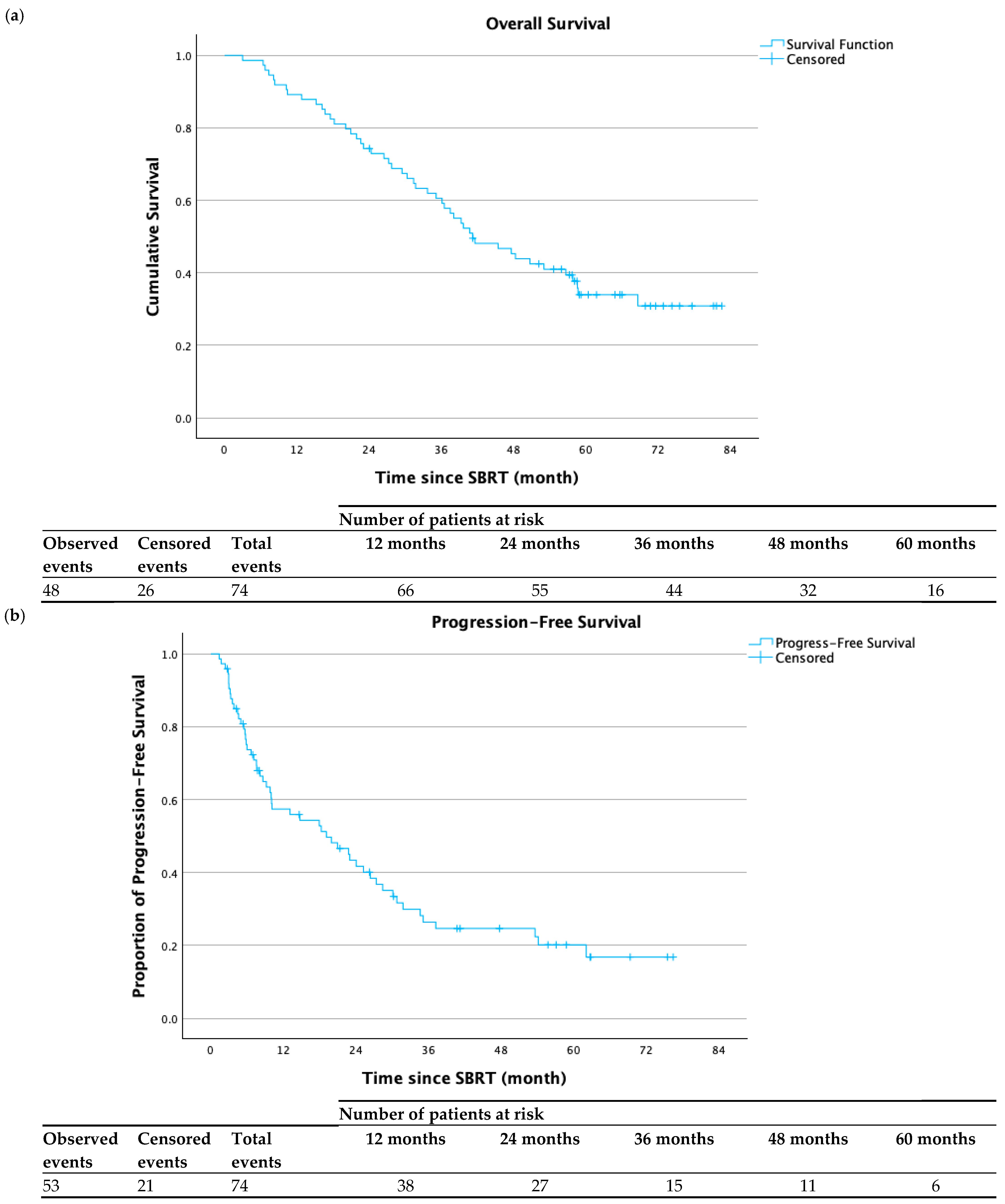
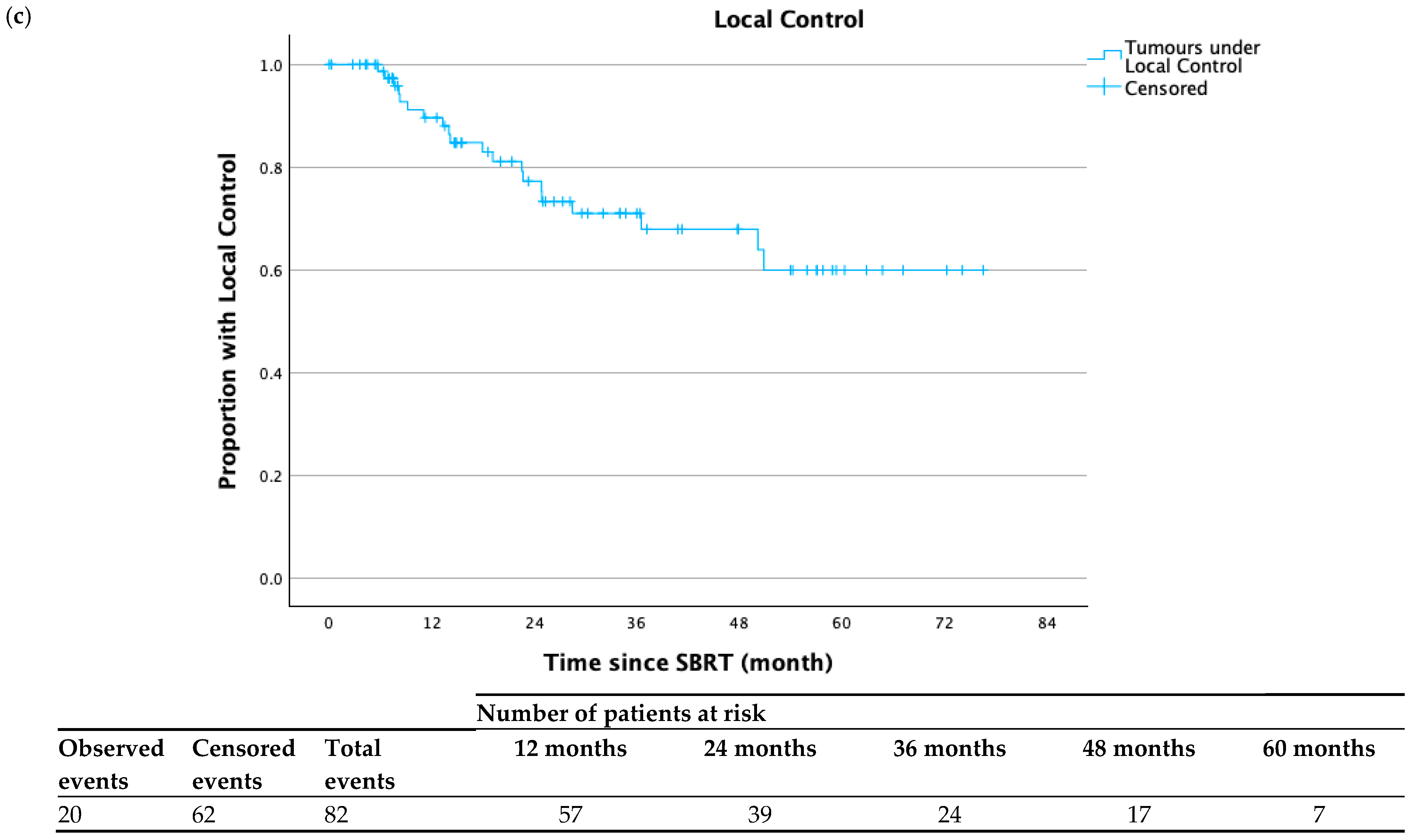
3.2. Clinical Outcomes
3.3. Predictors of Outcome
3.4. Toxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Bosetti, C.; Turati, F.; La Vecchia, C. Hepatocellular Carcinoma Epidemiology. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 753–770. [Google Scholar] [CrossRef]
- Andolino, D.L.; Johnson, C.S.; Maluccio, M.; Kwo, P.; Tector, A.J.; Zook, J.; Johnstone, P.A.S.; Cardenes, H.R. Stereotactic Body Radiotherapy for Primary Hepatocellular Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, e447–e453. [Google Scholar] [CrossRef]
- Bujold, A.; Massey, C.A.; Kim, J.J.; Brierley, J.; Cho, C.; Wong, R.K.S.; Dinniwell, R.E.; Kassam, Z.; Ringash, J.; Cummings, B.; et al. Sequential Phase I and II Trials of Stereotactic Body Radiotherapy for Locally Advanced Hepatocellular Carcinoma. J. Clin. Oncol. 2013, 31, 1631–1639. [Google Scholar] [CrossRef]
- Wang, Y.F.; Dai, Y.H.; Lin, C.S.; Chang, H.C.; Shen, P.C.; Yang, J.F.; Hsiang, C.W.; Lo, C.H.; Huang, W.Y. Clinical Outcome and Pathologic Correlation of Stereotactic Body Radiation Therapy as a Bridge to Transplantation for Advanced Hepatocellular Carcinoma: A Case Series. Radiat. Oncol. 2021, 16, 15. [Google Scholar] [CrossRef]
- Wang, F.; Numata, K.; Takeda, A.; Ogushi, K.; Fukuda, H.; Hara, K.; Chuma, M.; Eriguchi, T.; Tsurugai, Y.; Maeda, S. Safety and Efficacy Study: Short-Term Application of Radiofrequency Ablation and Stereotactic Body Radiotherapy for Barcelona Clinical Liver Cancer Stage 0-B1 Hepatocellular Carcinoma. PLoS ONE 2021, 16, e0245076. [Google Scholar] [CrossRef]
- Shen, P.C.; Chang, W.C.; Lo, C.H.; Yang, J.F.; Lee, M.S.; Dai, Y.H.; Lin, C.S.; Fan, C.Y.; Huang, W.Y. Comparison of Stereotactic Body Radiation Therapy and Transarterial Chemoembolization for Unresectable Medium-Sized Hepatocellular Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Kubo, K.; Kimura, T.; Aikata, H.; Takahashi, S.; Takeuchi, Y.; Takahashi, I.; Nishibuchi, I.; Murakami, Y.; Chayama, K.; Nagata, Y. Long-Term Outcome of Stereotactic Body Radiotherapy for Patients with Small Hepatocellular Carcinoma. Hepatol. Res. 2018, 48, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.I.; Bae, S.H.; Kim, M.-S.; Han, C.J.; Park, S.C.; Kim, S.B.; Cho, E.-H.; Choi, C.W.; Kim, K.S.; Hwang, S.; et al. A Phase 2 Multicenter Study of Stereotactic Body Radiotherapy for Hepatocellular Carcinoma: Safety and Efficacy. Cancer 2020, 126, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Huo, T.-I.; Liu, P.-H.; Ho, S.-Y. Treating Small Hepatocellular Carcinoma: Stereotactic Body Radiation Therapy Versus Radiofrequency Ablation. J. Gastroenterol. Hepatol. 2020, 35, 2020. [Google Scholar] [CrossRef]
- Yoon, S.M.; Kim, S.Y.; Lim, Y.S.; Kim, K.M.; Shim, J.H.; Lee, D.; An, J.; Jung, J.; Kim, J.H.; Lee, H.C. Stereotactic Body Radiation Therapy for Small (≤5 Cm) Hepatocellular Carcinoma Not Amenable to Curative Treatment: Results of a Single-Arm, Phase Ii Clinical Trial. Clin. Mol. Hepatol. 2020, 26, 506–515. [Google Scholar] [CrossRef]
- Pan, Y.X.; Fu, Y.Z.; Hu, D.D.; Long, Q.; Wang, J.C.; Xi, M.; Liu, S.L.; Xu, L.; Liu, M.Z.; Chen, M.S.; et al. Stereotactic Body Radiotherapy vs. Radiofrequency Ablation in the Treatment of Hepatocellular Carcinoma: A Meta-Analysis. Front. Oncol. 2020, 10, 1639. [Google Scholar] [CrossRef] [PubMed]
- Brunner, T.B.; Bettinger, D.; Schultheiss, M.; Maruschke, L.; Sturm, L.; Bartl, N.; Koundurdjieva, I.; Kirste, S.; Neeff, H.P.; Goetz, C.; et al. Efficacy of Stereotactic Body Radiotherapy in Patients with Hepatocellular Carcinoma Not Suitable for Transarterial Chemoembolization (HERACLES: HEpatocellular Carcinoma Stereotactic RAdiotherapy CLinical Efficacy Study). Front. Oncol. 2021, 11, 653141. [Google Scholar] [CrossRef]
- Murray, L.J.; Dawson, L.A. Advances in Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma. Semin. Radiat. Oncol. 2017, 27, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Takeda, A.; Sanuki, N.; Ariyoshi, K.; Yamaguchi, T.; Imagumbai, T.; Katoh, N.; Eriguchi, T.; Oku, Y.; Ozawa, S.; et al. Multicenter Prospective Study of Stereotactic Body Radiotherapy for Previously Untreated Solitary Primary Hepatocellular Carcinoma: The STRSPH Study. Hepatol. Res. 2021, 51, 461–471. [Google Scholar] [CrossRef]
- Prasetio, H.; Wölfelschneider, J.; Ziegler, M.; Serpa, M.; Witulla, B.; Bert, C. Dose Calculation and Verification of the Vero Gimbal Tracking Treatment Delivery. Phys. Med. Biol. 2018, 63, 035043. [Google Scholar] [CrossRef]
- Iizuka, Y.; Matsuo, Y.; Ishihara, Y.; Akimoto, M.; Tanabe, H.; Takayama, K.; Ueki, N.; Yokota, K.; Takashi, M.; Masaki, K.; et al. Dynamic Tumour-Tracking Radiotherapy with Real-Time Monitoring for Liver Tumours Using a Gimbal Mounted Linac. Radiother. Oncol. 2015, 117, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, Y.; Hiraoka, M.; Kokubo, M.; Sakamoto, T.; Karasawa, K.; Murofushi, K.; Nakamura, M.; Matsuo, Y.; Morita, S.; Inokuchi, H.; et al. Dynamic Tumour-Tracking Stereotactic Body Radiotherapy with Real-Time Monitoring of Liver Tumours Using a Gimbal-Mounted Linac: A Multi-Institutional Phase II Study. Clin. Transl. Radiat. Oncol. 2023, 39, 100591. [Google Scholar] [CrossRef]
- Depuydt, T.; Poels, K.; Verellen, D.; Engels, B.; Collen, C.; Buleteanu, M.; Van den Begin, R.; Boussaer, M.; Duchateau, M.; Gevaert, T.; et al. Treating Patients with Real-Time Tumor Tracking Using the VERO GIMBALED LINAC System: Implementation and First Review. Radiother. Oncol. 2014, 112, 343–351. [Google Scholar] [CrossRef]
- Ziegler, M.; Brandt, T.; Lettmaier, S.; Fietkau, R.; Bert, C. Performance of Gimbal-Based Dynamic Tumour Tracking for Treating Liver Carcinoma. Radiat. Oncol. 2018, 13, 242. [Google Scholar] [CrossRef]
- Shirato, H.; Seppenwoolde, Y.; Kitamura, K.; Onimura, R.; Shimizu, S. Intrafractional Tumour Motion: Lung and Liver. Semin. Radiat. Oncol. 2004, 14, 10–18. [Google Scholar] [CrossRef]
- Takayama, K.; Mizowaki, T.; Kokubo, M.; Kawada, N.; Nakayama, H.; Narita, Y.; Nagano, K.; Kamino, Y.; Hiraoka, M. Initial Validations for Pursuing Irradiation Using a Gimbals Tracking System. Radiother. Oncol. 2009, 93, 45–49. [Google Scholar] [CrossRef]
- Sapir, E.; Tao, Y.; Schipper, M.J.; Bazzi, L.; Novelli, P.M.; Devlin, P.; Owen, D.; Cuneo, K.C.; Lawrence, T.S.; Parikh, N.D.; et al. Stereotactic Body Radiation Therapy as an Alternative to Transarterial Chemoembolization for Hepatocellular Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 122–130. [Google Scholar] [CrossRef]
- Comito, T.; Loi, M.; Franzese, C.; Clerici, E.; Franceschini, D.; Badalamenti, M.; Teriaca, M.A.; Rimassa, L.; Pedicini, V.; Poretti, D.; et al. Stereotactic Radiotherapy after Incomplete Transarterial (Chemo-) Embolization (Tae\tace) versus Exclusive Tae or TACE for Treatment of Inoperable HCC: A Phase III Trial (NCT02323360). Curr. Oncol. 2022, 29, 8802–8813. [Google Scholar] [CrossRef]
- Chen, G.; Tai, A.; Keiper, T.D.; Lim, S.; Li, X.A. Technical Note: Comprehensive Performance Tests of the First Clinical Real-time Motion Tracking and Compensation System Using MLC and Jaws. Med. Phys. 2020, 47, 2814–2825. [Google Scholar] [CrossRef] [PubMed]
- Schweikard, A.; Shiomi, H.; Adler, J. Respiration Tracking in Radiosurgery. Med. Phys. 2004, 31, 2738–2741. [Google Scholar] [CrossRef] [PubMed]
- Wahl, D.R.; Stenmark, M.H.; Tao, Y.; Pollom, E.L.; Caoili, E.M.; Lawrence, T.S.; Schipper, M.J.; Feng, M. Outcomes after Stereotactic Body Radiotherapy or Radiofrequency Ablation for Hepatocellular Carcinoma. J. Clin. Oncol. 2016, 34, 452–459. [Google Scholar] [CrossRef]
- Bae, S.H.; Chun, S.J.; Chung, J.H.; Kim, E.; Kang, J.K.; Jang, W.I.; Moon, J.E.; Roquette, I.; Mirabel, X.; Kimura, T.; et al. Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma: Meta-Analysis and International Stereotactic Radiosurgery Society Practice Guidelines. Int. J. Radiat. Oncol. Biol. Phys. 2024, 118, 337–351. [Google Scholar] [CrossRef] [PubMed]
- American College of Radiology. CT/MRI Liver Imaging Reporting & Data System (LI-RADS) v2018; American College of Radiology: Reston, VA, USA, 2018. [Google Scholar] [CrossRef]
- Kitamura, K.; Shirato, H.; Seppenwoolde, Y.; Shimizu, T.; Kodama, Y.; Endo, H.; Onimaru, R.; Oda, M.; Fujita, K.; Shimizu, S.; et al. Tumour Location, Cirrhosis, and Surgical History Contribute to Tumour Movement in the Liver, as Measured during Stereotactic Irradiation Using a Real-Time Tumour-Tracking Radiotherapy System. Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 221–228. [Google Scholar] [CrossRef]
- Keall, P.J.; Mageras, G.S.; Balter, J.M.; Emery, R.S.; Forster, K.M.; Jiang, S.B.; Kapatoes, J.M.; Low, D.A.; Murphy, M.J.; Murray, B.R.; et al. The Management of Respiratory Motion in Radiation Oncology Report of AAPM Task Group 76. Med. Phys. 2006, 33, 3874–3900. [Google Scholar] [CrossRef]
- Bertholet, J.; Knopf, A.; Eiben, B.; McClelland, J.; Grimwood, A.; Harris, E.; Menten, M.; Poulsen, P.; Nguyen, D.T.; Keall, P.; et al. Real-Time Intrafraction Motion Monitoring in External Beam Radiotherapy. Phys. Med. Biol. 2019, 64, 15TR01. [Google Scholar] [CrossRef]
- Giraud, P.; Houle, A. Respiratory Gating for Radiotherapy: Main Technical Aspects and Clinical Benefits. ISRN Pulmonol. 2013, 2013, 519602. [Google Scholar] [CrossRef]
- Dunne, E.; Bergman, A.; Rodgerson, C.; Camborde, M.L.; Karan, T.; Liu, M.; Schellenberg, D.; Ma, R. Dynamic Tumour Tracking (DTT) for Hepatocellular Carcinoma using a Gimbaled Linac Stereotactic Body Radiation Therapy (SBRT) System. Radiother. Oncol. 2019, 139, S94. [Google Scholar] [CrossRef]
- Carpentier, E.E.; McDermott, R.L.; Dunne, E.M.; Camborde, M.L.A.; Bergman, A.M.; Karan, T.; Liu, M.C.C.; Ma, R.M.K.; Mestrovic, A. Four-Dimensional Dose Calculations for Dynamic Tumour Tracking with a Gimbal-Mounted Linear Accelerator. J. Appl. Clin. Med. Phys. 2021, 22, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Rostamzadeh, M.; Ishihara, Y.; Nakamura, M.; Popescu, I.A.; Mestrovic, A.; Gete, E.; Fedrigo, R.; Bergman, A.M. Monte Carlo Simulation of 6-MV Dynamic Wave VMAT Deliveries by VERO4DRT Linear Accelerator Using EGSNRC Moving Sources. J. Appl. Clin. Med. Phys. 2020, 21, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, E.E.; Mcdermott, R.L.; Su, S.; Rostamzadeh, M.; Popescu, I.A.; Bergman, A.M.; Mestrovic, A. Monte Carlo Modeling of Dynamic Tumor Tracking on a Gimbaled Linear Accelerator. J. Med. Phys. 2023, 48, 50–58. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Number (%) |
|---|---|
| Patient Characteristics | |
| Total patients | 74 |
| Total tumors treated | 82 |
| Median age at diagnosis—(range), y | 65.3 (36.0–87.5) |
| Median age at treatment—(range), y | 67.9 (36.4–91.0) |
| Sex | |
| Male | 54 (73.0%) |
| Female | 20 (27.0%) |
| Cause of Cirrhosis | |
| HCV | 34 (45.9%) |
| HBV | 21 (28.4%) |
| NASH | 9 (12.2%) |
| ALD | 7 (9.5%) |
| PBC | 2 (2.7%) |
| Cryptogenic | 1 (1.4%) |
| Child–Pugh Score Pre-SBRT | |
| A5 | 53 (71.6%) |
| A6 | 15 (20.3%) |
| B7 | 3 (4.1%) |
| B8 | 3 (4.1%) |
| Prior Local Liver Treatment | |
| Yes | 49 (66.2%) |
| No | 25 (33.8%) |
| Tumor Characteristics | |
| Tumor Max. Diameter | |
| Median (range), cm | 3.3 (1.1–10.1) |
| Gross Tumor Volume | |
| Median (range), cm3 | 15.8 (0.7–241.9) |
| Clinical Target Volume | |
| Median (range), cm3 | 39.5 (4.2–303.4) |
| Planning Target Volume | |
| Median (range), cm3 | 99.6 (15.1–563.2) |
| Prescribed Dose Fractionation (BEDGy10) | |
| 45 Gy, 3 fractions (112.5 Gy10) | 48 (58.6%) |
| 45 Gy, 5 fractions (85.5 Gy10) | 27 (33.0%) |
| 42.5 Gy, 5 fractions (78.6 Gy10) | 1 (1.2%) |
| 35 Gy, 3 fractions (75.8 Gy10) | 1 (1.2%) |
| 40 Gy, 5 fractions (72 Gy10) | 2 (2.4%) |
| 35 Gy, 5 fractions (59.5 Gy10) | 1 (1.2%) |
| 30 Gy, 5 fractions (48 Gy10) | 2 (2.4%) |
| 3 Fractions | 5 Fractions | |
|---|---|---|
| Spinal Cord PRV | Dmax ≤ 20.3 Gy | Dmax ≤ 25.3 Gy |
| Liver minus GTV | ≥700 cm3 ≤ 17 Gy | ≥700 cm3 ≤ 21 Gy |
| Duodenum | Dmax ≤ 22.2 Gy | Dmax ≤ 32 Gy |
| Stomach | Dmax ≤ 22.2 Gy | Dmax ≤ 32 Gy |
| Small Bowel | Dmax ≤ 25.2 Gy | Dmax ≤ 29 Gy |
| Large Bowel | Dmax ≤ 28.2 Gy | Dmax ≤ 38 Gy |
| Heart | Dmax ≤ 30 Gy V24 Gy ≤ 15 cm3 | Dmax ≤ 38 Gy V24 Gy ≤ 15 cm3 |
| Esophagus | Dmax ≤ 27 Gy | Dmax ≤ 35 Gy |
| Chest Wall | Dmax ≤ 44 Gy V30 Gy ≤ 30 cm3 | Dmax ≤ 55 Gy V37 Gy ≤ 30 cm3 |
| Great Vessels | Dmax ≤ 44 Gy | Dmax ≤ 51.5 Gy |
| Patient Number | GTV Volume (cm3) | Prescribed BED (Gy10) | CTV D99.5% (Gy10) | Time from SBRT to Transplant (Months) | OLT Pathology Report |
|---|---|---|---|---|---|
| 1 | 13.2 | 112.5 | 135.8 | 15.7 | No residual tumor |
| 2 | 17.6 | 85.5 | 101.8 | 8.6 | No residual tumor |
| 3 | 9.2 | 85.5 | 91.3 | 2.1 | 30% necrosis |
| 4 | 2.8 | 112.5 | 129.8 | 8.1 | No residual tumor |
| 5 | 3.1 | 112.5 | 130.6 | 50.1 | Friable necrotic center with 2.2 cm viable tumor |
| 6 | 31.4 | 112.5 | 121.5 | 10.1 | Partial necrosis |
| 7 | 5.7 | 85.5 | 75.2 | 5.9 | No residual tumor |
| 8 | 27.5 | 112.5 | 137.6 | 18.2 | Viable tumor present |
| 9 | 53.5 | 112.5 | 116.7 | 18.3 | 90% necrosis |
| 10 | 50.2 | 72 | 58.5 | 6.3 | 30% necrosis |
| 11 | 5.8 | 112.5 | 123.5 | 16.7 | 50% necrosis |
| 12 | 13.2 | 85.5 | 94.6 | 39.2 | Viable tumor present |
| Variable | p-Value | HR (95% CI) | p-Value | HR (95% CI) |
|---|---|---|---|---|
| Univariate (Cox Regression) | Multivariate (Cox Regression) | |||
| Age at treatment | 0.457 | 1.016 (0.974–1.061) | ||
| Gender (female vs. male) | 0.054 | 2.381 (0.984–5.764) | 0.233 | 1.781 (0.690–4.599) |
| Cause of cirrhosis (non-viral vs. HBV/HCV) | 0.013 | 3.138 (1.276–7.719) | 0.056 | 2.564 (0.976–6.733) |
| Pre-SBRT CPS (A6 or higher vs. A5) | 0.383 | 0.520 (0.120–2.258) | ||
| Prescribed dose, fractionation (45 Gy, 3 vs. other doses, fractionations) | 0.590 | 1.288 (0.513–3.231) | ||
| Prescribed BED10 (<112.5 Gy) | 0.590 | 0.777 (0.310–1.949) | ||
| Prior focal liver treatment | 0.700 | 0.835 (0.333–2.095) | ||
| Tumor diameter (≥3.3 cm) | 0.532 | 1.325 (0.548–3.205) | ||
| GTV volume (≥30 cm3) | 0.285 | 1.621 (0.669–3.925) | ||
| CTV D99.5% BED10 <100 Gy | 0.804 | 1.118 (0.462–2.704) | ||
| PTV volume (≥150 cm3) | 0.169 | 1.851 (0.770–4.453) | ||
| PTV D99.5% BED10 <80 Gy | 0.931 | 0.962 (0.398–2.326) | ||
| PTV D99.5% BED10 <100 Gy | 0.904 | 0.943 (0.361–2.459) | ||
| Variable | p-Value | HR (95% CI) | p-Value | HR (95% CI) |
|---|---|---|---|---|
| Univariate (Cox Regression) | Multivariate (Cox Regression) | |||
| Age at treatment | 0.210 | 1.030 (0.983–1.080) | ||
| Gender (female vs. male) | 0.177 | 2.052 (0.722–5.829) | ||
| Cause of cirrhosis (Non-viral vs. HBV/HCV) | 0.283 | 1.898 (0.589–6.123) | ||
| Pre-SBRT CPS (A6 or higher vs. A5) | 0.956 | 0.964 (0.268–3.474) | ||
| Prescribed BED10 (<112.5 Gy) | 0.232 | 1.910 (0.661–5.522) | ||
| Any prior focal liver treatment | 0.097 | 0.391 (0.129–1.186) | 0.628 | 0.565 (0.296–3.511) |
| Tumor diameter (≥3.3 cm) | 0.101 | 2.470 (0.838–7.278) | ||
| GTV volume (≥30 cm3) | 0.038 | 2.997 (1.063–8.452) | 0.886 | 0.890 (0.181–4.376) |
| PTV volume (≥150 cm3) | 0.010 | 4.097 (1.399–11.996) | 0.076 | 4.290 (0.858–21.443) |
| Pre-SBRT platelet count (<100,000/mm3) | 0.105 | 0.022 (0.000–2.217) | ||
| Volume of liver-GTV (<1000 cm3) | 0.554 | 1.574 (0.351–7.050) | ||
| Variable | p-Value | HR (95% CI) | p-Value | HR (95% CI) |
|---|---|---|---|---|
| Univariate (Cox Regression) | Multivariate (Cox Regression) | |||
| Age at treatment | 0.239 | 0.969 (0.921–1.021) | ||
| Gender (female vs. male) | 0.142 | 2.273 (0.759–6.806) | ||
| Cause of cirrhosis (non-viral vs. HBV/HCV) | 0.033 | 3.359 (1.103–10.228) | 0.579 | 1.452 (0.388–5.428) |
| Pre-SBRT CPS (A6 or higher vs. A5) | 0.010 | 4.992 (1.457–17.101) | 0.044 | 4.189 (1.040–16.882) |
| Prescribed BED10 (<112.5 Gy) | 0.267 | 1.926 (0.606–6.121) | ||
| Any prior focal liver treatment | 0.096 | 0.362 (0.109–1.196) | ||
| Tumor diameter (≥3.3 cm) | 0.521 | 1.433 (0.477–4.301) | ||
| GTV volume (≥30 cm3) | 0.265 | 1.873 (0.621–5.645) | ||
| PTV (≥150 cm3) | 0.612 | 1.337 (0.436–4.103) | ||
| Pre-SBRT platelet count (<100,000/mm3) | 0.017 | 4.335 (1.306–14.384) | 0.054 | 3.583 (0.979–13.116) |
| Volume of liver-GTV (<1000 cm3) | 0.093 | 3.069 (0.828–11.374) | 0.210 | 1.985 (0.658–5.411) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McDermott, R.L.; Dunne, E.M.; Ma, L.I.J.; Bergman, A.M.; Camborde, M.-L.A.; Karan, T.; Mestrovic, A.; Carpentier, E.E.; Liu, M.C.C.; Schellenberg, D.; et al. Dynamic Tumor Tracking (DTT) for Hepatocellular Carcinoma Using the Vero4DRT Gimbaled Linac Stereotactic Body Radiation Therapy (SBRT) System. Cancers 2025, 17, 2926. https://doi.org/10.3390/cancers17172926
McDermott RL, Dunne EM, Ma LIJ, Bergman AM, Camborde M-LA, Karan T, Mestrovic A, Carpentier EE, Liu MCC, Schellenberg D, et al. Dynamic Tumor Tracking (DTT) for Hepatocellular Carcinoma Using the Vero4DRT Gimbaled Linac Stereotactic Body Radiation Therapy (SBRT) System. Cancers. 2025; 17(17):2926. https://doi.org/10.3390/cancers17172926
Chicago/Turabian StyleMcDermott, Ronan L., Emma M. Dunne, Lok In Josephine Ma, Alanah M Bergman, Marie-Laure A. Camborde, Tania Karan, Ante Mestrovic, Emilie E. Carpentier, Mitchell C. C. Liu, Devin Schellenberg, and et al. 2025. "Dynamic Tumor Tracking (DTT) for Hepatocellular Carcinoma Using the Vero4DRT Gimbaled Linac Stereotactic Body Radiation Therapy (SBRT) System" Cancers 17, no. 17: 2926. https://doi.org/10.3390/cancers17172926
APA StyleMcDermott, R. L., Dunne, E. M., Ma, L. I. J., Bergman, A. M., Camborde, M.-L. A., Karan, T., Mestrovic, A., Carpentier, E. E., Liu, M. C. C., Schellenberg, D., & Ma, R. M. K. (2025). Dynamic Tumor Tracking (DTT) for Hepatocellular Carcinoma Using the Vero4DRT Gimbaled Linac Stereotactic Body Radiation Therapy (SBRT) System. Cancers, 17(17), 2926. https://doi.org/10.3390/cancers17172926






