Simple Summary
Liver transplantation (LT) is a curative treatment for patients with hepatocellular carcinoma (HCC), especially those with advanced liver disease. However, HCC can return after LT, with recurrence rates between 15–20% within the first two years. Managing small, localized recurrences effectively is key to improving patient outcomes. This study reviewed previous research comparing two treatments for HCC recurrence after LT: surgical resection and locoregional therapies (LRT). After following strict selection guidelines, ten studies from 2009 to 2024 were analyzed. Results showed that patients who had surgery had better outcomes than those treated with LRT. Specifically, one-year overall survival (OS) was higher in the surgery group (71% vs. 62%), and one-year disease-free survival (DFS) was also better (60% vs. 54%). It’s important to note that patients receiving LRT tended to have more aggressive cancer features before their transplant, such as microvascular invasion and higher alpha-fetoprotein levels. In conclusion, while surgery offers better survival chances, treatment decisions must consider each patient’s tumor features and liver condition. Future guidelines that include new treatments like immunotherapy and targeted therapies will help improve care for patients facing HCC recurrence after liver transplantation.
Abstract
Background: Liver transplantation (LT) is regarded as a curative approach for patients with hepatocellular carcinoma (HCC), especially those with underlying advanced liver disease. However, the recurrence of HCC post-LT poses significant challenges, with reported rates of 15–20% within the first two years following surgery. Effective management of single-nodule recurrence is critical to improving patient outcomes. Methods: This meta-analysis evaluates the efficacy of surgical resection versus locoregional therapies (LRT) in patients with localized HCC recurrence after LT. We adhered to the PRISMA Statement in conducting a thorough search of relevant studies published from 2009 to 2024, ultimately including ten studies that met our eligibility criteria. Results: The results indicate that patients undergoing surgical treatment displayed superior one-year overall survival (OS) rates compared to those receiving LRT (71% vs. 62%, p = 0.038), as well as higher one-year disease-free survival (DFS) rates (60% vs. 54%, p = 0.042). Notably, patients in the LRT group presented with more advanced HCC characteristics prior to transplantation, including higher rates of microvascular invasion and elevated alpha-fetoprotein levels. Conclusions: Our findings suggest that while surgical resection is associated with better survival outcomes, the choice between surgical and locoregional approaches must be individualized based on tumor characteristics and liver function. The ongoing development of standardized guidelines with the inclusion of immunotherapy or targeted agents will be essential in refining treatment pathways and improving outcomes for patients experiencing HCC recurrence following LT.
1. Introduction
Liver transplantation (LT) is the most effective treatment option for patients with hepatocellular carcinoma (HCC), especially in cases of advanced liver disease or neoplasms unsuitable for other local treatments like resection or ablation [1]. As one of the most prevalent malignancies globally, HCC typically arises from chronic liver conditions, such as hepatitis B and C, alcoholic liver disease, or metabolic-associated fatty liver disease (MAFLD), and is complicated by high morbidity and mortality [2]. Given these challenges, LT offers an effective dual solution: it not only removes the liver tumor but also replaces the damaged organ, reducing the risk of cancer recurrence associated with a damaged hepatic environment [3].
Nevertheless, transplantation offers a curative solution for patients with advanced disease or multifocal tumors within set criteria, which are aimed at selecting patients with a lower risk of recurrence.
Since the Milan criteria were introduced in the 1990s, patient selection for LT in HCC cases has become more precise. These criteria, which prioritize single tumors no larger than 5 cm or up to three tumors no larger than 3 cm each, aim to select candidates with a favorable prognosis [4]. Subsequent criteria, like the UCSF criteria, have sought to expand the pool of transplant candidates while maintaining acceptable post-transplant outcomes, offering an effective therapeutic pathway for patients who may not have qualified under Milan standards [5].
Despite the benefits, post-LT HCC recurrence remains a challenge, occurring in approximately 15–20% of cases within the first two years after surgery [6]. Standard recurrence prevention involves rigorous selection through the adoption of the aforementioned criteria, neoadjuvant treatments, and postoperative immunosuppressive management.
Understanding and managing recurrence is crucial to improve the outcomes [7]. The mechanisms driving HCC recurrence post-LT are multifactorial. Recurrence may result from residual microscopic disease not detected or removed at the time of LT or from metastatic dissemination that had occurred prior to the procedure. In some cases, the persistence of underlying oncogenic risk factors, such as viral hepatitis or metabolic syndrome, may contribute to the formation of new tumors [8].
The management of HCC recurrence after LT presents significant challenges. Due to the immunosuppressive environment required to prevent graft rejection, typical immune responses against cancer are weakened, complicating therapeutic options. Despite these challenges, several strategies have shown promise in managing both disseminated and localized recurrences [9].
The treatment strategies for a single-nodule HCC recurrence post-liver transplant can generally be categorized into surgical and local therapies. Surgical resection, which involves the removal of the recurrent tumor through surgery, is often considered when the patient is in good health and the lesion is deemed resectable. This approach can potentially achieve a clear margin of excision, which is associated with a lower risk of further recurrence. Surgical resection, however, is an invasive procedure that carries certain risks, especially in post-transplant patients who are often immunosuppressed and may have compromised liver function due to the cumulative effects of medications and underlying comorbidities. The invasiveness of surgery, coupled with the potential risk to the transplanted organ, often limits the applicability of resection in this population [10].
Locoregional therapies offer alternatives to resection, particularly in cases where the tumor is small, solitary, or where surgical resection would involve excessive risk. Transarterial Chemoembolization (TACE) delivers chemotherapy directly to the tumor site, reducing systemic exposure. Radiofrequency Ablation (RFA) and Microwave Ablation (MWA) use high-frequency radio waves to destroy cancer cells.
These therapies are less invasive than surgery and can often be repeated if recurrence occurs. However, they may not provide as definitive a solution as surgical resection, especially in cases where complete tumor removal is achievable through surgery [11].
The choice between surgical and local therapies in the setting of a single HCC recurrence after an LT requires a nuanced approach that accounts for multiple factors.
Our work aims to perform a literature review on the oncological and short-term survival outcomes in patients with localized recurrence of HCC after LT who are undergoing surgery or LRT.
2. Methods
This systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement criteria. The prospective review protocol was registered on PROSPERO with the following ID: 635506. If a reviewer was present in the authorship of a paper in evaluation, the decision to include that study was undertaken by other members of the review group.
As this was a review, ethics approval was not required.
2.1. Study Eligibility Criteria
Studies were included according to the following eligibility criteria: (1) Prospective or retrospective studies of HCC recurrence after LT; (2) publication within the last 15 years (2009–2024); (3) a liver malignancy histologically characterized as HCC, (4) an LT as prior surgical treatment, (5) a diagnosis of unifocal HCC recurrence, (6) a surgical treatment of the HCC recurrence as intervention; (7) a comparator of locoregional treatment (LRT), based on radiofrequency ablation (RFA), transarterial chemoembolization (TACE) or transarterial radioembolization (TARE), (8) one or more patient outcomes (1 year overall survival, 1 year disease-free survival).
We excluded papers without survival outcomes reported specifically. If studies presented overlapping patient populations, only the most up-to-date cohorts were included.
Randomized controlled trials (RCTs) and nonrandomized studies (nonrandomized controlled trials, interrupted time series, controlled before-and-after studies, cohort studies) were eligible for inclusion. Animal and preclinical studies were excluded, as were opinion manuscripts, conference abstracts, trial protocols, and grey literature.
2.2. Search Strategy and Selection of Studies
A systematic electronic search was performed on PubMed and Google Scholar up to November 2024.
The electronic search strategy was conducted by using a combination of the following MeSH terms and free text words: “Liver Transplantation* OR Carcinoma Hepatocellular*/pathology OR Carcinoma, Hepatocellular*/surgery OR Carcinoma, Hepatocellular/drug therapy* OR Liver Neoplasms*/surgery OR Liver Neoplasms/pathology OR Carcinoma, Hepatocellular/drug therapy* OR Liver Transplantation*/adverse effects AND Neoplasm Recurrence, Local OR Neoplasm Recurrence, Local/mortality* OR Graft Survival*”.
Two authors (M.M.P. and C.M.) independently extracted data from each included study.
2.3. Endpoints
The outcomes assessed in our work focused on fundamental indicators of transplant and oncological efficacy. The data limitations across cohorts, the high early mortality in this population, and the relevance of short-term survival in guiding clinical decision-making for recurrent HCC prompted us to choose a short but informative timing in daily clinical practice. These outcomes included the 1-year overall survival (OS) and 1-year disease-free survival (DFS). The data for each outcome were extracted directly, as reported in the source studies, without reclassifications of the rates.
2.4. Selection Process
Title and abstract screening and full-text screening were conducted by two researchers (M.M.P. and C.M.) in a standardized, independent manner using the Rayyan software program (https://rayyan.ai, accessed on 26 April 2025) [12]. If there was any disagreement, it was disclosed at the end of the independent individual evaluation and discussed until reaching a final decision. A manual search was also conducted on papers autonomously selected by the researchers because of their pertinence to the query.
In addition, reference lists of the included articles were manually searched in order to retrieve any possible full-length papers that could be included.
2.5. Data Collection
General information on the included papers (i.e., study design, year of publication, country, duration of the study) and data related to patients (i.e., age and gender, etiology underlying the HCC, inclusion in Milan criteria at time of transplant, histological and pathological characterization of the recurrences) were extracted from single papers and collected into a customized table.
2.6. Study Quality Assessment
The study quality assessment was performed throughout the modified Newcastle-Ottawa scale by 2 authors (M.M.P. and C.M.) [13] (Table 1). The methodological quality of the included studies was dishomogeneous. Seven articles out of seventeen reached a score equal to or higher than 7, such as Bodzin et al. [14], which, with a score of 8, represented the article with the highest methodological quality above those selected. Others, on the contrary, had an elevated risk of bias [15,16]. Nevertheless, we included their evidence in our qualitative analysis because we considered it as not neglectable. Furthermore, we tried to minimize the selection risk of bias by choosing strictly the inclusion criteria, such as including only studies performed on a population of patients whose histologically diagnosed HCC had been already treated by LT and had recurred. Additionally, we only included studies that described the treatment implemented for the recurrence and its efficacy in terms of survival. On top of that, we struck out case reports, conference abstracts, trial protocols, and grey literature, as well as any form of literature in languages other than English. Due to the limited availability of literature on the topic, the articles we selected may have a dishomogeneous description of the outcomes, a short follow-up, or patients could have received a combination of more than one treatment. The shortcomings mostly concerned the comparability and the outcomes domains: in fact, studies had different follow-up periods, some of them were not precise in the characterization of the recurrence features and the response to treatment, while others displayed a paucity of details when describing the population of the study and their clinical and pathological background.

Table 1.
Newcastle-Ottawa scale of the papers selected. On the left side are the categories of the scale. Every star stands for a point on the scale. In gray, the papers excluded after quality assessment.
2.7. Statistical Analysis
Pooled treatment effects for single-proportion outcomes were analyzed using a random-effects generalized linear mixed model with a 95% confidence interval (CI). Heterogeneity was evaluated with the tau-squared and I2 test by Higgins and Thompson. Heterogeneity was considered significant in the case of an I2 result of at least 40%. Subgroup analysis by type of treatment was performed in the case of 1 or more studies reporting an outcome. Subgroup analysis included pooling subgroup outcomes using a random-effects model, and subgroup effects were compared using the Q test, with a significant result defined as a p-value < 0.05. Jamovi® version 2.3.28 was used for statistical analysis.
3. Results
3.1. Study Selection
The electronic search provided 1174 records (PubMed: 968 papers, Scholar: 206 papers). Among them, 62 duplicates were detected and struck out. Then, 1034 papers were selected for full-paper evaluation. The manual search retrieved three additional articles, which underwent a full-text evaluation, providing a total of 1037 reviewed papers. Twelve articles fulfilled the inclusion criteria [14,15,16,17,18,19,20,21,22,23,24,25]. The subsequent study quality assessment rejected two articles. Ten articles, thus, were included in qualitative and quantitative synthesis [14,15,17,18,19,20,21,23,24,25]. The selection process is reported as a flow diagram, following the PRISMA guidelines, in Figure 1.
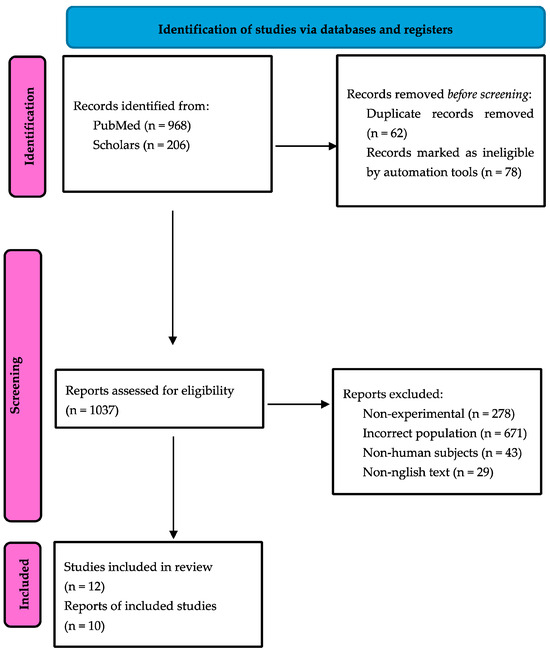
Figure 1.
PRISMA flow diagram.
3.2. Study Characteristics and Summary of Results
This systematic review includes ten retrospective cohort studies and a prospective study.
General information on the included papers (i.e., study design, year of publication, country, number of patients, study period) is reported in Table 2.

Table 2.
Main characteristics of the papers analyzed in the review.
The included study spanned from 2000 to 2024. Most of the studies (n = 6) came from China. One study was from Germany, one from France, two from the USA, and one from Korea.
The multifocal intrahepatic recurrence (more than three nodules) was present in the liver graft in the majority of the samples described by the studies we analyzed (in five of the studies, more than half of the population had more than three intrahepatic nodules at the time of recurrence). In eight of the studies, the majority of patients displayed both intra- and extrahepatic localizations of the recurrence.
For the sites of extrahepatic recurrence, in eight of the studies, the most frequently affected organ was the lung. Other common sites of recurrence were bone (in six studies), kidney (in one study), adrenal gland (in six studies), and brain (in two studies).
In all of the studies, the main outcomes were overall survival and disease-free survival after HCC recurrence was used as the principal outcome. Furthermore, in four of the studies, there was also a retrospective analysis of the characteristics generally displayed by patients with HCC recurrence suitable for surgical treatment. Six of the studies compared the efficacy of each of the three main therapeutic strategies (surgery, chemotherapy, and LRT), one only focused on the efficacy of microwave ablation, one on chemotherapy, and one on radiofrequency ablation.
A total of 5665 patients affected by HCC underwent LT. Among them, 791 patients displayed recurrence of the disease, either intra- or extrahepatic.
Overall, among these patients, 189 patients benefited from surgical treatment of the recurrence, whereas 273 were treated with nonsurgical approaches, be they systemic or locoregional. In the details, the LRT was found in 148 patients. Eleven patients underwent chemoembolization, 26 patients received a TACE treatment, 24 patients benefitted from RFA, and 87 from an MWA approach.
3.3. Overall Survival
Data for approximately 1-year OS were available in 10 studies for 265 patients (Figure 2).
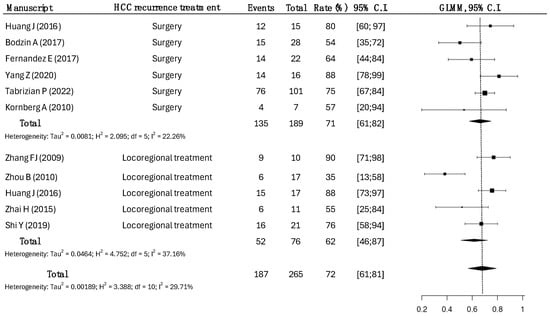
Figure 2.
One-year overall survival rates across types of HCC recurrence treatments. Surgical treatments had a survival rate of 71% (95% CI: 61–82), compared to the 62% (95% CI: 46–87) in LRT, with statistical significance (p = 0.0038) [14,15,17,18,19,20,21,22,23,25].
A total of 189 patients underwent surgical treatment, while 76 patients underwent LRT.
The overall survival at 1 year in the surgical group was higher, as opposed to the LRT group (71% vs. 62%, p = 0.038).
3.4. Disease-Free Survival
Data for approximately 1-year DFS were available in nine studies for 258 patients (Figure 3).
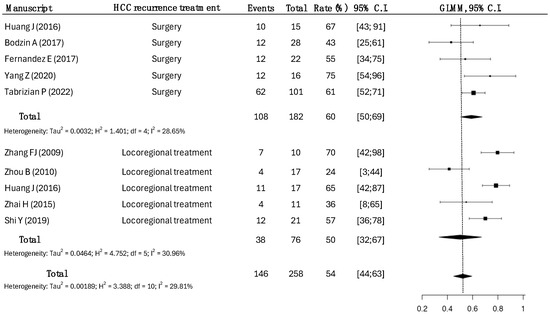
Figure 3.
One-year disease-free survival rates according to the treatment modalities of HCC recurrence. Surgical strategy had a disease-free survival rate of 60% (95% CI: 50–69), while the one-year disease-free survival rate in LRT was 50% (95% CI: 32–67), with a slight statistical significance (p = 0.048) [14,15,17,18,19,20,21,23,25].
A total of 182 patients underwent surgical treatment, while 76 patients underwent LRT.
In the surgical group, the DFS rate was 60%, compared to a DFS rate of 54% in the LRT group (p = 0.042).
3.5. Predictive Clinical-Pathological Variables Predictive of Outcomes
We compared the pre-LT and LT clinical-pathological characteristics of the patients with post-LT HCC recurrence who underwent surgical treatment or LRT.
Patients outside Milan criteria prior to LT were 42% in the LRT group and 18% in the surgical group (p = 0.031).
About the histological features of the HCC on the whole liver at the time of LT, the rate of the poor differentiation aspect was 22% in the surgical group and 61% in the LRT group (p = 0.018).
The microvascular invasion was more present in the HCC nodules of patients of the LRT group (38%) than in patients of the surgical group (21%).
Furthermore, the pre-LT analysis of oncological markers revealed a distinct pattern between the two groups. Specifically, the median alpha-fetoprotein level was more significant in the LRT group (342 ng/mL, IQR 128–560) compared to the surgical group (180 ng/mL, IQR 95–261) (p-value = 0.042).
4. Discussion
HCC is a major indication for LT in patients with cirrhosis and advanced liver disease. Despite stringent selection criteria for LT in cases of HCC, the recurrence of HCC after LT is a significant concern, with reported rates ranging from 10% to over 30%, depending on pre-LT tumor characteristics and treatment modalities [26]. A significant proportion of patients experience intrahepatic or extrahepatic HCC relapse post-transplant. Managing HCC recurrence is challenging due to the immunosuppressed state of the patient, which promotes tumor progression. Intrahepatic recurrences are particularly challenging due to the compromised liver function that often accompanies them [27]. Consequently, managing these recurrences requires a thorough understanding of treatment options available to optimize patient outcomes. Among the treatment options, surgical resection and LRT stand out as the most effective strategies for localized recurrences.
We performed a review of ten studies and 265 patients to compare outcomes of surgical treatment and LRT in patients with HCC recurrence after LT. The main results showed that the 1-year disease-free survival and 1-year overall survival rates were better in the surgically treated cohort. However, patients treated with LRT had more advanced pre-LT HCC characteristics in terms of clinical-radiological features (with Milan criteria), histopathological differentiation and microvascular invasion, and alpha-fetoprotein concentration.
Surgical resection represents a potentially curative approach for managing recurrent HCC after LT. Studies indicate that surgical resection can lead to improved survival rates among selected patients [28,29]. The ability to perform a resection depends heavily on the extent of recurrence, the location of the tumor, and the underlying function of the liver graft post-transplantation. Factors such as the number of lesions and the presence of extrahepatic metastases significantly influence eligibility for surgical management [30].
Recent studies have reported favorable outcomes for patients who underwent salvage hepatectomy after the failure of locoregional therapy [28]. Minagawa et al. demonstrated that repeat hepatectomy for locally recurrent HCC resulted in satisfactory oncological outcomes comparable to those seen with initial surgeries [28]. Patients undergoing salvage hepatectomy exhibited lower recurrence rates and better long-term survival than those limited to palliative care or untreatable by locoregional procedures.
However, surgical treatment is associated with complications and risks inherent to surgery, particularly in a hepatic context [30]. The liver graft, already compromised by the initial disease and transplantation process, may not tolerate further surgical interventions as effectively as a native liver does. Thus, careful patient selection and multidisciplinary evaluation are essential for optimizing outcomes.
LRT, which includes ablation techniques, transarterial chemoembolization (TACE), and radiotherapy, serves to target HCC with localized intervention while preserving liver function [31]. These treatments can often be repeated and used in combination with surgical options to manage recurrences effectively.
RFA is a widely used locoregional treatment for HCC, particularly in patients with small tumors. The technique is minimally invasive and can be repeated as necessary, which is particularly advantageous for managing recurrent disease [31]. Studies have demonstrated that RFA can effectively ablate small tumors and yield good local control rates, particularly when combined with other therapies [32]. Outcomes following RFA for recurrent HCC suggest that this approach may be less invasive than reoperation while still providing comparable survival benefits in selected patients [33].
TACE is another prominent locoregional therapy that combines the delivery of chemotherapy directly to the tumor with embolization to restrict blood flow [34]. During recurrence management, TACE may be employed for larger or multifocal lesions that are unsuitable for surgical excision, potentially increasing eligibility for subsequent transplantation by downstaging tumors.
LRT has also been studied for its potential role in downstaging HCC to meet the Milan criteria, allowing patients to become eligible for transplantation despite initially presenting beyond these strict thresholds [30,35]. Evidence suggests that TACE combined with locoregional therapies enhances survival rates compared to TACE alone, particularly in recurrent settings [36].
The choice between surgical and locoregional treatments for HCC recurrence following LT is complex, influenced by tumor burden, liver function, and prior treatments. Comparisons of OS and RFS between these modalities suggest that surgical options tend to offer better survival rates in select patient cohorts [26,30]. Foerster et al. noted similar survival rates for patients undergoing transplants for initially unresectable HCC and those undergoing salvage transplantation or resection following recurrence, underscoring the importance of timing and patient selection in maximizing surgical outcomes [29].
Contrastingly, locoregional treatments provide significant advantages through repeatability and lower overall risk profiles compared to extensive resection surgeries or transplantation. Patients who may not tolerate general anesthesia or have poor liver function may benefit more from locoregional approaches, which can also result in significant symptom relief and tumor control without the need for more extensive surgical interventions [31,37].
One of the major challenges in treating recurrent HCC after LT is the balance between eradicating the tumor and maintaining hepatic function. Surgical interventions inherently carry risks of further liver parenchyma loss, which can lead to postoperative liver failure in patients with limited functional reserve [30]. The presence of underlying cirrhosis and other chronic liver diseases complicates the prognosis significantly.
In contrast, locoregional therapies are generally more conservative, preserving liver function while allowing for continuous monitoring of disease progression. However, systemic therapies and locoregional treatments have yielded mixed results, particularly in the context of extrahepatic metastases, where surgical options may present limited benefits due to multifocal disease and a higher propensity for recurrence [38].
Deciding between surgical and locoregional treatment requires a case-by-case analysis, factoring in tumor characteristics, liver function, and the patient’s overall clinical situation [39]. A multidisciplinary approach involving hepatologists, surgical oncologists, and transplant surgeons is critical to tailor the best treatment pathway for each individual.
While LRT has seen advancements, including the potential for combination regimens that may enhance outcomes, the relative predictability of surgical interventions frequently underscores its perceived superiority in specific situations [36].
A fundamental limitation of our study is the absence of data about the concomitant or subsequent administration of chemotherapeutic drugs. Sorafenib and Regorafenib showed solid results also in HCC recurrence in patients who already undergone LT [40,41]. Immunotherapy and targeted agents are the other notable absence in our work. Immune checkpoint inhibitors like nivolumab and ipilimumab have shown promising results [42]. Anyway, all the systemic therapies exhibit more data in advanced HCC settings but are under-evaluated in post-transplant scenarios, despite some evidence suggesting tolerable side effects and the potential for meaningful outcomes in this cohort [43]. The absence of robust data on the use of systemic therapies poses a significant gap in the management strategies available for a single recurrence of HCC after LT.
Future research should aim to explore the integration of systemic therapies into the treatment regimen for HCC recurrence after liver transplantation. Combinations of locoregional therapies with immunotherapy may enhance management outcomes by addressing both local and systemic tumor activity [44,45]. Furthermore, establishing clear protocols for monitoring and early detection of recurrence using advanced imaging modalities, along with a deeper exploration of tumor biology and patient-specific factors, will be crucial in refining treatment approaches [46,47]. Addressing these gaps could lead to more personalized treatment strategies and potentially improved survival rates for transplant recipients facing HCC recurrence.
5. Conclusions
The management of recurrent HCC after LT presents significant challenges, necessitating an individualized approach that considers patient characteristics, tumor biology, and existing liver function. Both surgery and LRT play crucial roles in the therapeutic landscape, offering unique benefits and limitations. Understanding the nuances of these treatment options will ultimately lead to better decision-making and improved outcomes for patients facing HCC recurrences following LT.
Author Contributions
Conceptualization, M.M.P. and C.M.; methodology, E.N.; software, M.M.P.; validation, S.A. and F.F.; formal analysis, M.M.P.; investigation, C.M.; resources, M.M.P.; data curation, C.M.; writing—original draft preparation, C.M.; writing—review and editing, M.M.P.; visualization, M.M.P.; supervision, S.A.; project administration, E.N.; funding acquisition, M.M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cillo, U.; Gringeri, E.; D’Amico, F.E.; Lanari, J.; Furlanetto, A.; Vitale, A. Hepatocellular carcinoma: Revising the surgical approach in light of the concept of multiparametric therapeutic hierarchy. Dig. Liver Dis. 2025, 57, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Arab, J.P.; Díaz, L.A.; Rehm, J.; Im, G.; Arrese, M.; Kamath, P.S.; Lucey, M.R.; Mellinger, J.; Thiele, M.; Thursz, M.; et al. Metabolic dysfunction and alcohol-related liver disease (MetALD): Position statement by an expert panel on alcohol-related liver disease. J. Hepatol. 2025, 82, 744–756. [Google Scholar] [CrossRef]
- Mauro, E.; Rodríguez-Perálvarez, M.; D’Alessio, A.; Crespo, G.; Piñero, F.; De Martin, E.; Colmenero, J.; Pinato, D.J.; Forner, A. New Scenarios in Liver Transplantation for Hepatocellular Carcinoma. Liver Int. 2025, 45, e16142. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Chun, Y.S.; Poon, R.T.; Schwartz, M.E.; Yao, F.Y.; Marsh, J.W.; Bhoori, S.; Lee, S.G. Liver transplantation for hepatocellular carcinoma. Ann. Surg. Oncol. 2008, 15, 1001–1007. [Google Scholar] [CrossRef]
- Decaens, T.; Roudot-Thoraval, F.; Hadni-Bresson, S.; Meyer, C.; Gugenheim, J.; Durand, F.; Bernard, P.H.; Boillot, O.; Sulpice, L.; Calmus, Y.; et al. Impact of UCSF criteria according to pre- and post-OLT tumor features: Analysis of 479 patients listed for HCC with a short waiting time. Liver Transpl. 2006, 12, 1761–1769. [Google Scholar] [CrossRef]
- Rajendran, L.; Ivanics, T.; Claasen, M.P.; Muaddi, H.; Sapisochin, G. The management of post-transplantation recurrence of hepatocellular carcinoma. Clin. Mol. Hepatol. 2022, 28, 1–16. [Google Scholar] [CrossRef]
- Straś, W.A.; Wasiak, D.; Łągiewska, B.; Tronina, O.; Hreńczuk, M.; Gotlib, J.; Lisik, W.; Małkowski, P. Recurrence of Hepatocellular Carcinoma After Liver Transplantation: Risk Factors and Predictive Models. Ann. Transpl. 2022, 27, e934924. [Google Scholar] [CrossRef] [PubMed]
- Martínez Burgos, M.; González Grande, R.; López Ortega, S.; Santaella Leiva, I.; de la Cruz Lombardo, J.; Santoyo Santoyo, J.; Jiménez Pérez, M. Liver Transplantation for Hepatocarcinoma: Results over Two Decades of a Transplantation Programme and Analysis of Factors Associated with Recurrence. Biomedicines 2024, 12, 1302. [Google Scholar] [CrossRef] [PubMed]
- de’angelis, N.; Landi, F.; Carra, M.C.; Azoulay, D. Managements of recurrent hepatocellular carcinoma after liver transplantation: A systematic review. World J. Gastroenterol. 2015, 21, 11185–11198. [Google Scholar] [CrossRef]
- Pelizzaro, F.; Gambato, M.; Gringeri, E.; Vitale, A.; Cillo, U.; Farinati, F.; Burra, P.; Russo, F.P. Management of Hepatocellular Carcinoma Recurrence after Liver Transplantation. Cancers 2021, 13, 4882. [Google Scholar] [CrossRef]
- Enestvedt, C.K.; Orloff, S.L. Locoregional Therapy, Pathologic Response, and HCC Recurrence After Liver Transplantation: Highlighting Success and Looking Toward the Future. Ann. Surg. 2020, 271, 625–626. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Lo, C.K.; Mertz, D.; Loeb, M. Newcastle-Ottawa Scale: Comparing reviewers’ to authors’ assessments. BMC Med. Res. Methodol. 2014, 14, 45. [Google Scholar] [CrossRef]
- Bodzin, A.S.; Lunsford, K.E.; Markovic, D.; Harlander-Locke, M.P.; Busuttil, R.W.; Agopian, V.G. Predicting Mortality in Patients Developing Recurrent Hepatocellular Carcinoma After Liver Transplantation: Impact of Treatment Modality and Recurrence Characteristics. Ann. Surg. 2017, 266, 118–125. [Google Scholar] [CrossRef]
- Zhang, F.J.; Li, C.X.; Zhang, L.; Wu, P.H.; Jiao, D.C.; Duan, G.F. Short- to mid-term evaluation of CT-guided 125I brachytherapy on intra-hepatic recurrent tumors and/or extra-hepatic metastases after liver transplantation for hepatocellular carcinoma. Cancer Biol. Ther. 2009, 8, 585–590. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, H.R.; Cheon, S.H.; Rha, S.Y.; Lee, S.; Han, K.H.; Chon, C.Y.; Lee, J.D.; Sung, J.S.; Chung, H.C. Treatment of recurrent hepatocellular carcinoma after liver transplantation. Asia Pac. J. Clin. Oncol. 2011, 7, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Tabrizian, P.; Holzner, M.L.; Mehta, N.; Halazun, K.; Agopian, V.G.; Yao, F.; Busuttil, R.W.; Roberts, J.; Emond, J.C.; Samstein, B.; et al. Ten-Year Outcomes of Liver Transplant and Downstaging for Hepatocellular Carcinoma. JAMA Surg. 2022, 157, 779–788. [Google Scholar] [CrossRef]
- Zhou, B.; Shan, H.; Zhu, K.S.; Jiang, Z.B.; Guan, S.H.; Meng, X.C.; Zeng, X.C. Chemoembolization with lobaplatin mixed with iodized oil for unresectable recurrent hepatocellular carcinoma after orthotopic liver transplantation. J. Vasc. Interv. Radiol. 2010, 21, 333–338. [Google Scholar] [CrossRef]
- Shi, Y.; Chi, J.; Wang, T.; Cui, D.; Tang, X.; Ding, M.; Li, P.; Zhai, B. Mid-term outcome of percutaneous thermal ablation for intrahepatic recurrent hepatocellular carcinoma after liver transplantation. Clin. Radiol. 2019, 74, 735.e1–735.e7. [Google Scholar] [CrossRef]
- Zhai, H.; Liang, P.; Yu, X.L.; Cheng, Z.; Han, Z.Y.; Liu, F.; Yu, J. Microwave ablation in treating intrahepatic recurrence of hepatocellular carcinoma after liver transplantation: An analysis of 11 cases. Int. J. Hyperth. 2015, 31, 863–868. [Google Scholar] [CrossRef]
- Fernandez-Sevilla, E.; Allard, M.A.; Selten, J.; Golse, N.; Vibert, E.; Sa Cunha, A.; Cherqui, D.; Castaing, D.; Adam, R. Recurrence of hepatocellular carcinoma after liver transplantation: Is there a place for resection? Liver Transpl. 2017, 23, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Kornberg, A.; Küpper, B.; Tannapfel, A.; Katenkamp, K.; Thrum, K.; Habrecht, O.; Wilberg, J. Long-term survival after recurrent hepatocellular carcinoma in liver transplant patients: Clinical patterns and outcome variables. Eur. J. Surg. Oncol. 2010, 36, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, S.; Tian, X.Y.; Xie, Q.F.; Zhuang, L.; Li, Q.Y.; Chen, C.Z.; Zheng, S.S. Impact of treatment modalities on patients with recurrent hepatocellular carcinoma after liver transplantation: Preliminary experience. Hepatobiliary Pancreat. Dis. Int. 2020, 19, 365–370. [Google Scholar] [CrossRef]
- Ekpanyapong, S.; Philips, N.; Loza, B.L.; Abt, P.; Furth, E.E.; Tondon, R.; Khungar, V.; Olthoff, K.; Shaked, A.; Hoteit, M.A.; et al. Predictors, Presentation, and Treatment Outcomes of Recurrent Hepatocellular Carcinoma After Liver Transplantation: A Large Single Center Experience. J. Clin. Exp. Hepatol. 2020, 10, 304–315. [Google Scholar] [CrossRef]
- Huang, J.; Yan, L.; Wu, H.; Yang, J.; Liao, M.; Zeng, Y. Is radiofrequency ablation applicable for recurrent hepatocellular carcinoma after liver transplantation? Surg. Res. 2016, 200, 122–130. [Google Scholar] [CrossRef]
- Simsek, C.; Kim, A.; Ma, M.; Danis, N.; Gurakar, M.; Cameron, A.M.; Philosophe, B.; Garonzik-Wang, J.; Ottmann, S.; Gurakar, A.; et al. Recurrence of hepatocellular carcinoma following deceased donor liver transplantation: Case series. Hepatoma Res. 2020, 41, 217–220. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Hwang, S.; Lee, G.D.; Choi, S.H.; Kim, H.R.; Kim, Y.H.; Park, S.I.; Kim, D.K. Surgical outcome of pulmonary metastasectomy for hepatocellular carcinoma recurrence in liver transplant patients. Ann. Transplant. 2021, 26, e930383-1–e930383-9. [Google Scholar] [CrossRef]
- Minagawa, T.; Itano, O.; Kitago, M.; Abe, Y.; Yagi, H.; Hibi, T.; Shinoda, M.; Ojima, H.; Sakamoto, M.; Kitagawa, Y. Surgical and oncological outcomes of salvage hepatectomy for locally recurrent hepatocellular carcinoma after locoregional therapy: A single-institution experience. Cancers 2023, 15, 2320. [Google Scholar] [CrossRef] [PubMed]
- Foerster, F.; Hoppe-Lotichius, M.; Vollmar, J.; Marquardt, J.U.; Weinmann, A.; Wörns, M.A.; Otto, G.; Zimmermann, T.; Galle, P.R. Long-term observation of hepatocellular carcinoma recurrence after liver transplantation at a european transplantation centre. United Eur. Gastroenterol. J. 2019, 7, 838–849. [Google Scholar] [CrossRef]
- Vagefi, P.; Hirose, R. Downstaging of hepatocellular carcinoma prior to liver transplant: Is there a role for adjuvant sorafenib in locoregional therapy? J. Gastrointest. Cancer 2010, 41, 217–220. [Google Scholar] [CrossRef][Green Version]
- Isaac, A.; Mohamed, S.; Ahmed, O.; Hassan, A.; Rasmy, H. Amphiregulin as a novel diagnostic and prognostic biomarker of hepatocellular carcinoma before and after locoregional treatment. Egypt. J. Intern. Med. 2021, 33, 46. [Google Scholar] [CrossRef]
- Li, C.X.; Ling, C.C.; Shao, Y.; Xu, A.; Li, X.C.; Ng, K.T.; Liu, X.B.; Ma, Y.Y.; Qi, X.; Liu, H.; et al. Cxcl10/cxcr3 signaling mobilized-regulatory t cells promote liver tumor recurrence after transplantation. J. Hepatol. 2016, 65, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.; Yeh, H.; Lim, S.; Lin, W. Transcriptome analysis creates a new era of precision medicine for managing recurrent hepatocellular carcinoma. World J. Gastroenterol. 2023, 29, 780–799. [Google Scholar] [CrossRef]
- Makary, M.; Khandpur, U.; Cloyd, J.; Mumtaz, K.; Dowell, J. Locoregional therapy approaches for hepatocellular carcinoma: Recent advances and management strategies. Cancers 2020, 12, 1914. [Google Scholar] [CrossRef]
- Lee, K.; Lee, K.; Yi, N.; Suh, K.; Jang, J. Prognosis of hepatocellular carcinoma after liver transplantation: Comparative analysis with partial hepatectomy. J. Pathol. Transl. Med. 2017, 51, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Yong, C.C.; Tsai, M.C.; Lin, C.C.; Wang, C.C.; Lu, S.N.; Hung, C.H.; Hu, T.H.; Chen, C.L. Comparison of salvage living donor liver transplantation and local regional therapy for recurrent hepatocellular carcinoma. World J. Surg. 2016, 40, 2472–2480. [Google Scholar] [CrossRef]
- Finkenstedt, A.; Vikoler, A.; Portenkirchner, M.; Mülleder, K.; Maglione, M.; Margreiter, C.; Moser, P.; Vogel, W.; Bale, R.; Freund, M.; et al. Excellent post-transplant survival in patients with intermediate stage hepatocellular carcinoma responding to neoadjuvant therapy. Liver Int. 2015, 36, 688–695. [Google Scholar] [CrossRef]
- Chagas, A.L.; Felga, G.E.G.; Diniz, M.A.; Silva, R.F.; Mattos, A.A.; Silva, R.C.M.A.; Boin, I.F.S.F.; Garcia, J.H.P.; Lima, A.S.; Coelho, J.C.U.; et al. Hepatocellular carcinoma recurrence after liver transplantation in a brazilian multicenter study: Clinical profile and prognostic factors of survival. Eur. J. Gastroenterol. Hepatol. 2019, 31, 1148–1156. [Google Scholar] [CrossRef]
- Mauro, E.; Sanduzzi-Zamparelli, M.; Jutras, G.; Garcia, R.; Soler Perromat, A.; Llarch, N.; Holguin Arce, V.; Ruiz, P.; Rimola, J.; Lopez, E.; et al. Challenges in Liver Transplantation for Hepatocellular Carcinoma: A Review of Current Controversies. Cancers 2024, 16, 3059. [Google Scholar] [CrossRef]
- Sposito, C.; Mariani, L.; Germini, A.; Flores Reyes, M.; Bongini, M.; Grossi, G.; Bhoori, S.; Mazzaferro, V. Comparative efficacy of sorafenib versus best supportive care in recurrent hepatocellular carcinoma after liver transplantation: A case-control study. J. Hepatol. 2013, 59, 59–66. [Google Scholar] [CrossRef]
- Ozbay, M.F.; Harputluoglu, H.; Karaca, M.; Tekin, O.; Şendur, M.A.N.; Kaplan, M.A.; Sahin, B.; Geredeli, C.; Teker, F.; Tural, D.; et al. Sequential Use of Sorafenib and Regorafenib in Hepatocellular Cancer Recurrence After Liver Transplantation: Treatment Strategies and Outcomes. Cancers 2024, 16, 3880. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, N.; Patrono, D.; Colli, F.; Rizza, G.; Paraluppi, G.; Romagnoli, R. Liver Transplantation for Hepatocellular Carcinoma in the Era of Immune Checkpoint Inhibitors. Cancers 2024, 16, 2374. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Cummins, K.C.; Tsung, A. Immunotherapy as a Complement to Surgical Management of Hepatocellular Carcinoma. Cancers 2024, 16, 1852. [Google Scholar] [CrossRef]
- Avolio, A.W.; Agnes, S.; Barbarino, R.; Magalini, S.C.; Frongillo, F.; Pagano, L.; Larocca, L.M.; Pompili, M.; Caira, M.; Sollazzi, L.; et al. Posttransplant lymphoproliferative disorders after liver transplantation: Analysis of early and late cases in a 255 patient series. Transpl. Proc. 2007, 39, 1956–1960. [Google Scholar] [CrossRef] [PubMed]
- Himmelsbach, V.; Jeschke, M.; Lange, C.M.; Scheiner, B.; Pinter, M.; Sinner, F.; Venerito, M.; Queck, A.; Trojan, J.; Waidmann, O.; et al. Systemic Treatment of Recurrent Hepatocellular Carcinoma after Liver Transplantation: A Multicenter Trial. Cancers 2024, 16, 2442. [Google Scholar] [CrossRef]
- Avolio, A.W.; Spoletini, G.; Cillo, U.; Croome, K.; Oniscu, G.; Burra, P.; De Santibanes, M.; Egawa, H.; Gastaca, M.; Guo, Z.; et al. Protocol for an international multicenter, prospective, observational, non-competitive, study to validate and optimise prediction models of 90-day and 1-year allograft failure after liver transplantation: The global IMPROVEMENT Study. Updates Surg. 2025, 1–20. [Google Scholar] [CrossRef]
- Marrone, G.; Leone, M.S.; Biolato, M.; Liguori, A.; Bianco, G.; Spoletini, G.; Gasbarrini, A.; Miele, L.; Pompili, M. Therapeutic Approach to Post-Transplant Recurrence of Hepatocellular Carcinoma: Certainties and Open Issues. Cancers 2023, 15, 5593. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).

