Advancing CAR T-Cell Therapy in Solid Tumors: Current Landscape and Future Directions
Simple Summary
Abstract
1. Introduction
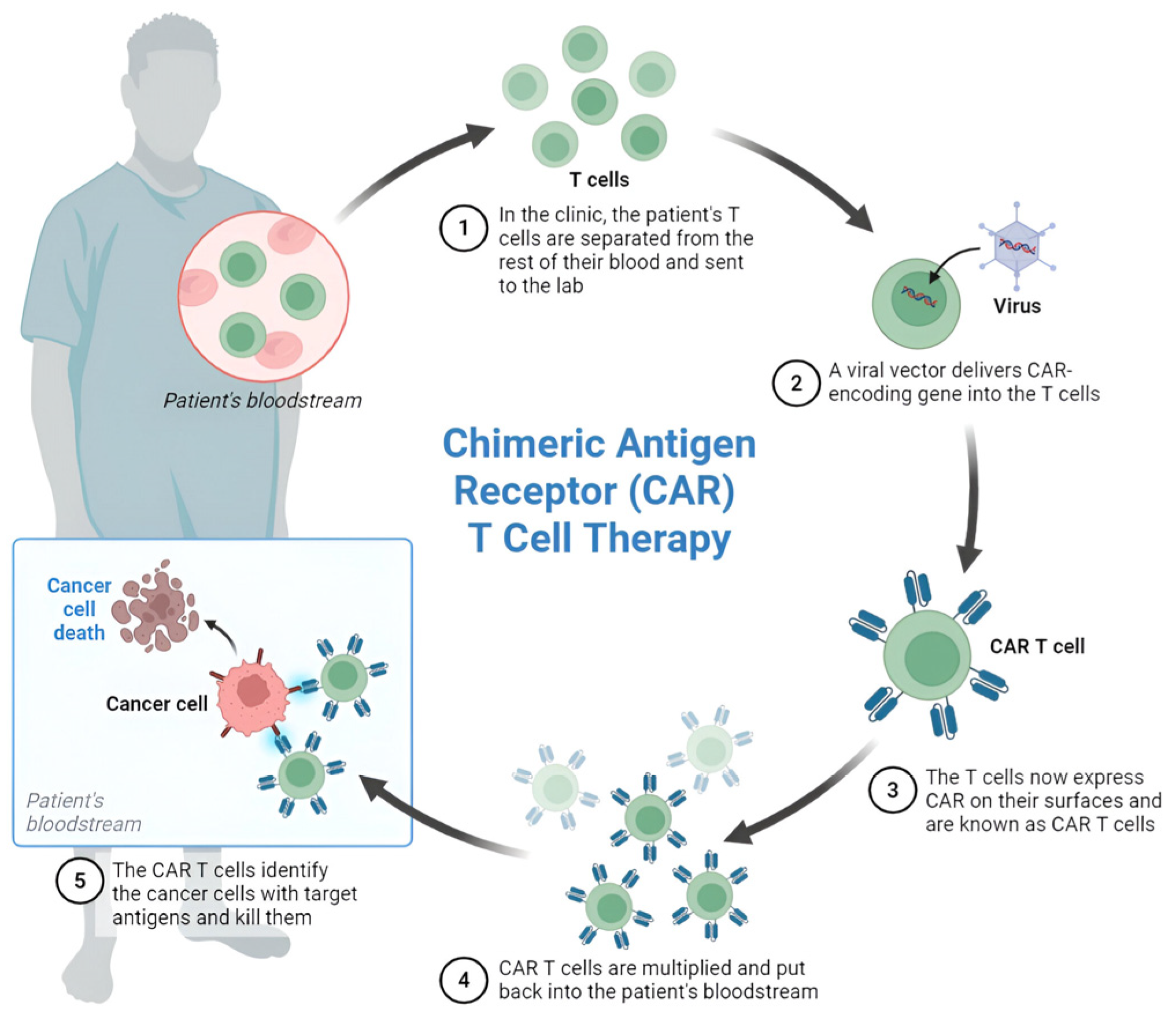
2. Principles of CAR T-Cell Therapy
- Leukapheresis: Collection of the patient’s peripheral blood mononuclear cells.
- Ex vivo T-cell isolation and activation: Selection and stimulation of T-cells.
- Genetic modification: Introduction of the CAR construct through viral vectors (typically retrovirus or lentivirus) or non-viral methods.
- Cell expansion: Cultivation of modified T-cells to achieve therapeutic quantities.
- Quality control: Testing for sterility, identity, and potency.
- Cryopreservation: Freezing for transportation and storage.
- Lymphodepletion conditioning: Pre-treatment conditioning of the patient (e.g., cyclophosphamide/fludarabine).
3. CAR T-Cell Construction
- Extracellular Antigen Recognition Domain: The recognition domain is derived from a monoclonal antibody and is responsible for targeting specific antigens on the surface of tumor cells. Commonly, single-chain variable fragments (scFvs) are utilized due to their ability to combine the specificity of antibodies with the advantages of smaller size and increased stability. Although traditionally derived from murine antibodies, efforts to humanize scFvs are underway to reduce immunogenicity and enhance in vivo persistence.
- Transmembrane Domain: This domain anchors the CAR molecule to the T-cell membrane, thereby enabling the transduction of signals upon antigen engagement. It plays a crucial role in maintaining the structural integrity and functionality of the CAR.
- 3.
- Intracellular Signaling Domains: The intracellular domain includes a combination of CD3ζ and co-stimulatory domains such as CD28, 4-1BB (CD137), or OX40 (CD134). These signaling domains are pivotal for T-cell activation, proliferation, and survival post-target recognition [15].
4. Mechanism of Action of CAR T-Cells
5. Evolution of CAR T-Cell Therapy
6. Approved CAR T-Cell Therapies in Hematological Cancers
6.1. Kymriah (Tisagenlecleucel)
6.2. Yescarta (Axicabtagene Ciloleucel)
6.3. Breyanzi (Lisocabtagene Maraleucel)
6.4. Abecma (Idecabtagene Vicleucel)
6.5. Carvykti (Ciltacabtagene Autoleucel)
6.6. Tecartus (Brexucabtagene Autoleucel)
6.7. Aucatzyl (Obecabtagene Autoleucel)
7. Recent Approvals of Engineered T-Cell Therapies for Solid Tumors
7.1. Synovial Sarcoma
7.2. Malignant Melanoma
8. CAR T-Cell Therapies in Development in Solid Malignancies
8.1. CAR T-Cell Therapy in Breast Cancer
8.2. HER2-Targeted CAR T-Cells in HER2-Positive Breast Cancerteraction Activates the T-Cells, Leading to the Release of Cy
8.3. Targeting Triple-Negative Breast Cancer (TNBC)
8.4. HER2 CAR T-Cell Therapy and PD-1 Inhibition Combination
8.5. Emerging Targets in Breast Cancer
9. CAR T-Cell Therapy in Lung Cancer
9.1. Non-Small Cell Lung Cancer (NSCLC)
9.2. Small Cell Lung Cancer (SCLC)
9.2.1. Targeting Neuroendocrine Markers in SCLC
9.2.2. Targeting DLL3 in SCLC
10. CAR T-Cell Therapy in Colorectal Cancer
11. CAR T-Cell Therapy in Esophageal, Gastroesophageal, and Gastric Cancers
12. CAR T-Cell Therapy in Urological Cancers
12.1. Renal Cell Carcinoma
12.1.1. Target Antigens and CAR Constructs
12.1.2. CD70 as an Emerging Target
13. Prostate Cancer
14. Bladder Cancer
15. CAR T-Cell Therapy in Gynecological Cancers
15.1. Ovarian Cancer
15.2. Uterine Cancer
15.3. Cervical Cancer
16. CAR T-Cell Therapy in Head and Neck Cancers
16.1. Targeted Approaches in Head and Neck Cancer
16.2. HPV-Positive Head and Neck Cancer
16.3. Future Directions in Head and Neck Cancer CAR T-Cell Therapy
17. CAR T-Cell Therapy in Pancreatic and Hepatobiliary Cancers
18. CAR T-Cell Therapy in Sarcomas
19. CAR T-Cell Therapy for Melanoma
20. CAR T-Cell Therapy for Glioblastoma
21. Integrating Artificial Intelligence for Precision CAR T-Cell Therapy in Solid Tumors
22. Barriers to CAR T-Cell Therapy in Solid Tumors
22.1. Tumor Heterogeneity
22.2. Tumor Microenvironment’s Role
22.3. Physical Barriers
22.4. Antigen Escape
22.5. “On-Target Off-Tumor” Toxicity in CAR T-Cell Therapy
- Affinity Tuning
- Lowering the binding affinity of CARs can enhance selectivity for tumor cells overexpressing the antigen while sparing normal cells with low antigen density. A study by Liu et al. (2022) demonstrated that reducing HER2-CAR affinity prevented off-tumor toxicity while maintaining anti-tumor efficacy [167].
- Humanized or Fully Human scFvs
- Murine-derived single-chain variable fragments (scFvs) can induce immunogenicity. Humanized or fully human CAR designs reduce immunogenicity and improve safety. As an example, a fully human CD19 CAR T-cell (lisocabtagene maraleucel) showed reduced immunogenicity compared to murine-based constructs [168].
- Logic-Gated CARs (AND, OR, NOT Gates)
- These CARs require multiple antigens for activation (AND gate) or inhibit activity in the presence of a normal tissue marker (NOT gate). As far back as 2013, a PSCA + PSMA AND-gated CAR T-cell demonstrated enhanced tumor specificity in prostate models [169].
- Transient CAR Expression (mRNA CARs, Switchable CARs)
- Short-lived CAR T-cells (via mRNA electroporation) or switchable CARs (controlled by an external antibody) can limit prolonged activity and mitigate toxicity. Zhao et al. demonstrated that mRNA-based CAR T-cells targeting mesothelin showed transient activity, thereby reducing toxicity [170].
- Inhibitory CARs (iCARs)
- iCARs co-express an inhibitory receptor (e.g., PD-1, CTLA-4) that suppresses CAR-T activity upon binding to normal tissue markers. This approach has been validated preclinically to prevent off-tumor toxicity, for example, in therapies targeting antigens with restricted expression on healthy tissues [171].
- Overall, mitigating “on-target off-tumor” toxicity requires a combination of affinity optimization, humanized designs, logic-gated systems, transient expression, and inhibitory mechanisms. Recent advances in synthetic biology and protein engineering are improving the safety profile of CAR T-cell therapies, particularly for solid tumors.
23. Development Strategies for Enhancing CAR T-Cell Therapy in Solid Cancers
23.1. Combining CAR T-Cell Therapy with Immune Checkpoint Inhibitors
23.2. Combination with Radiotherapy
Unique Challenges of “Homing” in CAR T-Cell Therapy with Radiotherapy
23.3. CRISPR-Cas9 Gene Editing to Optimize CAR T-Cell Efficacy and Safety
23.4. Other Development Strategies
23.4.1. Armored CAR T-Cells
23.4.2. Bispecific and Multi-Specific CAR T-Cells
23.5. Multi-Antigen CAR T-Cell Strategies
24. Quality-of-Life Considerations, Real-World Implementation Challenges Including Costs, Accessibility, and Scalability
25. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef] [PubMed]
- June, C.H.; O’Connor, R.S.; Kawalekar, O.U.; Ghassemi, S.; Milone, M.C. CAR T cell immunotherapy for human cancer. Science 2018, 359, 1361–1365. [Google Scholar] [CrossRef] [PubMed]
- Laetsch, T.W.; Maude, S.L.; Rives, S.; Hiramatsu, H.; Bittencourt, H.; Bader, P.; Baruchel, A.; Boyer, M.; De Moerloose, B.; Qayed, M.; et al. Three-Year Update of Tisagenlecleucel in Pediatric and Young Adult Patients with Relapsed/Refractory Acute Lymphoblastic Leukemia in the ELIANA Trial. J. Clin. Oncol. 2023, 41, 1664–1669. [Google Scholar] [CrossRef]
- D’Aloia, M.M.; Zizzari, I.G.; Sacchetti, B.; Falco, A.; Buoncervello, M.; De Angelis, B.; Venuta, M.C.; Di Rocco, G.; Alimandi, M.; Puca, R.; et al. CAR-T cells: The long and winding road to solid tumors. Cell Death Dis. 2018, 9, 282. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, A.; Palazon, A.; Noguera-Ortega, E.; Guedan, S.; Rouzaut, A.; Melero, I. CAR-T cells hit the tumor microenvironment: Strategies to overcome tumor escape. Front. Immunol. 2020, 11, 1109. [Google Scholar] [CrossRef]
- Liaqat Ali Khan, N.; Nafee, T.; Shao, T.; Hart, A.R.; Elliott, S.; Ola, B.; Heath, P.R.; Fazeli, A. Dysregulation in multiple transcriptomic endometrial pathways is associated with recurrent implantation failure and recurrent early pregnancy loss. Int. J. Mol. Sci. 2022, 23, 16051. [Google Scholar] [CrossRef]
- Sadelain, M.; Brentjens, R.; Riviere, I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013, 3, 388–398. [Google Scholar] [CrossRef]
- Sadelain, M.; Riviere, I.; Riddell, S. Therapeutic T cell engineering. Nature 2017, 545, 423–431. [Google Scholar] [CrossRef]
- Chmielewski, M.; Hombach, A.A.; Abken, H. Of CARs and TRUCKs: Chimeric antigen receptor (CAR) T cells engineered with an inducible cytokine to modulate the tumor stroma. Immunol. Rev. 2014, 257, 83–90. [Google Scholar] [CrossRef]
- Hiltensperger, M.; Krackhardt, A.M. Current and future concepts for the generation and application of genetically engineered CAR-T and TCR-T cells. Front. Immunol. 2023, 14, 1121030. [Google Scholar] [CrossRef]
- Wang, X.; Rivière, I. Clinical manufacturing of CAR-T cells: Foundation of a promising therapy. Mol. Ther. Oncolytics 2016, 3, 16015. [Google Scholar] [CrossRef]
- Levine, B.L.; Miskin, J.; Wonnacott, K.; Keir, C. Global manufacturing of CAR-T cell therapy. Mol. Ther. Methods Clin. Dev. 2017, 4, 92–101. [Google Scholar] [CrossRef]
- Fujiwara, K.; Tsunei, A.; Kusabuka, H.; Ogaki, E.; Tachibana, M.; Okada, N. Hinge and Transmembrane Domains of Chimeric Antigen Receptor Regulate Receptor Expression and Signaling Threshold. Cells 2020, 9, 1182. [Google Scholar] [CrossRef]
- Kochenderfer, J.N.; Rosenberg, S.A. Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nat. Rev. Clin. Oncol. 2013, 10, 267–276. [Google Scholar] [CrossRef]
- Abou-El-Enein, M.; Elsallab, M.; Feldman, S.A.; Fesnak, A.D.; Heslop, H.E.; Marks, P.; Till, B.G.; Bauer, G.; Savoldo, B. Scalable Manufacturing of CAR T Cells for Cancer Immunotherapy. Blood Cancer Discov. 2021, 2, 408–422. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Wei, R.; Ma, Q.; Shi, L.; He, F.; Shi, Z.; Jin, T.; Xie, R.; Wei, B.; Chen, J.; et al. In vivo expansion and antitumor activity of coinfused CD28- and 4-1BB-engineered CAR-T cells in patients with B cell leukemia. Mol. Ther. 2018, 26, 976–985. [Google Scholar] [CrossRef]
- Xiao, X.; He, Q.; Liu, Y.; Zhang, J.; Li, Y.; Fan, S.; Qi, J.; Wang, H.; Xie, J.; Yuan, Z.; et al. Mechanisms of cytokine release syn-drome and neurotoxicity of CAR T-cell therapy and associated prevention and management strategies. J. Exp. Clin. Cancer Res. 2021, 40, 367. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, T.F.; Schreiber, H.; Fu, Y.-X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013, 14, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; Barua, A.; Huang, L.; Ganguly, S.; Feng, Q.; He, B. From bench to bedside: The history and progress of CAR T cell therapy. Front. Immunol. 2023, 14, 1188049. [Google Scholar] [CrossRef]
- Shah, B.D.; Bishop, M.R.; Oluwole, O.O.; Logan, A.C.; Baer, M.R.; Donnellan, W.B.; O’Dwyer, K.M.; Holmes, H.; Arellano, M.L.; Ghobadi, A.; et al. KTE-X19 anti-CD19 CAR T-cell therapy in adult relapsed/refractory acute lymphoblastic leukemia: ZUMA-3 phase 1 results. Blood 2021, 138, 11–22. [Google Scholar] [CrossRef]
- Carpenito, C.; Milone, M.C.; Hassan, R.; Simonet, J.C.; Lakhal, M.; Suhoski, M.M.; Varela-Rohena, A.; Haines, K.M.; Heitjan, D.F.; Albelda, S.M.; et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc. Natl. Acad. Sci. USA 2009, 106, 3360–3365. [Google Scholar] [CrossRef] [PubMed]
- Yeku, O.O.; Brentjens, R.J. Armored CAR T-cells: Utilizing cytokines and pro-inflammatory ligands to enhance CAR T-cell anti-tumor efficacy. Biochem. Soc. Trans. 2016, 44, 412–418. [Google Scholar] [CrossRef]
- Morsut, L.; Roybal, K.T.; Xiong, X.; Gordley, R.M.; Coyle, S.M.; Thomson, M.; Lim, W.A. Engineering Customized Cell Sensing and Response Behaviors Using Synthetic Notch Receptors. Cell 2016, 164, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, J.; Zhong, J.F.; Zhang, X. Engineering CAR-T Cells. Biomark. Res. 2017, 5, 22. [Google Scholar] [CrossRef]
- Choe, J.H.; Watchmaker, P.B.; Simic, M.S.; Gilbert, R.D.; Li, A.W.; Krasnow, N.A.; Downey, K.M.; Yu, W.; Carrera, D.A.; Celli, A.; et al. SynNotch-CAR T cells overcome challenges of specificity, heterogeneity, and persistence in treating glioblastoma. Sci. Transl. Med. 2021, 13, eabe7378. [Google Scholar] [CrossRef]
- ASCO. Safety and Efficacy of Allogeneic CAR T Cells in B Cell Malignancies: A Systematic Review and Meta Analysis. J. Clin. Oncol. 2022, 40 (Suppl. S16), e19530. [Google Scholar] [CrossRef]
- Mullard, A. FDA approves first CAR-T therapy. Nat. Rev. Drug Discov. 2017, 16, 669. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. FDA Approves Tisagenlecleucel for Adults with Relapsed or Refractory Large B-Cell Lymphoma. 1 May 2018. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tisagenlecleucel-adults-relapsed-or-refractory-large-b-cell-lymphoma (accessed on 14 August 2025).
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jäger, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N. Engl. J. Med. 2019, 380, 45–56. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. FDA Approves New Treatment for Adults with Relapsed or Refractory Large B-Cell Lymphoma. 5 February 2021. Available online: https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-treatment-relapsed-or-refractory-diffuse-large-b-cell-lymphoma-and-high-grade-b-cell (accessed on 14 August 2025).
- U.S. Food and Drug Administration. FDA Approves First Cell-Based Gene Therapy for Adult Patients with Multiple Myeloma. 27 March 2021. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-cell-based-gene-therapy-adult-patients-multiple-myeloma (accessed on 14 August 2025).
- U.S. Food and Drug Administration. FDA Approves Ciltacabtagene Autoleucel for Relapsed or Refractory Multiple Myeloma. 28 February 2022. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-ciltacabtagene-autoleucel-relapsed-or-refractory-multiple-myeloma (accessed on 14 August 2025).
- American Society of Gene & Cell Therapy. FDA Approves First CAR T-Cell Therapy for ALL. ASGCT News. 14 October 2021. Available online: https://www.asgct.org/publications/news/october-2021/tecartus-first-cart-cell-therapy-all (accessed on 21 August 2025).
- U.S. Food and Drug Administration (FDA). FDA Approves Obecabtagene Autoleucel for Adults with Relapsed or Refractory B-Cell Precursor Acute Lymphoblastic Leukemia. FDA. 2022. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-obecabtagene-autoleucel-adults-relapsed-or-refractory-b-cell-precursor-acute (accessed on 20 August 2025).
- Schwartzentruber, D.J.; Lawson, D.H.; Richards, J.M.; Conry, R.M.; Miller, D.M.; Treisman, J.; Gailani, F.; Riley, L.; Conlon, K.; Pockaj, B.; et al. gp100 Peptide Vaccine and Interleukin-2 in Patients with Advanced Melanoma. N. Engl. J. Med. 2011, 364, 2119–2127. [Google Scholar] [CrossRef]
- D’Angelo, S.P.; Araujo, D.M.; Abdul Razak, A.R.; Blay, J.Y.; Jones, R.L.; Keohan, M.L.; Italiano, A.; Schoffski, P.; Attia, S.; Chawla, S.; et al. Afamitresgene autoleucel for advanced synovial sarcoma and myxoid round cell liposarcoma (SPEAR-HEAD-1): An international, open-label, phase 2 trial. Lancet 2024, 403, 1460–1471. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. FDA Approves First Gene Therapy to Treat Adults with Metastatic Synovial Sarcoma. 2 August 2024. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-gene-therapy-treat-adults-metastatic-synovial-sarcoma (accessed on 14 August 2025).
- U.S. Food and Drug Administration. FDA Grants Accelerated Approval to Lifileucel for Unresectable or Metastatic Melanoma. 2024. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-lifileucel-unresectable-or-metastatic-melanoma (accessed on 14 August 2025).
- Rohaan, M.W.; Kessels, R.; Haanen, J.B.A.G. Tumor-Infiltrating Lymphocyte Therapy in Advanced Melanoma. N. Engl. J. Med. 2023, 388, 859–860. [Google Scholar] [CrossRef] [PubMed]
- UICC. Breast Cancer. Union for International Cancer Control. 2025. Available online: https://www.uicc.org/what-we-do/thematic-areas/breast-cancer (accessed on 20 August 2025).
- Zhu, L.; Liu, J.; Li, J.; Wang, N.; Zhao, Y.; Su, H. Research Progress on HER2-Specific Chimeric Antigen Receptor T Cells for Immunotherapy of Solid Tumors. Front. Immunol. 2025, 16, 1514994. [Google Scholar] [CrossRef]
- Rahimmanesh, I.; Khanahmad, H. Chimeric antigen receptor-T cells immunotherapy for targeting breast cancer. Res. Pharm. Sci. 2021, 16, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Zhang, Y.; Zhang, Z.; Dai, H.; Zhu, G.; Li, S.; Yang, Z.; He, Y.; Wang, Y.; Liu, X.; et al. CAR-Based Immunotherapy for Breast Cancer: Peculiarities, Ongoing Investigations, and Future Strategies. Front. Immunol. 2024, 15, 1385571. [Google Scholar] [CrossRef]
- Alonso, M.R.; Grinyó-Escuer, A.; Duro-Sánchez, S.; Navarro-Barriuso, J.; Fernández, L.; Romero, A.; Limón-Mortés, M.C.; Hernández-Losa, J.; Villagrasa, P.; González-García, S.; et al. Generation of Chimeric Antigen Receptor T Cells Targeting p95HER2 in Solid Tumors. Nat. Commun. 2024, 15, 9589. [Google Scholar] [CrossRef]
- Li, H.; Huang, Y.; Jiang, D.Q.; Cui, L.Z.; He, Z.; Wang, C.; Zhang, Z.; Jiang, X.; Guo, T.; Xia, H.; et al. Overcome Trastuzumab Resistance of Breast Cancer Using Anti-HER2 Chimeric Antigen Receptor T Cells and PD1 Blockade. Am. J. Cancer Res. 2020, 10, 688–703. [Google Scholar]
- Wilkie, S.; van Schalkwyk, M.C.I.; Hobbs, S.; Davies, D.M.; van der Stegen, S.J.C.; Pereira, A.C.P.; Burbridge, S.E.; Box, C.; Eccles, S.A.; Maher, J. Dual Targeting of ErbB2 and MUC1 in Breast Cancer Using Chimeric Antigen Receptors Engineered to Provide Complementary Signaling. J. Clin. Immunol. 2012, 32, 1059–1070. [Google Scholar] [CrossRef]
- Hegde, M.; Mukherjee, M.; Grada, Z.; Pignata, A.; Landi, D.; Navai, S.A.; Wakefield, A.; Fousek, K.; Bielamowicz, K.; Chow, K.K.; et al. Tandem CAR T Cells Targeting HER2 and IL13Rα2 Mitigate Tumor Antigen Escape. J. Clin. Investig. 2016, 126, 3036–3052. [Google Scholar] [CrossRef]
- Chmielewski, M.; Abken, H. IL-12 Release by Engineered T Cells Expressing Chimeric Antigen Receptors Can Effectively Muster an Anti-Tumor Immune Response and Overcome the Immunosuppressive Tumor Microenvironment. J. Immunol. 2014, 193, 481–493. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, J.; Wu, X.; Zhang, M.; Luo, D.; Zhang, R.; Luo, J.; Lin, Z.; Qian, Q.; Zhu, M.; et al. Chimeric Antigen Receptor T Cell Targeting EGFRvIII for Metastatic Lung Cancer Therapy. Front. Med. 2019, 13, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huang, Y.; Jiang, D.Q.; Cui, L.Z.; He, Z.; Wang, C.; Zhang, Z.; Jiang, X.; Guo, T.; Xia, H.; et al. Antitumor Activity of EGFR-Specific CAR T Cells against Non-Small-Cell Lung Cancer Cells in Vitro and in Mice. Cell Death Dis. 2018, 9, 177. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Sferruzza, G.; Yang, L.; Zhou, L.; Chen, S. CAR-T and CAR-NK as Cellular Cancer Immunotherapy for Solid Tumors. J. Hematol. Oncol. 2022, 15, 118. [Google Scholar] [CrossRef]
- Ito, T.; Kudoh, S.; Fujino, K.; Sanada, M.; Tenjin, Y.; Saito, H.; Nakaishi-Fukuchi, Y.; Kameyama, H.; Ichimura, T.; Udaka, N.; et al. Pulmonary Neuroendocrine Cells and Small Cell Lung Carcinoma: Immunohistochemi-cal Study Focusing on Mechanisms of Neuroendocrine Differentiation. Acta Histochem. Cytochem. 2022, 55, 75–83. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- George, J.; Lim, J.S.; Jang, S.J.; Cun, Y.; Ozretic, L.; Kong, K.; Leenders, F.; Lu, X.; Fernandez-Cuesta, L.; Bosco, G.; et al. Comprehensive Genomic Profiles of Small Cell Lung Cancer. Nature 2015, 524, 47–53. [Google Scholar] [CrossRef]
- Crossland, D.L.; Denning, W.L.; Ang, S.; Olivares, S.; Mi, T.; Switzer, K.; Singh, H.; Huls, H.; Gold, K.S.; Glisson, B.S.; et al. Antitumor activity of CD56-chimeric antigen receptor T cells in neuroblastoma and SCLC models. Oncogene 2018, 37, 3686–3697. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, Y.; Zhang, Y.; Li, X.; Sun, Q.; Chen, H.; Liu, Z.; Xu, J.; Hu, W.; Zhao, Y.; et al. DLL3-Guided Therapies in Small-Cell Lung Cancer: From Antibody-Drug Conjugates to Chimeric Antigen Receptor T Cells. Mol. Cancer 2024, 23, 45. [Google Scholar] [CrossRef]
- Li, H.; Yang, C.; Cheng, H.; Huang, S.; Zheng, Y. CAR-T Cells for Colorectal Cancer: Target Selection and Strategies for Improved Activity and Safety. J. Cancer 2021, 12, 1804–1814. [Google Scholar] [CrossRef]
- Ye, K.; Yu, C.; Shen, Z. Severe Refractory Colitis after Intraperitoneal Infusion of CEA-Directed CAR T Cells in Patients with Colorectal Cancer. Ther. Adv. Med. Oncol. 2024, 16, 17588359241309825. [Google Scholar] [CrossRef]
- Ouladan, S.; Orouji, E. Chimeric Antigen Receptor-T Cells in Colorectal Cancer: Pioneering New Avenues in Solid Tumor Immunotherapy. J. Clin. Oncol. 2025, 43, 994–1005. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Z.; Yang, Z.; Wang, M.; Li, S.; Li, Y.; Chen, X.; Liu, J.; Zhao, H.; Sun, W.; et al. Phase I Escalating-Dose Trial of CAR-T Therapy Targeting CEA(+) Metastatic Colorectal Cancers. Mol. Ther. 2017, 25, 1248–1258. [Google Scholar] [CrossRef]
- Katz, S.C.; Hardaway, J.; Prince, E.; Guha, P.; Cunetta, M.; Moody, A.; Yao, V.; Grodman, H.; Liu, Z.; Sharma, P.; et al. HITM-SIR: Phase Ib Trial of Intraarterial Chimeric Antigen Receptor T-Cell Therapy and Selective Internal Radiation Therapy for CEA(+) Liver Metastases. Cancer Gene Ther. 2020, 27, 341–355. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, L.; Yu, G.; Zhang, T.; Wen, R.; Fan, H.; Li, Y.; Chen, J.; Liu, X.; Huang, Y.; et al. Safety and Efficacy of An-ti-CEA CAR-T Cells to Prolong Relapse-Free Survival of Colorectal Cancer Liver Metastases Patients after Radical Resection. J. Clin. Oncol. 2025, 43, 3541. [Google Scholar] [CrossRef]
- Snook, A.E.; Magee, M.S.; Schulz, S.; Smith, J.; Lee, K.; Patel, R.; Johnson, M.; Brown, T.; Davis, R.; Wilson, L.; et al. GUCY2C-Directed CAR-T Cells Oppose Colorectal Cancer Metastases Without Autoimmunity. Cancer Immunol. Res. 2016, 4, 582–592. [Google Scholar] [CrossRef]
- Zhang, B.L.; Li, D.; Gong, Y.L.; Huang, Y.; Qin, D.Y.; Jiang, L.; Chen, H.; Wang, X.; Liu, Y.; Sun, Z.; et al. Preclinical Evaluation of Chimeric Antigen Receptor-Modified T Cells Specific to Epithelial Cell Adhesion Molecule for Treating Colorectal Cancer. Hum. Gene Ther. 2019, 30, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Mathur, D.; Root, A.R.; Bugaj-Gaweda, B.; Chen, Y.; Li, X.; Wang, H.; Zhou, L.; Smith, J.; Lee, K.; Patel, R.; et al. A Novel GUCY2C-CD3 T-Cell Engaging Bispecific Construct (PF-07062119) for the Treatment of Gastrointestinal Cancers. Clin. Cancer Res. 2020, 26, 2188–2202. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, L.; Ping, Y.; Zhang, Z.; Yao, C.; Shen, C.; Li, F.; Wen, C.; Zhang, Y. CCR5 and IL-12 Co-Expression in CAR T Cells Improves Antitumour Efficacy by Reprogramming Tumour Microenvironment in Solid Tumours. Cancer Immunol. Immunother. 2025, 74, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, E.R.; D’Souza, R.R.; Klampatsa, A. Armored CAR T Cells: The Next Chapter in T Cell Cancer Immunotherapy. Biologics 2021, 15, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Lipatov, O.N.; Kim, H.R.; et al. First-Line Nivolumab Plus Chemotherapy versus Chemotherapy Alone for Advanced Gastric, Gastro-Oesophageal Junction, and Oesophageal Adenocarcinoma (CheckMate 649): A Randomised, Open-Label, Phase 3 Trial. Lancet 2021, 398, 27–40. [Google Scholar] [CrossRef]
- Sun, J.M.; Shen, L.; Shah, M.A.; Enzinger, P.; Adenis, A.; Doi, T.; Kato, K.; Piessen, G.; Kojima, T.; Cho, B.C.; et al. Pembrolizumab Plus Chemotherapy versus Chemotherapy Alone for First-Line Treatment of Advanced Oesophageal Cancer (KEY-NOTE-590): A Randomised, Placebo-Controlled, Phase 3 Study. Lancet 2021, 398, 759–771. [Google Scholar] [CrossRef]
- Cao, W.; Xing, H.; Li, Y.; Tian, W.; Song, Y.; Jiang, Z.; Yu, J. Claudin18.2 Is a Novel Molecular Biomarker for Tumor-Targeted Immunotherapy. Biomark. Res. 2022, 10, 38. [Google Scholar] [CrossRef]
- Qi, C.; Liu, C.; Peng, Z.; Li, X.; Zhang, Y.; Wang, H.; Chen, J.; Sun, W.; Zhao, H.; Liu, J.; et al. Claudin-18 Isoform 2-Specific CAR T-Cell Therapy (SATRICEL) versus Treatment of Physician’s Choice for Previously Treated Advanced Gastric or Gas-tro-Oesophageal Junction Cancer (CT041-ST-01): A Randomised, Open-Label, Phase 2 Trial. Lancet 2025, 405, 2049–2060. [Google Scholar] [CrossRef]
- Qi, C.; Liu, C.; Gong, J.; Zhang, Y.; Peng, Z.; Li, X.; Wang, H.; Chen, J.; Zhao, H.; Sun, W.; et al. Claudin18.2-Specific CAR T Cells in Gastrointestinal Cancers: Phase 1 Trial Final Results. Nat. Med. 2024, 30, 2224–2234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, D.; Yun, H.; Liu, W.; Chai, K.; Tong, J.; Wang, L.; Chen, Y.; Sun, W.; Zhao, H.; et al. CAR T Cells in the Treatment of Urologic Neoplasms: Present and Future. Front. Oncol. 2022, 12, 915171. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Frontera, O.A.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, D.J.; et al. Nivolumab Plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Powles, T.; Burotto, M.; Escudier, B.; Bourlon, M.T.; Zurawski, B.; Sharma, P.; Porta, C.; Robert, C.; Wang, E.; et al. Nivolumab Plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2021, 384, 829–841. [Google Scholar] [CrossRef]
- Motzer, R.; Alekseev, B.; Rha, S.Y.; Porta, C.; Eto, M.; Powles, T.; Grünwald, V.; Gillessen, S.; Kapoor, A.; Sternberg, C.N.; et al. Lenvatinib Plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N. Engl. J. Med. 2021, 384, 1289–1300. [Google Scholar] [CrossRef]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Sternberg, C.N.; Ni, J.; et al. Pembrolizumab Plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef]
- Shamis, S.A.K.; Edwards, J.; McMillan, D.C. The Relationship between Carbonic Anhydrase IX (CAIX) and Patient Survival in Breast Cancer: Systematic Review and Meta-Analysis. Diagn. Pathol. 2023, 18, 46. [Google Scholar] [CrossRef]
- Zhang, C.; Fang, L.; Wang, X.; Yuan, S.; Li, W.; Tian, W.; Xu, J.; Li, Y.; Chen, H.; Zhao, H.; et al. Oncolytic Adenovirus-Mediated Expression of Decorin Facilitates CAIX-Targeting CAR-T Therapy against Renal Cell Carcinoma. Mol. Ther. Oncolytics 2022, 24, 14–25. [Google Scholar] [CrossRef]
- Bradley, R.L.; Paul, E.; Singh, S.; Hutson, T.E. CD70-targeted allogeneic CAR T-cell therapy for clear cell renal cell carcinoma. Expert Rev. Anticancer Ther. 2025, 1–10. [Google Scholar] [CrossRef]
- Li, H.; Ding, J.; Lu, M.; Liu, H.; Miao, Y.; Li, L.; Wang, G.; Zheng, J.; Pei, D.; Zhang, Q. CAIX-specific CAR-T Cells and Sunitinib Show Synergistic Effects Against Metastatic Renal Cancer Models. J. Immunother. 2020, 43, 16–28. [Google Scholar] [CrossRef]
- Li, Q.; Yang, L.; Li, S.; Zhao, W.; Xue, Y.; Lu, Z.; Tang, J.; Gao, X.; Zheng, J.; Zhang, Q.; et al. Cabozantinib Enhances CAIX-Specific CAR T Cells against Renal Cancer by Improving Effector Functions and Augmenting the Tumor Immune Microenvironment. Biochem. Biophys. Res. Commun. 2024, 734, 150781. [Google Scholar] [CrossRef]
- Lanitis, E.; Kosti, P.; Ronet, C.; Cribioli, E.; Rota, G.; Spill, A.; Reichenbach, P.; Zoete, V.; Laniti, D.D.; Coukos, G.; et al. VEGFR-2 redirected CAR-T cells are functionally impaired by soluble VEGF-A competition for receptor binding. J. Immunother. Cancer 2021, 9, e002151. [Google Scholar] [CrossRef]
- Yousef, A.; Kim, S.S.; Krizova, A. CAIX Immunostaining in Non-Neoplastic Renal Diseases. Cancer Diagn. Progn. 2022, 2, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Lamers, C.H.J.; Sleijfer, S.; van Steenbergen, S.; van Elzakker, P.; van Krimpen, B.; Groot, C.; Vulto, A.; den Bakker, M.; Oost-erwijk, E.; Debets, R.; et al. Treatment of Metastatic Renal Cell Carcinoma with CAIX CAR-Engineered T Cells: Clinical Evaluation and Management of On-Target Toxicity. Mol. Ther. 2013, 21, 904–912. [Google Scholar] [CrossRef]
- Lo, A.W.I.; Chen, L.J. Affinity Fine Tuning Anti-CAIX CAR T Cells Mitigate On-Target Off-Tumor Toxicity in ccRCC by Differentiating between Tumor and Bile Duct Antigen Density. Mol. Cancer 2024, 23, 123. [Google Scholar] [CrossRef]
- Ruf, M.; Moch, H.; Schraml, P.; Zlobec, I.; Lugli, A.; Tornillo, L.; Komminoth, P.; Gasser, T.; Fankhauser, N.; Hofmann, M.; et al. Interaction of Tumor Cells with Infiltrating Lymphocytes via CD70 and CD27 in Clear Cell Renal Cell Carcinoma. Oncoim-Munology 2015, 4, e1049805. [Google Scholar] [CrossRef]
- Benhamouda, N.; Sam, I.; Epaillard, N.; Chabaud, S.; Martin, A.; Dupont, S.; Nguyen, L.; Boulle, N.; Moreau, P.; Petitprez, F.; et al. Plasma CD27, a Surrogate of the Intratumoral CD27–CD70 Interaction, Correlates with Immunotherapy Resistance in Renal Cell Carcinoma. Clin. Cancer Res. 2022, 28, 4983–4994. [Google Scholar] [CrossRef] [PubMed]
- Panowski, S.H.; Srinivasan, S.; Tan, N.; Chen, H.; Li, X.; Zhang, Y.; Wu, J.; Lee, K.; Smith, J.; Patel, R.; et al. Preclinical Development and Evaluation of Allogeneic CAR T Cells Targeting CD70 for the Treatment of Renal Cell Carcinoma. Cancer Res. 2022, 82, 2610–2624. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.K.; Tran, B.; Haanen, J.B.A.G.; Oudard, S.; Choueiri, T.K.; Albiges, L.; McDermott, D.F.; Powles, T.; Grivas, P.; Heng, D.Y.C.; et al. CD70-Targeted Allogeneic CAR T-Cell Therapy for Advanced Clear Cell Renal Cell Carcinoma. Cancer Discov. 2024, 14, 1234–1245. [Google Scholar] [CrossRef] [PubMed]
- Srour, S.A.; Chahoud, J.; Drakaki, A.; Pal, S.K.; Tran, B.; Haanen, J.B.A.G.; Oudard, S.; Choueiri, T.K.; Albiges, L.; McDermott, D.F.; et al. ALLO-316 in Advanced Clear Cell Renal Cell Carcinoma (ccRCC): Updated Results from the Phase 1 TRAVERSE Study. J. Clin. Oncol. 2025, 43 (Suppl. S16), 4508. [Google Scholar] [CrossRef]
- Bakht, M.K.; Beltran, H. Biological Determinants of PSMA Expression, Regulation and Heterogeneity in Prostate Cancer. Nat. Rev. Urol. 2025, 22, 26–45. [Google Scholar] [CrossRef]
- Saeki, N.; Gu, J.; Yoshida, T.; Wu, X. Prostate Stem Cell Antigen (PSCA): A Jekyll and Hyde Molecule? Clin. Cancer Res. 2010, 16, 3533–3538. [Google Scholar] [CrossRef]
- McKean, M.; Carabasi, M.H.; Stein, M.N.; Milowsky, M.I.; Vogelzang, N.J.; Kwon, E.D.; Kelly, W.K.; Kantoff, P.W.; Anto-narakis, E.S.; Pal, S.K.; et al. Safety and Early Efficacy Results from a Phase 1, Multicenter Trial of PSMA-Targeted Armored CAR T Cells in Patients with Advanced mCRPC. J. Clin. Oncol. 2022, 40 (Suppl. S6), 94. [Google Scholar] [CrossRef]
- Narayan, V.; Barber-Rotenberg, J.S.; Jung, I.Y.; Pal, S.K.; Milowsky, M.I.; Stein, M.N.; Antonarakis, E.S.; Carabasi, M.H.; Kelly, W.K.; Kantoff, P.W.; et al. PSMA-Targeting TGFβ-Insensitive Armored CAR T Cells in Metastatic Castration-Resistant Prostate Cancer: A Phase 1 Trial. Nat. Med. 2022, 28, 724–734. [Google Scholar] [CrossRef]
- Powles, T.; Assaf, C.; Balar, A.V.; Bellmunt, J.; Castellano, D.; Galsky, M.D.; Necchi, A.; O’Donnell, P.H.; Petrylak, D.P.; Sternberg, C.N.; et al. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Carcinoma. N. Engl. J. Med. 2023, 393, 1496–1506. [Google Scholar] [CrossRef]
- Sonpavde, G.; Pal, S.K.; Balar, A.V.; Bellmunt, J.; Castellano, D.; Galsky, M.D.; Necchi, A.; O’Donnell, P.H.; Petrylak, D.P.; Sternberg, C.N.; et al. Nivolumab Plus Gemcitabine–Cisplatin in Advanced Urothelial Carcinoma. N. Engl. J. Med. 2023, 393, 1434–1445. [Google Scholar] [CrossRef]
- Picarda, E.; Ohaegbulam, K.C.; Zang, X. Molecular Pathways: Targeting B7-H3 (CD276) for Human Cancer Immunotherapy. Clin. Cancer Res. 2016, 22, 3425–3431. [Google Scholar] [CrossRef]
- Du, H.; Liang, Y.; Zhang, W.; Sun, X.; Chen, J.; Li, Q.; Wang, Y.; Zhao, L.; Xu, P.; Li, R.; et al. Antitumor Responses in the Absence of Toxicity in Solid Tumors by Targeting B7-H3 via Chimeric Antigen Receptor T Cells. Cancer Cell 2019, 35, 221–237.e8. [Google Scholar] [CrossRef] [PubMed]
- Flieswasser, T.; Eynde, A.V.D.; Van Audenaerde, J.; De Waele, J.; Lardon, F.; Riether, C.; de Haard, H.; Smits, E.; Pauwels, P.; Jacobs, J. The CD70-CD27 axis in oncology: The new kids on the block. J. Exp. Clin. Cancer Res. 2022, 41, 1–15. [Google Scholar] [CrossRef]
- Zhao, M.; Li, N.; Wang, L.; Chen, J.; Wu, X.; Zhang, Y.; Li, H.; Xu, P.; Sun, S.; Huang, Y.; et al. Potential Therapeutic Targets for the Treatment of HPV-Associated Cancers. Cancers 2024, 16, 3474. [Google Scholar] [CrossRef]
- Liu, J.; Kang, Y.; Li, L.; Zang, L.; Luo, L.; Zhu, F.; Zhu, M.; Zhang, H.; Xu, Q. Efficacy and Safety of Immunotherapy in Cervical Cancer: A Review of Advances in Immune-Related Therapy for Cervical Cancer. Holist. Integr. Oncol. 2025, 4, 15. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, Y.; Zhou, Q.; Liu, H.; Chen, J.; Wang, L.; Li, X.; Sun, S.; Zhao, L.; Xu, P.; et al. T Cell Immunotherapy for Cervical Cancer: Challenges and Opportunities. BMC Cancer 2023, 23, 799. [Google Scholar] [CrossRef]
- Duan, Z.; Li, D.; Li, N.; Zhang, Y.; Chen, H.; Wang, L.; Sun, X.; Zhao, L.; Xu, P.; Li, R.; et al. CAR-T Cells Based on a TCR Mimic Nanobody Targeting HPV16 E6 Exhibit Antitumor Activity against Cervical Cancer. Mol. Ther. Oncol. 2024, 32, 200892. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Liu, J.; Chen, H.; Li, L.; Sun, S.; Zhao, L.; Xu, P.; Li, R.; Huang, Y.; et al. Potent, Selective CARs as Potential T-Cell Therapeutics for HPV-Positive Cancers. J. Immunother. 2021, 44, 292–306. [Google Scholar] [CrossRef]
- Doran, S.L.; Cai, Z.; Liu, C.; Zhang, X.; Peng, Y.; Wang, L.; Li, H.; Sun, S.; Xu, P.; Huang, Y.; et al. T-Cell Receptor Gene Therapy for Human Papillomavirus–Associated Epithelial Cancers: A First-in-Human, Phase I/II Study. J. Clin. Oncol. 2019, 37, 2759–2768. [Google Scholar] [CrossRef]
- Jin, B.Y.; Campbell, T.E.; Draper, L.M.; Wang, X.; Li, H.; Sun, S.; Zhao, L.; Xu, P.; Huang, Y.; Zhang, Y.; et al. Engineered T Cells Targeting E7 Mediate Regression of Human Papillomavirus Cancers in a Murine Model. JCI Insight 2018, 3, e99488. [Google Scholar] [CrossRef]
- Zhao, X.; Ran, J.; Li, S.; Chen, J. Advances and obstacles of T cell-based immunotherapy in gynecological malignancies. Mol. Cancer 2025, 24, 1–25. [Google Scholar] [CrossRef]
- Curiel, T.J.; Coukos, G.; Zou, L.; Alvarez, X.; Cheng, P.; Mottram, P.; Evdemon-Hogan, M.; Conejo-Garcia, J.R.; Zhang, L.; Burow, M.; et al. Specific Recruitment of Regulatory T Cells in Ovarian Carcinoma Fosters Immune Privilege and Predicts Reduced Survival. Nat. Med. 2004, 10, 942–949. [Google Scholar] [CrossRef]
- Sasaki, S.; Nishikawa, H.; Matsuda, M.; Aoki, Y.; Ishii, H.; Shimizu, K.; Takeda, K.; Sugiyama, H.; Ohnishi, T.; Nakagawa, S.; et al. The Presence of Tregs and High TGF-β Levels Correlate with Poor Prognosis in Cervical Cancer. Gynecol. Oncol. 2013, 128, 77–83. [Google Scholar] [CrossRef]
- Lee, E.H.J.; Tan, S.; Lee, W.; Choi, S.; Lee, J.; Lee, J.; Kim, Y.; Kim, S.; Kim, Y.; Lee, J.; et al. Antigen-Dependent IL-12 Signaling in CAR T Cells Enhances Tumor Immunity. Nat. Commun. 2023, 14, 4737. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, Z.; Zhang, G.; Fan, J. Interleukin-enhanced CAR-engineered immune cells in tumor immunotherapy: Current insights and future perspectives. Cytokine 2025, 192, 156973. [Google Scholar] [CrossRef]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R.; Ferris, R.L.; Guo, M.; Li, H.; Zhang, Y.; et al. Head and Neck Squamous Cell Carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Li, W.; Lui, V.W.Y.; Patel, J.J.; Grandis, J.R.; Ferris, R.L.; Bauman, J.E.; Johnson, D.E.; Zhang, Y.; Chen, X.; Sun, S.; et al. Recent Advances in Immunotherapy for Advanced HPV Negative Head and Neck Cancer: Challenges and Opportunities. Front. Oncol. 2021, 11, 727433. [Google Scholar] [CrossRef]
- Palumbo, C.; Benvenuto, M.; Focaccetti, C.; Albonici, L.; Cifaldi, L.; Rufini, A.; Nardozi, D.; Angiolini, V.; Bei, A.; Masuelli, L.; et al. Recent Findings on the Impact of ERBB Receptors Status on Prognosis and Therapy of Head and Neck Squamous Cell Carcinoma. Front. Med. 2023, 10, 1066021. [Google Scholar] [CrossRef]
- Dong, Y.-H.; Ding, Y.-M.; Guo, W.; Huang, J.-W.; Yang, Z.; Zhang, Y.; Chen, X.-H.; Li, H.; Wang, Y.; Sun, S.; et al. The Functional Verification of EGFR-CAR T Cells Targeted to Hypopharyngeal Squamous Cell Carcinoma. OncoTargets Ther. 2018, 11, 7053–7059. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.P.; Jin, L.; Bennett, K.B.; Wang, D.; Fredenburg, K.M.; Tseng, J.E.; Chang, L.J.; Huang, J.; Chan, E.K. CD70 as a Target for Chimeric Antigen Receptor T Cells in Head and Neck Squamous Cell Carcinoma. Oral Oncol. 2018, 78, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Zhang, K.; Lam, A.K.; Huang, J.; Qiu, F.; Qiao, B.; Zhang, Y.; Li, H.; Sun, S.; Zhao, L.; et al. MUC1 as a Target for CAR-T Therapy in Head and Neck Squamous Cell Carcinoma. Cancer Med. 2020, 9, 640–652. [Google Scholar] [CrossRef]
- Haist, C.; Schulte, E.; Bartels, N.; Bister, A.; Poschinski, Z.; Ibach, T.C.; Geipel, K.; Wiek, C.; Wagenmann, M.; Monzel, C.; et al. CD44v6-Targeted CAR T-Cells Specifically Eliminate CD44 Isoform 6 Expressing Head and Neck Squamous Cell Carcinoma Cells. Mol. Cancer Ther. 2022, 21, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Papa, S.; Adami, A.; Metoudi, M.; Achkova, D.; van Schalkwyk, M.; Parente Pereira, A.; Bosshard-Carter, L.; Whilding, L.; van der Stegen, S.; Davies, D.; et al. A Phase I Trial of T4 CAR T-Cell Immunotherapy in Head and Neck Squamous Cancer (HNSCC). J. Clin. Oncol. 2018, 36, 3046. [Google Scholar] [CrossRef]
- Lin, S.; Cheng, L.; Ye, W.; Li, S.; Zheng, D.; Qin, L.; Wu, Q.; Long, Y.; Lin, S.; Wang, S.; et al. Chimeric CTLA4-CD28-CD3z T Cells Potentiate Antitumor Activity Against CD80/CD86–Positive B Cell Malignancies. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Smith, E.L.; Mailankody, S.; Staehr, M.; Wang, X.; Senechal, B.; Purdon, T.J.; Daniyan, A.F.; Geyer, M.B.; Goldberg, A.D.; Mead, E.; et al. BCMA-Targeted CAR T Cell Therapy Plus Radiotherapy for the Treatment of Refractory Myeloma Reveals Potential Synergy. Cancer Immunol. Res. 2019, 7, 1047–1053. [Google Scholar] [CrossRef]
- Beatty, G.L.; Haas, A.R.; Maus, M.V.; Torigian, D.A.; Soulen, M.C.; Plesa, G.; Chew, A.; Zhao, Y.; Levine, B.L.; Albelda, S.M.; et al. Mesothelin-Specific Chimeric Antigen Receptor mRNA-Engineered T Cells Induce Anti-Tumor Activity in Solid Malignan-cies. Cancer Immunol. Res. 2014, 2, 112–120. [Google Scholar] [CrossRef]
- Watanabe, K.; Luo, Y.; Da, T.; Guedan, S.; Ruella, M.; Scholler, J.; Keith, B.; Young, R.M.; Engels, B.; Sorsa, S.; et al. Pancreatic Cancer Therapy with Combined Mesothelin-Redirected Chimeric Antigen Receptor T Cells and Cytokine-Armed Oncolytic Adenoviruses. JCI Insight 2018, 3, e99573. [Google Scholar] [CrossRef]
- Chen, Q.; Sun, Y.; Li, H. Application of CAR-T Cell Therapy Targeting Mesothelin in Solid Tumor Treatment. Discov. Oncol. 2024, 15, 289. [Google Scholar] [CrossRef] [PubMed]
- DeSelm, C.J.; Tano, Z.E.; Varghese, A.M.; Adusumilli, P.S. CAR T-cell therapy for pancreatic cancer. J. Surg. Oncol. 2017, 116, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.C.; Burga, R.A.; McCormack, E.; Wang, L.J.; Mooring, W.; Point, G.R.; Khare, P.D.; Thorn, M.; Ma, Q.; Stainken, B.F.; et al. Phase I Hepatic Immunotherapy for Metastases Study of Intrahepatic Infusion of CAR-T Cells Targeting CEA+ Liver Me-tastases. Clin. Cancer Res. 2015, 21, 3149–3159. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.; Sukumaran, S.; Bajgain, P.; Watanabe, N.; Heslop, H.E.; Rooney, C.M.; Brenner, M.K.; Fisher, W.E.; Leen, A.M.; Vera, J.F. Improving Chimeric Antigen Receptor-Modified T Cell Function by Reversing the Immunosuppressive Tumor Microenvironment of Pancreatic Cancer. Mol. Ther. 2017, 25, 249–258. [Google Scholar] [CrossRef]
- Foeng, J.; Comerford, I.; McColl, S.R. Harnessing the chemokine system to home CAR-T cells into solid tumors. Cell Rep. Med. 2022, 3, 100543. [Google Scholar] [CrossRef]
- Beatty, G.L.; O’Hara, M.H.; Lacey, S.F.; Melenhorst, J.J.; Levine, B.L.; Plesa, G.; Hamid, O.; Wang, C.; Andrechek, E.R.; Riches, J.C.; et al. Mesothelin-Specific CAR T Cells in Patients with Advanced Pancreatic Cancer: A Phase I Trial. Clin. Cancer Res. 2020, 26, 422–433. [Google Scholar]
- University of Pennsylvania. Phase I Study of Human Chimeric Antigen Receptor Modified T Cells (CAR T Cells) in Patients with Pancreatic Cancer. ClinicalTrials.gov Identifier: NCT03323944. Available online: https://clinicaltrials.gov/study/NCT03323944 (accessed on 21 August 2025).
- Bellicum Pharmaceuticals. Safety and Activity Study of PSCA-Targeted CAR-T Cells (BPX-601) in Subjects with Selected Advanced Solid Tumors. ClinicalTrials.gov Identifier: NCT02744287. Available online: https://clinicaltrials.gov/study/NCT02744287 (accessed on 21 August 2025).
- Xinqiao Hospital of Chongqing. Phase I Trial of Anti-GPC3 Chimeric T Cells for Subjects with GPC3-Positive Advanced Hepatocellular Carcinoma. ClinicalTrials.gov Identifier: NCT03084380. Available online: https://clinicaltrials.gov/study/NCT03084380 (accessed on 21 August 2025).
- Roth, J.; Brine, N.; Squiers, C.; Rathi, R.; Watanabe, M.; Brown, C.; Ramos, C.A.; Vaidya, A.; Dela Cruz, F.S.; Hingorani, P.; et al. GD2 Expression and Targeting in Osteosarcoma: Implications for CAR T Cell Therapy. Front. Immunol. 2023, 14, 1290762. [Google Scholar]
- Arnett, A.B.; Heczey, A. GD2-CAR CAR T Cells in Patients with Osteosarcoma and Neuroblastoma—It’s Not Only the T Cells That Matter. Cancer Cell 2024, 42, 8–10. [Google Scholar] [CrossRef]
- Kaczanowska, S.; Murty, T.; Alimadadi, A.; Lasek, W.; Fu, Y.; Xu, H.; Smith, D.; Lee, H.; Jones, M.; He, Y.; et al. Immune De-terminants of CAR-T Cell Expansion in Solid Tumor Patients Receiving GD2 CAR-T Cell Therapy. Cancer Cell 2024, 42, 35–51.e8. [Google Scholar] [CrossRef]
- Hegde, M.; Navai, S.; DeRenzo, C.; Wu, M.F.; Xu, H.; Turk, C.; Zhang, H.; Shea, M.; Bilgi, M.; Lin, Y.; et al. Autologous HER2-Specific CAR T Cells after Lymphodepletion for Advanced Sarcoma: A Phase 1 Trial. Nat. Cancer 2024, 5, 880–894. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.J.; Cowey, C.L.; Dummer, R.; Schadendorf, D.; Long, G.V.; Robert, C.; et al. Medical Needs and Therapeutic Options for Melanoma Patients Resistant to Anti–PD-1–Directed Im-mune Checkpoint Inhibition. Cancers 2023, 15, 3448. [Google Scholar]
- U.S. Food and Drug Administration (FDA). FDA Grants Accelerated Approval to Lifileucel (Amtagvi) for Unresectable or Metastatic Melanoma. FDA Press Release. 16 February 2024. Available online: https://www.ons.org/publications-research/voice/news-views/02-2024/fda-grants-accelerated-approval-lifileucel (accessed on 15 August 2025).
- Medina, T.; Chesney, J.A.; Whitman, E.; Kluger, H.M.; Thomas, S.; Sarnaik, A.; Kirkwood, J.M.; Larkin, J.M.G.; Weber, J.S.; Hamid, O.; et al. Long Term Efficacy and Safety of Lifileucel Tumor Infiltrating Lymphocyte (TIL) Cell Therapy in Patients with Advanced Melanoma: A 5 Year Analysis of the C 144 01 Study. J. Clin. Oncol. 2025. [Google Scholar] [CrossRef]
- Jilani, S.; Saco, J.D.; Mugarza, E.; Pujol Morcillo, A.; Chokry, J.; Ng, C.; Abril Rodriguez, G.; Berger Manerio, D.; Pant, A.; Hu, J.; et al. CAR T Cell Therapy Targeting Surface Expression of TYRP1 to Treat Cutaneous and Rare Melanoma Subtypes. Nat. Commun. 2024, 15, 1244. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; Curschmann, J.; et al. Radiotherapy Plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, F.; Ali, H.; Zhang, J.; Chen, X.; Li, Y.; Wang, Y.; Zhao, L.; Lin, Q.; Wu, H.; et al. Immunotherapy for Glioblastoma: Current State, Challenges, and Future Perspectives. Cell Mol. Immunol. 2024, 21, 1354–1375. [Google Scholar] [CrossRef]
- Bagley, S.J.; Desai, A.S.; Fraietta, J.A.; June, C.H.; Brown, C.; Smith, M.; Patel, S.; Chen, X.; Lee, D.; Anderson, K.; et al. In-tracerebroventricular Bivalent CAR T Cells Targeting EGFR and IL 13Rα2 in Recurrent Glioblastoma: A Phase 1 Trial. Nat. Med. 2025, 31, 2778–2787. [Google Scholar] [CrossRef]
- O’Rourke, D.M.; Nasrallah, M.P.; Desai, A.; Melenhorst, J.J.; Rooney, C.M.; June, C.H.; Brown, C.; Feng, M.; Smith, M.; Patel, S.; et al. A Single Dose of Peripherally Infused EGFRvIII Directed CAR T Cells Mediates Antigen Loss and Induces Adaptive Re-sistance in Patients with Recurrent Glioblastoma. Sci. Transl. Med. 2017, 9, eaaa0984. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.D.; Yu, X.; Castano, A.P.; Bouffard, A.A.; Schmidts, A.; Larson, R.C.; Bailey, S.R.; Boroughs, A.C.; Frigault, M.J.; Leick, M.B.; et al. CAR T Cells Secreting BiTEs Circumvent Antigen Escape Without Detectable Toxicity. Nat. Biotechnol. 2019, 37, 1049–1058. [Google Scholar] [CrossRef]
- Choi, B.D.; Gerstner, E.R.; Frigault, M.J.; Leick, M.B.; Mount, C.W.; Balaj, L.; Nikiforow, S.; Carter, B.S.; Curry, W.T.; Gallagher, K.; et al. Intraventricular CARv3 TEAM E T Cells in Recurrent Glioblastoma. N. Engl. J. Med. 2024, 390, 1290–1298. [Google Scholar] [CrossRef]
- Vitanza, N.A.; Johnson, A.J.; Wilson, A.L.; Brown, C.; Yokoyama, J.K.; Künkele, A.; Chang, C.A.; Rawlings-Rhea, S.; Huang, W.; Seidel, K.; et al. Locoregional Infusion of HER2-Specific CAR T Cells in Children and Young Adults with Recurrent or Refractory CNS Tumors: An Interim Analysis. Nat. Med. 2021, 27, 1544–1552. [Google Scholar] [CrossRef]
- Lo, C.M.; Iqbal, U.; Li, Y.C.J. Cancer Quantification from Data Mining to Artificial Intelligence. Comput. Methods Programs Biomed. 2017, 145, 104–115. [Google Scholar] [CrossRef]
- Shahzadi, M.; Rafique, H.; Waheed, A.; Iqbal, S.; Khan, R.; Ali, F.; Khan, M.; Ahmed, S.; Fatima, N.; Hussain, T.; et al. Artificial Intelligence for Chimeric Antigen Receptor Based Therapies: A Comprehensive Review of Current Applications and Future Perspectives. Ther. Adv. Vaccines Immunother. 2024, 12, 25151355241305856. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, Y.; Jing, Y.; Li, X.; Zhao, H.; Wang, Q.; Liu, Z.; Xu, Y.; Tan, L.; Sun, P.; et al. Advancing CAR T Cell Therapy through the Use of Multidimensional Omics Data. Nat. Rev. Clin. Oncol. 2023, 20, 211–228. [Google Scholar] [CrossRef]
- Dagar, G.; Gupta, A.; Masoodi, T.; Sharma, R.; Kumar, V.; Verma, P.; Singh, R.; Patel, S.; Choudhary, A.; Mehta, A.; et al. Harnessing the Potential of CAR-T Cell Therapy: Progress, Challenges, and Future Directions in Hematological and Solid Tumor Treatments. J. Transl. Med. 2023, 21, 449. [Google Scholar] [CrossRef] [PubMed]
- Bäckel, N.; Hort, S.; Kis, T.; Mayer, K.; Schmidt, L.; Weber, M.; Braun, F.; Keller, R.; Lang, H.; Zimmermann, S.; et al. Elaborating the Potential of Artificial Intelligence in Automated CAR-T Cell Manufacturing. Front. Mol. Med. 2023, 3, 1250508. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, J.; Jiang, H.; Zhou, M. Strategies to Overcome Antigen Heterogeneity in CAR-T Cell Therapy. Cells 2025, 14, 320. [Google Scholar] [CrossRef]
- Chen, N.; Li, X.; Chintala, N.K.; Tano, Z.E.; Adusumilli, P.S. Driving CARs on the Uneven Road of Antigen Heterogeneity in Solid Tumors. Curr. Opin. Immunol. 2018, 51, 103–110. [Google Scholar] [CrossRef]
- Huang, Y.; Kim, B.Y.S.; Chan, C.K.; Hahn, S.M.; Weissman, I.L.; Jiang, W. Improving Immune Vascular Crosstalk for Cancer Immunotherapy. Nat. Rev. Immunol. 2018, 18, 195–203. [Google Scholar] [CrossRef]
- Fonkoua, L.A.K.; Sirpilla, O.; Sakemura, R.; Siegler, E.L.; Kenderian, S.S. CAR T Cell Therapy and the Tumor Microenvironment: Current Challenges and Opportunities. Mol. Ther. Oncolytics 2022, 25, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Togashi, Y.; Shitara, K.; Nishikawa, H. Regulatory T Cells in Cancer Immunosuppression—Implications for Anticancer Therapy. Nat. Rev. Clin. Oncol. 2019, 16, 356–371. [Google Scholar] [CrossRef] [PubMed]
- Sterner, R.M.; Kenderian, S.S. Myeloid Cell and Cytokine Interactions with Chimeric Antigen Receptor T Cell Therapy: Im-plication for Future Therapies. Curr. Opin. Hematol. 2020, 27, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Singer, K.; Cheng, W.C.; Kreutz, M.; Ho, P.C.; Siska, P.J. Immunometabolism in Cancer at a Glance. Dis. Model Mech. 2018, 11, dmm034272. [Google Scholar] [CrossRef]
- Rojas-Quintero, J.; Díaz, M.P.; Palmar, J.; Galan-Freyle, N.J.; Morillo, V.; Escalona, D.; González-Torres, H.J.; Torres, W.; Navarro-Quiroz, E.; Rivera-Porras, D.; et al. CAR T Cells in Solid Tumors: Overcoming Obstacles. Int. J. Mol. Sci. 2024, 25, 4170. [Google Scholar] [CrossRef]
- Zhao, Y.; Shen, M.; Wu, L.; Li, X.; Zhang, H.; Chen, Y.; Wang, Q.; Liu, Z.; Sun, J.; Xu, Y.; et al. Stromal Cells in the Tumor Microenvironment: Accomplices of Tumor Progression? Cell Death Dis. 2023, 14, 587. [Google Scholar] [CrossRef]
- Lanitis, E.; Irving, M.; Coukos, G. Targeting the Tumor Vasculature to Enhance T Cell Activity. Curr. Opin. Immunol. 2015, 33, 55–63. [Google Scholar] [CrossRef]
- Lin, H.; Yang, X.; Ye, S.; Chen, L.; Zhao, Y.; Li, M.; Wang, H.; Zhou, J.; Sun, P.; Liu, Q.; et al. Antigen Escape in CAR-T Cell Therapy: Mechanisms and Overcoming Strategies. Biomed. Pharmacother. 2024, 178, 117252. [Google Scholar] [CrossRef]
- Zhai, Y.; Du, Y.; Li, G.; Wang, H.; Chen, X.; Zhang, L.; Sun, J.; Liu, Q.; Yang, P.; Zhao, M.; et al. Trogocytosis of CAR Molecule Regulates CAR T Cell Dysfunction and Tumor Antigen Escape. Signal Transduct. Target. Ther. 2023, 8, 383. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhao, F. Strategies to Overcome Tumour Relapse Caused by Antigen Escape after CAR T Therapy. Mol. Cancer 2025, 24, 126. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, S.; Fang, C.; Yang, S.; Olalere, D.; Pequignot, E.; Zhao, Y. Affinity-Tuned HER2 CAR-T Cells Demonstrate Reduced Off-Tumor Toxicity against Low HER2-Expressing Tissues While Maintaining Anti-Tumor Activity. Nat. Biotechnol. 2022, 40, 631–642. [Google Scholar] [CrossRef]
- Locke, F.L.; Miklos, D.B.; Jacobson, C.A.; Perales, M.-A.; Kersten, M.-J.; Oluwole, O.O.; Neelapu, S.S. Long-Term Safety and Activity of Axicabtagene Ciloleucel in Refractory Large B-Cell Lymphoma. Blood 2021, 137, 2306–2315. [Google Scholar] [CrossRef]
- Kloss, C.C.; Condomines, M.; Cartellieri, M.; Bachmann, M.; Sadelain, M. Combinatorial Antigen Recognition with Balanced Signaling Promotes Selective Tumor Eradication by Engineered T Cells. Nat. Biotechnol. 2013, 31, 71–75. [Google Scholar] [CrossRef]
- Zhao, Z.; Shi, L.; Zhang, W.; Han, J.; Zhang, S.; Fu, Z.; Wei, X. mRNA-Engineered CAR-T Cells with Transient Expression Reduce On-Target Off-Tumor Toxicity in Solid Tumors. Nat. Commun. 2023, 14, 1123. [Google Scholar] [CrossRef]
- Fedorov, V.D.; Themeli, M.; Sadelain, M. PD-1– and CTLA-4–Based Inhibitory Chimeric Antigen Receptors (iCARs) Divert Off-Target Immunotherapy Responses. Sci. Transl. Med. 2013, 5, 215ra172. [Google Scholar] [CrossRef]
- Grosser, R.; Cherkassky, L.; Chintala, N.; Adusumilli, P.S. Combination Immunotherapy with CAR T Cells and Checkpoint Blockade for the Treatment of Solid Tumors. Cancer Cell 2019, 36, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Luo, X.; Xie, Z.; Qiu, J.; Yang, J.; Deng, Y.; Long, R.; Tang, G.; Zhang, C.; Zuo, J. Prospects and Challenges of CAR T Cell Therapy Combined with ICIs. Front. Oncol. 2024, 14, 1368732. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Furlan, S.N.; Jaeger Ruckstuhl, C.A.; Li, X.; Zhang, H.; Chen, Y.; Wang, Q.; Liu, Z.; Sun, J.; Xu, Y.; et al. Immunogenic Chemotherapy Enhances Recruitment of CAR T Cells to Lung Tumors and Improves Antitumor Efficacy when Combined with Checkpoint Blockade. Cancer Cell 2021, 39, 193–208.e10. [Google Scholar] [CrossRef]
- He, S.; Zheng, L.; Qi, C. Myeloid Derived Suppressor Cells (MDSCs) in the Tumor Microenvironment and Their Targeting in Cancer Therapy. Mol. Cancer 2025, 24, 5. [Google Scholar] [CrossRef]
- Chong, E.A.; Melenhorst, J.J.; Lacey, S.F.; Ambrose, D.E.; Gonzalez, V.; Levine, B.L.; June, C.H.; Schuster, S.J. PD 1 Blockade Modulates Chimeric Antigen Receptor (CAR) Modified T Cells: Refueling the CAR. Blood 2017, 129, 1039–1041. [Google Scholar] [CrossRef]
- Heczey, A.; Louis, C.U.; Savoldo, B.; Dotti, G.; Pule, M.; Rooney, C.M.; Zhang, H.; Liu, H.; Heslop, H.E.; Brenner, M.K.; et al. CAR T Cells Administered in Combination with Lymphodepletion and PD 1 Inhibition to Patients with Neuroblastoma. Mol. Ther. 2017, 25, 2214–2224. [Google Scholar] [CrossRef]
- Jarosz Biej, M.; Smolarczyk, R.; Cichoń, T.; Kułach, N. Tumor Microenvironment as a ‘Game Changer’ in Cancer Radiotherapy. Int. J. Mol. Sci. 2019, 20, 3212. [Google Scholar] [CrossRef]
- Zhong, L.; Li, Y.; Muluh, T.A.; Wang, Y. Combination of CAR T Cell Therapy and Radiotherapy: Opportunities and Challenges in Solid Tumors. Oncol. Lett. 2023, 26, 281. [Google Scholar] [CrossRef] [PubMed]
- Ababneh, H.S.; Abramson, J.S.; Johnson, P.C.; Patel, C.G. Assessing the Role of Radiotherapy in Patients with Refractory or Relapsed High Grade B Cell Lymphomas Treated with CAR T Cell Therapy. Radiother. Oncol. 2022, 175, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Rafii, S.; Tashkandi, E.; Bukhari, N.; Al Shamsi, H.O. Current Status of CRISPR/Cas9 Application in Clinical Cancer Research: Opportunities and Challenges. Cancers 2022, 14, 947. [Google Scholar] [CrossRef]
- Tao, R.; Han, X.; Bai, X.; Liu, S.; Li, Z.; Zhang, Y.; Wang, H.; Chen, X.; Li, M.; Zhao, Y.; et al. Revolutionizing Cancer Treatment: Enhancing CAR T Cell Therapy with CRISPR/Cas9 Gene Editing Technology. Front. Immunol. 2024, 15, 1354825. [Google Scholar] [CrossRef] [PubMed]
- Dimitri, A.; Herbst, F.; Fraietta, J.A. Engineering the Next Generation of CAR T Cells with CRISPR Cas9 Gene Editing. Mol. Cancer 2022, 21, 78. [Google Scholar] [CrossRef]
- Shih, R.M.; Clubb, J.; Lam, K.; Shafer, A.; Vajragiri, S.; Bouren, A.; Chen, Y. IL 12/IL 18 Armored CAR T Cells Drive Pro Inflammatory Remodeling of the Glioblastoma Tumor Microenvironment [Abstract A027]. Cancer Immunol. Res. 2025, 13 (Suppl. S2), A027. [Google Scholar] [CrossRef]
- Yeku, O.O.; Purdon, T.J.; Koneru, M.; Spriggs, D.; Brentjens, R.J. Armored CAR T Cells Enhance Antitumor Efficacy and Overcome the Tumor Microenvironment. Sci. Rep. 2017, 7, 10541. [Google Scholar] [CrossRef]
- Svoboda, J.; Landsburg, D.J.; Gerson, J.; Nasta, S.D.; Barta, S.K.; Chong, E.A.; Cook, M.; Frey, N.V.; Shea, J.; Cervini, A.; et al. Enhanced CAR T-Cell Therapy for Lymphoma after Previous Failure. N. Engl. J. Med. 2025, 392, 1824–1835. [Google Scholar] [CrossRef]
- ClinicalTrials.Gov. Identifier: NCT06198296. Available online: https://clinicaltrials.gov/study/NCT06198296 (accessed on 20 August 2025).
- Marei, H.E.; Bedair, K.; Hasan, A.; Al-Mansoori, L.; Caratelli, S.; Sconocchia, G.; Gaiba, A.; Cenciarelli, C. Current Status and Innovative Developments of CAR T Cell Therapy for the Treatment of Breast Cancer. Cancer Cell Int. 2025, 25, 3. [Google Scholar] [CrossRef]
- Hardaway, J.C.; Prince, E.; Arepally, A.; Katz, S.C. Regional Infusion of Chimeric Antigen Receptor T Cells to Overcome Barriers for Solid Tumor Immunotherapy. J. Vasc. Interv. Radiol. 2018, 29, 1017–1021.e1. [Google Scholar] [CrossRef] [PubMed]
- Roybal, K.T.; Rupp, L.J.; Morsut, L.; Walker, W.J.; McNally, K.A.; Park, J.S.; Lim, W.A. Precision Tumor Recognition by T Cells With Combinatorial Antigen-Sensing Circuits. Cell 2016, 164, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Bangayan, N.J.; Wang, L.; Sojo, G.B.; Noguchi, M.; Cheng, D.; Ta, L.; Gunn, D.; Mao, Z.; Liu, S.; Yin, Q.; et al. Dual-inhibitory domain iCARs improve the efficiency of the AND-NOT gate CAR T strategy. Proc. Natl. Acad. Sci. USA 2023, 120, e2312374120. [Google Scholar] [CrossRef] [PubMed]
- Goodman, D.B.; Azimi, C.S.; Kearns, K.; Talbot, A.; Garakani, K.; Garcia, J.; Patel, N.; Hwang, B.; Lee, D.; Park, E.; et al. Pooled screening of CAR T cells identifies diverse immune signaling domains for next-generation immunotherapies. Sci. Transl. Med. 2022, 14, eabm1463. [Google Scholar] [CrossRef]
- Holtermann, A.; Gislon, M.; Angele, M.; Subklewe, M.; von Bergwelt-Baildon, M.; Lauber, K.; Kobold, S. Prospects of Synergy: Local Interventions and CAR T Cell Therapy in Solid Tumors. BioDrugs 2024, 38, 611–637. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Zheng, J.; Huang, Y.; Li, X.; Wang, H.; Chen, X.; Zhao, Y.; Liu, Z.; Sun, J.; Xu, Y.; et al. Nanoparticle Mediated Universal CAR T Therapy. Int. J. Pharm. 2024, 666, 124779. [Google Scholar] [CrossRef]
- Grosskopf, A.K.; Labanieh, L.; Klysz, D.D.; Roth, G.A.; Xu, P.; Adebowale, O.; Chen, X.; Li, Y.; Wang, H.; Zhao, Y.; et al. Delivery of CAR T Cells in a Transient Injectable Stimulatory Hydrogel Niche Improves Treatment of Solid Tumors. Sci. Adv. 2022, 8, eabn8264. [Google Scholar] [CrossRef] [PubMed]
- Sidana, S.; Dueck, A.C.; Thanarajasingam, G.; Griffin, J.M.; Thompson, C.; Durani, U.; Burtis, M.; Warsame, R.; Paludo, J.; Gertz, M.A.; et al. Longitudinal Patient Reported Outcomes with CAR-T Cell Therapy Versus Autologous and Allogeneic Stem Cell Transplant. Transplant. Cell Ther. 2022, 28, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.A.; Arshad, H. A Discount on the Cost of Cancer: India’s Homegrown CAR-T Cell Therapy. Blood Cell Ther. 2024, 7, 121–123. [Google Scholar] [PubMed]

| Signaling Component | Native T-Cell Function | CAR T-Cell Equivalent | Purpose |
|---|---|---|---|
| Signal 1 (Recognition) | TCR + CD3 complex sees peptide/MHC | scFv domain binds surface antigen | Target recognition and initial activation trigger |
| Signal 2 (Costimulation) | CD28 binds B7 on an APC | Costimulatory Domain (e.g., CD28, 4-1BB) | Full, robust activation; prevents anergy; promotes expansion and persistence |
| Activation Signal | ITAMs on CD3ζ chain transmit signal | ITAMs on CD3ζ domain transmit signal | Initiating the downstream signaling cascade for T-cell functions (killing, cytokine release) |
| NCT Number | Study Status | Study Title | Interventions | Design | Sponsor Country and Institution | Key Results |
|---|---|---|---|---|---|---|
| NCT05341492 | Recruiting (ongoing) | A Single-arm, Open, Exploratory Clinical Study Evaluating the Safety and Efficacy of EGFR/B7H3 CAR-T in Patients With EGFR/B7H3-positive Advanced Solid Tumors (Lung and Triple-negative Breast Cancer) | Biological: EGFR/B7H3 CAR-T | Early Phase 1, single arm, ~30 patients | Second Affiliated Hospital of Guangzhou Medical University, China | No formal outcome data yet available |
| NCT01837602 | Completed | Clinical Trial of Autologous cMet Redirected T Cells Administered Intratumorally in Patients With Breast Cancer | Biological: cMet RNA CAR T cells | Phase 1, interventional, ~6 evaluable patients | University of Pennsylvania, United States | Safe (≤Grade 1 toxicity); CAR T mRNA detected in blood/tumor; tumor necrosis and inflammatory response observed |
| NCT02580747 | start ~October 2015, possibly ended ~November 2017; current status unknown | Clinical Study of Chimeric Mesothelin Antigen Receptor-modified T Cells in Relapsed and/or Chemotherapy Refractory Malignancies | Biological: anti-meso-CAR vector transduced T cells | Phase 1, interventional, ~20 participants | Chinese PLA General Hospital, China | No outcomes publicly available as of now |
| NCT06682793 | Recruiting | A Seamless Phase 1/2 Study to Evaluate the Safety and Efficacy of A2B395, an Allogeneic Logic-gated Tmod™ CAR T, in Heterozygous HLA-A*02 Adults With Recurrent Unresectable, Locally Advanced, or Metastatic Solid Tumors That Express EGFR and Have Lost HLA-A*02 Expression | Biological: A2B395 Diagnostic Test: xT CDx with HLA-LOH assay | Seamless Phase 1/2; open-label, interventional, multi-center, ~240 participants | A2 Biotherapeutics, Inc., United States | The key milestone for this trial is that the first patient has been dosed (June 2025). No clinical outcome data (safety/efficacy) are available yet. |
| NCT04107142 | Not yet recruiting | A Phase I Dose-escalation Trial to Evaluate Haploidentical/Allogeneic Natural Killer Group 2D Ligand (NKG2DL)-Targeting Chimeric Antigen Receptor-grafted Gamma Delta (γδ) T Cells (CTM-N2D) in Subjects With Relapsed or Refractory Solid Tumour | Biological: Adoptive Cell Transfer of NKG2DL-targetting Chimeric Antigen Receptor-grafted Gamma Delta T cell | Open label, single-center, dose-escalation, Phase I study, ~10 participants | CytoMed Therapeutics Pte Ltd., Singapore | Results pending |
| NCT02587689 | Recruiting | Phase I/II Study of Anti-MUC1 CAR T Cells for Patients With MUC1+ Advanced Refractory Solid Tumor | Biological: anti-MUC1 CAR T Cells | Interventional, Phase 1/2, single-arm, open label, ~20 participants | PersonGen BioTherapeutics (Suzhou) Co., Ltd., China | Results pending |
| NCT02706392 | Terminated due to slow accruals. | Phase I Study of Adoptive Immunotherapy for Advanced ROR1+ Malignancies With Defined Subsets of Autologous T Cells Engineered to Express a ROR1-Specific Chimeric Antigen Receptor | Other: Laboratory Biomarker Analysis Biological: ROR1 CAR-specific Autologous T-Lymphocytes | Phase I, interventional, single-arm, open label, ~21 participants | Fred Hutchinson Cancer Center, United States | Safety Profile: No dose-limiting toxicities were observed. Efficacy Observations: In a subset of patients, evidence of CAR-T cell expansion and potential anti-tumor activity was noted. |
| NCT06347068 | Recruiting | Study of Administration of T Cells Expressing B7-H3 Specific Chimeric Antigen Receptors and Containing the Inducible Caspase 9 Safety Switch in Subjects With Triple Negative Breast Cancer | Biological: iC9-CAR.B7-H3 T Cell Therapy Drug: cyclophosphamide Drug: fludarabine | Interventional, Phase 1, single arm, open label, ~42 participants | UNC Lineberger Comprehensive Cancer Center, United States | Results pending |
| NCT02792114 | The trial is actively ongoing but not recruiting new participants. | A Phase I Clinical Trial to Evaluate the Safety and Tolerability of Mesothelin-Specific Chimeric Antigen Receptor-Positive T Cells in Patients With Metastatic Mesothelin-Expressing Breast Cancer | Drug: Cyclophosphamide Biological: Mesothelin-targeted T cells Drug: AP1903 | Interventional, Phase I, single arm, open label, ~186 participants | Memorial Sloan Kettering Cancer Center, United States | Results pending |
| NCT04025216 | The sponsor finds the risk/benefit analysis unfavorable and has terminated the study. | A Phase 1 Open-Label, Multi-Center First in Human Study of TnMUC1-Targeted Genetically-Modified Chimeric Antigen Receptor T Cells in Patients With Advanced TnMUC1-Positive Solid Tumors and Multiple Myeloma | Biological: CART-TnMUC1 Drug: Cyclophosphamide Drug: Fludarabine | Interventional, Phase 1, Parallel arms with sequential dose escalation, open label, ~16 participants | Kite, A Gilead Company, United States | As the study was terminated before completion, no results were posted. The termination was based on an unfavorable risk/benefit analysis by the sponsor. |
| NCT05483491 | The trial is actively recruiting participants. | T Cell Receptor Gene Therapy Targeting KK-LC-1 for Gastric, Breast, Cervical, Lung, and Other KK-LC-1 Positive Cancers | Biological: KK-LC-1 TCR-T cells Drug: Aldesleukin | Interventional, Phase 1, Sequential Assignment, open label, ~30 participants | The State University of New Jersey, United States | Results pending |
| NCT04981119 | The trial is actively recruiting participants. | An Observational Study Obtaining Solid Tumor Tissue From Participants and Apheresis for CAR T-Cell Therapy Manufacturing | Other: Apheresis Diagnostic Test: Next Generation Sequencing (NGS) Diagnostic Test: Long Range NGS HLA typing | Observational, ~200 participants | A2 Biotherapeutics Inc., United States | Results pending |
| NCT05694364 | The trial is active but not recruiting participants. | A Phase 1/1b Dose Escalation/Dose Expansion Study of PRGN-3007 UltraCAR-T Cells in Patients With Advanced Hematologic and Solid Tumor Malignancies | Drug: Fludarabine Drug: Cyclophosphamide Biological: PRGN-3007 | Interventional, Phase 1/1b, Dose Escalation/Dose Expansion, open label, 3 participants (actual) | H. Lee Moffitt Cancer Center and Research Institute, United States | Results pending |
| NCT05035407 | The trial is actively recruiting participants. | A Phase I Trial of T Cell Receptor Gene Therapy Targeting KK-LC-1 for Gastric, Breast, Cervical, Lung and Other KK-LC-1 Positive Epithelial Cancers | Drug: IL-2 (Aldesleukin) Drug: Cyclophosphamide Biological: KK-LC-1 TCR Drug: Fludarabine | Interventional, Phase 1, Sequential Assignment, open label, ~30 participants | National Institutes of Health Clinical Center (CC) (National Cancer Institute (NCI)), Unites States | Results pending |
| NCT Number | Study Status | Study Title | Interventions | Design | Sponsor Country and Institution | Key Results |
|---|---|---|---|---|---|---|
| NCT06972576 | Recruiting | Clinical Study of Combined EphA2-targeted CAR-DC and CAR-T Cell Therapy for Non-small Cell Lung Cancer | Biological: EphA2-targeted CAR-T Cells Biological: EphA2-targeted CAR-DCs | Interventional, Phase 1, open label, ~18 participants | Second Affiliated Hospital, School of Medicine, Zhejiang University, China | Results pending |
| NCT05060796 | Recruiting | A Single-arm, Open-label, Phase I Study to Evaluate the Safety and Efficacy of CXCR5 Modified EGFR Chimeric Antigen Receptor Autologous T Cells in EGFR-positive Patients With Advanced Non-small Cell Lung Cancer | Biological: CXCR5 modified EGFR Chimeric Antigen Receptor Autologous T cells | Interventional, Phase 3, Parallel Assignment, open Label, ~11 participants | Second Affiliated Hospital of Guangzhou Medical University, China | Results pending |
| NCT06043466 | Recruiting | Phase I Clinical Study of Chimeric Antigen Receptor T Cells (C-13-60) in the Treatment of Carcinoembryonic Antigen (CEA) Positive Advanced Malignant Solid Tumors | Biological: CEA-targeted CAR-T cells | Interventional, Phase 1, Sequential Assignment, open Label, ~30 participants | Chongqing Precision Biotech Co., Ltd., China | Results pending |
| NCT06653023 | Recruiting | A Clinical Study on the Safety and Efficacy of Universal CAR-T Cells (REVO-UWD-03) for Advanced Hepatocellular Carcinoma &Amp; Lung Cancer | Biological: Universal CAR-T cells injection for treating HCC and NSCLC | Interventional, Early Phase 1, open Label, ~60 participants | Wondercel Biotech (ShenZhen), China | Results pending |
| NCT06682793 | Recruiting | A Seamless Phase 1/2 Study to Evaluate the Safety and Efficacy of A2B395, an Allogeneic Logic-gated Tmod™ CAR T, in Heterozygous HLA-A*02 Adults With Recurrent Unresectable, Locally Advanced, or Metastatic Solid Tumors That Express EGFR and Have Lost HLA-A*02 Expression | Biological: A2B395 Diagnostic Test: xT CDx with HLA-LOH assay | Interventional, Phase 1 and 2, open Label, ~240 participants | A2 Biotherapeutics Inc., United States | The key result so far is that: The first patient has been successfully dosed with A2B395 Tmod™ CAR T cells on 26 June 2025, marking the inaugural human administration of this innovative therapy |
| NCT06051695 | Recruiting | A Seamless Phase 1/2 Study to Evaluate the Safety and Efficacy of A2B694, an Autologous Logic-gated Tmod™ CAR T, in Heterozygous HLA-A*02 Adults With Recurrent Unresectable, Locally Advanced, or Metastatic Solid Tumors That Express MSLN and Have Lost HLA-A*02 Expression | Biological: A2B694 Diagnostic Test: xT CDx with HLA-LOH Assay | Interventional, Phase 1 and 2, open Label, ~230 participants | A2 Biotherapeutics Inc., United States | First Patient Dosed: The trial successfully administered A2B694 to its first patient in April 2024, marking a major milestone in clinical translation. Dose Escalation: The dose-escalation phase is currently ongoing; no dosage outcomes or adverse event data have been published yet. |
| NCT05620342 | Recruiting | Administration of T Cells Expressing a 2nd Generation GD2 Chimeric Antigen Receptor, IL-15, and iCaspase9 Safety Switch in Subjects With Lung Cancer | Biological: iC9.GD2.CAR.IL-15 T Infusion | Interventional, Early Phase 1, open Label, ~24 participants | UNC Lineberger Comprehensive Cancer Center, United States | Results pending |
| NCT02587689 | Last known status was: Recruiting | Phase I/II Study of Anti-MUC1 CAR T Cells for Patients With MUC1+ Advanced Refractory Solid Tumor | Biological: anti-MUC1 CAR T-Cells | Interventional, Phase 1 and 2, open Label, ~20 participants | PersonGen BioTherapeutics (Suzhou) Co., Ltd., China | Results pending |
| NCT04503278 | Recruiting | Phase I/IIa, First-in-human (FIH), Open-label, Dose Escalation Trial With Expansion Cohorts to Evaluate Safety and Preliminary Efficacy of CLDN6 CAR-T With or Without CLDN6 RNA-LPX in Patients With CLDN6-positive Relapsed or Refractory Advanced Solid Tumors | Biological: CLDN6 CAR-T Biological: CLDN6 uRNA-LPX/CLDN6 modRNA-LPX | Interventional, Phase 1, open Label, ~214 participants | BioNTech Cell & Gene Therapies GmbH, Germany Study Locations: Australia, Germany, Netherlands, Sweden | Results pending |
| NCT04981119 | Recruiting | An Observational Study Obtaining Solid Tumor Tissue From Participants and Apheresis for CAR T-Cell Therapy Manufacturing | Other: Apheresis Diagnostic Test: Next Generation Sequencing (NGS) Diagnostic Test: Long Range NGS HLA typing | Observational, ~200 participants | A2 Biotherapeutics Inc., United States | About 4–5% had tumors with HLA-A02 LOH. Over 30 patients have successfully undergone leukapheresis to bank T-cells for future Tmod CAR-T trials (EVEREST studies). Recruitment efficiency improved with digital screening tools (AWARE system). |
| NCT Number | Study Status | Study Title | Interventions | Design | Sponsor Country and Institution | Key Results |
|---|---|---|---|---|---|---|
| NCT06586658 | Recruiting | An Exploratory Clinical Study of the Safety and Efficacy of Anti-CD70-CAR-T Cell Injection in Patients With Locally Advanced or Relapsed/Metastatic Renal Cell Carcinoma With CD70+ Inoperable | Biological: anti-CD70-CAR-T cells | Interventional, Early Phase 1, open Label, ~9 participants | Shanghai Changzheng Hospital, China | Results pending |
| NCT05420519 | Last known status was: Recruiting | A Phase I Clinical Study of CD70-targeted CAR-T Therapy for Advanced/Advanced Renal Cancer | Biological: CD70 CAR-T cells | Interventional, Phase 1, open Label, ~24 participants | Chongqing Precision Biotech Co., Ltd., China | Results pending |
| NCT04969354 | Recruiting | Clinical Study of CAIX-targeted CAR-T Cells in the Treatment of Advanced Renal Cell Carcinoma | Biological: CAR-T cell immunotherapy | Interventional, Phase 1, open Label, ~20 participants | The Affiliated Hospital of Xuzhou Medical University, China | Results pending |
| NCT06010875 | Recruiting | A Phase I Clinical Study to Assess the Safety and Efficacy of CD70-targeted CAR-T in the Treatment of CD70-positive Advanced/Metastatic Solid Tumors | Biological: CD70 CAR-T cells Biological: CD70 CAR-T cells | Interventional, Phase 1, open Label, ~48 participants | Chongqing Precision Biotech Co., Ltd., China | Results pending |
| NCT05518253 | Recruiting | A Phase I Clinical Study of CD70-targeting CAR-T Therapy in the Treatment of CD70-positive Advanced/Metastatic Solid Tumors | Biological: CD70 CAR-T cells Biological: CD70 CAR-T cells | Interventional, Phase 1, open Label, ~30 participants | Zhejiang University, China | Results pending |
| NCT05420545 | Last known status was: Recruiting | A Phase I Clinical Study of CD70-targeting CAR-T Therapy in the Treatment of CD70-positive Advanced/Metastatic Solid Tumors | Biological: CD70 CAR-T cells Biological: CD70 CAR-T cells | Interventional, Phase 1, open Label, ~36 participants | Chongqing Precision Biotech Co., Ltd., China | Results pending |
| NCT05468190 | Last known status was: Recruiting | A Phase I Clinical Study to Assess the Safety and Tolerability of CD70-targeting CAR-T in the Treatment of CD70-positive Advanced/Metastatic Solid Tumors | Biological: CD70 CAR-T cells Biological: CD70 CAR-T cells | Interventional, Phase 1, open Label, ~48 participants | Chongqing Precision Biotech Co., Ltd., China | Results pending |
| NCT03393936 | Terminated, Adjustment of study strategy | A Dose Escalation and Dose Expansion Trial to Assess the Safety, Tolerability and Anti-tumor Activity of Autologous T Cell Modified Chimeric Antigen Receptor (CAR) CCT 301-38 or CCT 301-59 in Patients With Recurrent or Refractory Stage IV Renal Cell Carcinoma | Biological: CCT301-38 Biological: CCT301-59 | Interventional, Phase 1 and 2, open Label, ~66 participants | Shanghai PerHum Therapeutics Co., Ltd., China | Results pending |
| NCT06480565 | Active, not recruiting | A Phase 1/2 Trial of ADI-270 (Engineered γδ Chimeric Receptor [CAR] Vδ1 T Cells Targeting CD70) in Adults With Relapsed or Refractory (R/R) Clear Cell Renal Cell Carcinoma (ccRCC) | Drug: ADI-270 Drug: Fludarabine Drug: Cyclophosphamide | Interventional, Phase 1 and 2, open Label, ~60 participants | Adicet Therapeutics, United States | Results pending |
| NCT04438083 | Terminated, Patients to be followed up in the CRSP-ONC-LTF study | A Phase 1 Dose Escalation and Cohort Expansion Study of the Safety and Efficacy of Allogeneic CRISPR-Cas9-Engineered T Cells (CTX130) in Subjects With Advanced, Relapsed or Refractory Renal Cell Carcinoma With Clear Cell Differentiation | Biological: CTX130 | Interventional, Phase 1, open Label, ~19 participants | CRISPR Therapeutics AG, Study Locations: United States, Australia, Canada, Netherlands | Results pending |
| NCT01218867 | Terminated, No objective responses were observed | Phase I/II Study of Metastatic Cancer Using Lymphodepleting Conditioning Followed by Infusion of Anti-VEGFR2 Gene Engineered CD8+ Lymphocytes | Biological: Anti-VEGFR2 CAR CD8 plus PBL Drug: Cyclophosphamide Biological: Aldesleukin Drug: Fludarabine | Interventional, Phase 1 and 2, open Label, ~24 participants | National Cancer Institute (NCI), United States | Terminated Phase I/II; showed early signs of antitumor activity—9 responses, with up to 12 patients achieving their best response within one month post-infusion. |
| NCT04696731 | Recruiting | A Phase 1 Multicenter Study Evaluating the Safety and Efficacy of ALLO-316 Following ALLO-647 Containing Conditioning Regimen in Subjects With Advanced or Metastatic Clear Cell Renal Cell Carcinoma | Genetic: ALLO-316 Biological: ALLO-647 Drug: Fludarabine Drug: Cyclophosphamide | Interventional, Phase 1, open Label, ~120 participants | Allogene Therapeutics, United States | Results pending |
| NCT05795595 | Recruiting | A Phase 1/2, Open-label, Multicenter, Dose Escalation and Cohort Expansion Study of the Safety and Efficacy of Anti-CD70 Allogeneic CRISPR-Cas9-Engineered T Cells (CTX131) in Adult Subjects With Relapsed or Refractory Solid Tumors | Biological: CTX131 | Interventional, Phase 1 and 2, open Label, ~250 participants | CRISPR Therapeutics AG, United States | Results pending |
| NCT02830724 | Recruiting | A Phase I/II Study Administering Peripheral Blood Lymphocytes Transduced With a CD70-Binding Chimeric Antigen Receptor to Patients With CD70-Expressing Cancers | Drug: Cyclophosphamide Drug: Fludarabine Drug: Aldesleukin Biological: Anti-hCD70 CAR transduced PBL | Interventional, Phase 1 and 2, open Label, ~124 participants | National Cancer Institute (NCI), United States | Results pending |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafii, S.; Mukherji, D.; Komaranchath, A.S.; Khalil, C.; Iqbal, F.; Abdelwahab, S.I.; Abyad, A.; Abuhelwa, A.Y.; Gandikota, L.; Al-Shamsi, H.O. Advancing CAR T-Cell Therapy in Solid Tumors: Current Landscape and Future Directions. Cancers 2025, 17, 2898. https://doi.org/10.3390/cancers17172898
Rafii S, Mukherji D, Komaranchath AS, Khalil C, Iqbal F, Abdelwahab SI, Abyad A, Abuhelwa AY, Gandikota L, Al-Shamsi HO. Advancing CAR T-Cell Therapy in Solid Tumors: Current Landscape and Future Directions. Cancers. 2025; 17(17):2898. https://doi.org/10.3390/cancers17172898
Chicago/Turabian StyleRafii, Saeed, Deborah Mukherji, Ashok Sebastian Komaranchath, Charbel Khalil, Faryal Iqbal, Siddig Ibrahim Abdelwahab, Amin Abyad, Ahmad Y. Abuhelwa, Lakshmikanth Gandikota, and Humaid O. Al-Shamsi. 2025. "Advancing CAR T-Cell Therapy in Solid Tumors: Current Landscape and Future Directions" Cancers 17, no. 17: 2898. https://doi.org/10.3390/cancers17172898
APA StyleRafii, S., Mukherji, D., Komaranchath, A. S., Khalil, C., Iqbal, F., Abdelwahab, S. I., Abyad, A., Abuhelwa, A. Y., Gandikota, L., & Al-Shamsi, H. O. (2025). Advancing CAR T-Cell Therapy in Solid Tumors: Current Landscape and Future Directions. Cancers, 17(17), 2898. https://doi.org/10.3390/cancers17172898





