Comparative Analysis of Clinical, Dermoscopic, and Confocal Microscopy Scores for Assessing Severity of Actinic Keratosis
Simple Summary
Abstract
1. Introduction
2. Non-Invasive Diagnosis of Actinic Keratosis
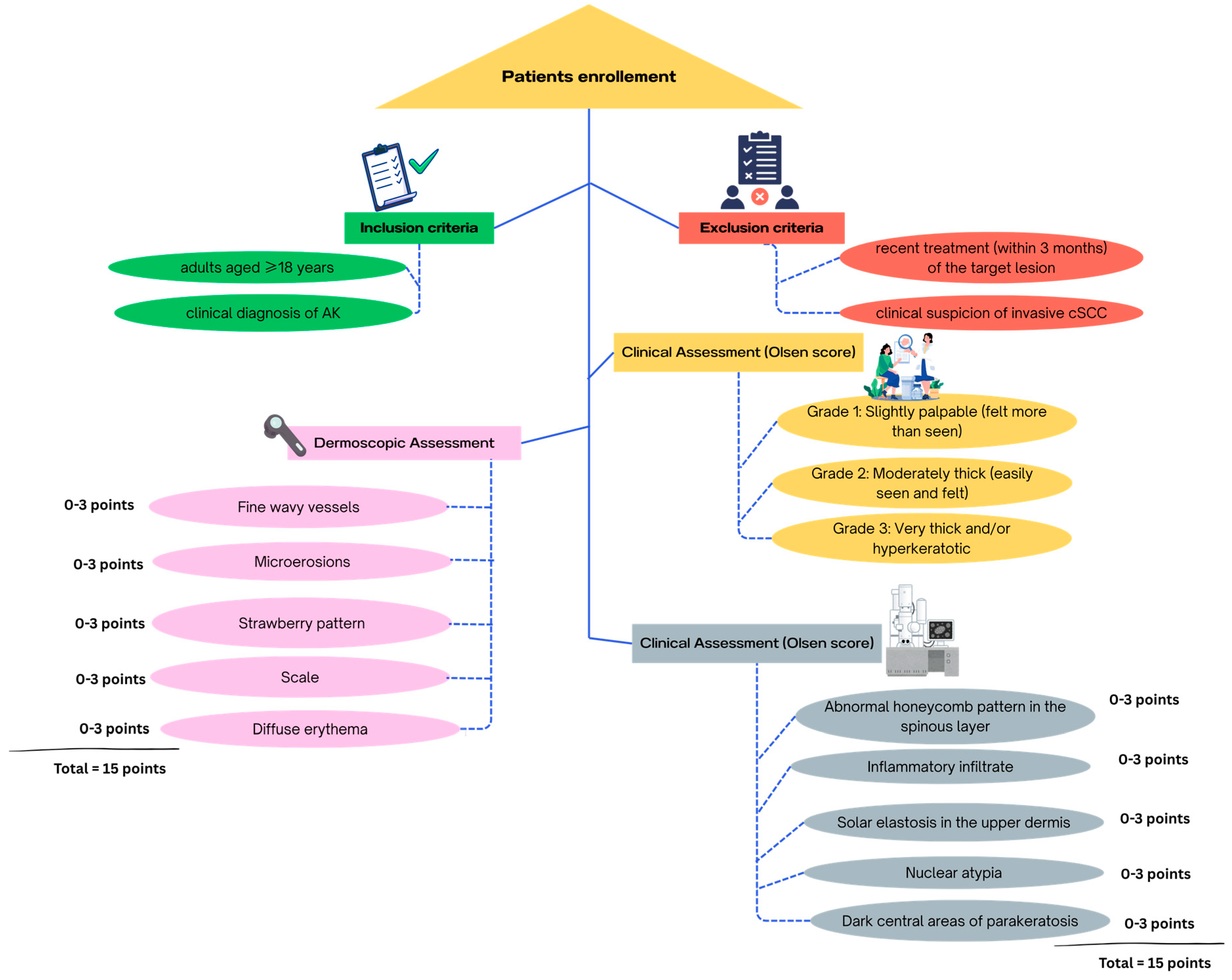
3. Materials and Methods
3.1. Study Design and Ethical Aspects
3.2. Study Protocol
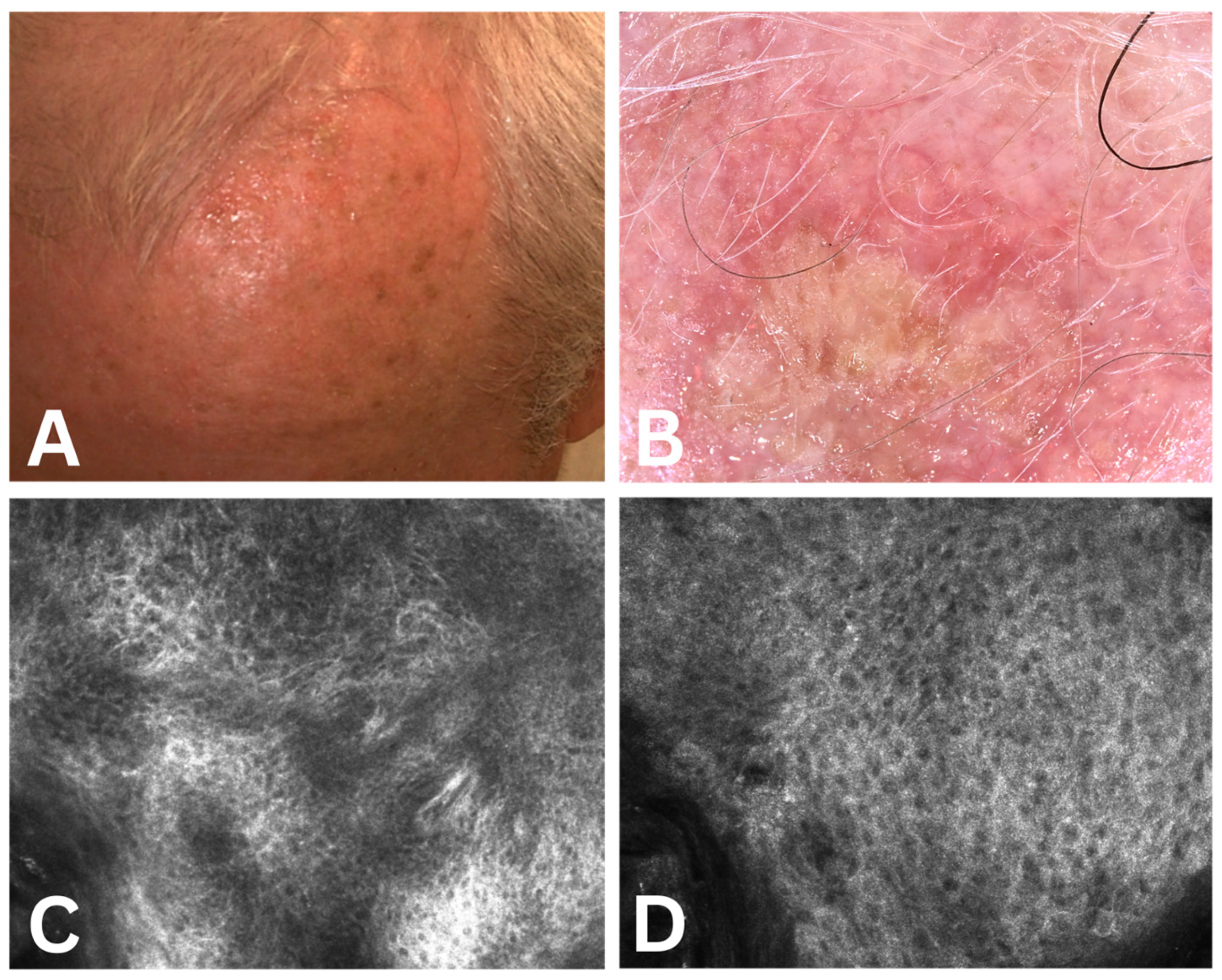
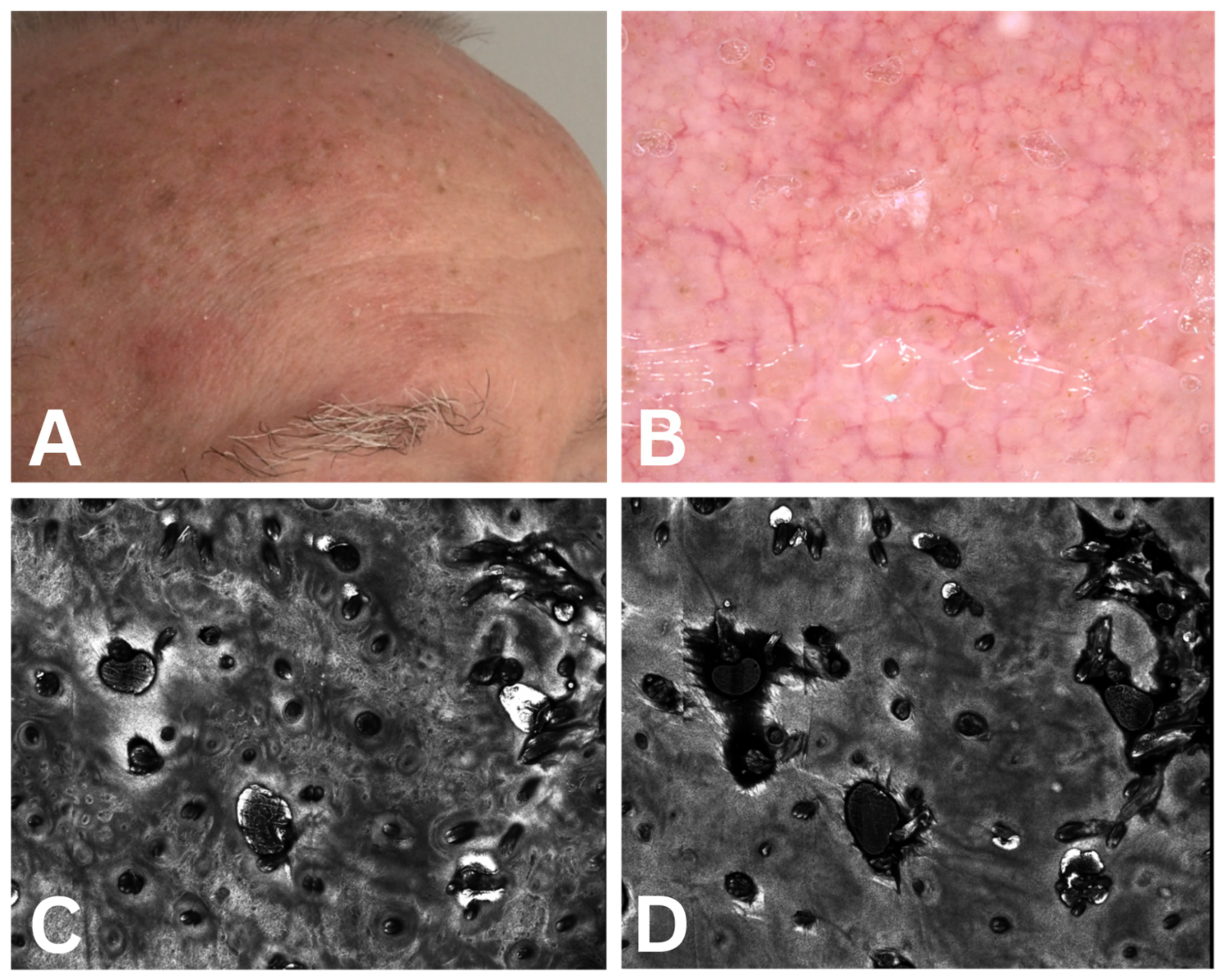
3.2.1. Dermoscopic Assessment
3.2.2. Confocal Microscopy Assessment
3.3. Data Analysis
4. Results
4.1. Association Between Clinical Severity and Dermoscopic Features
4.2. Association Between Clinical Severity and Confocal Microscopy Features
4.3. Association Between Overall Dermoscopic Score and Overall Confocal Microscopy Score
4.4. Relationship Between Confocal Microscopy Features and Overall Dermoscopic Classification
4.5. Relationship Between Dermoscopic Features and Overall Confocal Microscopy Classification
4.6. Association Between Dermoscopy Severity Score, RCM Severity Score, and Sex
5. Discussion
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AK | actinic keratosis |
| RCM | reflectance confocal microscopy |
| OCT | optical coherence tomography |
| cSCC | cutaneous squamous cell carcinoma |
References
- Ferreira, I.G.; Godoi, D.F.; Perugini, E.R. Nosological Profile of Dermatological Diseases in Primary Health Care and Dermatology Secondary Care in Florianópolis (2016–2017). An. Bras. Dermatol. 2020, 95, 428–438. [Google Scholar] [CrossRef]
- Glogau, R.G. The Risk of Progression to Invasive Disease. J. Am. Acad. Dermatol. 2000, 42 Pt 2, 23–24. [Google Scholar] [CrossRef]
- Oliveira, V.M.; Fontenelle, L.F.V.; Costa, L.H.; Santos, R.A.; Borges, M.V.R.; Morais, I.M.d.A. Rastreamento e Caracterização de Lesões de Pele Pré-Cancerosas: Uma Revisão Integrativa. E-Acadêmica 2022, 3, e2033300. [Google Scholar] [CrossRef]
- Farnetani, F.; Scope, A.; Braun, R.P.; Gonzalez, S.; Guitera, P.; Malvehy, J.; Manfredini, M.; Marghoob, A.A.; Moscarella, E.; Oliviero, M.; et al. Skin Cancer Diagnosis with Reflectance Confocal Microscopy: Reproducibility of Feature Recognition and Accuracy of Diagnosis: Reproducibility of Feature Recognition and Accuracy of Diagnosis. JAMA Dermatol. 2015, 151, 1075–1080. [Google Scholar] [CrossRef]
- Salasche, S.J. Epidemiology of Actinic Keratoses and Squamous Cell Carcinoma. J. Am. Acad. Dermatol. 2000, 42 Pt 2, 4–7. [Google Scholar] [CrossRef]
- Fu, W.; Cockerell, C.J. The Actinic (Solar) Keratosis: A 21st-Century Perspective. Arch. Dermatol. 2003, 139, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Heaphy, M.R., Jr.; Ackerman, A.B. The Nature of Solar Keratosis: A Critical Review in Historical Perspective. J. Am. Acad. Dermatol. 2000, 43 Pt 1, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.J. Actinic Keratosis: Facts and Controversies. Clin. Dermatol. 2010, 28, 249–253. [Google Scholar] [CrossRef]
- Lober, B.A.; Lober, C.W.; Accola, J. Actinic Keratosis Is Squamous Cell Carcinoma. J. Am. Acad. Dermatol. 2000, 43 Pt 1, 881–882. [Google Scholar] [CrossRef]
- Röwert-Huber, J.; Patel, M.J.; Forschner, T.; Ulrich, C.; Eberle, J.; Kerl, H.; Sterry, W.; Stockfleth, E. Actinic Keratosis Is an Early in Situ Squamous Cell Carcinoma: A Proposal for Reclassification. Br. J. Dermatol. 2007, 156 (Suppl. 3), 8–12. [Google Scholar] [CrossRef] [PubMed]
- Cockerell, C.J. Histopathology of Incipient Intraepidermal Squamous Cell Carcinoma (“actinic Keratosis”). J. Am. Acad. Dermatol. 2000, 42 Pt 2, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Reinehr, C.P.H.; Bakos, R.M. Actinic Keratoses: Review of Clinical, Dermoscopic, and Therapeutic Aspects (Corrigir Nos Altos de Página Onde Tem o Título). An. Bras. Dermatol. 2019, 94, 637–657. [Google Scholar] [CrossRef]
- Werner, R.N.; Jacobs, A.; Rosumeck, S.; Erdmann, R.; Sporbeck, B.; Nast, A. Methods and Results Report–Evidence and Consensus-Based (S3) Guidelines for the Treatment of Actinic Keratosis-International League of Dermatological Societies in Cooperation with the European Dermatology Forum. J. Eur. Acad. Dermatol. Venereol. 2015, 29, e1–e66. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.N.; Stockfleth, E.; Connolly, S.M.; Correia, O.; Erdmann, R.; Foley, P.; Gupta, A.K.; Jacobs, A.; Kerl, H.; Lim, H.W.; et al. International League of Dermatological Societies; European Dermatology Forum. Evidence- and Consensus-Based (S3) Guidelines for the Treatment of Actinic Keratosis–International League of Dermatological Societies in Cooperation with the European Dermatology Forum–Short Version. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2069–2079. [Google Scholar] [CrossRef]
- Ackerman, A.B.; Mones, J.M. Solar (Actinic) Keratosis Is Squamous Cell Carcinoma: Solar (Actinic) Keratosis Is Squamous Cell Carcinoma. Br. J. Dermatol. 2006, 155, 9–22. [Google Scholar] [CrossRef]
- Jonason, A.S.; Kunala, S.; Price, G.J.; Restifo, R.J.; Spinelli, H.M.; Persing, J.A.; Leffell, D.J.; Tarone, R.E.; Brash, D.E. Frequent Clones of P53-Mutated Keratinocytes in Normal Human Skin. Proc. Natl. Acad. Sci. USA 1996, 93, 14025–14029. [Google Scholar] [CrossRef]
- Denat, L.; Kadekaro, A.L.; Marrot, L.; Leachman, S.A.; Abdel-Malek, Z.A. Melanocytes as Instigators and Victims of Oxidative Stress. J. Investig. Dermatol. 2014, 134, 1512–1518. [Google Scholar] [CrossRef]
- Bf, M. Dioxygen and Reactive Oxygen Species’ Paramagnetic Properties Are Important Factors in Dermatology. Int. J. Dermatol. Clin. Res. 2022, 8, 016–023. [Google Scholar] [CrossRef]
- Ulrich, M.; Krueger-Corcoran, D.; Roewert-Huber, J.; Sterry, W.; Stockfleth, E.; Astner, S. Reflectance Confocal Microscopy for Noninvasive Monitoring of Therapy and Detection of Subclinical Actinic Keratoses. Dermatology 2010, 220, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Willenbrink, T.J.; Ruiz, E.S.; Cornejo, C.M.; Schmults, C.D.; Arron, S.T.; Jambusaria-Pahlajani, A. Field Cancerization: Definition, Epidemiology, Risk Factors, and Outcomes. J. Am. Acad. Dermatol. 2020, 83, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Stockfleth, E. The Importance of Treating the Field in Actinic Keratosis. J. Eur. Acad. Dermatol. Venereol. 2017, 31 (Suppl. 2), 8–11. [Google Scholar] [CrossRef]
- Braakhuis, B.J.M.; Tabor, M.P.; Kummer, J.A.; Leemans, C.R.; Brakenhoff, R.H. A Genetic Explanation of Slaughter’s Concept of Field Cancerization: Evidence and Clinical Implications. Cancer Res. 2003, 63, 1727–1730. [Google Scholar]
- Micali, G.; Verzì, A.E.; Barresi, S.; Dirschka, T.; Lacarrubba, F. Field Cancerization in Clinically Solitary Actinic Keratosis: A Pilot Study. Dermatol. Ther. 2021, 34, e14607. [Google Scholar] [CrossRef]
- Vatve, M.; Ortonne, J.-P.; Birch-Machin, M.A.; Gupta, G. Management of Field Change in Actinic Keratosis: Management of Field Change in AK. Br. J. Dermatol. 2007, 157 (Suppl. 2), 21–24. [Google Scholar] [CrossRef] [PubMed]
- Russo, T.; Piccolo, V.; Lallas, A.; Giacomel, J.; Moscarella, E.; Alfano, R.; Argenziano, G. Dermoscopy of Malignant Skin Tumours: What’s New? Dermatology 2017, 233, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Prow, T.W.; Tan, J.-M.; Pellacani, G. Reflectance Confocal Microscopy: Hallmarks of Keratinocyte Cancer and Its Precursors. Curr. Probl. Dermatol. 2015, 46, 85–94. [Google Scholar] [CrossRef]
- Flohil, S.C.; van der Leest, R.J.T.; Dowlatshahi, E.A.; Hofman, A.; de Vries, E.; Nijsten, T. Prevalence of Actinic Keratosis and Its Risk Factors in the General Population: The Rotterdam Study. J. Investig. Dermatol. 2013, 133, 1971–1978. [Google Scholar] [CrossRef]
- Olsen, E.A.; Abernethy, M.L.; Kulp-Shorten, C.; Callen, J.P.; Glazer, S.D.; Huntley, A.; McCray, M.; Monroe, A.B.; Tschen, E.; Wolf, J.E., Jr. A Double-Blind, Vehicle-Controlled Study Evaluating Masoprocol Cream in the Treatment of Actinic Keratoses on the Head and Neck. J. Am. Acad. Dermatol. 1991, 24 Pt 1, 738–743. [Google Scholar] [CrossRef]
- Butani, A.K.; Arbesfeld, D.M.; Schwartz, R.A. Premalignant and Early Squamous Cell Carcinoma. Clin. Plast. Surg. 2005, 32, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Venna, S.S.; Lee, D.; Stadecker, M.J.; Rogers, G.S. Clinical Recognition of Actinic Keratoses in a High-Risk Population: How Good Are We?: How Good Are We? Arch. Dermatol. 2005, 141, 507–509. [Google Scholar] [CrossRef]
- Zalaudek, I.; Argenziano, G. Dermoscopy of Actinic Keratosis, Intraepidermal Carcinoma and Squamous Cell Carcinoma. Curr. Probl. Dermatol. 2015, 46, 70–76. [Google Scholar] [CrossRef]
- Li, X.; Sun, J.; Zhang, H.; Zhang, C.; Li, W. Dermoscopy and Pathological Correlation in Different Grades of Actinic Keratosis and Squamous Cell Carcinoma. Clin. Cosmet. Investig. Dermatol. 2025, 18, 1359–1373. [Google Scholar] [CrossRef]
- Jindal, R.; Chauhan, P.; Shirazi, N. Rosette or Four Dot Signs in Dermoscopy: A Non-Specific Observation. Dermatol. Pract. Concept. 2022, 12, e2022069. [Google Scholar] [CrossRef]
- Peris, K.; Micantonio, T.; Piccolo, D.; Fargnoli, M.C. Dermoscopic Features of Actinic Keratosis. J. Dtsch. Dermatol. Ges. 2007, 5, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Sgouros, D.; Theofili, M.; Zafeiropoulou, T.; Lallas, A.; Apalla, Z.; Zaras, A.; Liopyris, K.; Pappa, G.; Polychronaki, E.; Kousta, F.; et al. Dermoscopy of Actinic Keratosis: Is There a True Differentiation between Non-Pigmented and Pigmented Lesions? J. Clin. Med. 2023, 12, 1063. [Google Scholar] [CrossRef]
- Conforti, C.; Ambrosio, L.; Retrosi, C.; Cantisani, C.; Di Lella, G.; Fania, L.; Rotunno, R.; Zalaudek, I.; Pellacani, G. Clinical and Dermoscopic Diagnosis of Actinic Keratosis. Dermatol. Pract. Concept. 2024, 14, e2024147S. [Google Scholar] [CrossRef] [PubMed]
- Kelati, A.; Baybay, H.; Moscarella, E.; Argenziano, G.; Gallouj, S.; Mernissi, F.Z. Dermoscopy of Pigmented Actinic Keratosis of the Face: A Study of 232 Cases. Actas Dermosifiliogr. 2017, 108, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Lallas, A.; Tschandl, P.; Kyrgidis, A.; Stolz, W.; Rabinovitz, H.; Cameron, A.; Gourhant, J.Y.; Giacomel, J.; Kittler, H.; Muir, J.; et al. Dermoscopic Clues to Differentiate Facial Lentigo Maligna from Pigmented Actinic Keratosis. Br. J. Dermatol. 2016, 174, 1079–1085. [Google Scholar] [CrossRef]
- Braghiroli, N.F.; Sugerik, S.; Freitas, L.A.R.d.; Oliviero, M.; Rabinovitz, H. The Skin through Reflectance Confocal Microscopy–Historical Background, Technical Principles, and Its Correlation with Histopathology. An. Bras. Dermatol. 2022, 97, 697–703. [Google Scholar] [CrossRef]
- Ulrich, M.; Lange-Asschenfeldt, S.; González, S. In Vivo Reflectance Confocal Microscopy for Early Diagnosis of Nonmelanoma Skin Cancer. Actas Dermosifiliogr. 2012, 103, 784–789. [Google Scholar] [CrossRef]
- Moscarella, E.; Rabinovitz, H.; Zalaudek, I.; Piana, S.; Stanganelli, I.; Oliviero, M.C.; Lallas, A.; Ardigo, M.; Cota, C.; Catricalà, C.; et al. Dermoscopy and Reflectance Confocal Microscopy of Pigmented Actinic Keratoses: A Morphological Study. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 307–314. [Google Scholar] [CrossRef]
- Combalia, A.; Fustà-Novell, X.; Alejo, B.; Domínguez, M.; Barreiro, A.; Carrera, C. Actinic Keratosis—Can Dermoscopy or RCM Differentiate AK (Not Full Thickness Atypia) from Full-Thickness Atypia/Invasive SCC? Curr. Dermatol. Rep. 2018, 7, 75–83. [Google Scholar] [CrossRef]
- Nascimento Cavalleiro Macedo Mota, A.; Piñeiro-Maceira, J.M.; Baptista Barcaui, C. Evaluation of Diagnostic Criteria of Actinic Keratosis through Reflectance Confocal Microscopy. Skin Res. Technol. 2020, 26, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ran, H.; Zhao, Y.; Liang, X.; Gu, Z.; Xue, Y. The Clinical Value of Reflectance Confocal Microscopy in Monitoring Treatment of Actinic Keratosis: A Systematic Review. Photodiagn. Photodyn. Ther. 2025, 53, 104539. [Google Scholar] [CrossRef]
- Mazur, E.; Kwiatkowska, D.; Reich, A. Reflectance Confocal Microscopy and Dermoscopy of Facial Pigmented and Non-Pigmented Actinic Keratosis Features before and after Photodynamic Therapy Treatment. Cancers 2023, 15, 5598. [Google Scholar] [CrossRef] [PubMed]
- Segurado-Miravalles, G.; Jiménez-Gómez, N.; Muñoz Moreno-Arrones, O.; Alarcón-Salazar, I.; Alegre-Sánchez, A.; Saceda-Corralo, D.; Jaén-Olasolo, P.; González-Rodríguez, S. Assessment of the Effect of 3% Diclofenac Sodium on Photodamaged Skin by Means of Reflectance Confocal Microscopy. Acta Derm. Venereol. 2018, 98, 963–969. [Google Scholar] [CrossRef]
- Guerra, L.O.; Leão Santos, A.C.; Cortinoz, J.R.; Magalhães, R.F.; Vasques, L.I.; Leonardi, G.R. Photographic Scale for the Characterization of Actinic Keratosis through Reflectance Confocal Microscopy: A Quantitative Approach to Cellular Transformation. Front. Med. 2024, 11, 1391859. [Google Scholar] [CrossRef]
- Lee, D.Y.; Kim, B.R.; Yang, S.; Kim, M.; Yoon, T.Y.; Youn, S.W. Histopathological Predictor of the Progression from Actinic Keratosis to Squamous Cell Carcinoma: Quantitative Computer-Aided Image Analysis. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 116–122. [Google Scholar] [CrossRef]
- Huang, A.; Nguyen, J.K.; Austin, E.; Mamalis, A.; Jagdeo, J. Updates on Treatment Approaches for Cutaneous Field Cancerization. Curr. Dermatol. Rep. 2019, 8, 122–132. [Google Scholar] [CrossRef]
- Maier, T.; Braun-Falco, M.; Laubender, R.P.; Ruzicka, T.; Berking, C. Actinic Keratosis in the En-Face and Slice Imaging Mode of High-Definition Optical Coherence Tomography and Comparison with Histology: Actinic Keratosis in High-Definition Optical Coherence Tomography. Br. J. Dermatol. 2013, 168, 120–128. [Google Scholar] [CrossRef]
- Cinotti, E.; Bertello, M.; Cartocci, A.; Fiorani, D.; Tognetti, L.; Solmi, V.; Cappilli, S.; Peris, K.; Perrot, J.L.; Suppa, M.; et al. Comparison of Reflectance Confocal Microscopy and Line-Field Optical Coherence Tomography for the Identification of Keratinocyte Skin Tumours. Skin Res. Technol. 2023, 29, e13215. [Google Scholar] [CrossRef]
- Jambusaria-Pahlajani, A.; Jeanselme, V.; Wang, D.M.; Ran, N.A.; Granger, E.E.; Cañueto, J.; Brodland, D.G.; Carr, D.R.; Carter, J.B.; Carucci, J.A.; et al. riSCC: A Personalized Risk Model for the Development of Poor Outcomes in Cutaneous Squamous Cell Carcinoma. J. Am. Acad. Dermatol. 2025, 93, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Ostertag, J.U.; Quaedvlieg, P.J.F.; van der Geer, S.; Nelemans, P.; Christianen, M.E.M.C.; Neumann, M.H.A.M.; Krekels, G.A.M. A Clinical Comparison and Long-Term Follow-up of Topical 5-Fluorouracil versus Laser Resurfacing in the Treatment of Widespread Actinic Keratoses. Lasers Surg. Med. 2006, 38, 731–739. [Google Scholar] [CrossRef]
- Kato, J.; Horimoto, K.; Sato, S.; Minowa, T.; Uhara, H. Dermoscopy of Melanoma and Non-Melanoma Skin Cancers. Front. Med. 2019, 6, 180. [Google Scholar] [CrossRef] [PubMed]
- Feldman, S.R.; Fleischer, A.B., Jr. Progression of Actinic Keratosis to Squamous Cell Carcinoma Revisited: Clinical and Treatment Implications. Cutis 2011, 87, 201–207. [Google Scholar] [PubMed]
- Smoller, B.R. Squamous Cell Carcinoma: From Precursor Lesions to High-Risk Variants. Mod. Pathol. 2006, 19 (Suppl. 2), S88–S92. [Google Scholar] [CrossRef]
- Falkenberg, C.; Dirschka, T.; Gilbert, G.; Stockfleth, E.; Homey, B.; Schmitz, L. Basal Proliferation and Acantholysis May Represent Histological High-Risk Factors for Progression into Invasive Squamous Cell Carcinoma: A Comparison Study in Solid Organ Transplant Recipients and Matched Immunocompetent Patients. Cancers 2023, 15, 1765. [Google Scholar] [CrossRef]
- Li, Z.; Lu, F.; Zhou, F.; Song, D.; Chang, L.; Liu, W.; Yan, G.; Zhang, G. From Actinic Keratosis to Cutaneous Squamous Cell Carcinoma: The Key Pathogenesis and Treatments. Front. Immunol. 2025, 16, 1518633. [Google Scholar] [CrossRef]

| Score | Dermoscopy 1 | Dermoscopy 2 | Dermoscopy 3 | p-Value | |
|---|---|---|---|---|---|
| Abnormal honeycomb | 0 | 0 (0%) | 0 (0%) | 0 (0%) | <0.001 ** |
| 1 | 26 (89.65%) | 3 (10.35%) | 0 (0%) | ||
| 2 | 1 (5.88%) | 16 (94.12%) | 0 (0%) | ||
| 3 | 0 (0%) | 2 (50%) | 2 (50%) | ||
| Nuclear atypia | 0 | 25 (65.79%) | 12 (31.58%) | 1 (2.63%) | 0.012 ** |
| 1 | 2 (16.67%) | 9 (75%) | 1 (8.33%) | ||
| 2 | 0 (0%) | 0 (0%) | 0 (0%) | ||
| 3 | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Inflammation | 0 | 1 (100%) | 0 (0%) | 0 (0%) | <0.001 ** |
| 1 | 25 (73.53%) | 8 (23.53%) | 1 (2.94%) | ||
| 2 | 1 (6.67%) | 13 (86.67%) | 1 (6.67%) | ||
| 3 | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Elastosis | 0 | 0 (0%) | 0 (0%) | 0 (0%) | <0.001 ** |
| 1 | 24 (77.42%) | 7 (22.58%) | 0 (0%) | ||
| 2 | 3 (18.75%) | 11 (68.75%) | 2 (12.5%) | ||
| 3 | 0 (0%) | 3 (100%) | 0 (0%) | ||
| Parakeratosis | 0 | 14 (93.33%) | 1 (6.67%) | 0 (0%) | <0.001 ** |
| 1 | 13 (48.15%) | 14 (51.85%) | 0 (0%) | ||
| 2 | 0 (0%) | 6 (75%) | 2 (25%) | ||
| 3 | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Overall RCM | 1 | 26 (86.67%) | 4 (13.33%) | 0 (0%) | <0.001 ** |
| 2 | 1 (5%) | 17 (85%) | 2 (10%) | ||
| 3 | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Score | MCR 1 | MCR 2 | MCR 3 | p-Value | |
|---|---|---|---|---|---|
| Vessels | 0 | 3 (100%) | 0 (0%) | 0 (0%) | 0.296 |
| 1 | 15 (62.5%) | 9 (37.5%) | 0 (0%) | ||
| 2 | 9 (47.37%) | 10 (52.63%) | 0 (0%) | ||
| 3 | 3 (75%) | 1 (25%) | 0 (0%) | ||
| Erosions | 0 | 25 (78.12%) | 7 (21.88%) | 0 (0%) | <0.001 ** |
| 1 | 5 (62.5%) | 3 (37.5%) | 0 (0%) | ||
| 2 | 0 (0%) | 7 (100%) | 0 (0%) | ||
| 3 | 0 (0%) | 3 (100%) | 0 (0%) | ||
| Strawberry pattern | 0 | 11 (84.62%) | 2 (15.38%) | 0 (0%) | 0.132 |
| 1 | 14 (53.85%) | 12 (46.15%) | 0 (0%) | ||
| 2 | 5 (50%) | 5 (50%) | 0 (0%) | ||
| 3 | 0 (0%) | 3 (100%) | 0 (0%) | ||
| Erythema | 0 | 0 (0%) | 0 (0%) | 0 (0%) | <0.001 ** |
| 1 | 18 (90%) | 2 (10%) | 0 (0%) | ||
| 2 | 12 (48%) | 13 (52%) | 0 (0%) | ||
| 3 | 0 (0%) | 5 (100%) | 0 (0%) | ||
| Scales | 0 | 12 (100%) | 0 (0%) | 0 (0%) | <0.001 ** |
| 1 | 15 (68.18%) | 7 (31.82%) | 0 (0%) | ||
| 2 | 2 (22.22%) | 7 (77.78%) | 0 (0%) | ||
| 3 | 1 (14.28%) | 6 (85.72%) | 0 (0%) | ||
| Overall dermoscopy | 1 (0–5) | 26 (96.3%) | 1 (3.7%) | 0 (0%) | <0.001 ** |
| 2 (6–10) | 4 (19.05%) | 17 (80.95%) | 0 (0%) | ||
| 3 (11–15) | 0 (0%) | 2 (100%) | 0 (0%) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soare, C.; Cozma, E.C.; Giurcăneanu, C.; Voiculescu, V.M. Comparative Analysis of Clinical, Dermoscopic, and Confocal Microscopy Scores for Assessing Severity of Actinic Keratosis. Cancers 2025, 17, 2899. https://doi.org/10.3390/cancers17172899
Soare C, Cozma EC, Giurcăneanu C, Voiculescu VM. Comparative Analysis of Clinical, Dermoscopic, and Confocal Microscopy Scores for Assessing Severity of Actinic Keratosis. Cancers. 2025; 17(17):2899. https://doi.org/10.3390/cancers17172899
Chicago/Turabian StyleSoare, Cristina, Elena Codruța Cozma, Călin Giurcăneanu, and Vlad Mihai Voiculescu. 2025. "Comparative Analysis of Clinical, Dermoscopic, and Confocal Microscopy Scores for Assessing Severity of Actinic Keratosis" Cancers 17, no. 17: 2899. https://doi.org/10.3390/cancers17172899
APA StyleSoare, C., Cozma, E. C., Giurcăneanu, C., & Voiculescu, V. M. (2025). Comparative Analysis of Clinical, Dermoscopic, and Confocal Microscopy Scores for Assessing Severity of Actinic Keratosis. Cancers, 17(17), 2899. https://doi.org/10.3390/cancers17172899








