Eliminating REMS for CAR T-Cell Therapies: An Opportunity to Improve Access
Abstract
Simple Summary
Abstract
1. Introduction
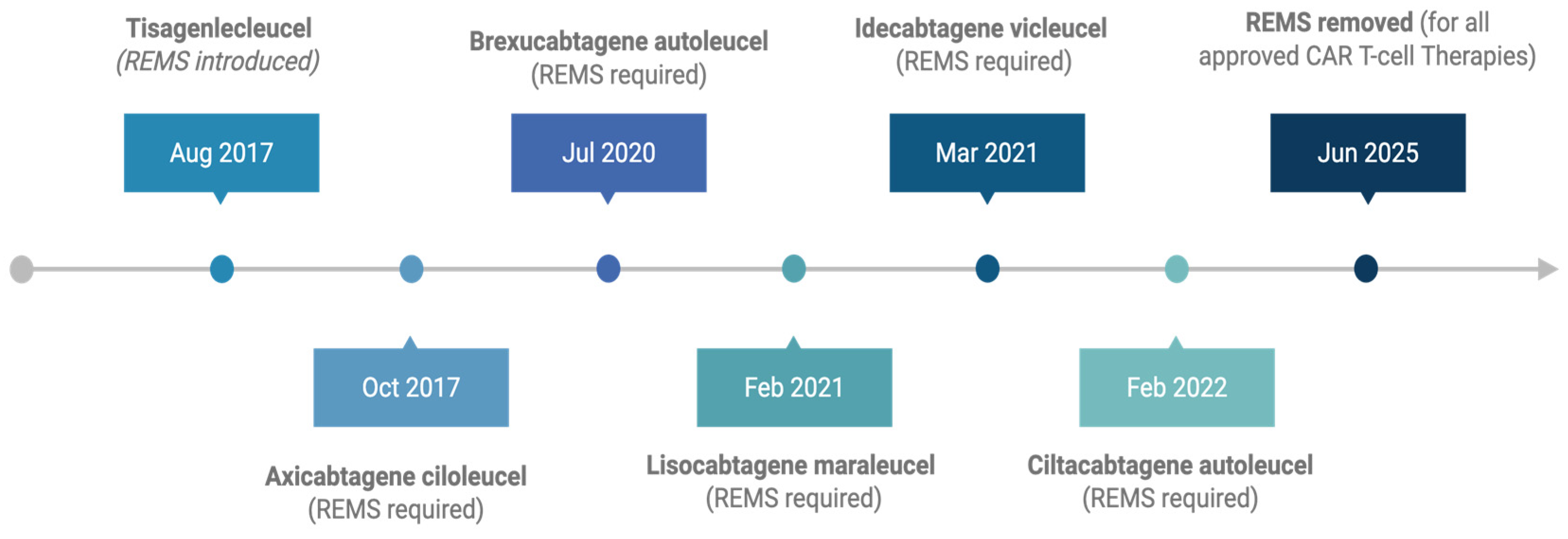
2. Early CAR T-Cell Therapy Approvals and REMS Implementation
3. CRS and ICANS Onset Across CAR T-Cell Therapies
4. Additional Safety Considerations Beyond CRS and ICANS
5. Regulatory Reform: The June 2025 FDA Decision
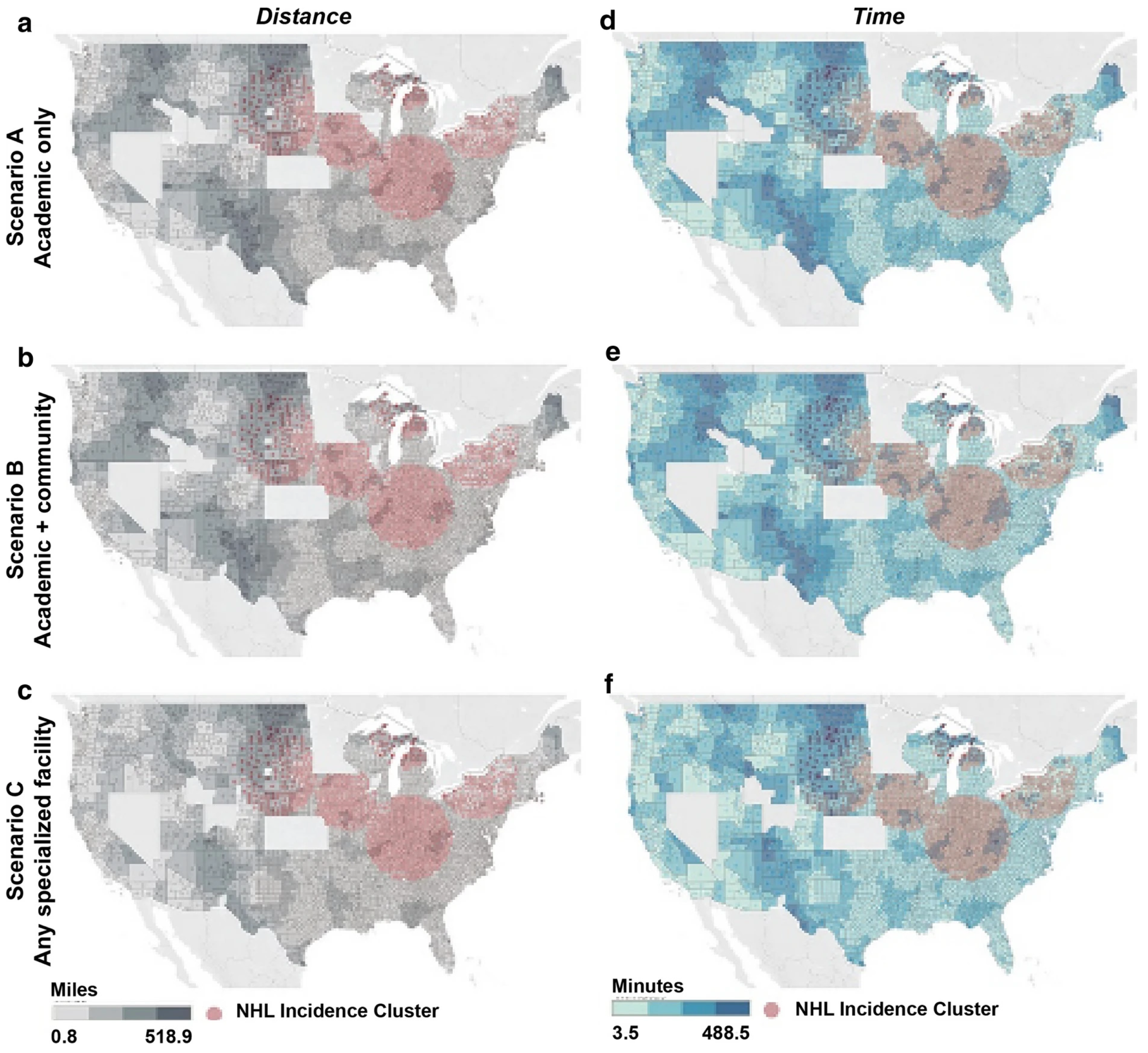
6. Expanding Access and Addressing Health Equity
7. Adapting Delivery Models in Response to REMS Elimination
7.1. Shared-Care Approach
7.2. Organizational Support
7.3. Accreditation Challenges
7.4. Telehealth Integration
7.5. Future Delivery Models
8. Recommendations and Equity-Driven Implementation Models
8.1. Expand Treatment Sites
8.2. Revise Payer Policies
8.3. Standardize Patient and Caregiver Education
8.4. Integrate Telehealth and Remote Monitoring
8.5. Educate Community Providers
8.6. Strengthen Equity Monitoring Systems
9. Challenges in Maintaining Safety After REMS Removal
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACCC | Association for Community Cancer Centers |
| ALL | Acute lymphoblastic leukemia |
| ASTCT | American Society for Transplantation and Cellular Therapy |
| BCMA | B-cell maturation antigen |
| B-ALL | B-cell acute lymphoblastic leukemia |
| CAR T | Chimeric antigen receptor T cell |
| CI | Confidence interval |
| CIBMTR | Center for International Blood and Marrow Transplant Research |
| CIDR | Cellular Immunotherapy Data Resource |
| CLL/SLL | Chronic lymphocytic leukemia/small lymphocytic lymphoma |
| CRS | Cytokine release syndrome |
| DEPICT | Diverse and Equitable Participation in Clinical Trials |
| FACT | Foundation for Accreditation of Cellular Therapies |
| FDA | Food and Drug Administration |
| FL | Follicular lymphoma |
| ICANS | Immune effector cell-associated neurotoxicity syndrome |
| LBCL | Large B-cell lymphoma |
| MCL | Mantle cell lymphoma |
| MM | Multiple Myeloma |
| NIH | National Institutes of Health |
| OR | Odds ratio |
| OS | Overall Survival |
| PFS | Progression-free survival |
| REMS | Risk Evaluation and Mitigation Strategies |
| RPM | Remote patient monitoring |
| SEER | Surveillance, Epidemiology, and End Results Program. |
| VTE | Venous thromboembolism |
References
- U.S. Food and Drug Administration. What’s in a REMS? U.S. Food and Drug Administration. 2022. Available online: https://www.fda.gov/drugs/risk-evaluation-and-mitigation-strategies-rems/whats-rems (accessed on 29 September 2025).
- U.S. Food and Drug Administration. FDA Eliminates Risk Evaluation and Mitigation Strategies (REMS) for Autologous Chimeric Antigen Receptor (CAR) T-Cell Immunotherapies. Available online: https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/fda-eliminates-risk-evaluation-and-mitigation-strategies-rems-autologous-chimeric-antigen-receptor (accessed on 10 August 2025).
- KYMRIAH (Tisagenlecleucel) Prescribing Information. East Hanover (NJ): Novartis Pharmaceuticals Corporation. 2025. Available online: https://www.fda.gov/media/107296/download (accessed on 9 August 2025).
- YESCARTA (Axicabtagene Ciloleucel) Prescribing Information. Santa Monica (CA): Kite Pharma, Inc. 2025. Available online: https://www.fda.gov/media/108377/download (accessed on 9 August 2025).
- TECARTUS (Brexucabtagene Autoleucel) Prescribing Information. Santa Monica (CA): Kite Pharma, Inc. 2025. Available online: https://www.fda.gov/media/140409/download (accessed on 9 August 2025).
- BREYANZI (Lisocabtagene Maraleucel) Prescribing Information. Summit (NJ): Bristol-Myers Squibb. 2025. Available online: https://www.fda.gov/media/187950/download (accessed on 9 August 2025).
- ABECMA (Idecabtagene Vicleucel) Prescribing Information. Summit (NJ): Bristol-Myers Squibb. 2025. Available online: https://www.fda.gov/media/147055/download (accessed on 9 August 2025).
- CARVYKTI (Ciltacabtagene Autoleucel) Prescribing Information. Horsham (PA): Janssen Biotech, Inc. 2025. Available online: https://www.fda.gov/media/156560/download (accessed on 9 August 2025).
- Ahmed, N.; Wesson, W.; Lutfi, F.; Porter, D.L.; Bachanova, V.; Nastoupil, L.J.; Perales, M.-A.; Maziarz, R.T.; Brower, J.; Shah, G.L.; et al. Optimizing the post-CAR T monitoring period in recipients of axicabtagene ciloleucel, tisagenlecleucel, and lisocabtagene maraleucel. Blood Adv. 2024, 8, 5346–5353. [Google Scholar] [CrossRef]
- Ventin, M.; Cattaneo, G.; Maggs, L.; Arya, S.; Wang, X.; Ferrone, C.R. Implications of High Tumor Burden on Chimeric Antigen Receptor T-Cell Immunotherapy: A Review. JAMA Oncol. 2024, 10, 115–121. [Google Scholar] [CrossRef]
- Hill, J.A.; Seo, S.K. How I prevent infections in patients receiving CD19-targeted chimeric antigen receptor T cells for B-cell malignancies. Blood 2020, 136, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Little, J.S.; Aleissa, M.M.; Beluch, K.; Gonzalez-Bocco, I.H.; Marty, F.M.; Manne-Goehler, J.; Koo, S.; Hammond, S.P.; Jacobson, C.A. Low incidence of invasive fungal disease following CD19 chimeric antigen receptor T-cell therapy for non-Hodgkin lymphoma. Blood Adv. 2022, 6, 4821–4830. [Google Scholar] [CrossRef]
- Jain, T.; Knezevic, A.; Pennisi, M.; Chen, Y.; Ruiz, J.D.; Purdon, T.J.; Devlin, S.M.; Smith, M.; Shah, G.L.; Halton, E.; et al. Hematopoietic recovery in patients receiving chimeric antigen receptor T-cell therapy for hematologic malignancies. Blood Adv. 2020, 4, 3776–3787. [Google Scholar] [CrossRef] [PubMed]
- Melody, M.; Gandhi, S.; Saunders, H.; Abdel-Rahman, Z.; Hastings, J.; Lengerke Diaz, P.; Gannon, N.; Truong, T.; Hathcock, M.; Khurana, A.; et al. Incidence of thrombosis in relapsed/refractory B-cell lymphoma treated with axicabtagene ciloleucel: Mayo Clinic experience. Leuk. Lymphoma 2022, 63, 1363–1368. [Google Scholar] [CrossRef] [PubMed]
- Tix, T.; Alhomoud, M.; Shouval, R.; Scheffer Cliff, E.R.; Perales, M.-A.; Cordas dos Santos, D.M.; Rejeski, K. Second Primary Malignancies after CAR T-Cell Therapy: A Systematic Review and Meta-Analysis of 5,517 Lymphoma and Myeloma Patients. Clin. Cancer Res. 2024, 30, 4690–4700. [Google Scholar] [CrossRef]
- Ellithi, M.; Elsallab, M.; Lunning, M.A.; Holstein, S.A.; Sharma, S.; Trinh, J.Q.; Ma, J.; Maus, M.V.; Frigault, M.J.; D’Angelo, C.R. Neurotoxicity and Rare Adverse Events in BCMA-Directed CAR T Cell Therapy: A Comprehensive Analysis of Real-World FAERS Data. Transplant. Cell. Ther. 2025, 31, 71.e1–71.e14. [Google Scholar] [CrossRef]
- Graham, C.E.; Velasco, R.; Alarcon Tomas, A.; Stewart, O.P.; Dachy, G.; Del Bufalo, F.; Doglio, M.; Henter, J.I.; Ortí, G.; Peric, Z.; et al. Non-ICANS neurological complications after CAR T-cell therapies: Recommendations from the EBMT Practice Harmonisation and Guidelines Committee. Lancet Oncol. 2025, 26, e203–e213. [Google Scholar] [CrossRef]
- American Society of Hematology. FDA-Mandated CAR-T Monitoring Period Could Be Halved. 2024. Available online: https://www.hematology.org/newsroom/press-releases/2024/fda-mandated-car-t-monitoring-period-could-be-halved (accessed on 10 August 2025).
- Berberabe, T. CAR T-Cell Therapy Remains Underutilized, Despite Improvements in Access. Targeted Ther. Oncol. 2024, 13, 16. [Google Scholar]
- Ahmed, N.; Shahzad, M.; Shippey, E.; Bansal, R.; Mushtaq, M.U.; Mahmoudjafari, Z.; Faisal, M.S.; Hoffmann, M.; Abdallah, A.O.; Divine, C.; et al. Socioeconomic and Racial Disparity in Chimeric Antigen Receptor T Cell Therapy Access. Transplant. Cell Ther. 2022, 28, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Badr, H.; Rouce, R.; Scheurer, M.E.; Lulla, P.; Mims, M.; Reddy, P. Bringing CAR T Cell Therapy Trials to Underserved Populations. Cancer Cell 2023, 41, 2007–2010. [Google Scholar] [CrossRef]
- Islam, N.; Budvytyte, L.; Khera, N.; Hilal, T. Disparities in clinical trial enrollment- focus on CAR-T and bispecific antibody therapies. Curr. Hematol. Malig. Rep. 2025, 20, 1. [Google Scholar] [CrossRef]
- U.S. Congress. Diverse and Equitable Participation in Clinical Trials (DEPICT) Act of 2022, H.R.6584, 117th Congress. Available online: https://www.congress.gov/bill/117th-congress/house-bill/6584 (accessed on 4 September 2025).
- U.S. Congress. NIH Clinical Trial Diversity Act of 2023, H.R.3503 / S.1701, 118th Congress. Available online: https://www.congress.gov/bill/118th-congress/senate-bill/1701 (accessed on 4 September 2025).
- Emole, J.; Lawal, O.; Lupak, O.; Dias, A.; Shune, L.; Yusuf, K. Demographic Differences among Patients Treated with Chimeric Antigen Receptor T-Cell Therapy in the United States. Cancer Med. 2022, 11, 4440–4448. [Google Scholar] [CrossRef]
- Ahmed, N.; Sun, F.; Teigland, C.; Kilgore, K.M.; Mohammadi, I.; Chambers, J.; Dieyi, C.; Feng, C.; Osborn, J.; Fu, C.; et al. Chimeric Antigen Receptor T-Cell Access in Patients with Relapsed/Refractory Large B-Cell Lymphoma: Association of Access with Social Determinants of Health and Travel Time to Treatment Centers. Transpl. Cell. Ther. 2024, 30, 714–725. [Google Scholar] [CrossRef]
- Karmali, R.; Machhi, R.; Epperla, N.; Shouse, G.; Romancik, J.; Moyo, T.K.; Kenkre, V.; Ollila, T.A.; Fitzgerald, L.; Hess, B.; et al. Impact of Race and Social Determinants of Health on Outcomes in Patients with Aggressive B-Cell NHL Treated with CAR-T Therapy. Blood Adv. 2024, 8, 2592–2599. [Google Scholar] [CrossRef] [PubMed]
- Snyder, S.; Chung, K.C.; Jun, M.P.; Gitlin, M. Access to Chimeric Antigen Receptor T Cell Therapy for Diffuse Large B Cell Lymphoma. Adv. Ther. 2021, 38, 4659–4674. [Google Scholar] [CrossRef]
- Davis, E.S.; Franks, J.A.; Bhatia, S.; Kenzik, K.M. Urban-rural differences in cancer mortality: Operationalizing rurality. J. Rural. Health 2024, 40, 268–271. [Google Scholar] [CrossRef]
- Khare, S.; Williamson, S.; O’Barr, B.; Schachter, L.; Chen, A.; Hayes-Lattin, B.; Leonard, J.; Desai, A.; Ferreira-Gandolfo, P.; Christmas, K.; et al. Sociodemographic Factors Influencing Access to Chimeric Antigen Receptor T-Cell Therapy for Patients with Non-Hodgkin Lymphoma. Clin. Lymphoma Myeloma Leuk. 2025, 25, e120–e125. [Google Scholar] [CrossRef]
- Borgert, R. Improving outcomes and mitigating costs associated with CAR T-cell therapy. Am. J. Manag. Care 2021, 27 (Suppl. 13), S253–S261. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.S.; Hunter, B.D.; Cobb, P.W.; Varela, J.C.; Muñoz, J. Overcoming barriers to referral for chimeric antigen receptor T-cell therapy in patients with relapsed/refractory diffuse large B-cell lymphoma. Transpl. Cell Ther. 2023, 29, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Ram, R.; Grisariu, S.; Shargian-Alon, L.; Amit, O.; Bar-On, Y.; Stepensky, P.; Yeshurun, M.; Avni, B.; Hagin, D.; Perry, C.; et al. Toxicity and efficacy of chimeric antigen receptor T-cell therapy in patients with diffuse large B-cell lymphoma above the age of 70 years compared to younger patients- a matched control multicenter cohort study. Haematologica 2022, 107, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Chihara, D.; Liao, L.; Tkacz, J.; Franco, A.; Lewing, B.; Kilgore, K.M.; Nastoupil, L.J.; Chen, L. Real-world experience of CAR T-cell therapy in older patients with relapsed/refractory diffuse large B-cell lymphoma. Blood 2023, 142, 1047–1054. [Google Scholar] [CrossRef]
- Kharfan-Dabaja, M.A.; Mohty, R.; Easwar, N.; Johnston, P.; Iqbal, M.; Epperla, N.; Yared, J.; Ahmed, N.; Hamadani, M.; Beitinjaneh, A.; et al. Chimeric antigen receptor T cell therapy in octogenarians with B-cell lymphoma: A real-world US multicenter collaborative study. Bone Marrow Transpl. 2025, 60, 632–639. [Google Scholar] [CrossRef]
- Shouse, G.; Danilov, A.V.; Artz, A. CAR T-Cell Therapy in the Older Person: Indications and Risks. Curr. Oncol. Rep. 2022, 24, 1189–1199. [Google Scholar] [CrossRef]
- Stolz, S.M.; Musa, A.; Bachofner, A.; Bankova, A.K.; Gourri, E.; Manz, M.G.; Rieger, M.J.; Schneidawind, D.; Wolfensberger, N.; Zenz, T.; et al. Prophylactic Tocilizumab Prior to Infusion of CD19 CAR T-Cells Reduces Therapy-Related Complications in Older Lymphoma Patients. Ann. Hematol. 2025, 104, 4149–4155. [Google Scholar] [CrossRef]
- Association of Community Cancer Centers. Bringing CAR T-Cell Therapies to Community Oncology. Available online: https://www.accc-cancer.org/home/learn/precision-medicine/treatment/immunotherapy/car-t-cell-therapy/bringing-car-t-cell-therapies-to-community-oncology (accessed on 10 August 2025).
- Byrne, M.T.; Lyss, A.J.; Mullangi, S. Key Challenges in CAR T-Cell Therapy Access in Community Oncology. JAMA Oncol. 2025, 11, 481–482. [Google Scholar] [CrossRef]
- Sumarsono, A.; Case, M.; Kassa, S.; Moran, B. Telehealth as a Tool to Improve Access and Reduce No-Show Rates in a Large Safety-Net Population in the USA. J. Urban Health 2023, 100, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Ojinnaka, C.O.; Johnstun, L.; Dunnigan, A.; Nordstrom, L.; Yuh, S. Telemedicine Reduces Missed Appointments but Disparities Persist. Am. J. Prev. Med. 2024, 67, 90–96. [Google Scholar] [CrossRef]
- Paludo, J.; Bansal, R.; Holland, A.T.; Haugen, K.L.; Spychalla, M.T.; McClanahan, A.L.; Truong, T.A.; Hathcock, M.A.; Khurana, A.; Alkhateeb, H.B.; et al. Pilot implementation of remote patient monitoring program for outpatient management of CAR-T cell therapy. Blood 2021, 138 (Suppl. 1), 568–570. [Google Scholar] [CrossRef]
- Devine, S.M.; Horowitz, M.M. Building a Fit for Purpose Clinical Trials Infrastructure to Accelerate the Assessment of Novel Hematopoietic Cell Transplantation Strategies and Cellular Immunotherapies. J. Clin. Oncol. 2021, 39, 534–546. [Google Scholar] [CrossRef] [PubMed]
| CAR T-Cell Therapy | Target | Indications | CRS Onset Median (Range) | ICANS Onset Median (Range) |
|---|---|---|---|---|
| Tisagenlecleucel | CD19 | B-ALL (≤25 y), LBCL, FL | 3 days (1–51) | 5 days (1–368) |
| Axicabtagene ciloleucel | CD19 | LBCL, FL | 3 days (1–20) | 5 days (1–133) |
| Brexucabtagene autoleucel | CD19 | MCL, B-ALL | 4 days (1–13) | 6 days (1–51) |
| Lisocabtagene maraleucel | CD19 | LBCL, FL, CLL/SLL, MCL | 5 days (1–63) | 8 days (1–63) |
| Idecabtagene vicleucel | BCMA | MM | 1 day (1–27) | 2 days (1–148) |
| Ciltacabtagene autoleucel | BCMA | MM | 7 days (1–23) | 8 days (1–28) |
| Policy Requirement | Before June 2025 | After June 2025 |
|---|---|---|
| Institutional certification | Required at REMS-approved sites | Not required |
| Tocilizumab availability | On-site and immediately accessible | Not required |
| Prescriber/staff training | REMS-specific certification | Integrated into product labeling |
| Patient proximity after infusion | 4 weeks within 2 h radius (often stricter in practice) | 2 weeks within reasonable proximity to a healthcare facility |
| Driving restriction | 8 weeks | 2 weeks |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orosco-Ttamina, A.L.; Arana Yi, C.; Tsang, M.; Hilal, T.; Rosenthal, A.; Munoz, J. Eliminating REMS for CAR T-Cell Therapies: An Opportunity to Improve Access. Cancers 2025, 17, 3216. https://doi.org/10.3390/cancers17193216
Orosco-Ttamina AL, Arana Yi C, Tsang M, Hilal T, Rosenthal A, Munoz J. Eliminating REMS for CAR T-Cell Therapies: An Opportunity to Improve Access. Cancers. 2025; 17(19):3216. https://doi.org/10.3390/cancers17193216
Chicago/Turabian StyleOrosco-Ttamina, Angel Luis, Cecilia Arana Yi, Mazie Tsang, Talal Hilal, Allison Rosenthal, and Javier Munoz. 2025. "Eliminating REMS for CAR T-Cell Therapies: An Opportunity to Improve Access" Cancers 17, no. 19: 3216. https://doi.org/10.3390/cancers17193216
APA StyleOrosco-Ttamina, A. L., Arana Yi, C., Tsang, M., Hilal, T., Rosenthal, A., & Munoz, J. (2025). Eliminating REMS for CAR T-Cell Therapies: An Opportunity to Improve Access. Cancers, 17(19), 3216. https://doi.org/10.3390/cancers17193216






