Optimizing Motion Management and Baseline Shifts in Magnetic Resonance-Guided Spine Stereotactic Body Radiation Therapy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. MRI Motion Data Acquisition and Data Processing
2.3. Optimization and Motion Analysis of Registration Structures
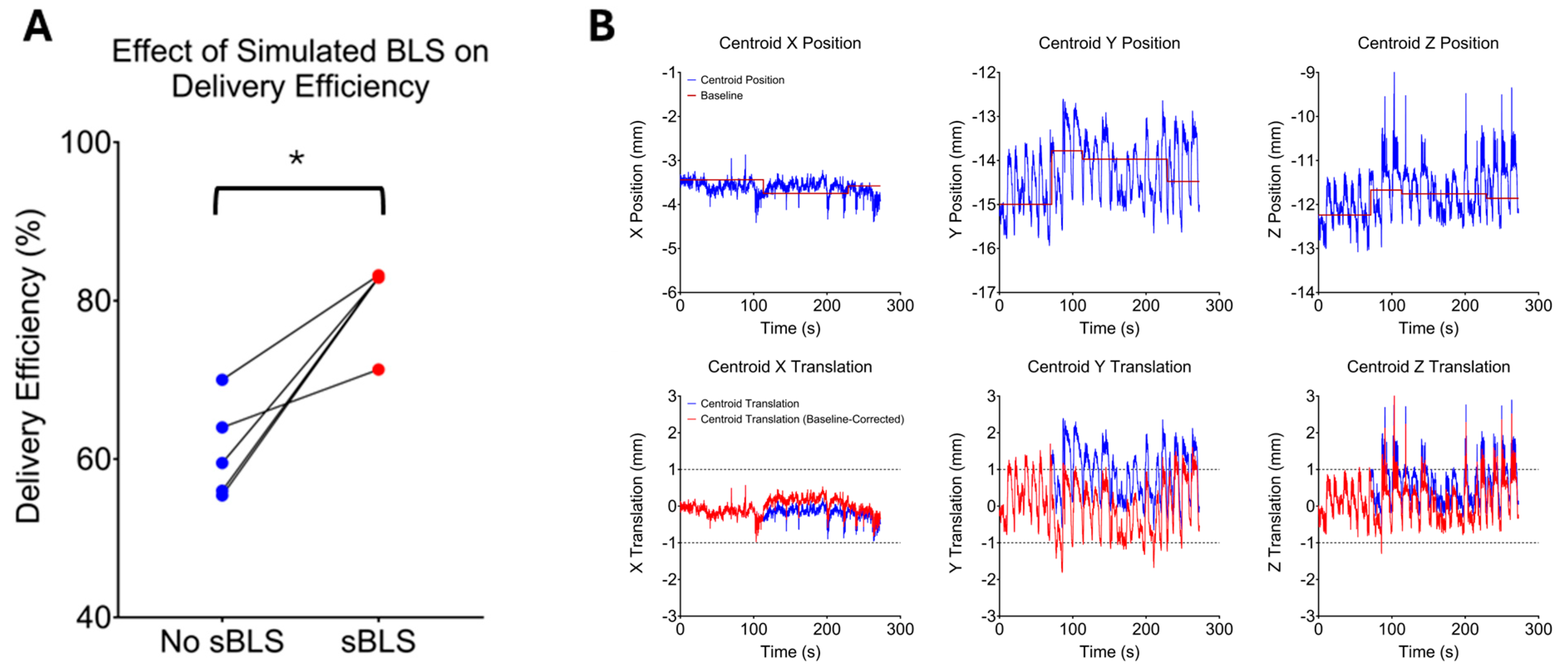
2.4. Delivery Efficiency and Baseline Shift Correction
- More than 1 min had passed since the start of the cine acquisition or the last baseline correction;
- Calculated delivery efficiency was below 80% for the prior minute;
- More than 10 s remained in the cine acquisition session.
2.5. Statistical Analysis
3. Results
3.1. Optimization of the Registration Structure
3.2. Range of Intrafraction Motion
3.3. Delivery Efficiency
3.4. Quality of Simulated Baseline Shift-Corrected Plans
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SBRT | stereotactic body radiation therapy |
| MR | magnetic resonance |
| Linac | linear accelerator |
| MRL | MR linac |
| CMM | comprehensive motion management |
| MMRP | motion management research package |
| GTV | gross tumor volume |
| CTV | clinical target volume |
| PTV | planning target volume |
| SAM | segment aperture morphing |
| ATP | adapt-to-position |
| ATS | adapt-to-shape |
| VB | vertebral body |
| RL | right-left |
| AP | anterior–posterior |
| SI | superior–inferior |
| BLS | baseline shift |
| sBLS | simulated BLS |
References
- Zhang, X.; Larsen, A.G.; Kharas, N.; Bilsky, M.H.; Newman, W.C. Separation surgery for metastatic spine tumors: How less became more. Neuro-Oncol. Adv. 2024, 6 (Suppl. S3), iii94–iii100. [Google Scholar] [CrossRef]
- Bilsky, M.H.; Yamada, Y.; Yenice, K.M.; Lovelock, M.; Hunt, M.; Gutin, P.H.; Leibel, S.A. Intensity-modulated Stereotactic Radiotherapy of Paraspinal Tumors: A Preliminary Report. Neurosurgery 2004, 54, 823–831. [Google Scholar] [CrossRef]
- Gerszten, P.C.; Burton, S.A.; Ozhasoglu, C.; Welch, W.C. Radiosurgery for Spinal Metastases. Spine 2007, 32, 193–199. [Google Scholar] [CrossRef]
- Boyce-Fappiano, D.; Gjyshi, O.; Pezzi, T.A.; Allen, P.K.; Solimman, M.; Taku, N.; Bernstein, M.B.; Cabanillas, M.E.; Amini, B.; Tatsui, C.E.; et al. Spine stereotactic radiosurgery for metastatic thyroid cancer: A single-institution experience. J. Neurosurg. Spine 2020, 32, 941–949. [Google Scholar] [CrossRef]
- Yamada, Y.; Bilsky, M.H.; Lovelock, D.M.; Venkatraman, E.S.; Toner, S.; Johnson, J.; Zatcky, J.; Zelefsky, M.J.; Fuks, Z. High-Dose, Single-Fraction Image-Guided Intensity-Modulated Radiotherapy for Metastatic Spinal Lesions. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 484–490. [Google Scholar] [CrossRef]
- Wu, J.S.-Y.; Wong, R.; Johnston, M.; Bezjak, A.; Whelan, T. Meta-analysis of dose-fractionation radiotherapy trials for the palliation of painful bone metastases. Int. J. Radiat. Oncol. Biol. Phys. 2003, 55, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Soltys, S.G.; Grimm, J.; Milano, M.T.; Xue, J.; Sahgal, A.; Yorke, E.; Yamada, Y.; Ding, G.X.; Li, X.A.; Lovelock, D.M.; et al. Stereotactic Body Radiation Therapy for Spinal Metastases: Tumor Control Probability Analyses and Recommended Reporting Standards. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 112–123. [Google Scholar] [CrossRef]
- Zelefsky, M.J.; Greco, C.; Motzer, R.; Magsanoc, J.M.; Pei, X.; Lovelock, M.; Mechalakos, J.; Zatcky, J.; Fuks, Z.; Yamada, Y. Tumor Control Outcomes After Hypofractionated and Single-Dose Stereotactic Image-Guided Intensity-Modulated Radiotherapy for Extracranial Metastases From Renal Cell Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 1744–1748. [Google Scholar] [CrossRef] [PubMed]
- Lovelock, D.M.; Zhang, Z.; Jackson, A.; Keam, J.; Bekelman, J.; Bilsky, M.; Lis, E.; Yamada, Y. Correlation of Local Failure With Measures of Dose Insufficiency in the High-Dose Single-Fraction Treatment of Bony Metastases. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 1282–1287. [Google Scholar] [CrossRef] [PubMed]
- Laufer, I.; Iorgulescu, J.B.; Chapman, T.; Lis, E.; Shi, W.; Zhang, Z.; Cox, B.W.; Yamada, Y.; Bilsky, M.H. Local disease control for spinal metastases following ‘separation surgery’ and adjuvant hypofractionated or high-dose single-fraction stereotactic radiosurgery: Outcome analysis in 186 patients. J. Neurosurg. Spine 2013, 18, 207–214. [Google Scholar] [CrossRef]
- Katsoulakis, E.; Jackson, A.; Cox, B.; Lovelock, M.; Yamada, Y. A Detailed Dosimetric Analysis of Spinal Cord Tolerance in High-Dose Spine Radiosurgery. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 598–607. [Google Scholar] [CrossRef]
- Diao, K.; Song, J.; Thall, P.F.; McGinnis, G.J.; Boyce-Fappiano, D.; Amini, B.; Brown, P.D.; Yeboa, D.N.; Bishop, A.J.; Li, J.; et al. Low risk of radiation myelopathy with relaxed spinal cord dose constraints in de novo, single fraction spine stereotactic radiosurgery. Radiother. Oncol. 2020, 152, 49–55. [Google Scholar] [CrossRef]
- Ghia, A.J.; Guha-Thakurta, N.; Hess, K.; Yang, J.N.; Settle, S.H.; Sharpe, H.J.; Li, J.; McAleer, M.; Chang, E.L.; Tatsui, C.E.; et al. Phase 1 Study of Spinal Cord Constraint Relaxation With Single Session Spine Stereotactic Radiosurgery in the Primary Management of Patients With Inoperable, Previously Unirradiated Metastatic Epidural Spinal Cord Compression. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 1481–1488. [Google Scholar] [CrossRef]
- Tseng, C.-L.; Sussman, M.S.; Atenafu, E.G.; Letourneau, D.; Ma, L.; Soliman, H.; Thibault, I.; Cho, B.C.J.; Simeonov, A.; Yu, E.; et al. Magnetic Resonance Imaging Assessment of Spinal Cord and Cauda Equina Motion in Supine Patients With Spinal Metastases Planned for Spine Stereotactic Body Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 995–1002. [Google Scholar] [CrossRef]
- Oztek, M.A.; Mayr, N.A.; Mossa-Basha, M.; Nyflot, M.; Sponseller, P.A.; Wu, W.; Hofstetter, C.P.; Saigal, R.; Bowen, S.R.; Hippe, D.S.; et al. The Dancing Cord: Inherent Spinal Cord Motion and Its Effect on Cord Dose in Spine Stereotactic Body Radiation Therapy. Neurosurgery 2020, 87, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Raaymakers, B.W.; Lagendijk, J.J.W.; Overweg, J.; Kok, J.G.M.; Raaijmakers, A.J.E.; Kerkhof, E.M.; van der Put, R.W.; Meijsing, I.; Crijns, S.P.M.; Benedosso, F.; et al. Integrating a 1.5 T MRI scanner with a 6 MV accelerator: Proof of concept. Phys. Med. Biol. 2009, 54, N229–N237. [Google Scholar] [CrossRef]
- Lagendijk, J.J.; Raaymakers, B.W.; Raaijmakers, A.J.; Overweg, J.; Brown, K.J.; Kerkhof, E.M.; van der Put, R.W.; Hårdemark, B.; van Vulpen, M.; van der Heide, U.A. MRI/linac integration. Radiother. Oncol. 2008, 86, 25–29. [Google Scholar] [CrossRef]
- Hall, W.A.; Paulson, E.; Li, X.A.; Erickson, B.; Schultz, C.; Tree, A.; Awan, M.; Low, D.A.; McDonald, B.A.; Salzillo, T.; et al. Magnetic resonance linear accelerator technology and adaptive radiation therapy: An overview for clinicians. CA Cancer J. Clin. 2022, 72, 34–56. [Google Scholar] [CrossRef] [PubMed]
- Joint Head and Neck Radiotherapy-MRI Development Cooperative; Salzillo, T.C.; Dresner, M.A.; Way, A.; Wahid, K.A.; McDonald, B.A.; Mulder, S.; Naser, M.A.; He, R.; Ding, Y.; et al. Development and implementation of optimized endogenous contrast sequences for delineation in adaptive radiotherapy on a 1.5T MR-linear-accelerator: A prospective R-IDEAL stage 0-2a quantitative/qualitative evaluation of in vivo site-specific quality-assurance using a 3D T2 fat-suppressed platform for head and neck cancer. J. Med. Imaging 2023, 10, 065501. [Google Scholar] [CrossRef]
- Han, E.Y.; Aima, M.; Hughes, N.; Briere, T.M.; Yeboa, D.N.; Castillo, P.; Wang, J.; Yang, J.; Vedam, S. Feasibility of spinal stereotactic body radiotherapy in Elekta Unity® MR-Linac. J. Radiosurg SBRT 2020, 7, 127–134. [Google Scholar]
- Han, E.Y.; Yeboa, D.N.; Briere, T.M.; Yang, J.; Wang, H. Dosimetric analysis of MR-LINAC treatment plans for salvage spine SBRT re-irradiation. J. Appl. Clin. Med. Phys. 2022, 23, e13752. [Google Scholar] [CrossRef]
- Lee, Y.K.; Munawar, I.; Mashouf, S.; Sahgal, A.; Ruschin, M. Dosimetric comparison of two treatment planning systems for spine SBRT. Med. Dosim. 2020, 45, 77–84. [Google Scholar] [CrossRef]
- Brown, K.; Corbett, J. Elekta Unity Comprehensive Motion Management—Explained. 2023. Available online: https://www.elekta.com/products/radiation-therapy/unity/assets/Elekta%20Unity%20Comprehensive%20Motion%20Management%20Explained.pdf (accessed on 2 February 2025).
- Jassar, H.; Tai, A.; Chen, X.; Keiper, T.D.; Paulson, E.; Lathuilière, F.; Bériault, S.; Hébert, F.; Savard, L.; Cooper, D.T.; et al. Real-time motion monitoring using orthogonal cine MRI during MR-guided adaptive radiation therapy for abdominal tumors on 1.5T MR-Linac. Med. Phys. 2023, 50, 3103–3116. [Google Scholar] [CrossRef]
- Keiper, T.D.; Tai, A.; Chen, X.; Paulson, E.; Lathuilière, F.; Bériault, S.; Hébert, F.; Cooper, D.T.; Lachaine, M.; Li, X.A. Feasibility of real-time motion tracking using cine MRI during MR-guided radiation therapy for abdominal targets. Med. Phys. 2020, 47, 3554–3566. [Google Scholar] [CrossRef] [PubMed]
- van den Dobbelsteen, M.; Hackett, S.L.; van Asselen, B.; Oolbekkink, S.; Raaymakers, B.W.; de Boer, J.C.J. Treatment planning evaluation and experimental validation of the magnetic resonance-based intrafraction drift correction. Phys. Imaging Radiat. Oncol. 2024, 30, 100580. [Google Scholar] [CrossRef]
- Grimbergen, G.; Hackett, S.L.; van Ommen, F.; van Lier, A.L.; Borman, P.T.; Meijers, L.T.; de Groot-van Breugel, E.N.; de Boer, J.C.; Raaymakers, B.W.; Intven, M.P.; et al. Gating and intrafraction drift correction on a 1.5 T MR-Linac: Clinical dosimetric benefits for upper abdominal tumors. Radiother. Oncol. 2023, 189, 109932. [Google Scholar] [CrossRef]
- Rusu, S.D.; Smith, B.R.; St-Aubin, J.J.; Shaffer, N.; Hyer, D.E. Surrogate gating strategies for the Elekta Unity MR-Linac gating system. J. Appl. Clin. Med. Phys. 2025, 26, e14566. [Google Scholar] [CrossRef]
- Redler, G.; Stevens, T.; Cammin, J.; Malin, M.; Green, O.; Mutic, S.; Pitroda, S.; Aydogan, B. Dosimetric Feasibility of Utilizing the ViewRay Magnetic Resonance Guided Linac System for Image-guided Spine Stereotactic Body Radiation Therapy. Cureus 2019, 11, e6364. [Google Scholar] [CrossRef]
- Grimbergen, G.; Eijkelenkamp, H.; Heerkens, H.D.; Raaymakers, B.W.; Intven, M.P.W.; Meijer, G.J. Dosimetric impact of intrafraction motion under abdominal compression during MR-guided SBRT for (Peri-) pancreatic tumors. Phys. Med. Biol. 2022, 67, 185016. [Google Scholar] [CrossRef]
- Seiffert, S.; Weber, S.; Sack, U.; Keller, T. Use of logit transformation within statistical analyses of experimental results obtained as proportions: Example of method validation experiments and EQA in flow cytometry. Front. Mol. Biosci. 2024, 11, 1335174. [Google Scholar] [CrossRef]
- Quan, E.; Krafft, S.P.; Briere, T.M.; Vaccarelli, M.J.; Ghia, A.J.; Bishop, A.J.; Yeboa, D.N.; Swanson, T.A.; Han, E.Y. Comparison of setup accuracy and efficiency between the Klarity system and BodyFIX system for spine stereotactic body radiation therapy. J. Appl. Clin. Med. Phys. 2022, 23, e13804. [Google Scholar] [CrossRef]
- Mesko, S.; Wang, H.; Tung, S.; Wang, C.; Pasalic, D.; Ning, M.S.; Pezzi, T.A.; Moreno, A.C.; Reddy, J.P.; Garden, A.S.; et al. SABR for Skull Base Malignancies: A Systematic Analysis of Set-Up and Positioning Accuracy. Pract. Radiat. Oncol. 2020, 10, 363–371. [Google Scholar] [CrossRef]
- Chow, V.U.Y.; Cheung, M.L.M.; Kan, M.W.K.; Chan, A.T.C. Clinical Experience of Intrafractional Motion Monitoring of Patients Under Head and Neck Radiation Therapy Using ExacTrac Dynamic System. Adv. Radiat. Oncol. 2024, 9, 101390. [Google Scholar] [CrossRef]
- Henni, A.H.; Gensanne, D.; Bulot, G.; Roge, M.; Mallet, R.; Colard, E.; Daras, M.; Hanzen, C.; Thureau, S. ExacTrac X-Ray 6D Imaging During Stereotactic Body Radiation Therapy of Spinal and Nonspinal Metastases. Technol. Cancer Res. Treat. 2023, 22, 15330338231210786. [Google Scholar] [CrossRef]
- Corradini, S.; Alongi, F.; Andratschke, N.; Azria, D.; Bohoudi, O.; Boldrini, L.; Bruynzeel, A.; Hörner-Rieber, J.; Jürgenliemk-Schulz, I.; Lagerwaard, F.; et al. ESTRO-ACROP recommendations on the clinical implementation of hybrid MR-linac systems in radiation oncology. Radiother. Oncol. 2021, 159, 146–154. [Google Scholar] [CrossRef]
- Westerhoff, J.M.; Borman, P.T.; Rutgers, R.H.; Raaymakers, B.W.; Winchester, N.; Verkooijen, H.M.; Fast, M.F. On Patient Experience and Anxiety During Treatment With Magnetic Resonance–Guided Radiation Therapy. Adv. Radiat. Oncol. 2024, 9, 101537. [Google Scholar] [CrossRef]
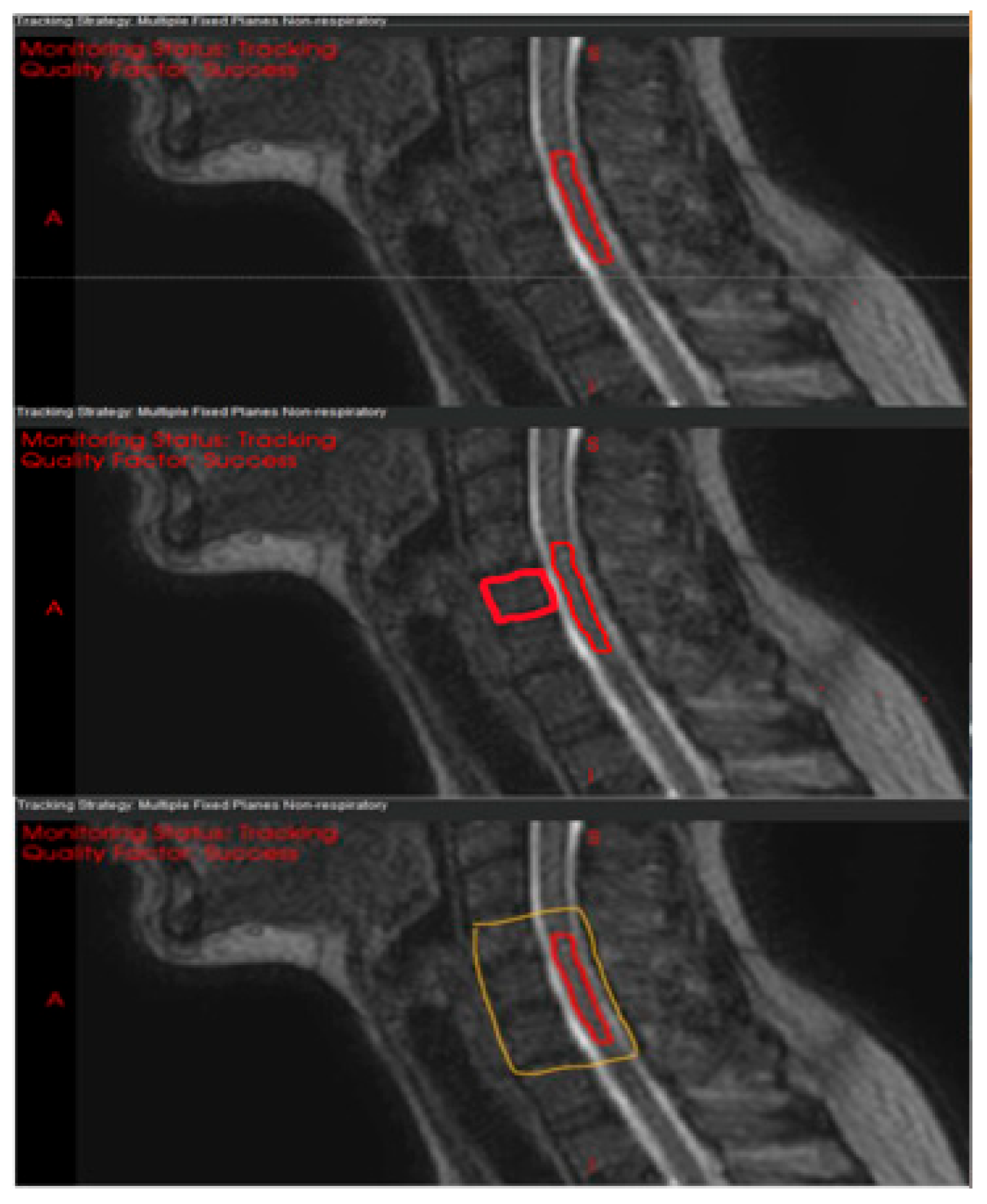

| Patient | Target | Immobilization | Rx (GTV/CTV) | 1st Cine, min | 2nd Cine, min |
|---|---|---|---|---|---|
| 1 | C5 | Masked | 24/16 (1FX) | 20.2 | 16.2 * |
| 2 | T1 | Masked | 27/21 (3FX) | 20.2 | 14.6 * |
| 3 | T2 | Masked | 18/16 (1FX) | 20.2 | 19.7 |
| 4 | T2–T3 | Masked | 24/16 (1FX) | 20.2 | NA |
| 5 | T3 | Masked | 24/16 (1FX) | 20.2 | 20.2 |
| 6 | T3 | Masked | 18/16 (1FX) | 20.2 | 20.2 |
| 7 | T5 | Masked | 27/21 (3FX) | 20.2 | 20.2 |
| 8 | T6 | Un-Masked | 24/16 (1FX) | 20.2 | 20.2 |
| 9 | T7 | Un-Masked | 18/16 (1FX) | 5.2 * | 20.2 |
| 10 | T12 | Un-Masked | 18/16 (1FX) | 11.9 * | 20.2 |
| 11 | L1 | Un-Masked | 18/16 (1FX) | 20.2 | 6.6 * |
| 12 | L2 | Un-Masked | 18/16 (1FX) | 11.2 * | 20.2 |
| Patient 3 | Patient 4 | Patient 12 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cord Only | Cord + CTV | Canal + 3VB | Cord Only | Cord + CTV | Canal + 3VB | Cord Only | Cord + CTV | Canal + 3VB | |
| LR, mm | 2.0 | 1.8 | 1.5 | 2.5 | 2.9 | 2.9 | 0.9 | 0.8 | 0.8 |
| AP, mm | 3.4 | 3.7 | 3.3 | 2.2 | 2.5 | 2.8 | 1.5 | 1.1 | 1.2 |
| SI, mm | 2.3 | 1.4 | 1.1 | 4.5 | 1.7 | 1.5 | 1.6 | 0.7 | 0.7 |
| Pt1 | Pt2 | Pt3 | Pt4 | Pt5 | Pt6 | Pt7 | Pt8 | Pt9 | Pt10 | Pt11 | Pt12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st Cine | 99.4 | 99.0 | 59.5 (82.9, 3) | 56.0 (83.0, 3) | 99.9 | 99.2 | 55.4 (82.9, 4) | 94.1 | 70.0 (83.2, 1) | 97.2 | 99.9 | 99.3 |
| 2nd Cine | 99.8 | 98.0 | 88.2 | NA | 99.9 | 64.0 (71.3, 5) | 91.0 | 88.0 | 99.8 | 99.9 | 99.3 | 99.9 |
| Dosimetric Changes Following SAM-Based ATP Plan Adaptation | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt 3 | Pt 4 | Pt 6 | Pt 7 | Pt 9 | |||||||||||
| Descriptors of | (0.0 mm LR, 0.0 SI, 1.8 AP) | (1.5 mm LR, 0.5 SI, 2.0 AP) | (0.8 mm LR, 0.5 SI, 0.4 AP) | (0.5 mm LR, 1.5 SI, 2.1 AP) | (1.0 mm LR, 0.8 SI, 1.0 AP) | ||||||||||
| Plan Quality | Original | sBLS | Diff | Original | sBLS | Diff | Original | sBLS | Diff | Original | sBLS | Diff | Original | sBLS | Diff |
| Cord | |||||||||||||||
| D0.01cc, cGy | 1140.5 | 1354 | 18.7% | 1164.5 | 1524.5 | 30.9% | 1195.5 | 1550.5 | 29.7% | 1398.5 | 2264.5 | 61.9% | 1202 | 1288.5 | 7.2% |
| CTV | |||||||||||||||
| V100, cGy | 91.75 | 93.98 | 2.2% | 96.78 | 97.28 | 0.5% | 93.8 | 77.26 | −16.5% | 94.78 | 92.78 | −2.0% | 96.02 | 95.2 | −0.8% |
| GTV | |||||||||||||||
| Dmin, cGy | 1723.3 | 1730.9 | 0.4% | 1167.8 | 1224.6 | 4.9% | 1657.6 | 1662.8 | 0.3% | 1755.3 | 1970.2 | 12.2% | 1629.4 | 1653.3 | 1.5% |
| V100, % | 97.13 | 97.04 | −0.1% | 85 | 84.99 | 0.0% | 98.02 | 98 | 0.0% | 82.63 | 82.64 | 0.0% | 97.58 | 97.58 | 0.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Y.; Salzillo, T.C.; Yeboa, D.N.; Tom, M.C.; Wang, Z.; Diagaradjane, P.; Subashi, E.; Yang, J.; Swanson, T.; Beckham, T.; et al. Optimizing Motion Management and Baseline Shifts in Magnetic Resonance-Guided Spine Stereotactic Body Radiation Therapy. Cancers 2025, 17, 2697. https://doi.org/10.3390/cancers17162697
Ding Y, Salzillo TC, Yeboa DN, Tom MC, Wang Z, Diagaradjane P, Subashi E, Yang J, Swanson T, Beckham T, et al. Optimizing Motion Management and Baseline Shifts in Magnetic Resonance-Guided Spine Stereotactic Body Radiation Therapy. Cancers. 2025; 17(16):2697. https://doi.org/10.3390/cancers17162697
Chicago/Turabian StyleDing, Yao, Travis C. Salzillo, Debra N. Yeboa, Martin C. Tom, Zhiheng Wang, Parmeswaran Diagaradjane, Ergys Subashi, Jinzhong Yang, Todd Swanson, Thomas Beckham, and et al. 2025. "Optimizing Motion Management and Baseline Shifts in Magnetic Resonance-Guided Spine Stereotactic Body Radiation Therapy" Cancers 17, no. 16: 2697. https://doi.org/10.3390/cancers17162697
APA StyleDing, Y., Salzillo, T. C., Yeboa, D. N., Tom, M. C., Wang, Z., Diagaradjane, P., Subashi, E., Yang, J., Swanson, T., Beckham, T., Wang, C., Ghia, A. J., Briere, T., Wang, J., Lathuilière, F., Cloake, S., & Han, E. Y. (2025). Optimizing Motion Management and Baseline Shifts in Magnetic Resonance-Guided Spine Stereotactic Body Radiation Therapy. Cancers, 17(16), 2697. https://doi.org/10.3390/cancers17162697






