Clinical Integration of NIR-II Fluorescence Imaging for Cancer Surgery: A Translational Evaluation of Preclinical and Intraoperative Systems
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
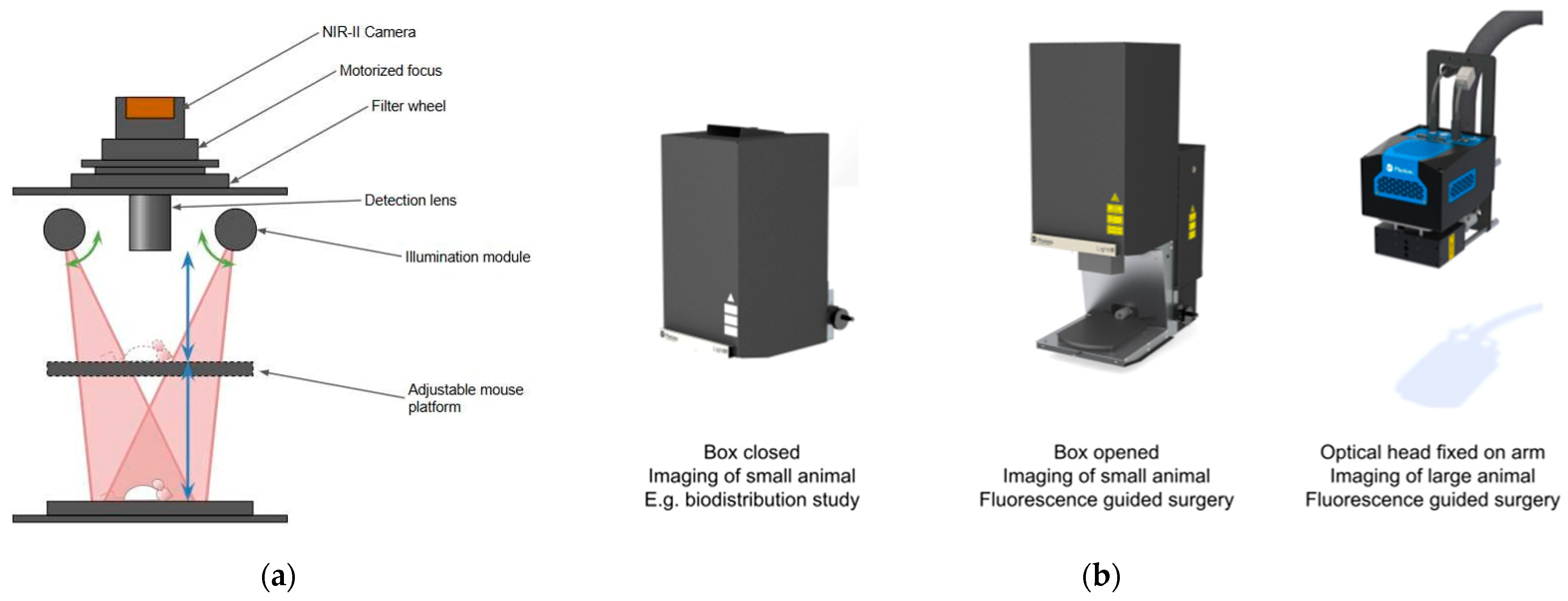
2.1. Imaging Systems
2.1.1. Preclinical Imaging—IR VIVO (Photon, etc.)
2.1.2. Intraoperative Imaging—LightIR (Kaer Labs)
2.2. Phantom Fabrication
2.2.1. QUEL Imaging Phantoms
2.2.2. IR-1048 Depth Phantoms
2.3. Imaging and Analysis Protocols
2.3.1. Sensitivity and Depth Imaging with ICG
2.3.2. Resolution Testing
2.3.3. Depth Imaging and Surgical Guidance with IR-1048
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NIR | Near-Infrared |
| NIR-I | First Near-Infrared Window (700–950 nm) |
| NIR-II | Second Near-Infrared Window (1000–1700 nm) |
| FGS | Fluorescence-Guided Surgery |
| ICG | Indocyanine Green |
| SBR | Signal-to-Background Ratio |
| ROI | Region of Interest |
| LP | Long-Pass |
| InGaAs | Indium Gallium Arsenide |
Appendix A
Appendix A.1

Appendix A.2

Appendix A.3
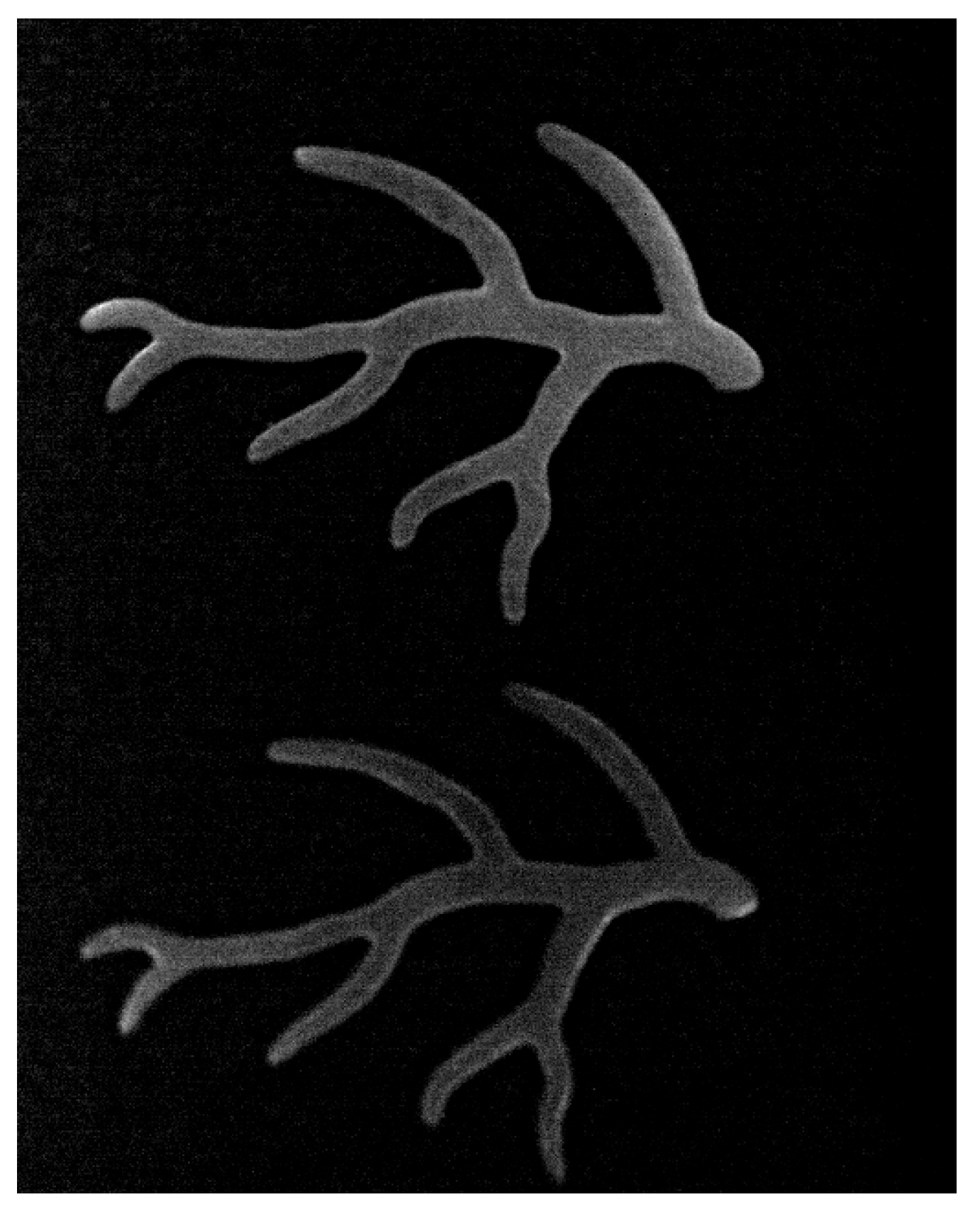
Appendix A.4
| Power Density in mW/mm2 | |||||
|---|---|---|---|---|---|
| Excitation Laser | 760 nm | 808 nm | 890 nm | 940 nm | |
| Stage Position | |||||
| 3 mice | 1.00 | 1.10 | 0.84 | 1.15 | |
| 2 mice | 1.63 | 1.80 | 1.59 | 2.14 | |
| 1 mouse wide | 2.00 | 2.33 | 1.94 | 2.66 | |
| 1 mouse macro | 1.35 | 1.44 | 1.18 | 1.65 | |
| Macro View | 1.93 | 2.26 | 1.94 | 2.64 | |
| Super Macro View | 2.02 | 2.37 | 2.02 | 2.80 | |
References
- Zhang, R.R.; Schroeder, A.B.; Grudzinski, J.J.; Rosenthal, E.L.; Warram, J.M.; Pinchuk, A.N.; Eliceiri, K.W.; Kuo, J.S.; Weichert, J.P. Beyond the margins: Real-time detection of cancer using targeted fluorophores. Nat. Rev. Clin. Oncol. 2017, 14, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, B.; Mieog, J.; Hutteman, M.; Vorst, J.; Kuppen, P.; Löwik, C.; Vahrmeijer, A. The clinical use of indocyanine green as a Near-Infrared fluorescent contrast agent for image-guided oncologic surgery. J. Surg. Oncol. 2011, 104, 323–332. [Google Scholar] [CrossRef]
- Gioux, S.; Choi, H.; Frangioni, J. Image-guided surgery using invisible Near-Infrared light: Fundamentals of clinical translation. Mol. Imaging 2010, 9. [Google Scholar] [CrossRef]
- Chi, C.; Du, Y.; Ye, J.; Kou, D.; Qiu, J.; Wang, J.; Chen, X. Intraoperative imaging-guided cancer surgery: From current fluorescence molecular imaging methods to future multi-modality imaging technology. Theranostics 2014, 4, 1072–1084. [Google Scholar] [CrossRef]
- Lee, J.Y.; Thawani, J.P.; Pierce, J.; Zeh, R.; Martinez-Lage, M.; Chanin, M.; Venegas, O.; Nims, S.; Learned, K.; Keating, J.; et al. Intraoperative Near-Infrared Optical Imaging Can Localize Gadolinium-Enhancing Gliomas During Surgery. Neurosurgery 2016, 79, 856–871. [Google Scholar] [CrossRef]
- Okusanya, O.T.; Holt, D.; Heitjan, D.; Deshpande, C.; Venegas, O.; Jiang, J.; Judy, R.; DeJesus, E.; Madajewski, B.; Oh, K.; et al. Intraoperative near-infrared imaging can identify pulmonary nodules. Ann. Thorac. Surg. 2014, 98, 1223–1230. [Google Scholar] [CrossRef]
- Holt, D.; Okusanya, O.; Judy, R.; Venegas, O.; Jiang, J.; DeJesus, E.; Eruslanov, E.; Quatromoni, J.; Bhojnagarwala, P.; Deshpande, C.; et al. Intraoperative near-infrared imaging can distinguish cancer from normal tissue but not inflammation. PLoS ONE 2014, 9, e103342. [Google Scholar] [CrossRef] [PubMed]
- Madajewski, B.; Judy, B.F.; Mouchli, A.; Kapoor, V.; Holt, D.; Wang, M.D.; Nie, S.; Singhal, S. Intraoperative near-infrared imaging of surgical wounds after tumor resections can detect residual disease. Clin. Cancer Res. 2012, 18, 5741–5751. [Google Scholar] [CrossRef]
- Vahrmeijer, A.L.; Hutteman, M.; van der Vorst, J.R.; van de Velde, C.J.; Frangioni, J.V. Image-guided cancer surgery using near-infrared fluorescence. Nat. Rev. Clin. Oncol. 2013, 10, 507–518. [Google Scholar] [CrossRef] [PubMed]
- van der Vorst, J.R.; Schaafsma, B.E.; Hutteman, M.; Verbeek, F.P.; Liefers, G.J.; Hartgrink, H.H.; Smit, V.T.; Lowik, C.W.; van de Velde, C.J.; Frangioni, J.V.; et al. Near-infrared fluorescence-guided resection of colorectal liver metastases. Cancer 2013, 119, 3411–3418. [Google Scholar] [CrossRef]
- Gao, R.W.; Teraphongphom, N.T.; van den Berg, N.S.; Martin, B.A.; Oberhelman, N.J.; Divi, V.; Kaplan, M.J.; Hong, S.S.; Lu, G.; Ertsey, R.; et al. Determination of Tumor Margins with Surgical Specimen Mapping Using Near-Infrared Fluorescence. Cancer Res. 2018, 78, 5144–5154. [Google Scholar] [CrossRef] [PubMed]
- Lauwerends, L.J.; van Driel, P.; Baatenburg de Jong, R.J.; Hardillo, J.A.U.; Koljenovic, S.; Puppels, G.; Mezzanotte, L.; Lowik, C.; Rosenthal, E.L.; Vahrmeijer, A.L.; et al. Real-time fluorescence imaging in intraoperative decision making for cancer surgery. Lancet Oncol. 2021, 22, e186–e195. [Google Scholar] [CrossRef]
- Wang, L.G.; Barth, C.W.; Kitts, C.H.; Mebrat, M.D.; Montano, A.R.; House, B.J.; McCoy, M.E.; Antaris, A.L.; Galvis, S.N.; McDowall, I.; et al. Near-infrared nerve-binding fluorophores for buried nerve tissue imaging. Sci. Transl. Med. 2020, 12, eaay0712. [Google Scholar] [CrossRef]
- Metildi, C.A.; Kaushal, S.; Luiken, G.A.; Talamini, M.A.; Hoffman, R.M.; Bouvet, M. Fluorescently labeled chimeric anti-CEA antibody improves detection and resection of human colon cancer in a patient-derived orthotopic xenograft (PDOX) nude mouse model. J. Surg. Oncol. 2014, 109, 451–458. [Google Scholar] [CrossRef]
- Bou-Samra, P.; Muhammad, N.; Chang, A.; Karsalia, R.; Azari, F.; Kennedy, G.; Stummer, W.; Tanyi, J.; Martin, L.; Vahrmeijer, A.; et al. Intraoperative molecular imaging: 3rd biennial clinical trials update. J. Biomed. Opt. 2023, 28, 050901. [Google Scholar] [CrossRef]
- Azari, F.; Zhang, K.; Kennedy, G.T.; Chang, A.; Nadeem, B.; Delikatny, E.J.; Singhal, S. Precision Surgery Guided by Intraoperative Molecular Imaging. J. Nucl. Med. 2022, 63, 1620–1627. [Google Scholar] [CrossRef] [PubMed]
- Hart, M.C.; Isuri, R.K.; Ramos, D.; Osharovich, S.A.; Rodriguez, A.E.; Harmsen, S.; Dudek, G.C.; Huck, J.L.; Holt, D.E.; Popov, A.V.; et al. Non-Small Cell Lung Cancer Imaging Using a Phospholipase A2 Activatable Fluorophore. Chem. Biomed. Imaging 2024, 2, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Keereweer, S.; Driel, P.; Snoeks, T.; Kerrebijn, J.; Jong, R.; Vahrmeijer, A.; Löwik, C. Optical image-guided cancer surgery: Challenges and limitations. Clin. Cancer Res. 2013, 19, 3745–3754. [Google Scholar] [CrossRef]
- Mieog, J.; Troyan, S.; Hutteman, M.; Donohoe, K.; Vorst, J.; Stockdale, A.; Vahrmeijer, A. Toward optimization of imaging system and lymphatic tracer for near-infrared fluorescent sentinel lymph node mapping in breast cancer. Ann. Surg. Oncol. 2011, 18, 2483–2491. [Google Scholar] [CrossRef]
- Manen, L.; Handgraaf, H.; Diana, M.; Dijkstra, J.; Ishizawa, T.; Vahrmeijer, A.; Mieog, J. A practical guide for the use of indocyanine green and methylene blue in fluorescence-guided abdominal surgery. J. Surg. Oncol. 2018, 118, 283–300. [Google Scholar] [CrossRef]
- Egloff-Juras, C.; Bezdetnaya, L.; Dolivet, G.; Lassalle, H. NIR fluorescence-guided tumor surgery: New strategies for the use of indocyanine green. Int. J. Nanomed. 2019, 14, 7823–7838. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Lee, J.; Robinson, J.; Raaz, U.; Xie, L.; Huang, N.; Dai, H. Multifunctional in vivo vascular imaging using Near-Infrared II fluorescence. Nat. Med. 2012, 18, 1841–1846. [Google Scholar] [CrossRef]
- Kenry; Duan, Y.; Liu, B. Recent advances of optical imaging in the second Near-Infrared window. Adv. Mater. 2018, 30, 1802394. [Google Scholar] [CrossRef]
- Furtjes, G.; Reinecke, D.; von Spreckelsen, N.; Meissner, A.K.; Ruess, D.; Timmer, M.; Freudiger, C.; Ion-Margineanu, A.; Khalid, F.; Watrinet, K.; et al. Intraoperative microscopic autofluorescence detection and characterization in brain tumors using stimulated Raman histology and two-photon fluorescence. Front. Oncol. 2023, 13, 1146031. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhong, X.; Dennis, A.M. Minimizing near-infrared autofluorescence in preclinical imaging with diet and wavelength selection. J. Biomed. Opt. 2023, 28, 094805. [Google Scholar] [CrossRef]
- Upputuri, P.; Pramanik, M. Recent advances toward preclinical and clinical translation of photoacoustic tomography: A review. J. Biomed. Opt. 2016, 22, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Yung, B.; Chandra, S.; Niu, G.; Antaris, A.; Chen, X. Near-Infrared-II (NIR-II) bioimaging via off-peak NIR-I fluorescence emission. Theranostics 2018, 8, 4141–4151. [Google Scholar] [CrossRef]
- Zhu, S.; Tian, R.; Antaris, A.; Chen, X.; Dai, H. Near-Infrared-II molecular dyes for cancer imaging and surgery. Adv. Mater. 2019, 31, 24. [Google Scholar] [CrossRef]
- Shou, K.; Qu, C.; Sun, Y.; Chen, H.; Chen, S.; Zhang, L.; Cheng, Z. Multifunctional biomedical imaging in physiological and pathological conditions using a NIR-II probe. Adv. Funct. Mater. 2017, 27, 23. [Google Scholar] [CrossRef]
- Harun, A.; Bendele, N.; Khalil, M.; Vasquez, I.; Djuanda, J.; Posey, R.; Srivastava, I. 3d tumor-mimicking phantom models for assessing NIR I/II nanoparticles in fluorescence-guided surgical interventions. bioRxiv 2025. [Google Scholar] [CrossRef]
- Li, D.; Liu, Q.; Qi, Q.; Shi, H.; Hsu, E.; Chen, W.; Cheng, Z. Gold nanoclusters for NIR-II fluorescence imaging of bones. Small 2020, 16, 43. [Google Scholar] [CrossRef]
- Antaris, A.; Chen, H.; Diao, S.; Ma, Z.; Zhang, Z.; Zhu, S.; Cheng, Z. A high quantum yield molecule-protein complex fluorophore for Near-Infrared II imaging. Nat. Commun. 2017, 8, 15269. [Google Scholar] [CrossRef]
- Tian, R.; Ma, H.; Yang, Q.; Wan, H.; Zhu, S.; Chandra, S.; Chen, X. Rational design of a super-contrast NIR-II fluorophore affords high-performance NIR-II molecular imaging guided microsurgery. Chem. Sci. 2019, 10, 326–332. [Google Scholar] [CrossRef]
- Mondal, S.; Gao, S.; Zhu, N.; Liang, R.; Gruev, V.; Achilefu, S. Real-time fluorescence image-guided oncologic surgery. Imaging Surg. 2014, 124, 171–211. [Google Scholar] [CrossRef]
- Xu, D.; Li, L.; Chu, C.; Zhang, X.; Liu, G. Advances and perspectives in Near-Infrared fluorescent organic probes for surgical oncology. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, 5. [Google Scholar] [CrossRef]
- Wang, P.; Fan, Y.; Lu, L.; Liu, L.; Fan, L.; Zhao, M.; Xie, Y.; Xu, C.; Zhang, F. NIR-II Nanoprobes in-vivo Assembly to Improve Image-Guided Surgery for Metastatic Ovarian Cancer. Nat. Commun. 2018, 9, 2898. [Google Scholar] [CrossRef]
- Pu, T.; Liu, Y.; Pei, Y.; Peng, J.; Wang, Z.; Du, M.; Zhang, X. NIR-II fluorescence imaging for the detection and resection of cancerous foci and lymph nodes in early-stage orthotopic and advanced-stage metastatic ovarian cancer models. ACS Appl. Mater. Interfaces 2023, 15, 32226–32239. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Song, J.; Qu, J.; Cheng, Z. Crucial breakthrough of second Near-Infrared biological window fluorophores: Design and synthesis toward multimodal imaging and theranostics. Chem. Soc. Rev. 2018, 47, 4258–4278. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Liu, X.; Zhu, S. Near-Infrared-II Cyanine/Polymethine Dyes, Current State and Perspective. Front. Chem. 2021, 9, 718709. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Lee, J.; Kim, J.; Kim, J.; Wi, J.S.; Park, J.Y.; Lee, T.G.; Choi, B.G.; Lee, K.G.; Kim, Y.H.; et al. Janus Gold Nanodiscs with an Asymmetrically Positioned Polyaniline Nano-Urchin for Photothermal Therapy and Multimodal Imaging in the Second Near-Infrared Window. ACS Appl. Mater. Interfaces 2025, 17, 31799–31809. [Google Scholar] [CrossRef]
- Lee, K.W.; Gao, Y.; Wei, W.C.; Tan, J.H.; Wan, Y.; Feng, Z.; Zhang, Y.; Liu, Y.; Zheng, X.; Cao, C.; et al. Anti-Quenching NIR-II J-Aggregates of Benzo[c]thiophene Fluorophore for Highly Efficient Bioimaging and Phototheranostics. Adv. Mater. 2023, 35, e2211632. [Google Scholar] [CrossRef]
- Yan, T.; Wang, X.; Liu, S.; Fan, D.; Xu, X.; Zeng, Q.; Xie, H.; Yang, X.; Zhu, S.; Ma, X.; et al. Confocal Laser Scanning Microscopy Based on a Silicon Photomultiplier for Multicolor In Vivo Imaging in Near-Infrared Regions I and II. Small Methods 2022, 6, e2201105. [Google Scholar] [CrossRef]
- Suo, Y.; Wu, F.; Xu, P.; Shi, H.; Wang, T.; Liu, H.; Cheng, Z. NIR-II Fluorescence Endoscopy for Targeted Imaging of Colorectal Cancer. Adv. Healthc. Mater. 2019, 8, e1900974. [Google Scholar] [CrossRef]
- Azari, F.; Kennedy, G.; Bernstein, E.; Delikatny, J.; Lee, J.Y.K.; Kucharczuk, J.; Low, P.S.; Singhal, S. Evaluation of OTL38-Generated Tumor-to-Background Ratio in Intraoperative Molecular Imaging-Guided Lung Cancer Resections. Mol. Imaging Biol. 2023, 25, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.W.; Cho, S.S.; Singh, Y.; De Ravin, E.; Somers, K.; Buch, L.; Brem, S.; Singhal, S.; Delikatny, J.; Lee, J.Y.K. Second Window ICG Predicts Gross Total Resection and Progression Free Survival During Brain Metastasis Surgery. Neurosurgery 2020, 67, 312. [Google Scholar] [CrossRef]
- Chen, K.; Teng, X.; Zhou, N.; Cheng, W. Rising sun or strangled in the cradle? A narrative review of near-infrared fluorescence imaging-guided surgery for pancreatic tumors. Int. J. Surg. 2024, 110, 7929–7947. [Google Scholar] [CrossRef]
- Bhavane, R.; Starosolski, Z.; Stupin, I.; Ghaghada, K.B.; Annapragada, A. NIR-II Fluorescence Imaging Using Indocyanine Green Nanoparticles. Sci. Rep. 2018, 8, 14455. [Google Scholar] [CrossRef]
- Valimukhametova, A.R.; Fannon, O.; Topkiran, U.C.; Dorsky, A.; Sottile, O.; Gonzalez-Rodriguez, R.; Coffer, J.; Naumov, A.V. Five near-infrared-emissive graphene quantum dots for multiplex bioimaging. 2D Mater 2024, 11, 025009. [Google Scholar] [CrossRef] [PubMed]
- Vivier, D.; Hautière, M.; Pineau, D.; Dancer, P.A.; Herbet, A.; Hugnot, J.P.; Bernhard, C.; Goncalves, V.; Truillet, C.; Boquet, D.; et al. Synthesis and Preclinical Fluorescence Imaging of Dually Functionalized Antibody Conjugates Targeting Endothelin Receptor-Positive Tumors. Bioconjug. Chem. 2023, 34, 2144–2153. [Google Scholar] [CrossRef]
- Lee, B.; Stokes, G.A.; Valimukhametova, A.; Nguyen, S.; Gonzalez-Rodriguez, R.; Bhaloo, A.; Coffer, J.; Naumov, A.V. Automated Approach to In Vitro Image-Guided Photothermal Therapy with Top-Down and Bottom-Up-Synthesized Graphene Quantum Dots. Nanomaterials 2023, 13, 805. [Google Scholar] [CrossRef]
- Hautiere, M.; Vivier, D.; Dorval, P.; Pineau, D.; Kereselidze, D.; Denis, C.; Herbet, A.; Costa, N.; Bernhard, C.; Goncalves, V.; et al. Preoperative PET imaging and fluorescence-guided surgery of human glioblastoma using dual-labeled antibody targeting ET(A) receptors in a preclinical mouse model: A theranostic approach. Theranostics 2024, 14, 6268–6280. [Google Scholar] [CrossRef] [PubMed]
- Privat, M.; Massot, A.; Hermetet, F.; Al Sabea, H.; Racoeur, C.; Mabrouk, N.; Cordonnier, M.; Moreau, M.; Collin, B.; Bettaieb, A.; et al. Development of an Immuno-SPECT/Fluorescent Bimodal Tracer Targeting Human or Murine PD-L1 on Preclinical Models. J. Med. Chem. 2024, 67, 2188–2201. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.F.; Zhong, Y.T.; Bruns, O.; Liang, Y.Y.; Dai, H.J. In vivo NIR-II fluorescence imaging for biology and medicine. Nat. Photonics 2024, 18, 535–547. [Google Scholar] [CrossRef]
- Lwin, T.; Cox, K.E.; Amirfakhri, S.; Schnermann, M.J.; Yazaki, P.J.; Bouvet, M. Optimization of Fluorophore and Imaging Devices for Fluorescence-Guided Surgery. J. Am. Coll. Surg. 2024, 239, S451. [Google Scholar]
- Bandi, V.G.; Luciano, M.P.; Saccomano, M.; Patel, N.L.; Bischof, T.S.; Lingg, J.G.P.; Tsrunchev, P.T.; Nix, M.N.; Ruehle, B.; Sanders, C.; et al. Targeted multicolor in vivo imaging over 1,000 nm enabled by nonamethine cyanines. Nat. Methods 2022, 19, 353–358. [Google Scholar] [CrossRef] [PubMed]
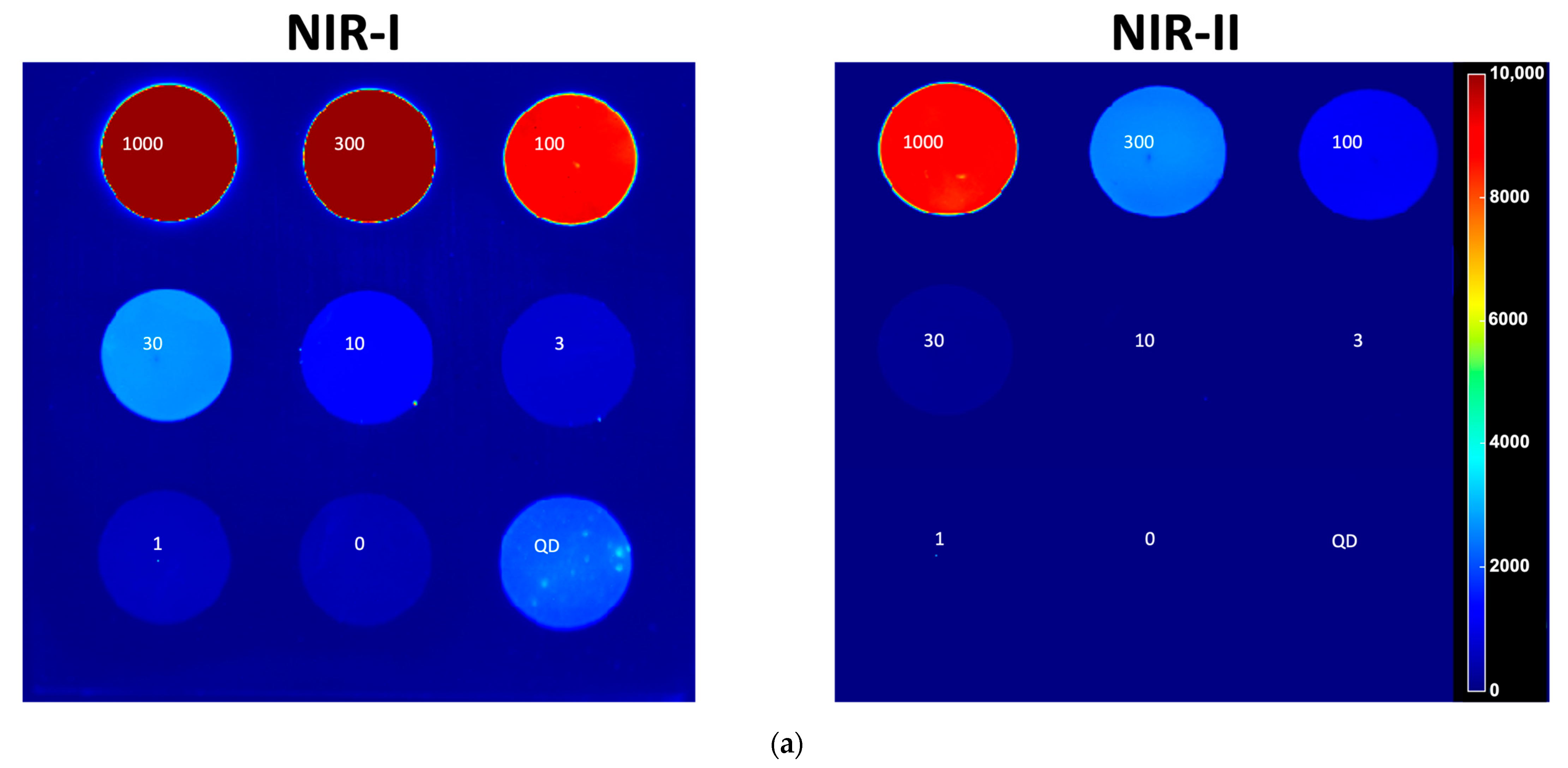
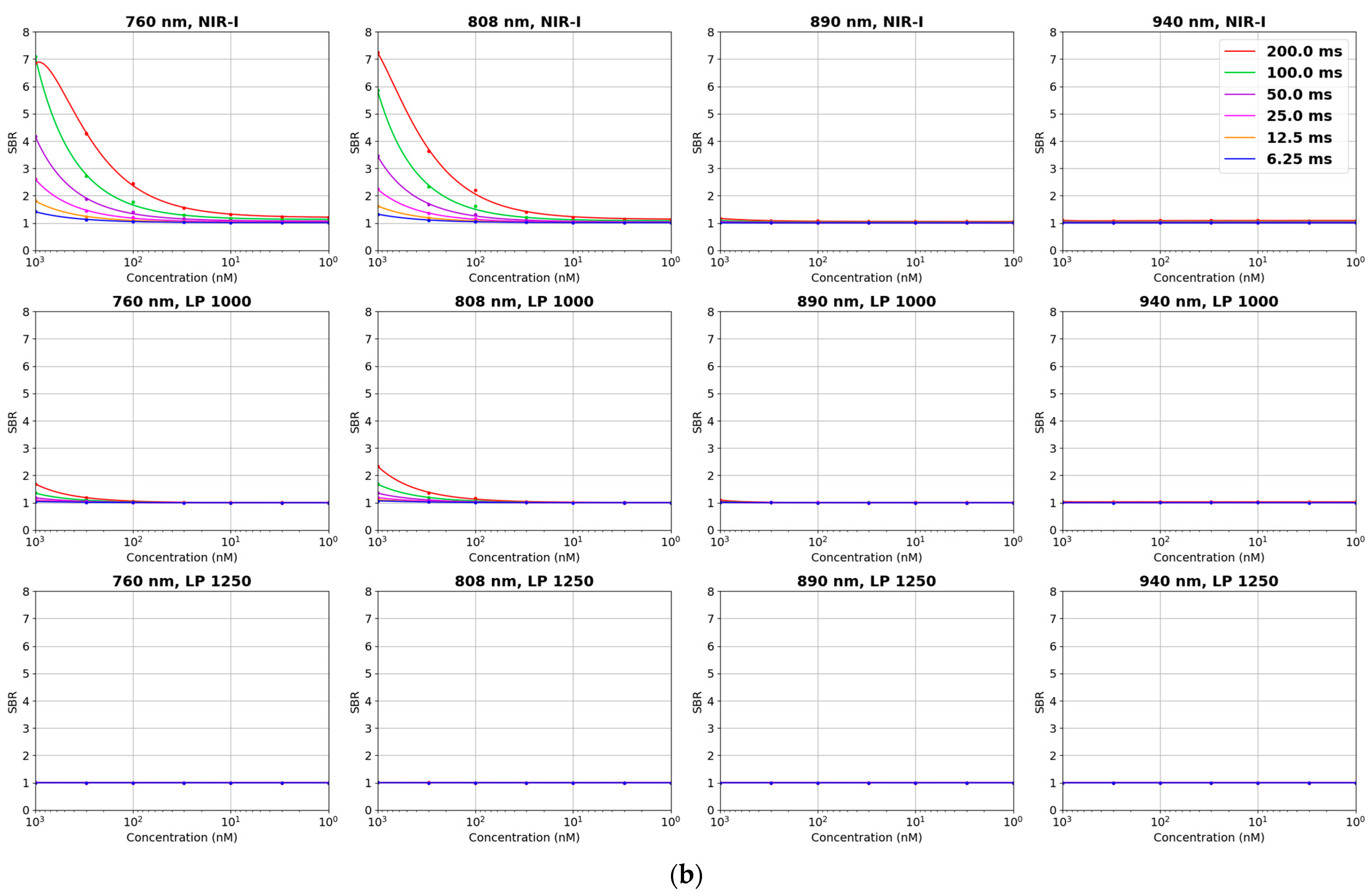
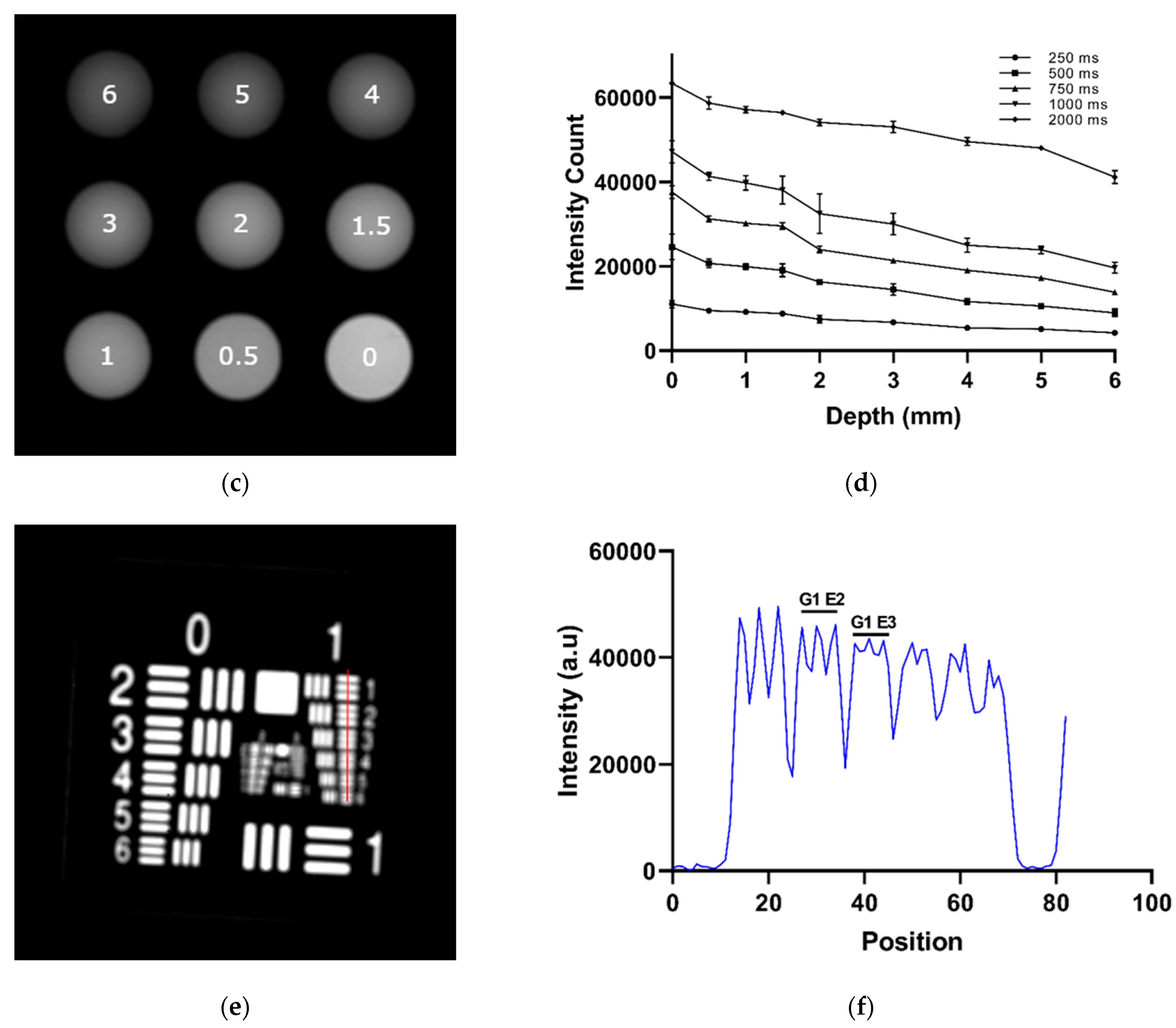
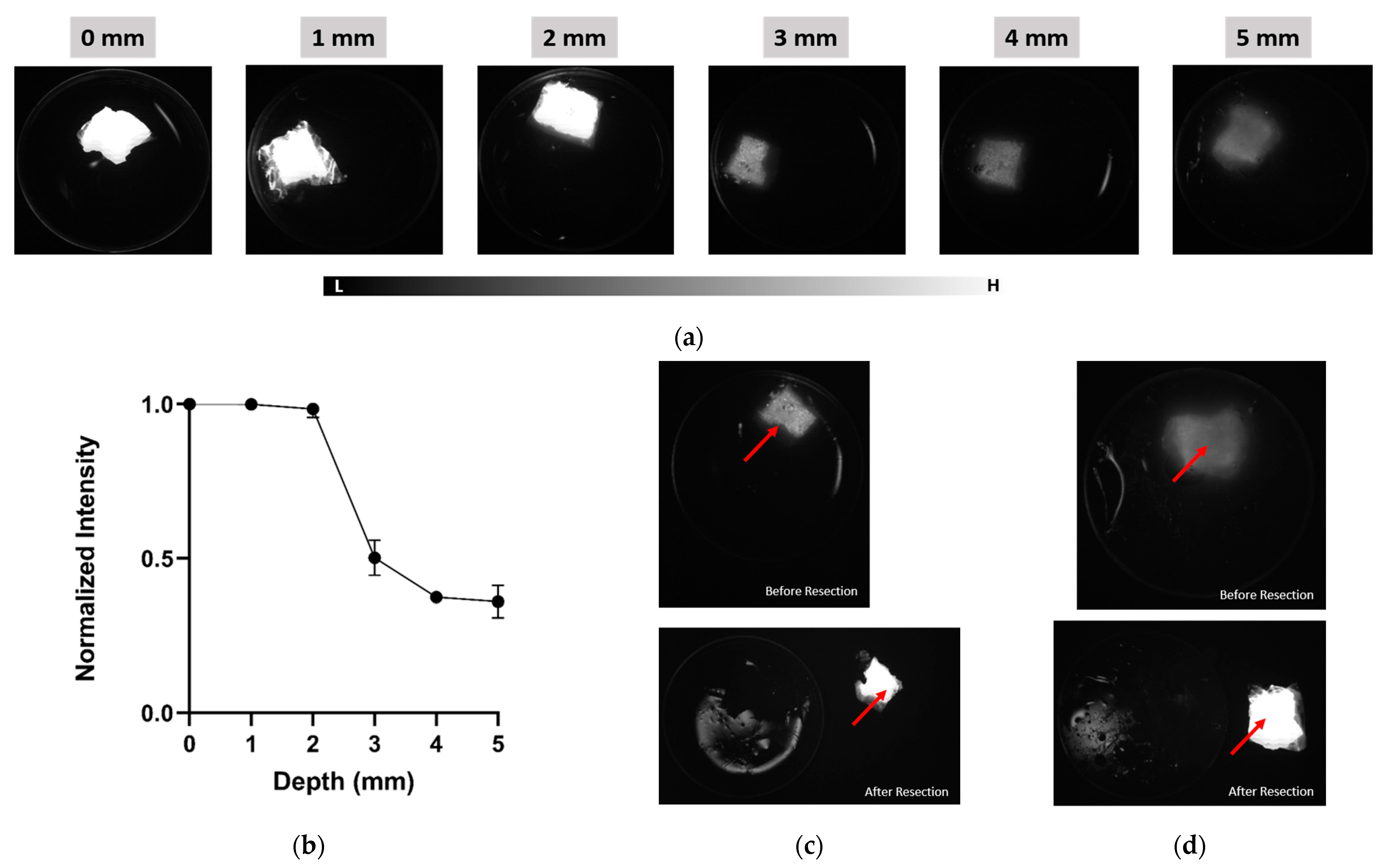
| Parameter | IR VIVO | LightIR 1 |
|---|---|---|
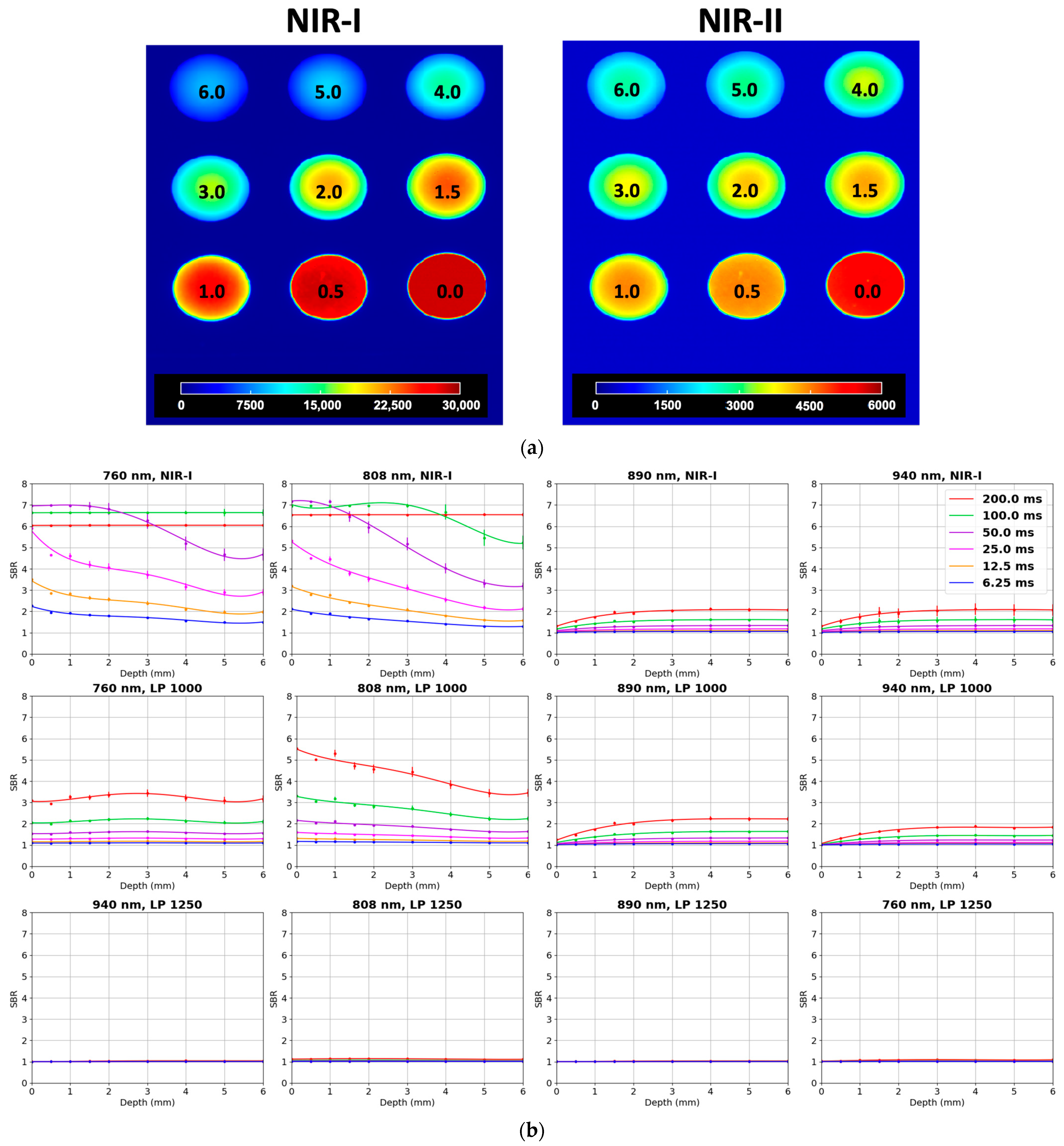
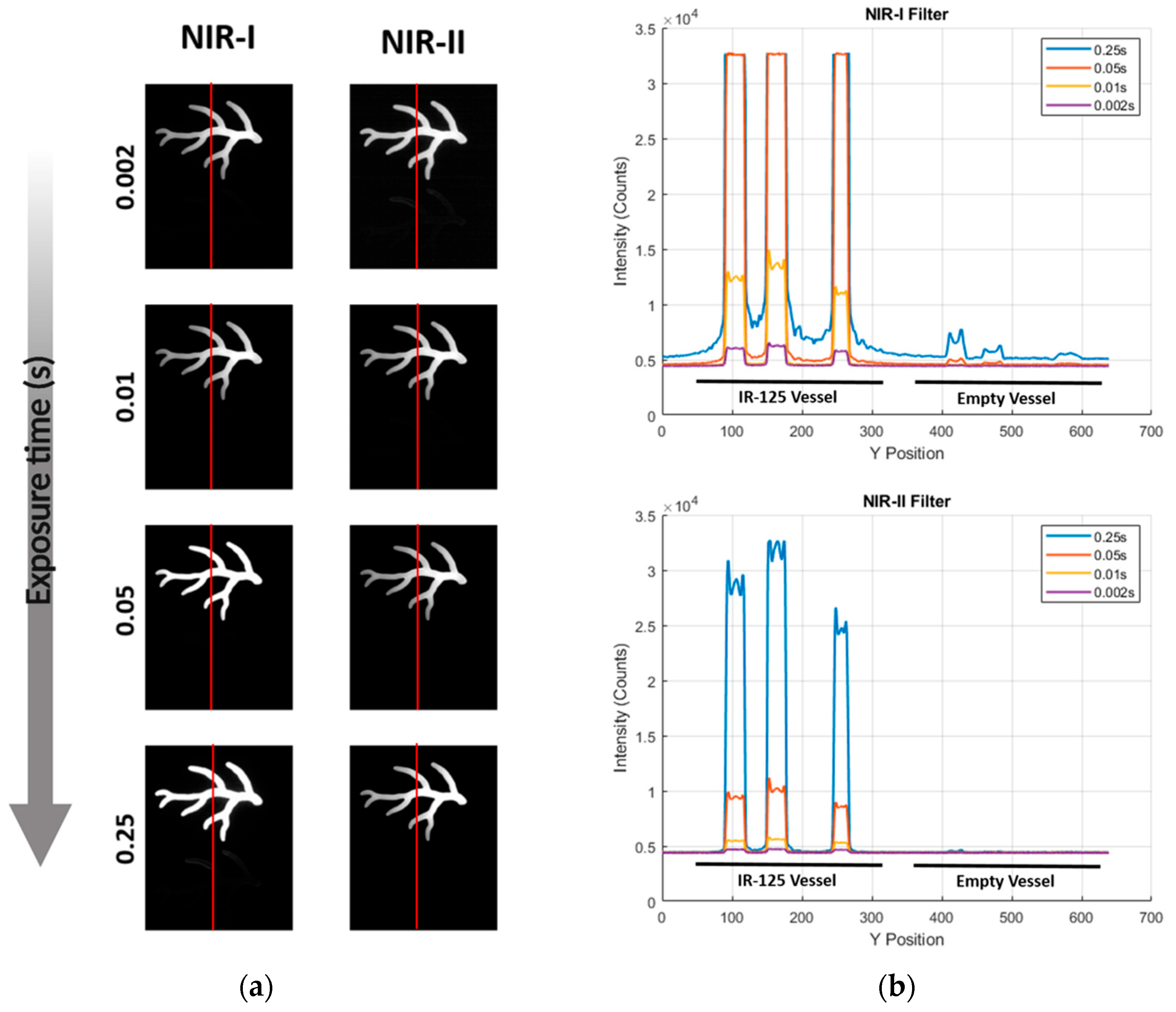
| Depth Sensitivity | 808 nm LP1000 (NIR-II) maintained SBRs of 2.0–2.5 from 0 to 6 mm with sharper boundaries, while 808 nm NIR-I showed higher initial SBR (6.5 at 0 mm) but dropped below 2.0 beyond 4 mm with increased blurring (Exposure time: 200 ms) | Detectable ICG signal to 4 mm or more with LP1050 at 808 nm excitation (Exposure time: 750 ms) |
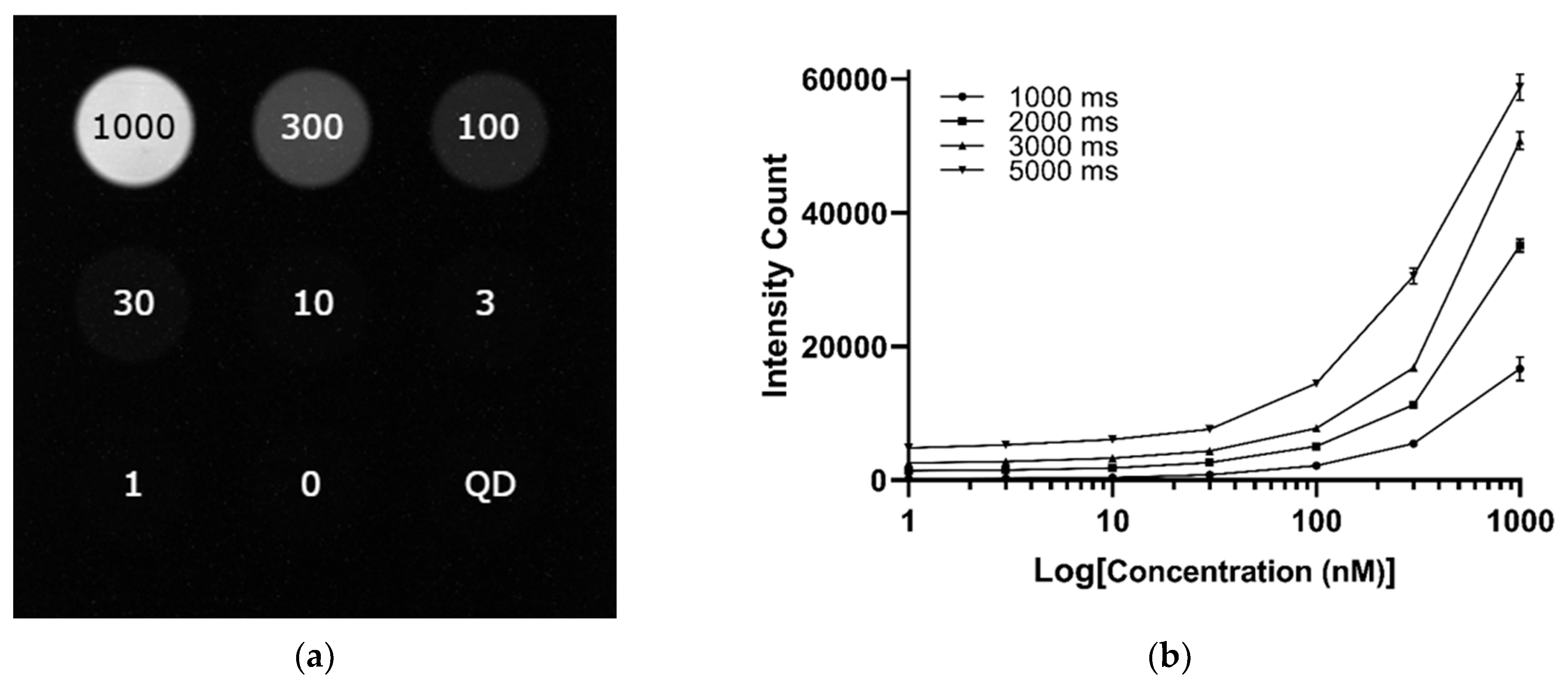
| Concentration Sensitivity | Detectable ICG down to approximately 30 nM in NIR-I; limit of detection in NIR-II approximately 300 nM (Exposure time: 200 ms) | Detectable ICG down to 100 nM in NIR-II (Exposure time: 1000 ms) |
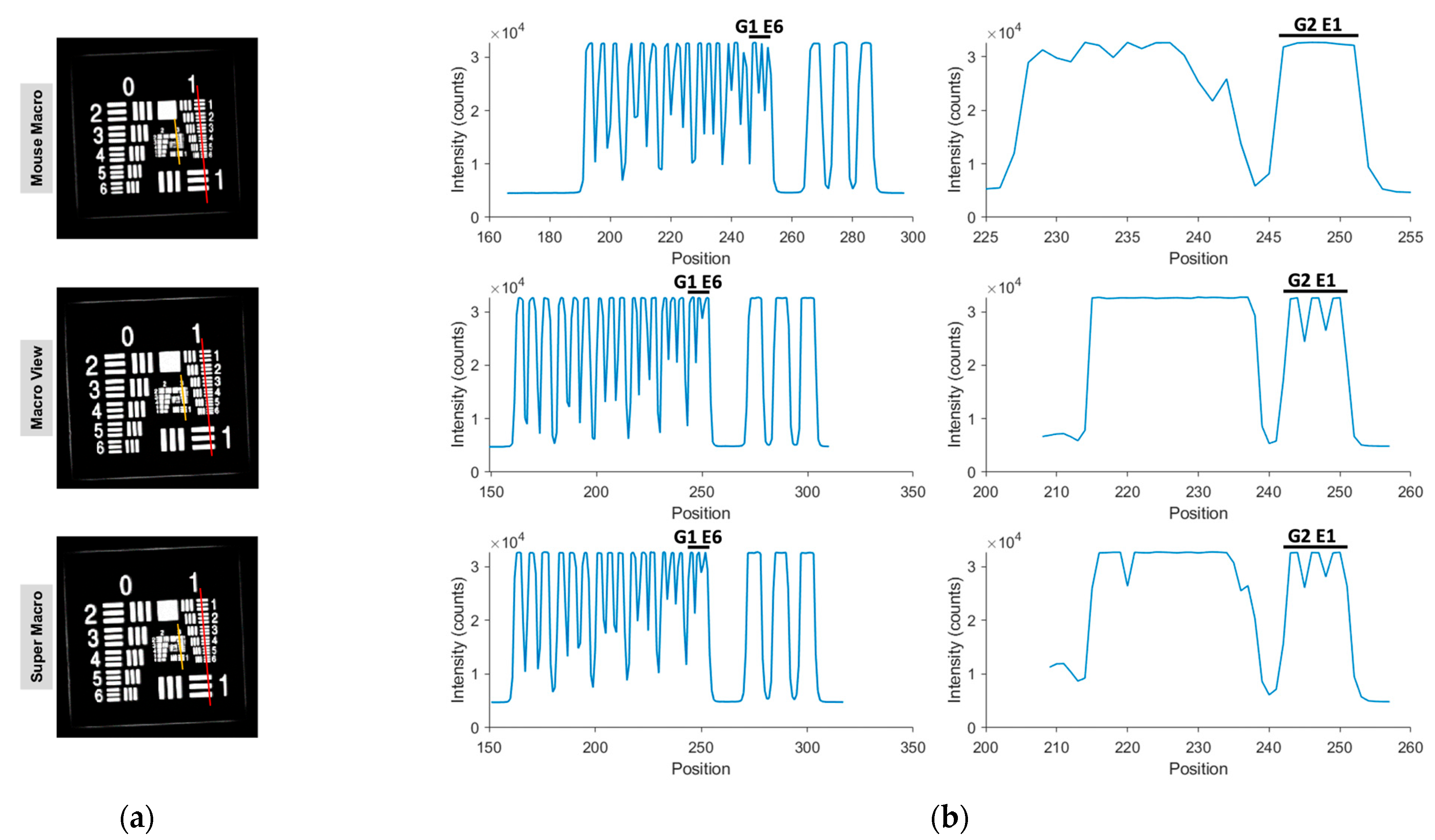
| Spatial Resolution | Resolved Group 2, Element 1 (4.00 lp/mm; approximately 125 μm) with Macro/Super Macro lens (Exposure time: 50 ms) | Resolved Group 1, Element 2 (1.78 lp/mm; approximately 281 μm); partial resolution of Element 3 (approximately 250 μm) (Exposure time: 850 ms) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isuri, R.K.; Williams, J.; Rioux, D.; Dorval, P.; Chung, W.; Dancer, P.-A.; Delikatny, E.J. Clinical Integration of NIR-II Fluorescence Imaging for Cancer Surgery: A Translational Evaluation of Preclinical and Intraoperative Systems. Cancers 2025, 17, 2676. https://doi.org/10.3390/cancers17162676
Isuri RK, Williams J, Rioux D, Dorval P, Chung W, Dancer P-A, Delikatny EJ. Clinical Integration of NIR-II Fluorescence Imaging for Cancer Surgery: A Translational Evaluation of Preclinical and Intraoperative Systems. Cancers. 2025; 17(16):2676. https://doi.org/10.3390/cancers17162676
Chicago/Turabian StyleIsuri, Ritesh K., Justin Williams, David Rioux, Paul Dorval, Wendy Chung, Pierre-Alix Dancer, and Edward J. Delikatny. 2025. "Clinical Integration of NIR-II Fluorescence Imaging for Cancer Surgery: A Translational Evaluation of Preclinical and Intraoperative Systems" Cancers 17, no. 16: 2676. https://doi.org/10.3390/cancers17162676
APA StyleIsuri, R. K., Williams, J., Rioux, D., Dorval, P., Chung, W., Dancer, P.-A., & Delikatny, E. J. (2025). Clinical Integration of NIR-II Fluorescence Imaging for Cancer Surgery: A Translational Evaluation of Preclinical and Intraoperative Systems. Cancers, 17(16), 2676. https://doi.org/10.3390/cancers17162676







