High-Resolution Quantitative Reconstruction of Microvascular Architectures in Mouse Hepatocellular Carcinoma Models
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
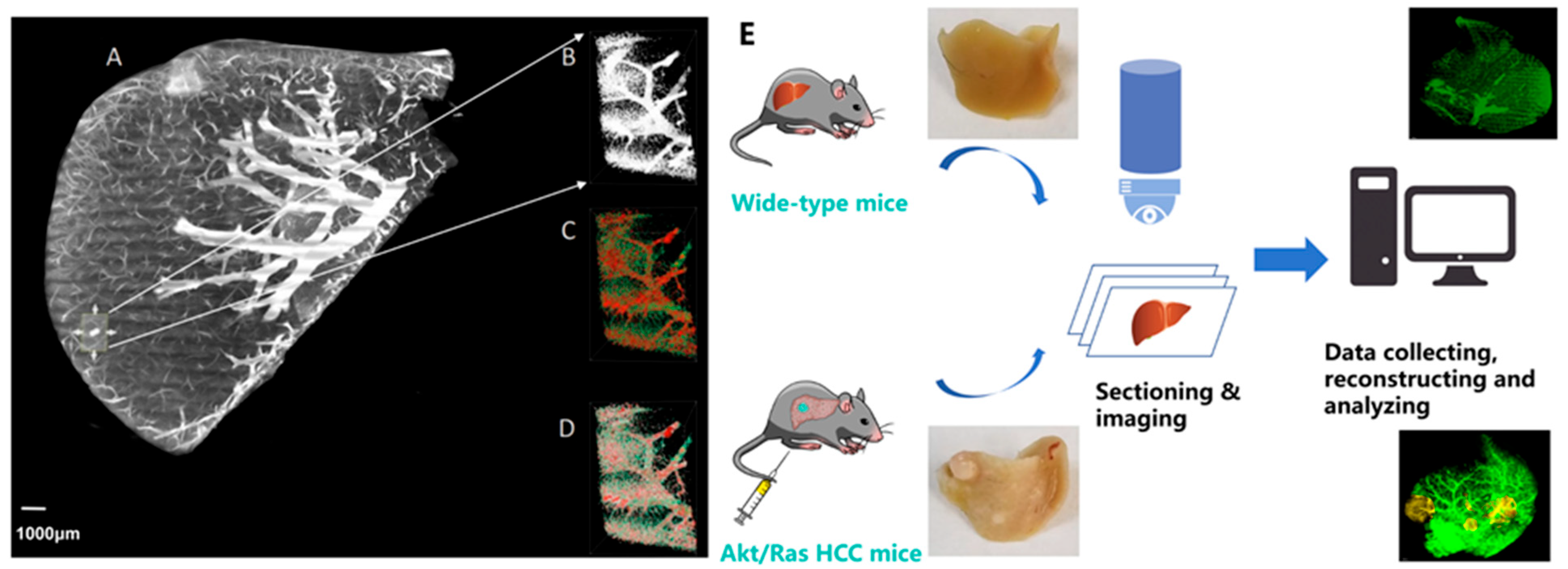
2.1. Animal Sample Preparation
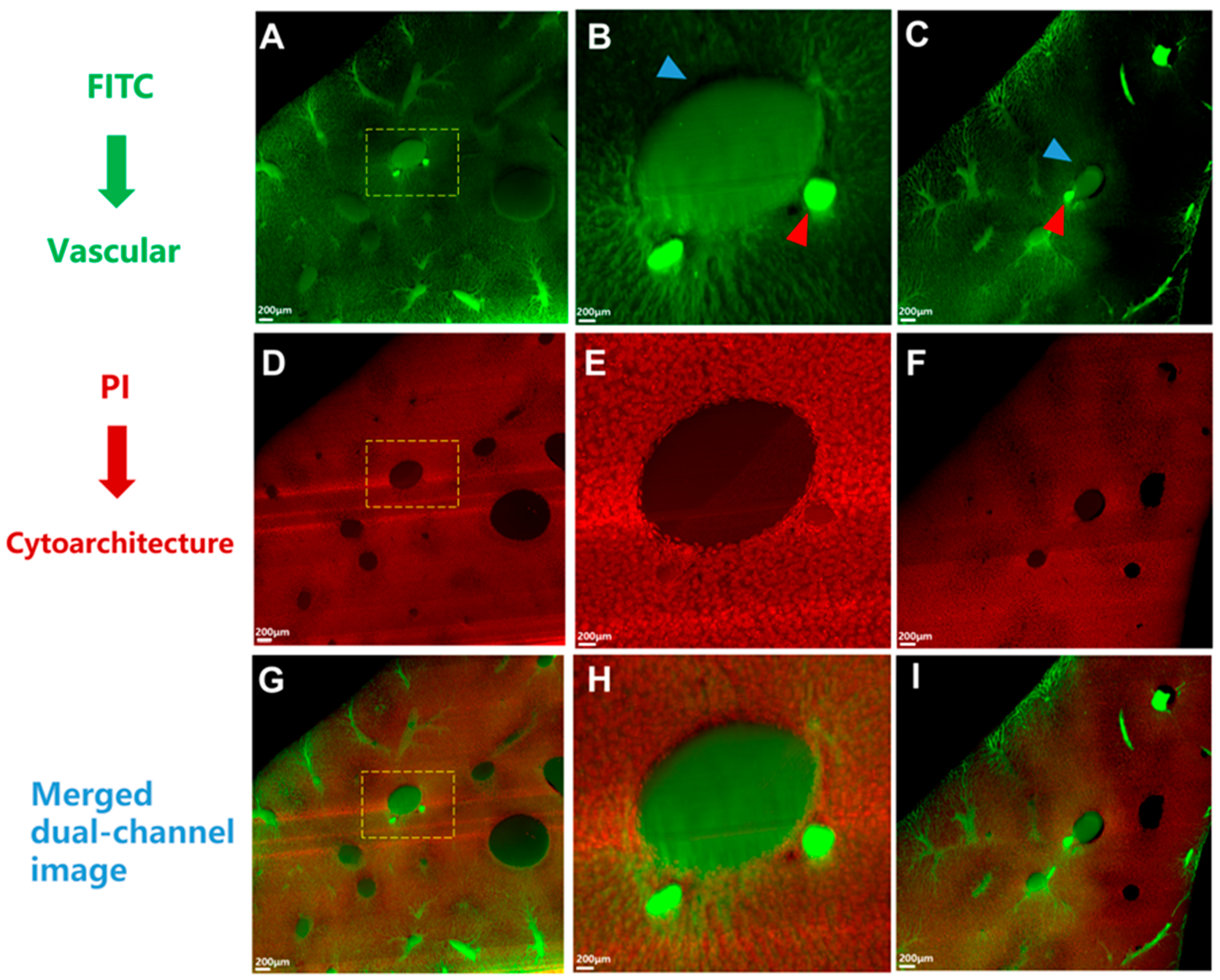
2.2. Imaging
2.3. Image Preprocessing and Vessel Reconstruction
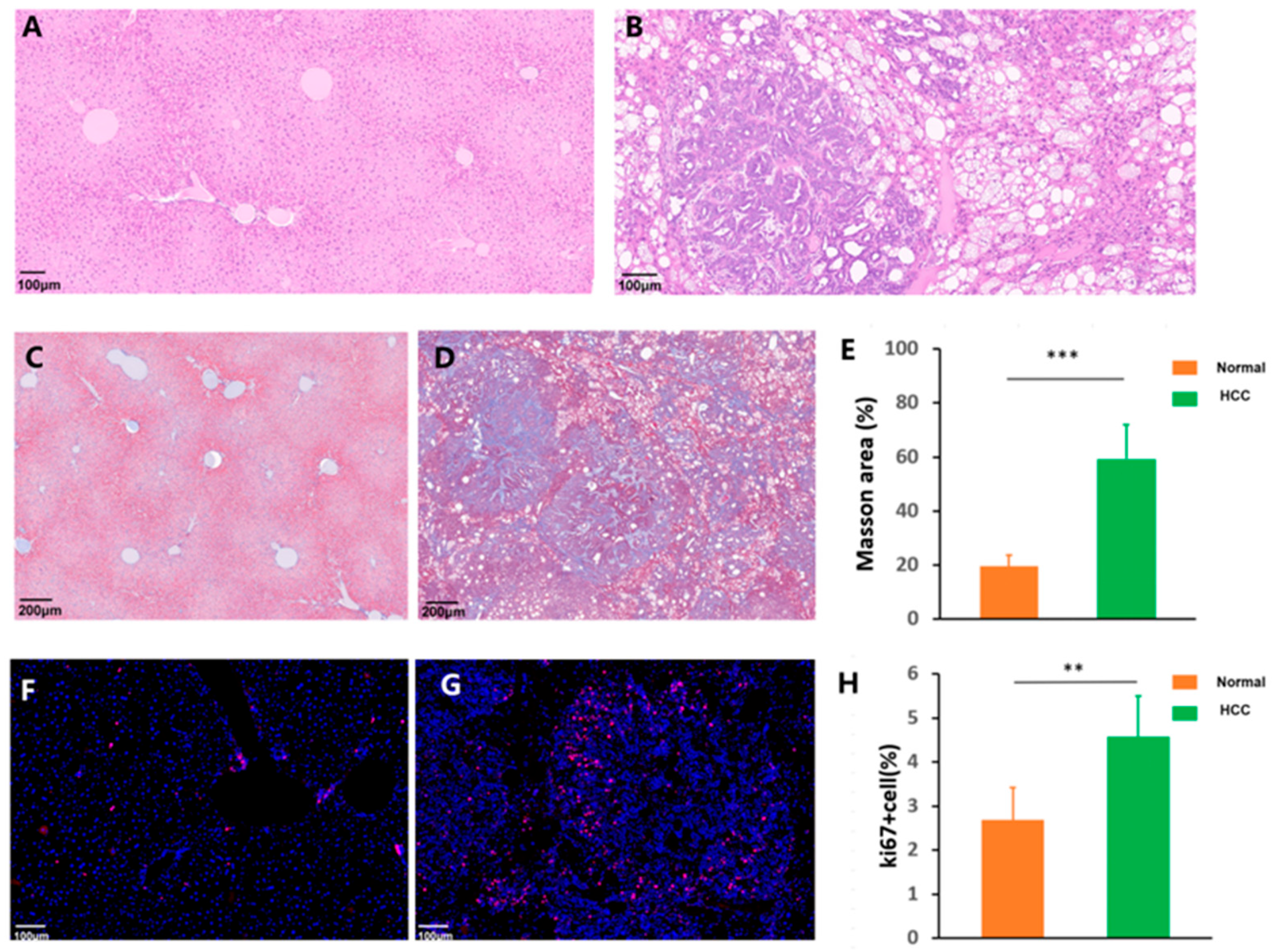
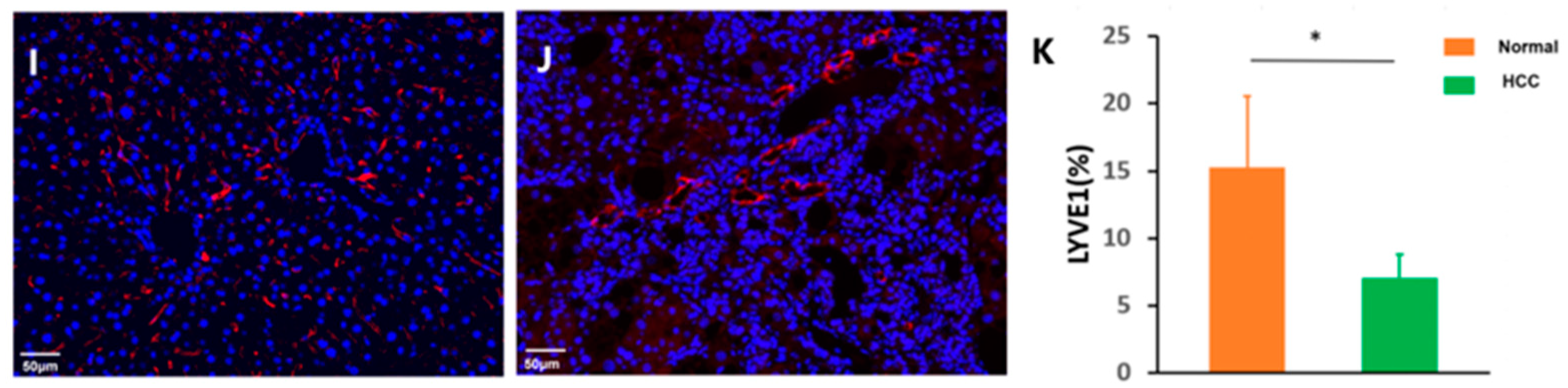
2.4. Histology and Immunohistochemistry
2.5. Statistical Data Analysis
3. Results
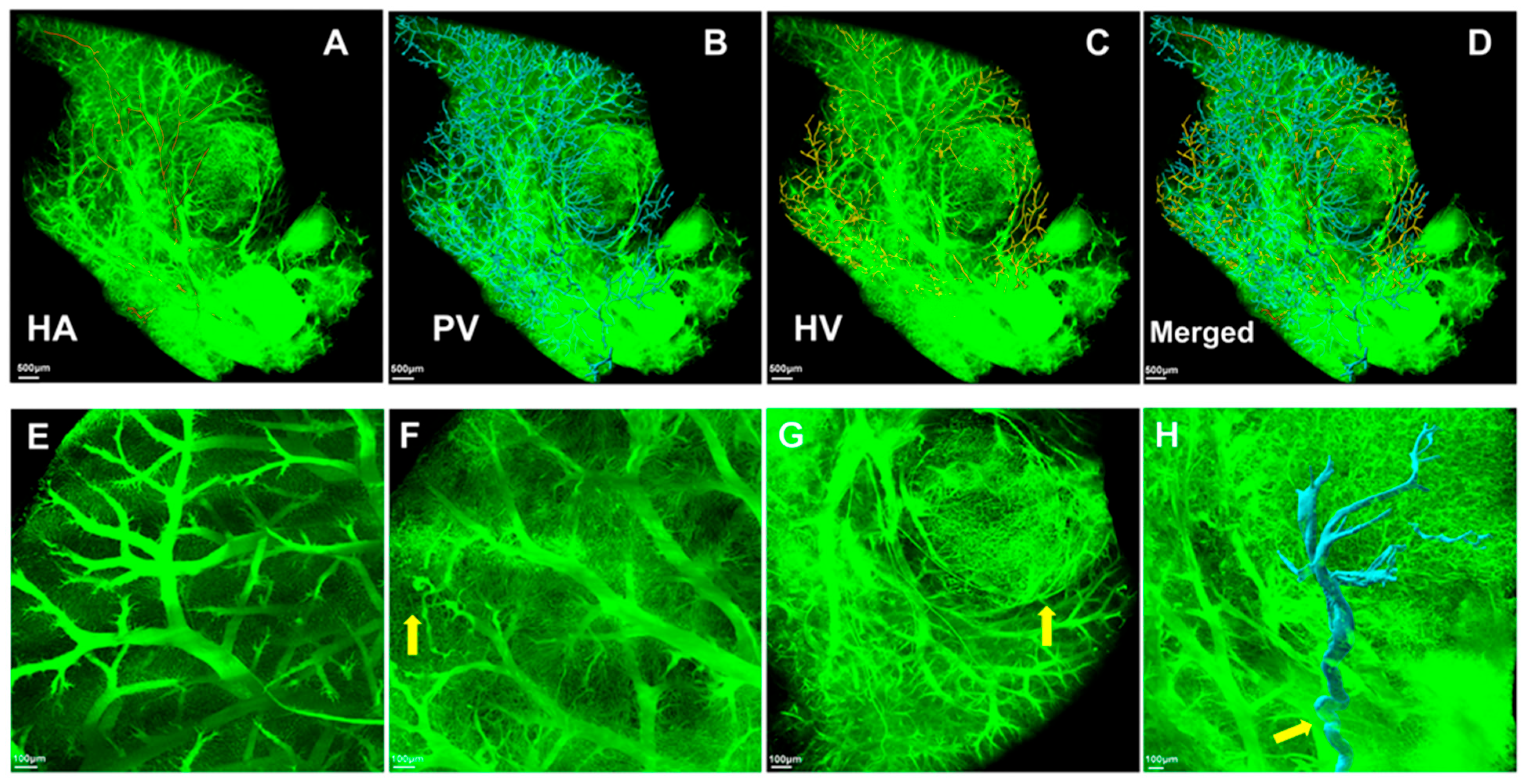
3.1. Validation of the Gelatin-FTIC Perfusion Method for Liver Vasculature Acquisition
3.2. Histopathology Analysis
3.3. Three-Dimensional Morphological Features of the Microvascular Systems
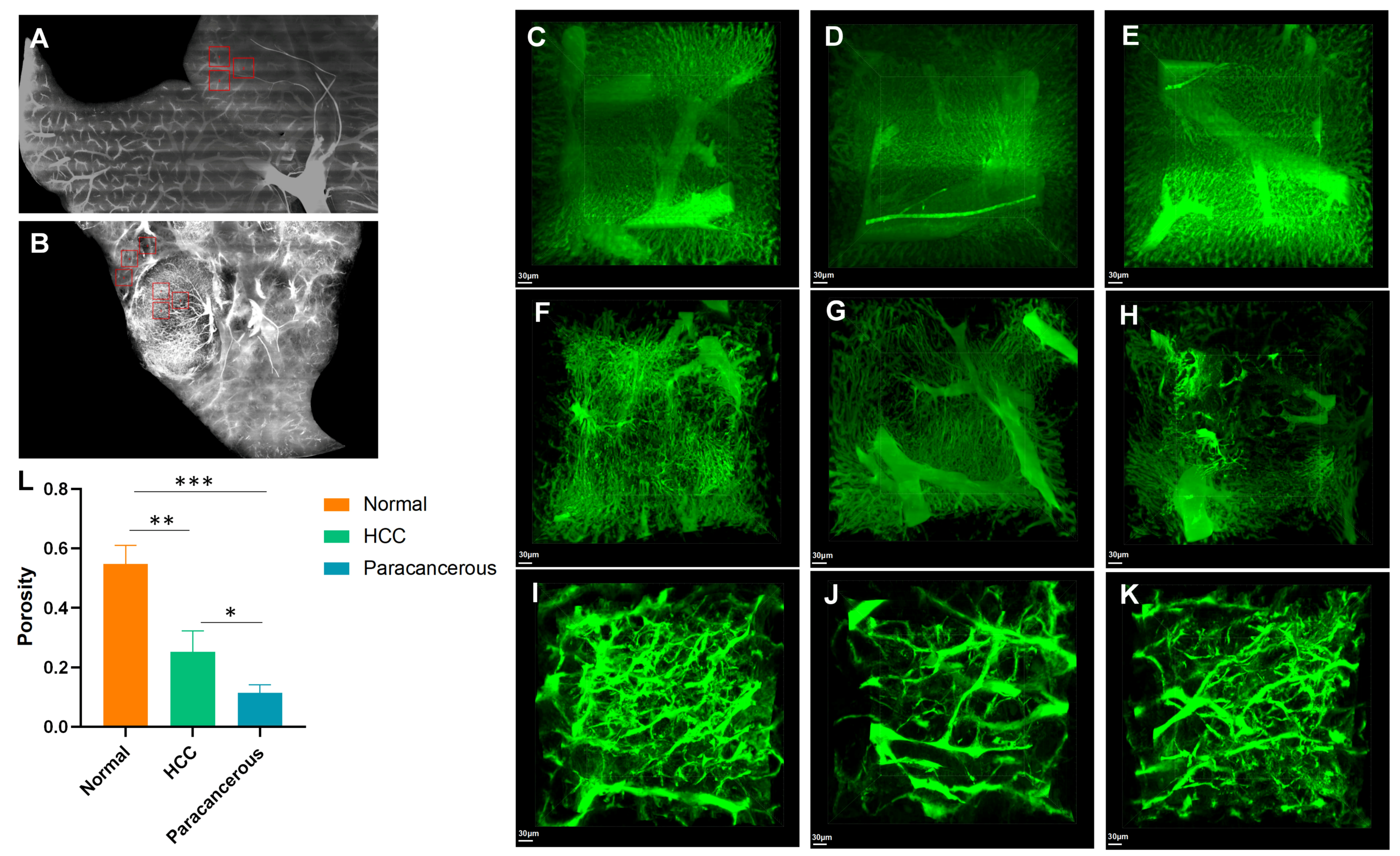
3.4. Cirrhosis and Tumors Specifically Affected the Sinusoidal Network Zone
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CT | computed tomography |
| FITC | fluorescein isothiocyanate |
| H&E | hematoxylin and eosin |
| HA | hepatic artery |
| HCC | hepatocellular carcinoma |
| HD-fMOST | high-definition fluorescence micro-optical sectioning tomography |
| HV | hepatic vein |
| LYVE1 | lymphatic endothelial receptor-1 |
| PBS | phosphate-buffered saline |
| PI | propidium iodide |
| PV | portal vein |
| TACE | transarterial chemoembolization |
| WT | wild-type |
References
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global burden of liver disease: 2023 update. J. Hepatol. 2023, 79, 516–537. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA A Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Vogel, A.; Chan, S.L.; Dawson, L.A.; Kelley, R.K.; Llovet, J.M.; Meyer, T.; Ricke, J.; Rimassa, L.; Sapisochin, G.; Vilgrain, V.; et al. Hepatocellular carcinoma: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2025, 36, 491–506. [Google Scholar] [CrossRef]
- Morse, M.A.; Sun, W.; Kim, R.; He, A.R.; Abada, P.B.; Mynderse, M.; Finn, R.S. The Role of Angiogenesis in Hepatocellular Carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 912–920. [Google Scholar] [CrossRef]
- Lei, L.; Ei Mourabit, H.; Housset, C.; Cadoret, A.; Lemoinne, S. Role of Angiogenesis in the Pathogenesis of NAFLD. J. Clin. Med. 2021, 10, 1338. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Ueshima, K.; Ikeda, M.; Torimura, T.; Tanabe, N.; Aikata, H.; Izumi, N.; Yamasaki, T.; Nojiri, S.; Hino, K.; et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut 2020, 69, 1492–1501. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Kudo, M.; Bruix, J. Breakthroughs in Hepatocellular Carcinoma Therapies. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2023, 21, 2135–2149. [Google Scholar] [CrossRef]
- Peeters, G.; Debbaut, C.; Friebel, A.; Cornillie, P.; De Vos, W.H.; Favere, K.; Vander Elst, I.; Vandecasteele, T.; Johann, T.; Van Hoorebeke, L.; et al. Quantitative analysis of hepatic macro- and microvascular alterations during cirrhogenesis in the rat. J. Anat. 2018, 232, 485–496. [Google Scholar] [CrossRef]
- Xie, C.; Schwen, L.O.; Wei, W.; Schenk, A.; Zafarnia, S.; Gremse, F.; Dahmen, U. Quantification of Hepatic Vascular and Parenchymal Regeneration in Mice. PLoS ONE 2016, 11, e0160581. [Google Scholar] [CrossRef]
- Cai, G.; Lu, Y.; Chen, J.; Yang, D.; Yan, R.; Ren, M.; He, S.; Wu, S.; Zhao, Y. Brain-wide mapping of c-Fos expression with fluorescence micro-optical sectioning tomography in a chronic sleep deprivation mouse model. Neurobiol. Stress 2022, 20, 100478. [Google Scholar] [CrossRef]
- Cao, Z.; Zhao, Y.; Sun, H.; Sun, X.; Zhang, Y.; Zhang, S.; Wang, C.; Xiong, T.; Naeem, A.; Zhang, J.; et al. Cross-scale tracing of nanoparticles and tumors at the single-cell level using the whole-lung atlas. Sci. Adv. 2023, 9, eadh7779. [Google Scholar] [CrossRef]
- Li, A.; Gong, H.; Zhang, B.; Wang, Q.; Yan, C.; Wu, J.; Liu, Q.; Zeng, S.; Luo, Q. Micro-optical sectioning tomography to obtain a high-resolution atlas of the mouse brain. Science 2010, 330, 1404–1408. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, X.; Ren, X.; Sun, H.; Wu, L.; Wang, C.; Ye, X.; York, P.; Gao, Z.; Jiang, H.; et al. Multiscale Co-reconstruction of Lung Architectures and Inhalable Materials Spatial Distribution. Adv. Sci. 2021, 8, 2003941. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, X.; Zhang, J.; Li, A.; Gong, H.; Luo, Q.; Zhang, H.; Gao, Z.; Jiang, H. High-resolution mapping of brain vasculature and its impairment in the hippocampus of Alzheimer’s disease mice. Nat. Sci. Rev. 2019, 6, 1223–1238. [Google Scholar] [CrossRef]
- Tang, Q.; Fan, B.; Cai, X.; Shen, Z.; Zhang, J.; Hu, J.; Li, J.; Zhu, Y. High-Resolution Single-Neuron Reconstruction Analysis in Golgi-Stained Brain Tissues. Cell Prolif. 2025, e70092. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, A.; Chen, S.; Yuan, J.; Jiang, T.; Li, X.; Luo, Q.; Feng, Z.; Gong, H. Multiscale reconstruction of various vessels in the intact murine liver lobe. Commun. Biol. 2022, 5, 260. [Google Scholar] [CrossRef]
- Yu, A.; Yu, P.; Zhu, Y.; Zhu, R.; Sun, R.; Ye, D.; Yu, F.X. Glucose-induced and ChREBP: MLX-mediated lipogenic program promotes hepatocellular carcinoma development. Oncogene 2023, 42, 3182–3193. [Google Scholar] [CrossRef]
- Liu, N.; Chang, C.W.; Steer, C.J.; Wang, X.W.; Song, G. MicroRNA-15a/16-1 Prevents Hepatocellular Carcinoma by Disrupting the Communication Between Kupffer Cells and Regulatory T Cells. Gastroenterology 2022, 162, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Yang, Y.; Lai, S.; Jeong, J.; Jung, Y.; McConnell, M.; Utsumi, T.; Iwakiri, Y. Single-Cell Transcriptomics Reveals Zone-Specific Alterations of Liver Sinusoidal Endothelial Cells in Cirrhosis. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 1139–1161. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on the management of hepatocellular carcinoma. J. Hepatol. 2025, 82, 315–374. [Google Scholar] [CrossRef] [PubMed]
- Ke, K.; Pillai, K.; Mekkawy, A.H.; Akhter, J.; Badar, S.; Valle, S.J.; Morris, D.L. Physical and chemical factors affecting the loading and release of bromelain from DC beads. Am. J. Transl. Res. 2022, 14, 7135–7146. [Google Scholar] [PubMed]
- Mikhailov, O.V. Gelatin as It Is: History and Modernity. Int. J. Mol. Sci. 2023, 24, 3583. [Google Scholar] [CrossRef]
- Liang, X.; Liu, H.; Chen, H.; Peng, X.; Li, Z.; Teng, M.; Peng, Y.; Li, J.; Ding, L.; Mao, J.; et al. Rhein-based Pickering emulsion for hepatocellular carcinoma: Shaping the metabolic signaling and immunoactivation in transarterial chemoembolization. Aggregate 2024, 5, 20. [Google Scholar] [CrossRef]
- Gou, L.; Wang, Y.; Gao, L.; Liu, S.; Wang, M.; Chai, Q.; Fang, J.; Zhan, L.; Shen, X.; Jiang, T.; et al. Single-neuron projectomes of macaque prefrontal cortex reveal refined axon targeting and arborization. Cell 2025, 188, 3806–3822.e3824. [Google Scholar] [CrossRef]
- Zhang, Y.; Xing, X.; Long, B.; Cao, Y.; Hu, S.; Li, X.; Yu, Y.; Tian, D.; Sui, B.; Luo, Z.; et al. A spatial and cellular distribution of rabies virus infection in the mouse brain revealed by fMOST and single-cell RNA sequencing. Clin. Transl. Med. 2022, 12, e700. [Google Scholar] [CrossRef]
- Markel, M.; Ginzel, M.; Peukert, N.; Schneider, H.; Haak, R.; Mayer, S.; Suttkus, A.; Lacher, M.; Kluth, D.; Gosemann, J.H. High resolution three-dimensional imaging and measurement of lung, heart, liver, and diaphragmatic development in the fetal rat based on micro-computed tomography (micro-CT). J. Anat. 2020, 238, 1042–1054. [Google Scholar] [CrossRef] [PubMed]
- Zaw Thin, M.; Moore, C.; Snoeks, T.; Kalber, T.; Downward, J.; Behrens, A. Micro-CT acquisition and image processing to track and characterize pulmonary nodules in mice. Nat. Protoc. 2023, 18, 990–1015. [Google Scholar] [CrossRef]
- Debbaut, C.; Segers, P.; Cornillie, P.; Casteleyn, C.; Dierick, M.; Laleman, W.; Monbaliu, D. Analyzing the human liver vascular architecture by combining vascular corrosion casting and micro-CT scanning: A feasibility study. J. Anat. 2014, 224, 509–517. [Google Scholar] [CrossRef]
- Peeters, G.; Debbaut, C.; Laleman, W.; Monbaliu, D.; Vander Elst, I.; Detrez, J.R.; Vandecasteele, T.; De Schryver, T.; Van Hoorebeke, L.; Favere, K.; et al. A multilevel framework to reconstruct anatomical 3D models of the hepatic vasculature in rat livers. J. Anat. 2017, 230, 471–483. [Google Scholar] [CrossRef]
- Madani, S.P.; Mirza-Aghazadeh-Attari, M.; Mohseni, A.; Pawlik, T.; Kamel, I.R. Diffuse infiltrative hepatocellular carcinoma: Multimodality imaging manifestations. J. Surg. Oncol. 2023, 127, 385–393. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Zhao, H.; Wang, X.; Dai, W.; Ren, X.; Wang, J.; Cai, G. High-Resolution Quantitative Reconstruction of Microvascular Architectures in Mouse Hepatocellular Carcinoma Models. Cancers 2025, 17, 2653. https://doi.org/10.3390/cancers17162653
Zhao Y, Zhao H, Wang X, Dai W, Ren X, Wang J, Cai G. High-Resolution Quantitative Reconstruction of Microvascular Architectures in Mouse Hepatocellular Carcinoma Models. Cancers. 2025; 17(16):2653. https://doi.org/10.3390/cancers17162653
Chicago/Turabian StyleZhao, Yan, Haogang Zhao, Xin Wang, Wei Dai, Xuhua Ren, Jing Wang, and Guohong Cai. 2025. "High-Resolution Quantitative Reconstruction of Microvascular Architectures in Mouse Hepatocellular Carcinoma Models" Cancers 17, no. 16: 2653. https://doi.org/10.3390/cancers17162653
APA StyleZhao, Y., Zhao, H., Wang, X., Dai, W., Ren, X., Wang, J., & Cai, G. (2025). High-Resolution Quantitative Reconstruction of Microvascular Architectures in Mouse Hepatocellular Carcinoma Models. Cancers, 17(16), 2653. https://doi.org/10.3390/cancers17162653




