Lung Cancer Under Siege in Spain: Timeliness, Treatment, and Survival Before and After the COVID-19 Pandemic
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion and Exclusion Criteria
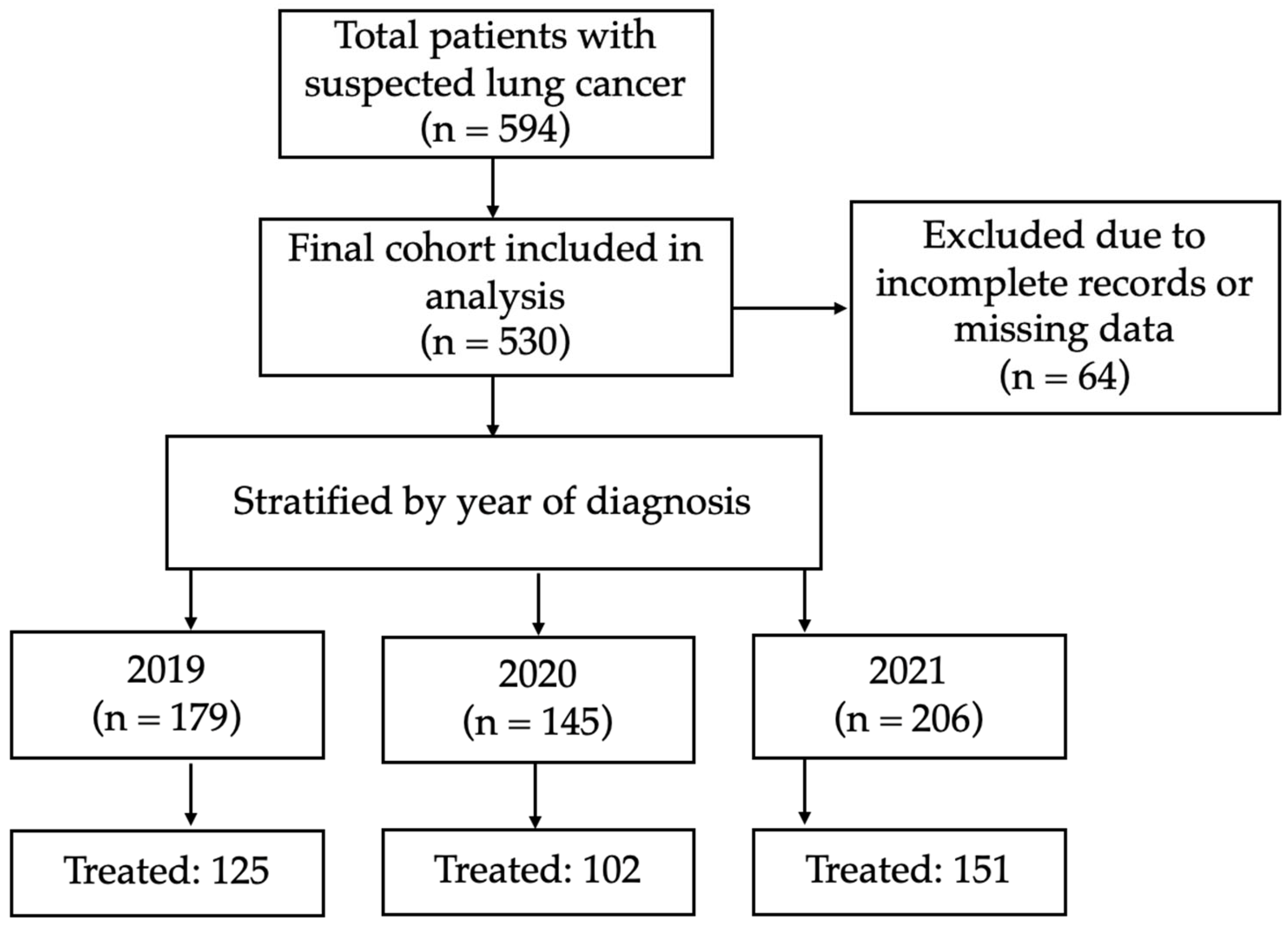
2.3. Study Population
2.4. Data Collection and Assessment of Lung Cancer Care Timelines
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Lung Cancer Diagnoses and Diagnostic Efficiency
3.3. Treatment Rates and Modalities
3.4. Timeliness of Diagnoses and Treatment
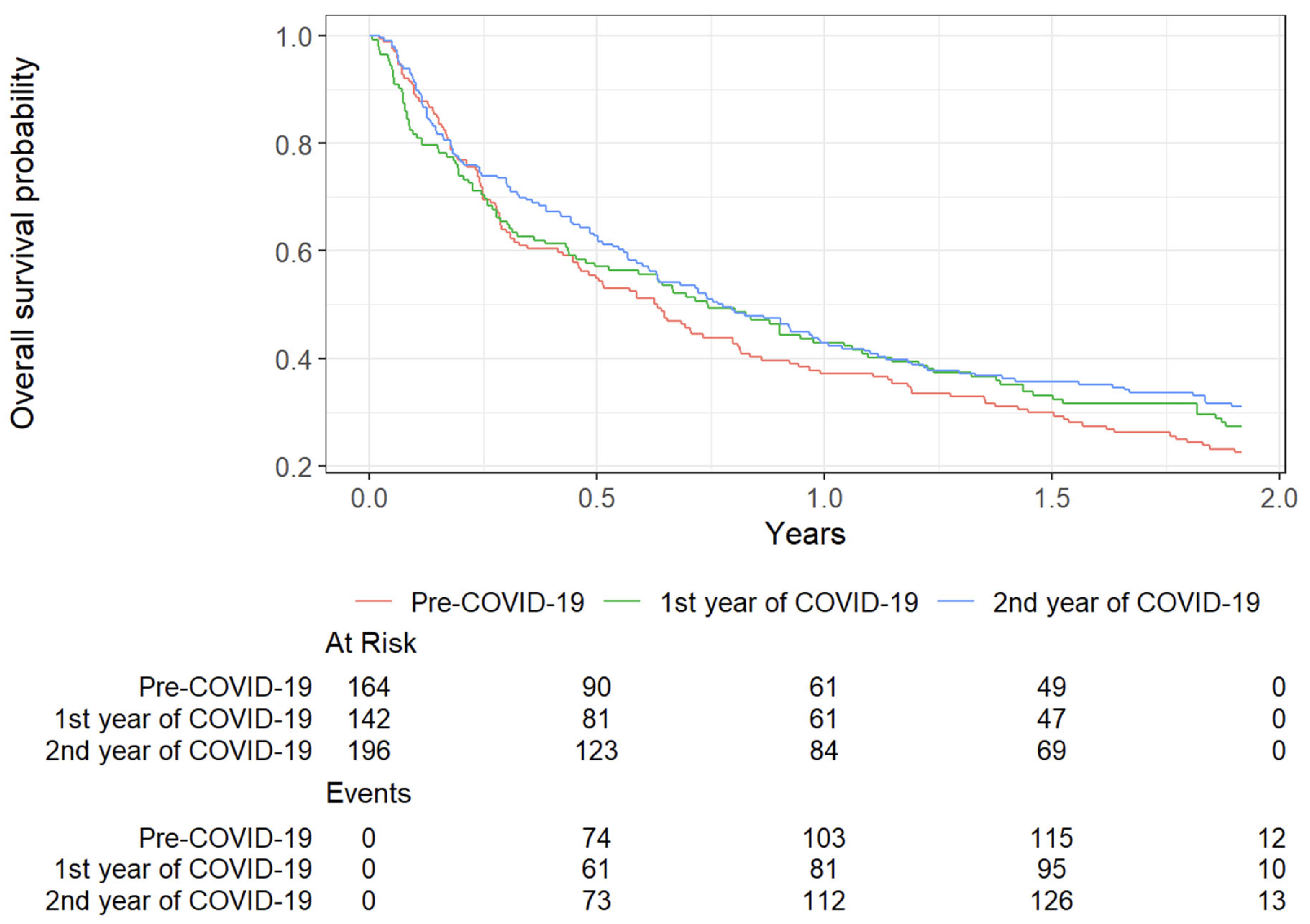
3.5. Survival Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LC | Lung Cancer |
| NSCLC | Non-Small Cell Lung Cancer |
| SCLC | Small Cell Lung Cancer |
| ECOG | Eastern Cooperative Oncology Group |
| FDT | First Definitive Treatment |
| SBRT | Stereotactic Body Radiation Therapy |
| CT | Computed Tomography |
| PET | Positron Emission Tomography |
| EGFR | Epidermal Growth Factor Receptor |
| ALK | Anaplastic Lymphoma Kinase |
| PD-L1 | Programmed Death-Ligand 1 |
| HR | Hazard Ratio |
| CI | Confidence Interval |
| LCCA | Lung Cancer Care Andalusia (Andalusian Clinical Practice Guidelines) |
| BTS | British Thoracic Society |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Dolan, D.P.; Lee, D.N.; Polhemus, E.; Kucukak, S.; De León, L.E.; Wiener, D.; Jaklitsch, M.T.; Swanson, S.J.; White, A. Report on lung cancer surgery during COVID-19 pandemic at a high volume US institution. J. Thorac. Dis. 2022, 14, 2874–2879. [Google Scholar] [CrossRef] [PubMed]
- Mitjà, P.S.; Àvila, M.; García-Olivé, I. Impact of the COVID-19 pandemic on lung cancer diagnosis and treatment. Med. Clínica (Engl. Ed.) 2022, 158, 138–139. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Hernández, N.J.; Silva, U.C.; Sánchez, A.C.; de la Cruz, J.L.C.-C.; Carillo, A.O.; Sarceda, J.R.J.; López, S.S.; Ramos, Á.C.; Díaz, J.L.R.; Call, S.; et al. Effect of COVID-19 on Thoracic Oncology Surgery in Spain: A Spanish Thoracic Surgery Society (SECT) Survey. Cancers 2021, 13, 2897. [Google Scholar] [CrossRef]
- Conibear, J.; Nossiter, J.; Foster, C.; West, D.; Cromwell, D.; Navani, N. The National Lung Cancer Audit: The Impact of COVID-19. Clin. Oncol. 2022, 34, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Malapelle, U.; Pisapia, P.; Iaccarino, A.; Barberis, M.; Bellevicine, C.; Brunnström, H.; De Biase, D.; De Maglio, G.; Ericson Lindquist, K.; Fassan, M.; et al. Predictive molecular pathology in the time of coronavirus disease (COVID-19) in Europe. J. Clin. Pathol. 2020, 74, 391–395. [Google Scholar] [CrossRef]
- Kasymjanova, G.; Anwar, A.; Cohen, V.; Sultanem, K.; Pepe, C.; Sakr, L.; Friedmann, J.; Agulnik, J.S. The Impact of COVID-19 on the Diagnosis and Treatment of Lung Cancer at a Canadian Academic Center: A Retrospective Chart Review. Curr. Oncol. 2021, 28, 4247–4255. [Google Scholar] [CrossRef]
- Depypere, L.P.; Daddi, N.; Gooseman, M.R.; Batirel, H.F.; Brunelli, A. The impact of coronavirus disease 2019 on the practice of thoracic oncology surgery: A survey of members of the European Society of Thoracic Surgeons (ESTS). Eur. J. Cardio-Thorac. Surg. 2020, 58, 752–762. [Google Scholar] [CrossRef]
- Samson, P.; Patel, A.; Garrett, T.; Crabtree, T.; Kreisel, D.; Krupnick, A.S.; Patterson, G.A.; Broderick, S.; Meyers, B.F.; Puri, V. Effects of Delayed Surgical Resection on Short-Term and Long-Term Outcomes in Clinical Stage I Non-Small Cell Lung Cancer. Ann. Thorac. Surg. 2015, 99, 1906–1913. [Google Scholar] [CrossRef]
- Elkrief, A.; Kazandjian, S.; Bouganim, N. Changes in Lung Cancer Treatment as a Result of the Coronavirus Disease 2019 Pandemic. JAMA Oncol. 2020, 6, 1805–1806. [Google Scholar] [CrossRef]
- Blanco-Villar, M.L.; Expósito-Hernández, J.; Navarro-Moreno, E.; Martín, J.M.L.; Mota, A.A.; Couñago, F. Analyzing diagnostic and treatment wait times for lung cancer Patients: Key insights from a provincial registry study. Lung Cancer 2024, 194, 107867. [Google Scholar] [CrossRef]
- The Lung Cancer Working Party of The British Thoracic Society Standards of Care Committee BTS recommendations to respiratory physicians for organising the care of patients with lung cancer. Thorax 1998, 53, S1–S8. [CrossRef]
- Cancer Quality Council of Andalucia. Andalucia Integrated Lung Cancer Care Process. Available online: https://www.juntadeandalucia.es/export/drupaljda/salud_5af1956e967b0_cancer_pulmon_diciembre_2014.pdf (accessed on 5 August 2025).
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Kasymjanova, G.; Rizzolo, A.; Pepe, C.; Friedmann, J.E.; Small, D.; Spicer, J.; Lecavalier-Barsoum, M.; Sultanem, K.; Wang, H.; Spatz, A.; et al. The Impact of COVID-19 on the Diagnosis and Treatment of Lung Cancer over a 2-Year Period at a Canadian Academic Center. Curr. Oncol. 2022, 29, 8677–8685. [Google Scholar] [CrossRef] [PubMed]
- Orelaru, F.; Edwards, M.; Raleigh, R.; Abunayla, A.; Bush, R.; Porter, S.; Schumaker, K.; Albright, J.; Adams, K.N. Short-Term Outcomes of COVID-19 Pandemic on Non-Small Cell Lung Cancer Screening and Management. Surg. J. 2023, 9, e156–e161. [Google Scholar] [CrossRef] [PubMed]
- Melocchi, L.; Mengoli, M.C.; Bogina, G.S.; Facchetti, M.; Migliorati, F.; Gandolfi, L.; Rossi, G. COVID-19 and lung cancer. Pathologica 2023, 115, 284–291. [Google Scholar] [CrossRef]
- Agulnik, J.; Kasymjanova, G.; Pepe, C.; Friedmann, J.; Small, D.; Sakr, L.; Wang, H.; Spatz, A.; Sultanem, K.; Cohen, V. Real-World Evidence of the Impact of the COVID-19 Pandemic on Lung Cancer Survival: Canadian Perspective. Curr. Oncol. 2024, 31, 1562–1571. [Google Scholar] [CrossRef]
- Ocanto, A.; Mielgo-Rubio, X.; Tirado, J.L.; Mesa, N.L.; Valcárcel, M.L.; Pedraza, S.; Barragan, V.V.; Nieto, P.V.; Martín, J.Z.; Couñago, F. Coronavirus disease 2019 and lung cancer: Where are we? Explor. Target. Anti-Tumor Ther. 2023, 4, 1082–1094. [Google Scholar] [CrossRef]
- Bungaro, M.; Passiglia, F.; Scagliotti, G.V. COVID-19 and Lung Cancer: A Comprehensive Overview from Outbreak to Recovery. Biomedicines 2022, 10, 776. [Google Scholar] [CrossRef]
- Mylonakis, A.; Kalfoutzou, A.; Panagakis, A.; Despotidis, M.; Yfantopoulos, J. The Impact of the COVID-19 Pandemic on Surgical Activities: A Single-Center Experience and Literature Review. Cureus 2022, 14, e30785. [Google Scholar] [CrossRef]
- Terashima, T.; Tsutsumi, A.; Iwami, E.; Kuroda, A.; Nakajima, T.; Eguchi, K. Delayed visit and treatment of lung cancer during the coronavirus disease 2019 pandemic in Japan: A retrospective study. J. Int. Med. Res. 2022, 50. [Google Scholar] [CrossRef]
- Zhang, M.; Tierney, P.; Brennan, A.; Murray, D.; Mullooly, M.; Bennett, K. Modelling the impact of the COVID-19 pandemic on cancer stage migration and excess mortality in Ireland. Prev. Med. Rep. 2025, 52, 103020. [Google Scholar] [CrossRef]
- Bouza, E.; Martin, M.; Alés, J.E.; Aragonés, N.; Barragán, B.; de la Cámara, R.; Del Pozo, J.L.; García-Gutiérrez, V.; García-Sanz, R.; Gracia, D.; et al. Impact of the COVID-19 pandemic on the diagnosis and treatment of onco-hematologic patients: A discussion paper. Rev. Esp. De Quimioter. 2022, 36, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Bian, D.J.H.; Sabri, S.; Abdulkarim, B.S. Interactions between COVID-19 and Lung Cancer: Lessons Learned during the Pandemic. Cancers 2022, 14, 3598. [Google Scholar] [CrossRef] [PubMed]
- Cantini, L.; Mentrasti, G.; Russo, G.; Signorelli, D.; Pasello, G.; Rijavec, E.; Russano, M.; Antonuzzo, L.; Rocco, D.; Giusti, R.; et al. Evaluation of COVID-19 impact on DELAYing diagnostic-therapeutic pathways of lung cancer patients in Italy (COVID-DELAY study): Fewer cases and higher stages from a real-world scenario. ESMO Open 2022, 7, 100406. [Google Scholar] [CrossRef] [PubMed]
- Qsous, G.; Korelidis, G.; Vianna, T.; Chambers, A.; Will, M.; Zamvar, V. The Effect of Pandemic on Lung Cancer Waiting Time—Tertiary Center Experience. Asian Pac. J. Cancer Prev. 2024, 25, 1643–1647. [Google Scholar] [CrossRef]
- Mostafaei, A.; Sadeghi-Ghyassi, F.; Kabiri, N.; Hajebrahimi, S. Experiences of patients and providers while using telemedicine in cancer care during COVID-19 pandemic: A systematic review and meta-synthesis of qualitative literature. Support. Care Cancer 2022, 30, 10483–10494. [Google Scholar] [CrossRef]
- Johnson, W.V.; Hsu, M.L.; Gupta, A. A Fifth of Their Days: The Time Commitments of Advanced Cancer and Its Care. JCO Oncol. Pract. 2025, OP2401085. [Google Scholar] [CrossRef]
- Allahqoli, L.; Mazidimoradi, A.; Salehiniya, H.; Alkatout, I. Impact of COVID-19 on cancer screening: A global perspective. Curr. Opin. Support. Palliat. Care 2022, 16, 102–109. [Google Scholar] [CrossRef]
| Characteristics | 2019 n = 179 | 2020 n = 145 | 2021 n = 206 | p-Value | |
|---|---|---|---|---|---|
| Median age (years) | 65 | 64 | 67 | 0.0368 | |
| Sex [n (%)] | Male | 146 (81.56%) | 114 (78.62%) | 160 (77.67%) | NS |
| Female | 33 (18.44%) | 31 (21.38%) | 46 (22.33%) | ||
| Smoking history [n (%)] | Former/current smoker | 168 (94.92%) | 130 (90.91%) | 187 (91.22%) | NS |
| Non-smoker | 9 (5.08%) | 13 (9.09%) | 18 (8.78%) | ||
| ECOG performance status [n (%)] | 0–1 | 104 (65.41% | 96 (78.80%) | 143 (76.06%) | 0.0411 |
| >1 | 55 (34.58%) | 29 (23.20%) | 45 (23.94%) | ||
| Subtype/Histology [n (%)] | NSCLC | 135 (79.88%) | 116 (85.29%) | 169 (84.08%) | NS |
| SCLC | 34 (20.12%) | 20 (14.71%) | 32 (15.92%) | ||
| Unknown | 10 (5.59%) | 9 (6.21%) | |||
| NSCLC Subtype (n/%) | Adenocarcinoma | 88 (66.17%) | 63 (55.26%) | 95 (56.89%) | NS |
| Squamous | 33 (24.81%) | 39 (34.21%) | 64 (38.32%) | ||
| Others * | 12 (9.02%) | 12 (10.53%) | 8 (4.79%) | ||
| Biomarkers | EGFR Mutation | NS | |||
| Positive Negative | 9 (12%) 66 (88%) | 6 (10%) 54 (90%) | 6 (7.89%) 70 (92.1%) | ||
| ALK translocation | NS | ||||
| Positive Negative | 0 (0%) 72 (100%) | 2 (3.4%) 58 (96.6%) | 2 (2.7%) 71 (97.3%) | ||
| PD-L1 expression | NS | ||||
| <1% 1–49% >50% | 39 (40.2%) 28 (28.8%) 30 (31%) | 50 (54.4%) 21 (22.8%) 21 (22.8%) | 51 (48.5%) 28 (26.7%) 26 (24.8%) | ||
| Cancer Stage [n (%)] | Early stage 1 | 28 (15.64%) | 23 (15.86%) | 37 (17.96%) | NS |
| Locorregional 2 | 28 (15.64%) | 34 (23.45%) | 53 (25.73%) | ||
| Advanced/metastatic stage 3 | 123 (68.72%) | 88 (60.69%) | 116 (56.31%) | ||
| Treatment type [n (%)] | FDT 4 | 125 (69.83%) | 102 (70.34%) | 151 (73.30%) | NS |
| PT 5 | 54 (30.17%) | 43 (29.66%) | 55 (26.70%) | ||
| First consultation with pulmonary specialist [n (%)] | Outpatient referral from GP | 37 (20.67%) | 30 (20.69%) | 44 (21.36%) | NS |
| Inpatient consult | 37 (20.67%) | 37 (25.52%) | 58 (28.16%) | ||
| Referral from emergency department | 105 (58.66%) | 78 (53.79%) | 104 (50.59%) | ||
| Variables | 2019 | 2020 | 2021 |
|---|---|---|---|
| Number of new lung cancer diagnoses | 179 | 145 | 206 |
| Average per month | 15 | 12 | 17 |
| Change vs. previous year | − | −19% | +42% |
| Type of FTD | Total | 2019 n = 125 | 2020 n = 102 | 2021 n = 151 | p-Value |
|---|---|---|---|---|---|
| Surgery (n/%) | 76 (14.34%) | 22 (12.29%) | 23 (15.86%) | 31 (15.05%) | NS |
| Definitive radiation (n/%) | 22 (4.15%) | 7 (3.91%) | 4 (2.76%) | 11 (5.34%) | NS |
| Conventional | 12 (2.26%) | 4 (2.23%) | 0 (0.00%) | 8 (3.88%) | |
| SBRT | 10 (1.89%) | 3 (1.68%) | 4 (2.76%) | 3 (1.46%) | |
| Definitive chemoradiation (n/%) | 79 (14.90%) | 20 (11.17%) | 22 (15.18%) | 37 (17.96%) | NS |
| Concurrent chemoradiation | 44 (8.30%) | 13 (7.26%) | 14 (9.66%) | 17 (8.25%) | |
| Sequential chemoradiation | 35 (6.60%) | 7 (3.91%) | 8 (5.52%) | 20 (9.71%) | |
| Palliative radiation (n/%) | 38 (7.17%) | 22 (12.29%) | 7 (4.83%) | 9 (4.37%) | 0.0048 |
| Systemic (n/%) | 163 (30.75%) | 54 (30.17%) | 46 (31.72%) | 63 (30.58%) | NS |
| Standard systemic chemotherapy | 111 (20.94%) | 42 (23.46%) | 32 (22.07%) | 37 (17.96%) | |
| Immunotherapy ± Chemotherapy | 41 (7.74%) | 9 (5.03%) | 9 (6.21%) | 23 (11.17%) | |
| Targeted therapy | 11 (2.08%) | 3 (1.68%) | 5 (3.45%) | 3 (1.46%) | |
| Symptom-directed (palliative care) | 152 (28.68%) | 54 (30.17%) | 43 (29.66%) | 55 (26.70%) | NS |
| Interval | 2019 | 2020 | 2021 | p-Value |
|---|---|---|---|---|
| Referral → LC specialist (≤7 days) | 2 (1.0; 7.0) | 1 (1.0; 5.0) | 1 (1.0; 6.0) | NS |
| Referral → Diagnosis (≤30 days) | 19 (14.0; 29.0) | 14 (11.0; 22.0) | 14 (11.0; 22.2) | <0.0001 |
| Pathology report (≤7 days) | 7 (6.0; 9.5) | 6 (6.0; 8.0) | 6 (6.0; 8.0) | 0.0001 |
| Molecular results (≤14 days) | 11 (8.0; 18.0) | 13 (8.0; 21.0) | 15 (10.5; 22.0) | 0.0226 |
| Diagnosis → FDT 2 (≤42 days) | 33 (20.0; 50.5) | 34.5 (23.25; 49.0) | 35.5 (23.0; 54.0) | NS |
| Interval (Days) | 2019 | 2020 | 2021 | p-Value |
|---|---|---|---|---|
| Referral → LC specialist (≤7 days) | 77.69% | 81.44% | 80.54% | 0.7518 |
| Referral → Diagnosis (≤30 days) | 80.98% | 84.80% | 83.70% | 0.6630 |
| Pathology report (≤7 days) | 55.21% | 74.40% | 73.37% | 0.0002 |
| Molecular results (≤14 days) | 44.59% | 64.15% | 40.91% | 0.0278 |
| Diagnosis → Treatment (≤42 days) | 65.25% | 66.67% | 61.43% | 0.6839 |
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age (vs ≤ 70 years) | 1.15 (0.86, 1.53) | NS | 1.23 (0.89–1.70) | NS |
| Female sex (vs. male) | 0.64 (0.47, 0.96) | 0.027 | 0.84 (0.57–1.23) | NS |
| Ever-smoker (vs. never) | 1.84 (1.21–2.86) | 0.008 | 1.79 (0.90–3.57) | NS |
| ECOG performance status (vs 0–1) ≥2 | 3.97 (2.87, 5.50) | <0.001 | 3.34 (2.28, 4.90) | <0.001 |
| Histology (vs. SCLC) | 0.51 (0.07, 3.68) | <0.001 | 0.70 (0.49, 0.98) | 0.038 |
| Stage (vs. Early stage-NSLC) Locally-Advanced + Limited Advanced + Extended | 3.87 (2.21, 6.76) 9.29 (5.53, 15.6) | <0.001 <0.001 | 3.28 (1.26, 8.52) 6.62 (2.53, 17.3) | 0.015 <0.001 |
| First treatment (vs. Surgery) Definitive radiation Definitive chemoradiation Palliative Radiation Systemic (chemo and/or immunotherapy) | 2.08 (0.95, 4.55) 2.88 (1.64, 5.08) 17 (9.34, 30.93) 7.12 (4.28, 11.8) | NS <0.001 <0.001 <0.001 | 0.89 (0.36, 2.21) 0.72 (0.30, 1.74) 1.92 (0.77, 4.75) 0.94 (0.40, 2.19) | NS NS NS NS |
| Year of diagnosis (vs. Pre-COVID-19) 1st year of COVID-19 2nd year of COVID-19 | 0.74 (0.53, 1.04) 0.78 (0.58, 1.06) | NS NS | 0.98 (0.68, 1.41) 1.18 (0.84, 1.64) | NS NS |
| Wait times 1st appointment → Diagnosis ≥30 days Diagnosis → 1st treatment ≥42 days | 0.74 (0.51, 1.06) 0.82 (0.61, 1.16) | NS NS | 0.78 (0.54, 1.14) 0.90 (0.65, 1.24) | NS NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco-Villar, M.L.; Expósito-Hernández, J.; Navarro-Moreno, E.; Aparicio Mota, A.; López Martín, J.M. Lung Cancer Under Siege in Spain: Timeliness, Treatment, and Survival Before and After the COVID-19 Pandemic. Cancers 2025, 17, 2655. https://doi.org/10.3390/cancers17162655
Blanco-Villar ML, Expósito-Hernández J, Navarro-Moreno E, Aparicio Mota A, López Martín JM. Lung Cancer Under Siege in Spain: Timeliness, Treatment, and Survival Before and After the COVID-19 Pandemic. Cancers. 2025; 17(16):2655. https://doi.org/10.3390/cancers17162655
Chicago/Turabian StyleBlanco-Villar, Manuel Luis, José Expósito-Hernández, Eulalia Navarro-Moreno, Adrián Aparicio Mota, and José María López Martín. 2025. "Lung Cancer Under Siege in Spain: Timeliness, Treatment, and Survival Before and After the COVID-19 Pandemic" Cancers 17, no. 16: 2655. https://doi.org/10.3390/cancers17162655
APA StyleBlanco-Villar, M. L., Expósito-Hernández, J., Navarro-Moreno, E., Aparicio Mota, A., & López Martín, J. M. (2025). Lung Cancer Under Siege in Spain: Timeliness, Treatment, and Survival Before and After the COVID-19 Pandemic. Cancers, 17(16), 2655. https://doi.org/10.3390/cancers17162655






