Endocrine Adverse Events Induced by Cancer Treatments: The Role of 18F-Fluorodeoxyglucose Positron Emission Tomography
Simple Summary
Abstract
1. Introduction
2. Treatment-Induced Adverse Effects in Oncological Patients
Prevalence, Pathogenesis, and Clinical Presentation
3. Clinical Management and PET/CT Imaging
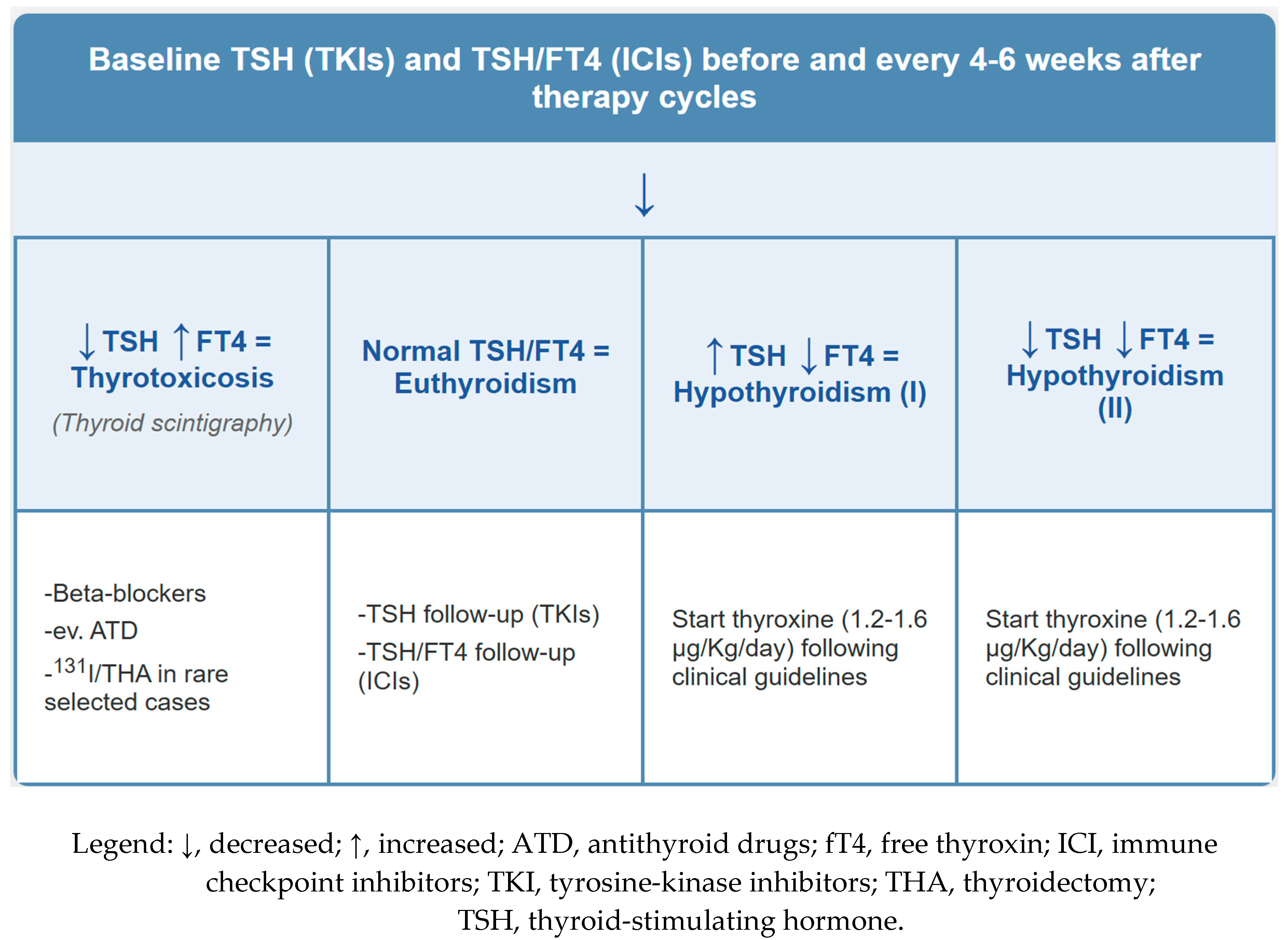
3.1. Thyroid Adverse Effects
3.2. Immunotherapy-Induced Pituitary Diseases
3.3. Immunotherapy-Induced Adrenal Diseases
3.4. Immunotherapy-Induced Pancreatitits and Diabetes Mellitus
4. Pros and Cons
5. Conclusions
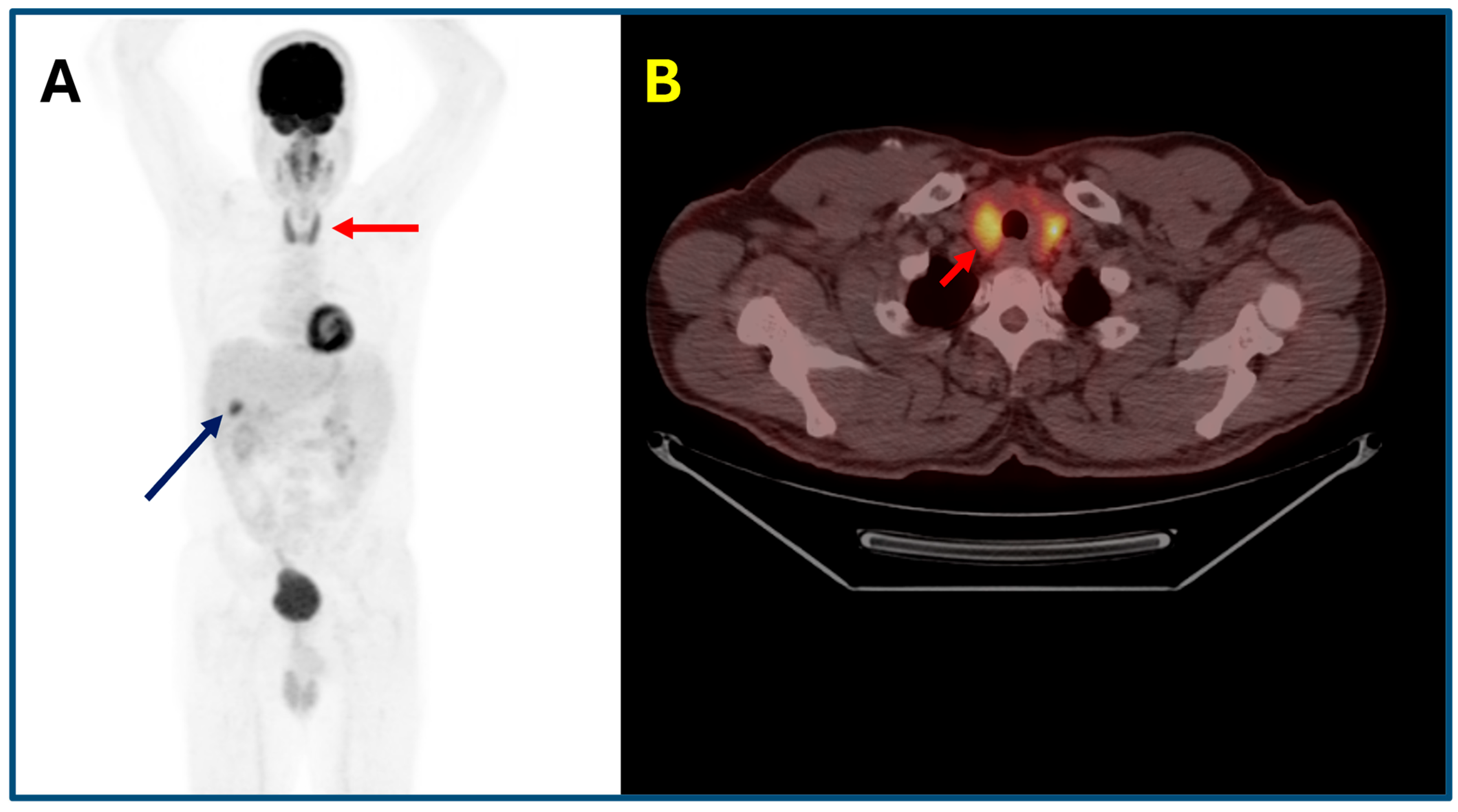
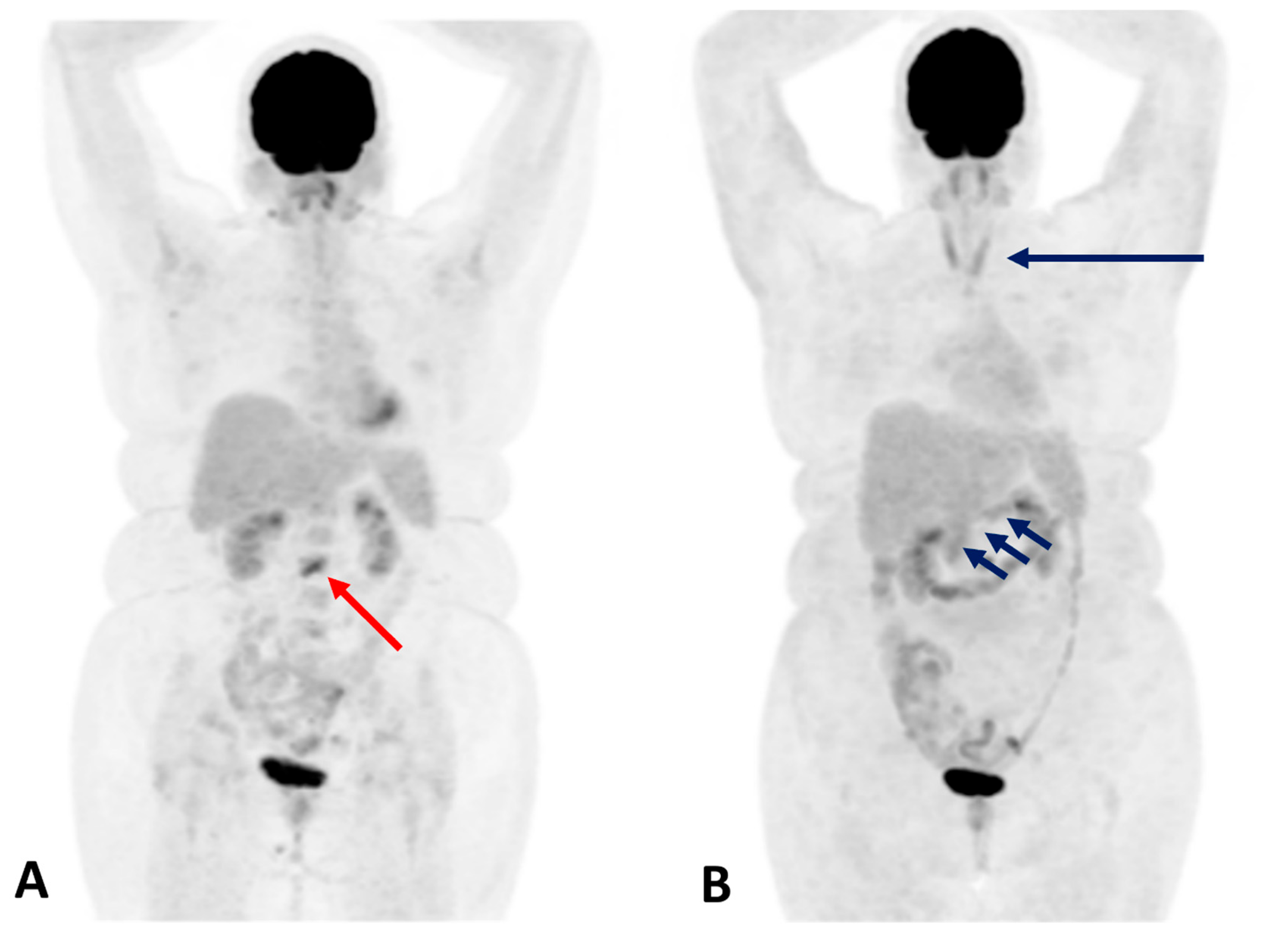
- Thyroiditis: Diffuse or heterogeneous increased thyroid uptake, often corresponding to a transition from thyrotoxicosis to hypothyroidism. Focal uptake requires careful evaluation for nodular disease or metastasis.
- Hypophysitis: Homogeneous or asymmetric pituitary uptake associated with symptoms such as headache, visual disturbances, and hypopituitarism.
- Adrenalitis: Symmetric, diffuse adrenal uptake with slight gland enlargement, frequently accompanied by adrenal insufficiency—a potentially life-threatening condition.
- Pancreatitis: Diffuse or focal pancreatic uptake correlating with asymptomatic enzyme elevation or clinical pancreatitis, necessitating differentiation from metastatic disease.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Puliani, G.; Appetecchia, M. Endocrine Toxicities of Antineoplastic Therapy. Cancers 2021, 13, 294. [Google Scholar] [CrossRef]
- Lu, T.; Gao, X.; Yu, Z.; Liu, L.; Chen, X.; Xiao, Y.; Zhong, F.; Dong, Q.; Xie, H.; Tu, Z.; et al. Reducing Radiation-Induced Hypothyroidism by Modified Delineation of Cervical Lymphatic Drainage Area for Nasopharyngeal Carcinoma Treated by Intensity-Modulated Radiation Therapy: 3 Years’ Experience. Radiother. Oncol. 2025, 204, 110713. [Google Scholar] [CrossRef]
- Randhawa, A.S.; Yadav, H.P.; Singh Banipal, R.P.; Goyal, G.; Garg, P.; Marcus, S. Functional and Biochemical Changes in the Thyroid Gland Following Exposure to Therapeutic Doses of External Beam Radiotherapy in the Head-and-Neck Cancer Patients. J. Cancer Res. Ther. 2021, 17, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Fiore, A.R.; Lourenço, G.J.; Pereira, E.B.; dos Anjos, L.F.D.A.; Lima, C.S.P.; Zantut-Wittmann, D.E. Early and Long-Term Thyroid Dysfunction in Patients with Head and Neck Squamous Cell Carcinoma After External Radiotherapy: Clinicopathological Risk Factors. Head Neck 2025, 47, 2336–2347. [Google Scholar] [CrossRef]
- Vankoevering, K.K.; Sabetsarvestani, K.; Sullivan, S.E.; Barkan, A.; Mierzwa, M.; McKean, E.L. Pituitary Dysfunction after Radiation for Anterior Skull Base Malignancies: Incidence and Screening. J. Neurol. Surg. Part B Skull Base 2020, 81, 75–81. [Google Scholar] [CrossRef]
- Petranović Ovčariček, P.; Deandreis, D.; Giovanella, L. Thyroid Dysfunctions Induced by Molecular Cancer Therapies: A Synopsis for Nuclear Medicine Thyroidologists. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3355–3360. [Google Scholar] [CrossRef]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef]
- Nogueira, E.; Newsom-Davis, T.; Morganstein, D.L. Immunotherapy-Induced Endocrinopathies: Assessment, Management and Monitoring. Ther. Adv. Endocrinol. Metab. 2019, 10, 2042018819896182. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.-S.; Barroso-Sousa, R.; Tolaney, S.M.; Hodi, F.S.; Kaiser, U.B.; Min, L. Endocrine Toxicity of Cancer Immunotherapy Targeting Immune Checkpoints. Endocr. Rev. 2019, 40, 17–65. [Google Scholar] [CrossRef] [PubMed]
- Byun, D.J.; Wolchok, J.D.; Rosenberg, L.M.; Girotra, M. Cancer Immunotherapy—Immune Checkpoint Blockade and Associated Endocrinopathies. Nat. Rev. Endocrinol. 2017, 13, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Eshghi, N.; Garland, L.L.; Nia, E.; Betancourt, R.; Krupinski, E.; Kuo, P.H. 18F-FDG PET/CT Can Predict Development of Thyroiditis Due to Immunotherapy for Lung Cancer. J. Nucl. Med. Technol. 2018, 46, 260–264. [Google Scholar] [CrossRef]
- Chemotherapy Side Effects | American Cancer Society. Available online: https://www.cancer.org/cancer/managing-cancer/treatment-types/chemotherapy/chemotherapy-side-effects.html (accessed on 1 July 2025).
- Torino, F.; Barnabei, A.; Paragliola, R.; Baldelli, R.; Appetecchia, M.; Corsello, S.M. Thyroid Dysfunction as an Unintended Side Effect of Anticancer Drugs. Thyroid 2013, 23, 1345–1366. [Google Scholar] [CrossRef] [PubMed]
- Lugat, A.; Drui, D.; Baron, S.; Thebaud, E.; Supiot, S.; Jouglar, E.; Doré, M. Endocrine Side Effects of Radiation Therapy: Diagnosis, Prevention, and Treatment. Cancer Radiother. 2022, 26, 1078–1089. [Google Scholar] [CrossRef] [PubMed]
- Castinetti, F.; Albarel, F.; Archambeaud, F.; Bertherat, J.; Bouillet, B.; Buffier, P.; Briet, C.; Cariou, B.; Caron, P.; Chabre, O.; et al. Endocrine Side-Effects of New Anticancer Therapies: Overall Monitoring and Conclusions. Ann. d’Endocrinol. 2018, 79, 591–595. [Google Scholar] [CrossRef]
- Lodish, M.B. Kinase Inhibitors: Adverse Effects Related to the Endocrine System. J. Clin. Endocrinol. Metab. 2013, 98, 1333–1342. [Google Scholar] [CrossRef] [PubMed]
- Ferro, A. Mechanistic Target of Rapamycin Modulation: An Emerging Therapeutic Approach in a Wide Variety of Disease Processes. Br. J. Clin. Pharmacol. 2016, 82, 1156. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Brahmer, J.R.; Callahan, M.K.; Flores-Chávez, A.; Keegan, N.; Khamashta, M.A.; Lambotte, O.; Mariette, X.; Prat, A.; Suárez-Almazor, M.E. Immune-Related Adverse Events of Checkpoint Inhibitors. Nat. Rev. Dis. Primers 2020, 6, 38. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Horita, N.; Adib, E.; Zhou, S.; Nassar, A.H.; Asad, Z.U.A.; Cortellini, A.; Naqash, A.R. Treatment-Related Adverse Events, Including Fatal Toxicities, in Patients with Solid Tumours Receiving Neoadjuvant and Adjuvant Immune Checkpoint Blockade: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Lancet Oncol. 2024, 25, 62–75. [Google Scholar] [CrossRef]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse Effects of Immune-Checkpoint Inhibitors: Epidemiology, Management and Surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef]
- Postow, M.A.; Chesney, J.; Pavlick, A.C.; Robert, C.; Grossmann, K.; McDermott, D.; Linette, G.P.; Meyer, N.; Giguere, J.K.; Agarwala, S.S.; et al. Nivolumab and Ipilimumab versus Ipilimumab in Untreated Melanoma. N. Engl. J. Med. 2015, 372, 2006–2017. [Google Scholar] [CrossRef]
- Spagnolo, C.C.; Campo, I.; Campennì, A.; Cardile, D.; Cannavò, S.; Silvestris, N.; Santarpia, M.; Ruggeri, R.M. Challenges and Pitfalls in the Management of Endocrine Toxicities from Immune Checkpoint Inhibitors: A Case Presentation of Synchronous Thyrotoxicosis and Primary Adrenal Insufficiency in a Melanoma Patient. Hormones 2024, 23, 759–764. [Google Scholar] [CrossRef]
- Wright, J.J.; Powers, A.C.; Johnson, D.B. Endocrine Toxicities of Immune Checkpoint Inhibitors. Nat. Rev. Endocrinol. 2021, 17, 389–399. [Google Scholar] [CrossRef]
- Van der Kooij, M.K.; Suijkerbuijk, K.P.M.; Aarts, M.J.B.; Van den Berkmortel, F.W.P.J.; Blank, C.U.; Boers-Sonderen, M.J.; Van Breeschoten, J.; Van den Eertwegh, A.J.M.; De Groot, J.W.B.; Haanen, J.B.A.G.; et al. Safety and Efficacy of Checkpoint Inhibition in Patients with Melanoma and Preexisting Autoimmune Disease: A Cohort Study. Ann. Intern. Med. 2021, 174, 641–648. [Google Scholar] [CrossRef]
- Ruggeri, R.M.; Campennì, A.; Giuffrida, G.; Trimboli, P.; Giovanella, L.; Trimarchi, F.; Cannavò, S. Endocrine and Metabolic Adverse Effects of Immune Checkpoint Inhibitors: An Overview (What Endocrinologists Should Know). J. Endocrinol. Investig. 2019, 42, 745–756. [Google Scholar] [CrossRef]
- Simoni, L.J.C.; Brandão, S.C.S. New Imaging Methods for Detection of Drug-Induced Cardiotoxicity in Cancer Patients. Curr. Cardiovasc. Imaging Rep. 2017, 10, 18. [Google Scholar] [CrossRef]
- Borde, C.; Kand, P.; Basu, S. Enhanced Myocardial Fluorodeoxyglucose Uptake Following Adriamycin-Based Therapy: Evidence of Early Chemotherapeutic Cardiotoxicity? World J. Radiol. 2012, 4, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.A.; Bhalla, S.; Ganeshan, D.; Felder, G.J.; Itani, M. Side Effects of Oncologic Treatment in the Chest: Manifestations at FDG PET/CT. Radiographics 2021, 41, 2071–2089. [Google Scholar] [CrossRef] [PubMed]
- Dejanovic, D.; Specht, L.; Czyzewska, D.; Kiil Berthelsen, A.; Loft, A. Response Evaluation Following Radiation Therapy With 18F-FDG PET/CT: Common Variants of Radiation-Induced Changes and Potential Pitfalls. Semin. Nucl. Med. 2022, 52, 681–706. [Google Scholar] [CrossRef] [PubMed]
- Antoch, G.; Kanja, J.; Bauer, S.; Kuehl, H.; Renzing-Koehler, K. Comparison of PET, CT, and Dual-Modality PET/CT Imaging for Monitoring of Imatinib (STI571) Therapy in Patients with Gastrointestinal Stromal Tumors. J. Nucl. Med. 2004, 45, 357–365. [Google Scholar]
- Lopci, E.; Hicks, R.J.; Dimitrakopoulou-Strauss, A.; Dercle, L.; Iravani, A.; Seban, R.D.; Sachpekidis, C.; Humbert, O.; Gheysens, O.; Glaudemans, A.W.J.M.; et al. Joint EANM/SNMMI/ANZSNM Practice Guidelines/Procedure Standards on Recommended Use of [18F]FDG PET/CT Imaging during Immunomodulatory Treatments in Patients with Solid Tumors Version 1. 0. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2323–2341. [Google Scholar] [CrossRef]
- Fischer, A.; Martínez-Gómez, J.M.; Mangana, J.; Dummer, R.; Erlic, Z.; Nölting, S.; Beuschlein, F.; Maurer, A.; Messerli, M.; Huellner, M.W.; et al. 18F-FDG PET/CT for Detection of Immunotherapy-Induced Hypophysitis—A Case-Control Study. Clin. Nucl. Med. 2024, 49, e656–e663. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Salem, J.E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal Toxic Effects Associated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef]
- Abdel-Rahman, O.; Fouad, M. Risk of Thyroid Dysfunction in Patients with Solid Tumors Treated with VEGF Receptor Tyrosine Kinase Inhibitors: A Critical Literature Review and Meta Analysis. Expert Rev. Anticancer. Ther. 2014, 14, 1063–1073. [Google Scholar] [CrossRef]
- Jannin, A.; Penel, N.; Ladsous, M.; Vantyghem, M.C.; Cao, C. Do Tyrosine Kinase Inhibitors and Immune Checkpoint Inhibitors-Induced Thyroid Disorders. Crit. Rev. Oncol. Hematol. 2019, 141, 23–35. [Google Scholar] [CrossRef]
- Barroso-Sousa, R.; Barry, W.T.; Garrido-Castro, A.C.; Hodi, F.S.; Min, L.; Krop, I.E.; Tolaney, S.M. Incidence of Endocrine Dysfunction Following the Use of Different Immune Checkpoint Inhibitor Regimens: A Systematic Review and Meta-Analysis. JAMA Oncol. 2018, 4, 173–182. [Google Scholar] [CrossRef]
- Hamnvik, O.-P.R.; Larsen, P.R.; Marqusee, E. Thyroid Dysfunction from Antineoplastic Agents. J. Natl. Cancer Inst. 2011, 103, 1572–1587. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Goyal, A.; Kaur, P.; Singh, R.; Kalra, S. Anticancer Drug-Induced Thyroid Dysfunction. Eur. Endocrinol. 2020, 16, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Galligan, A.; Wallace, R.; Krishnamurthy, B.; Kay, T.W.H.; Sachithanandan, N.; Chiang, C.; Sandhu, S.; Hicks, R.J.; Iravani, A. Increased Thyroidal Activity on Routine FDG-PET/CT after Combination Immune Checkpoint Inhibition: Temporal Associations with Clinical and Biochemical Thyroiditis. Cancers 2023, 15, 5803. [Google Scholar] [CrossRef] [PubMed]
- Spielvogel, C.P.; Lazarevic, A.; Zisser, L.; Haberl, D.; Eseroglou, C.; Beer, L.; Hacker, M.; Calabretta, R. Artificial Intelligence-Enabled Opportunistic Identification of Immune Checkpoint Inhibitor-Related Adverse Events Using [18F]FDG PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2025, 1–9. [Google Scholar] [CrossRef]
- Iravani, A.; Galligan, A.; Lasocki, A.; Wallace, R.; Weppler, A.; Au Yeung, G.; Akhurst, T.; Sachithanandan, N.; Chiang, C.; Sandhu, S.; et al. FDG PET in the Evaluation of Immune-Related Hypophysitis and Thyroiditis Following Combination Ipilimumab and Nivolumab in Advanced Melanoma. J. Nucl. Med. 2020, 61, 482. [Google Scholar]
- Bacanovic, S.; Burger, I.A.; Stolzmann, P.; Hafner, J.; Huellner, M.W. Ipilimumab-Induced Adrenalitis: A Possible Pitfall in 18F-FDG-PET/CT. Clin. Nucl. Med. 2015, 40, e518–e519. [Google Scholar] [CrossRef] [PubMed]
- Bluestone, J.A.; Anderson, M.; Herold, K.C.; Stamatouli, A.M.; Quandt, Z.; Perdigoto, A.L.; Clark, P.L.; Kluger, H.; Weiss, S.A.; Gettinger, S.; et al. Collateral Damage: Insulin-Dependent Diabetes Induced with Checkpoint Inhibitors. Diabetes 2018, 67, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.A.; Kurra, V.; Bradley, W.; Kilcoyne, A.; Mojtahed, A.; Lee, S.I. Abdominal Immune-Related Adverse Events: Detection on Ultrasonography, CT, MRI and 18F-Fluorodeoxyglucose Positron Emission Tomography. Br. J. Radiol. 2020, 94, 20200663. [Google Scholar] [CrossRef]
- Hoadley, A.; Sandanayake, N.; Long, G.V. Atrophic Exocrine Pancreatic Insufficiency Associated with Anti-PD1 Therapy. Ann. Oncol. 2017, 28, 434–435. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, W.; Akily, L.; Mierzejewska, M.; Teodorczyk, J.; Bandura, A.; Zaucha, R.; Cytawa, W. Is 18F-FDG-PET/CT an Optimal Imaging Modality for Detecting Immune-Related Adverse Events after Immune-Checkpoint Inhibitor Therapy? Pros and Cons. Cancers 2024, 16, 1990. [Google Scholar] [CrossRef]
- Das, J.P.; Postow, M.A.; Friedman, C.F.; Do, R.K.; Halpenny, D.F. Imaging findings of immune checkpoint inhibitor associated pancreatitis. Eur. J. Radiol. 2020, 131, 109250. [Google Scholar] [CrossRef]
- Das, J.P.; Halpenny, D.; Do, R.K.; Ulaner, G.A. Focal Immunotherapy-Induced Pancreatitis Mimicking Metastasis on FDG PET/CT. Clin. Nucl. Med. 2019, 44, 836–837. [Google Scholar] [CrossRef]


| Treatment Type | Endocrine Adverse Events | Prevalence (%) |
|---|---|---|
| PD-1/PD-L1 inhibitors | Thyroiditis, hypothyroidism, adrenalitis, diabetes | 10–35% |
| CTLA-4 inhibitors | Hypophysitis, adrenalitis, diabetes | 5–10% |
| Combined ICIs (PD-1 + CTLA-4) | Hypophysitis, multi-endocrine events | 30–50% |
| Pelvic radiotherapy | Gonadal failure, infertility | 40–60% |
| Aromatase inhibitors | Osteoporosis, hypothyroidism | 10–30% |
| Androgen deprivation therapy | Hypogonadism, osteoporosis, metabolic syndrome | 50–70% |
| Tyrosine kinase inhibitors | Hypothyroidism, thyroiditis, Hyperglycemia, Hypoglycemia, | 40–70% 15–40% |
| mTOR inhibitors | Hyperglycemia, insulin resistance, dyslipidemia | 10–25% |
| Chemotherapy (alkylating agents) | Ovarian/testicular failure | 30–60% |
| Tumor Type | Primary Treatment | Common Endocrine AEs | Estimated Prevalence (%) |
|---|---|---|---|
| Non-small cell lung cancer | PD-1/PD-L1 inhibitors | Hypothyroidism, | 15–35% |
| Adrenalitis | <2% | ||
| Diabetes | <1% | ||
| Melanoma | CTLA-4 + PD-1 inhibitors | Thyroiditis | 25–40% |
| Hypophysitis | ~10% | ||
| Diabetes | Rare | ||
| Breast cancer | AIs, GnRH analogs, and chemotherapy | Ovarian failure | 20–50% |
| Osteoporosis | 5–25% | ||
| Prostate cancer | ADT (GnRH agonists/antagonists) | Hypogonadism | >90% |
| Osteoporosis | 50–70% | ||
| Insulin resistance | 30–50% | ||
| Renal cell carcinoma | TKIs (e.g., sunitinib) | Hypothyroidism | 50–70% |
| Head/Neck cancer | External beam radiation therapy | Primary hypothyroidism | 20–50% (hypothyroidism) |
| Brain tumors (Pediatric/Adult) | Cranial irradiation | Hypopituitarism -central hypothyroidism, -central hypogonadism -central adrenal failure -GH deficit | 30–70% |
| Hematologic malignancies | Chemotherapy, TBI, HSCT | Gonadal failure | 40–60% (gonadal failure), |
| GH/TSH/ACTH deficiency | 30–50% (HPA axis) | ||
| Triple-negative breast cancer | PD-L1 inhibitors (e.g., atezolizumab) | Hypothyroidism, | 10–20% |
| adrenalitis (rare) | <1% |
| Organ/Gland | Prevalence (PET Studies) | Causes/Etiologies |
|---|---|---|
| Thyroid | ~0.1 to 4.5 % (mean ~1.6 to 1.9 %) | Mainly autoimmune thyroiditis (e.g., Hashimoto’s), less often Graves’; associated with risk of evolving hypo- or hyperthyroidism |
| Pituitary | Extremely rare: ~0.073 % focal uptake, but truly diffuse pattern not well characterized | Mostly pituitary adenomas, hypophysitis, metastases, Langerhans cell histiocytosis |
| Adrenal glands | Diffuse bilateral adrenal uptake is uncommon (studies focus on focal/incidental unilateral lesions: ~7.6 % of scans report adrenal uptake) | Focal adrenal uptake more often: benign adenomas, metastases, adrenal carcinoma, pheochromocytoma; truly diffuse uptake likely physiologic or inflammatory changes, but rare |
| Pancreas | Diffuse pancreatic uptake is not well described in the literature; usually, only focal uptake is noted (pancreatic inflammation, neoplasm) | Diffuse uptake is likely physiologic or related to inflammatory changes (e.g., pancreatitis), but little formal data exist. |
| Gland | FDG Uptake Pattern | Associated EAE | Imaging Features | Clinical Presentation |
|---|---|---|---|---|
| Thyroid | Diffuse increased FDG uptake | Autoimmune thyroiditis (most common irAE) | Symmetrical, homogeneous thyroid uptake; often associated with thyroid atrophy in late stages | Often presents as painless thyroiditis, progressing to hypothyroidism; elevated TPO antibodies are common. May present as transient hyperthyroidism followed by hypothyroidism (biphasic course) |
| Pituitary | Diffuse or focal increased uptake in the sella turcica | Hypophysitis (especially with anti-CTLA-4) | Mild-to-moderate diffuse uptake; pituitary may be enlarged; can mimic pituitary adenoma | Symptoms: headache, fatigue, nausea, visual changes; lab: ↓ACTH, ↓TSH, ↓LH/FSH, ↓cortisol |
| Adrenal glands | Mild diffuse bilateral uptake (rarely focal) | Adrenalitis (immune-related adrenal insufficiency) | Bilateral symmetric uptake or volume loss (if late phase); often subtle | Primary adrenal insufficiency: ↓cortisol, ↑ACTH, fatigue, hypotension |
| Pancreas | Diffuse increased uptake | Immune-related pancreatitis | Pancreatic enlargement, homogeneous increased uptake | Elevated lipase/amylase; symptoms may be absent or include abdominal pain; may require steroids |
| Pros | |
|---|---|
| Advantages | Description |
| Incidental early detection | PET/CT is often performed for oncologic staging/restaging, allowing opportunistic identification of endocrine AEs even before symptoms occur. |
| Whole-body assessment | Enables simultaneous evaluation of multiple endocrine organs (thyroid, adrenal, pituitary, pancreas). Useful in systemic immune-related events. |
| Non-invasive functional imaging | Highlights metabolic changes (inflammation, hyperactivity) before structural changes appear on CT or MRI. |
| Correlates with inflammation | FDG uptake often corresponds to immune-related inflammation, helping differentiate autoimmune from metastatic or degenerative causes |
| Potential prognostic relevance | Immune-related AEs, visible on PET, may correlate with better response to immunotherapy (ongoing area of research). |
| Cons | |
| Limitations | Description |
| Not specific or diagnostic | FDG uptake is nonspecific: cannot distinguish between inflammation, infection, or malignancy without clinical/lab correlation. |
| Low sensitivity for some AEs | Many endocrine AEs are biochemically silent or not metabolically active enough to be detected (e.g., central hypothyroidism). |
| Not routinely indicated | PET/CT is not recommended for diagnosis or follow-up of endocrine AEs—labs and clinical signs usually monitor these. |
| Risk of overinterpretation | Incidental uptake may lead to unnecessary testing or anxiety if not carefully contextualized. |
| Radiation and cost | Repeated PET scans have radiation exposure and financial costs, not justifiable solely for AE detection. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giovanella, L.; Tuncel, M.; Campennì, A.; Ruggeri, R.M.; Huellner, M.; Petranović Ovčariček, P. Endocrine Adverse Events Induced by Cancer Treatments: The Role of 18F-Fluorodeoxyglucose Positron Emission Tomography. Cancers 2025, 17, 2651. https://doi.org/10.3390/cancers17162651
Giovanella L, Tuncel M, Campennì A, Ruggeri RM, Huellner M, Petranović Ovčariček P. Endocrine Adverse Events Induced by Cancer Treatments: The Role of 18F-Fluorodeoxyglucose Positron Emission Tomography. Cancers. 2025; 17(16):2651. https://doi.org/10.3390/cancers17162651
Chicago/Turabian StyleGiovanella, Luca, Murat Tuncel, Alfredo Campennì, Rosaria Maddalena Ruggeri, Martin Huellner, and Petra Petranović Ovčariček. 2025. "Endocrine Adverse Events Induced by Cancer Treatments: The Role of 18F-Fluorodeoxyglucose Positron Emission Tomography" Cancers 17, no. 16: 2651. https://doi.org/10.3390/cancers17162651
APA StyleGiovanella, L., Tuncel, M., Campennì, A., Ruggeri, R. M., Huellner, M., & Petranović Ovčariček, P. (2025). Endocrine Adverse Events Induced by Cancer Treatments: The Role of 18F-Fluorodeoxyglucose Positron Emission Tomography. Cancers, 17(16), 2651. https://doi.org/10.3390/cancers17162651





