Discoidin Domain Receptor 2 Contributes to Breast Cancer Progression and Chemoresistance by Interacting with Collagen Type I
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Tissues
2.2. Immunohistochemistry
2.3. Scoring of Immunohistochemistry
2.4. Cell Line and Chemicals
2.5. Plasmid Construction, siRNAs, and Transfection
2.6. Immunoblotting
2.7. RT-qPCR
2.8. Cell Proliferation Assay
2.9. Caspase 3/7 Assay
2.10. Statistical Analysis
3. Results
3.1. Immunolocalization of DDR2 and Collagen Type I in Human Breast Cancer
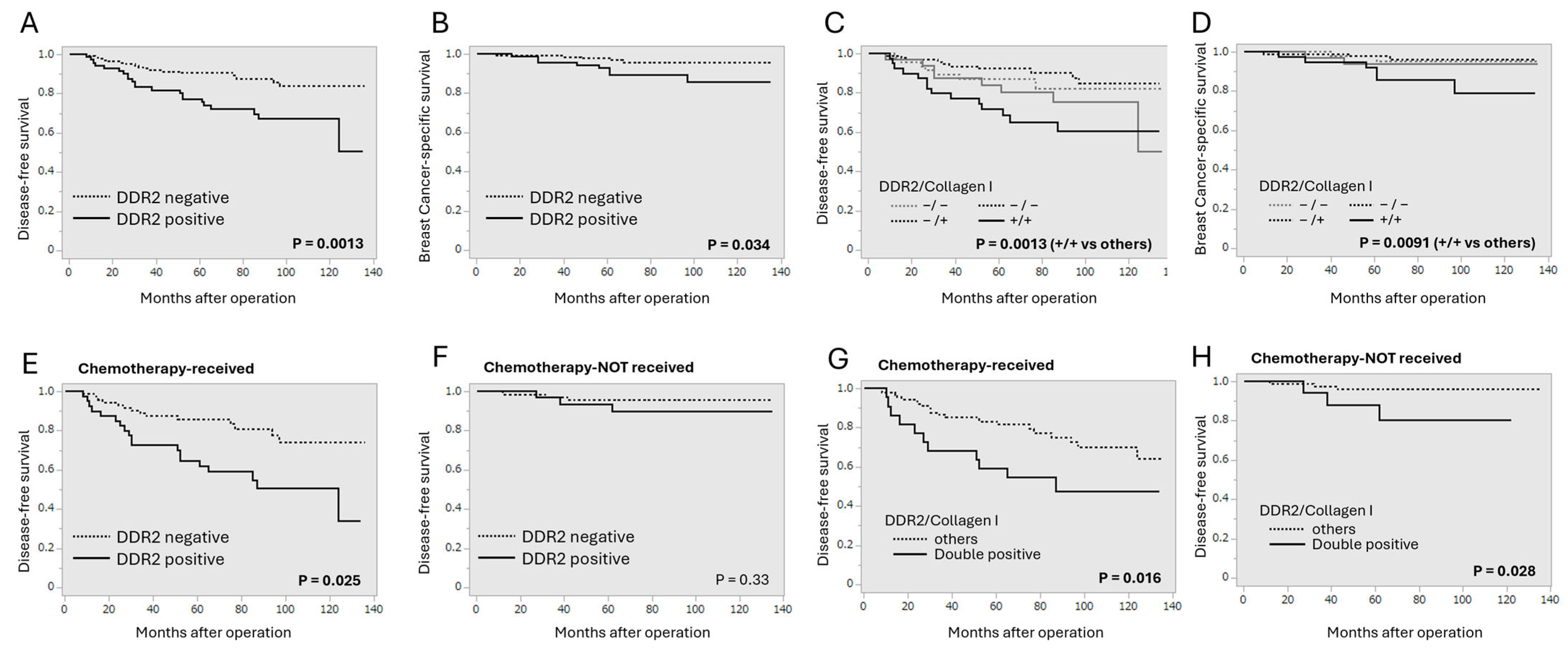
3.2. Correlation Between DDR2 and Collagen Type I Immunoreactivity and Clinical Outcome of Breast Cancer Patients
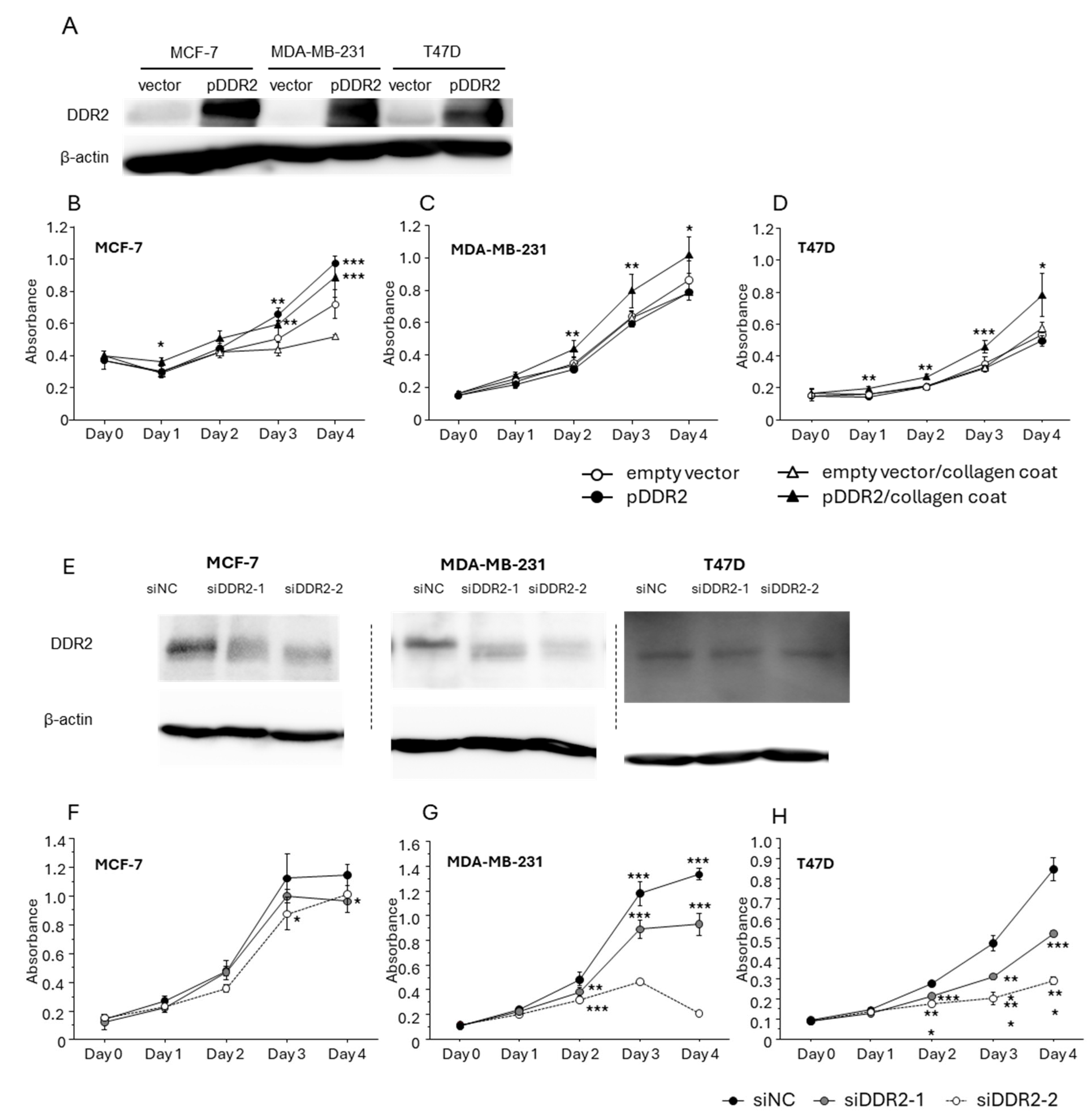
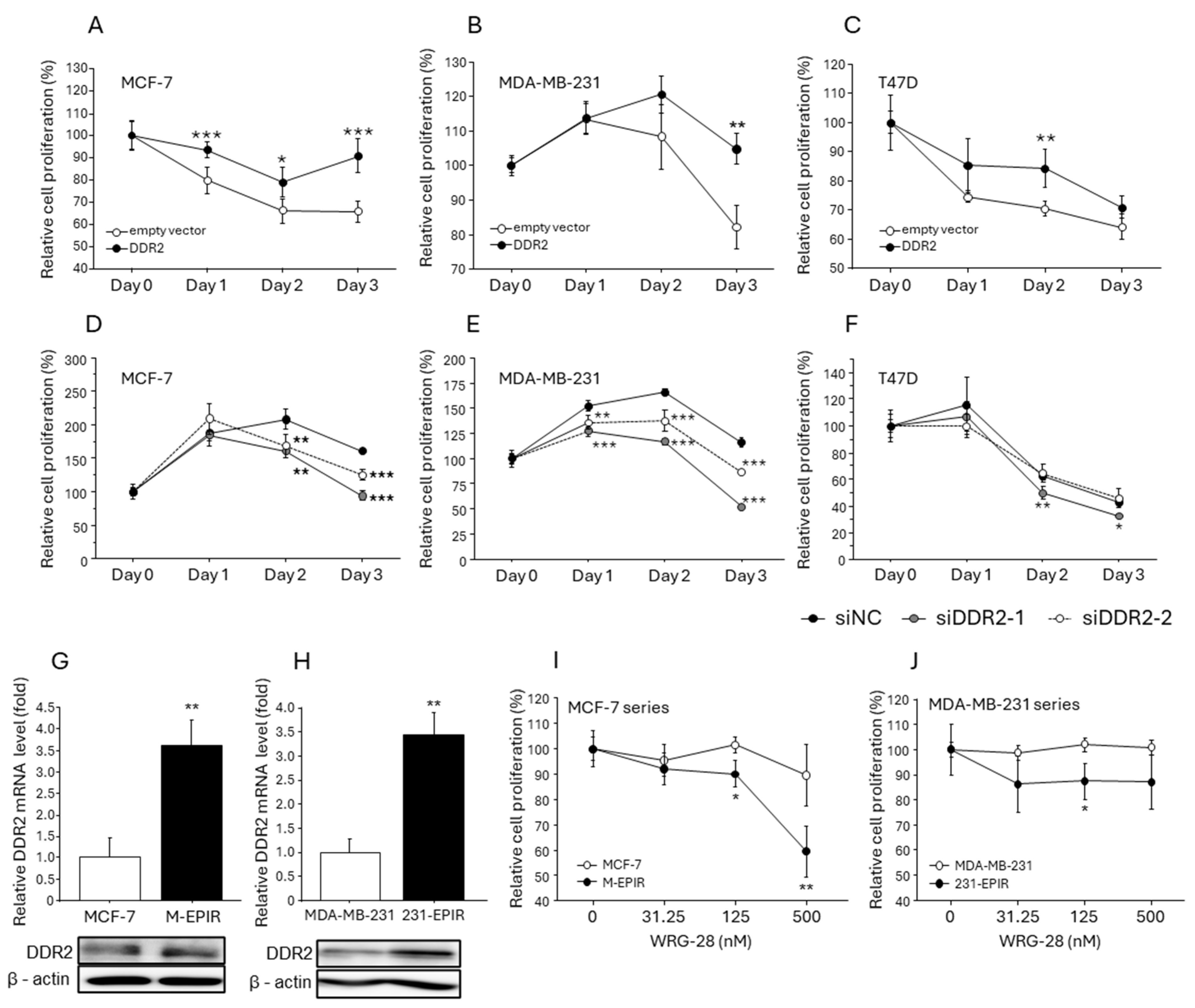
3.3. Effect of DDR-2 on the Proliferation of Breast Cancer Cells
3.4. Increased DDR2 Expression in EPI-Resistant Breast Cancer Cells and the Effect of DDR2 on Chemotherapy Resistance in Breast Cancer Cells
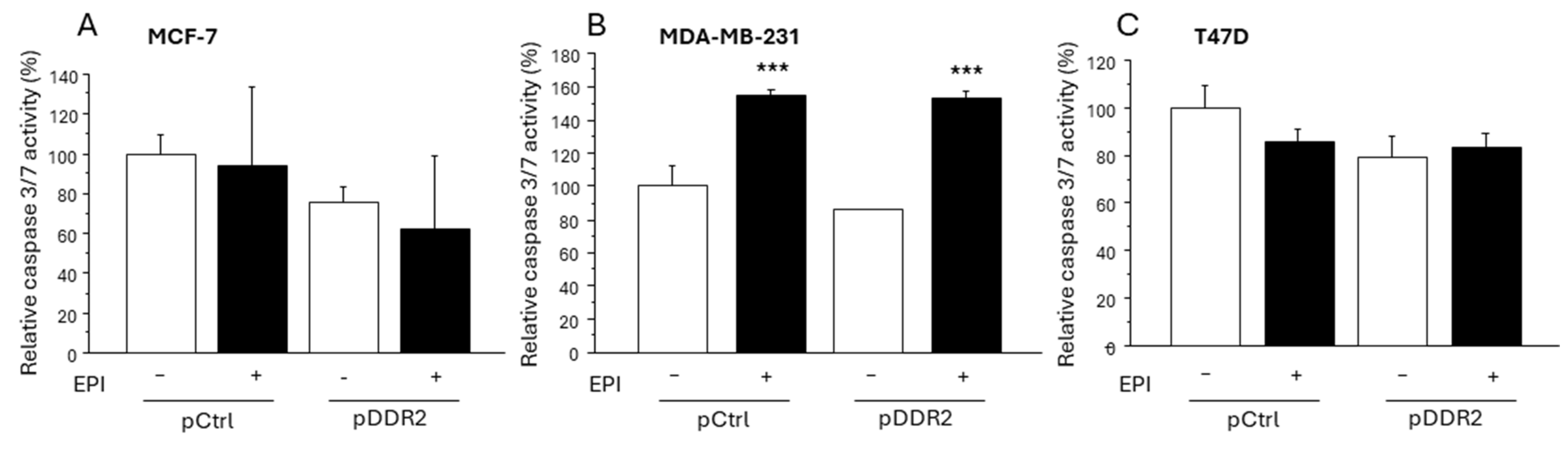
3.5. Effect of DDR-2 on the Apoptosis of Breast Cancer Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today (Version 1.1); International Agency for Research on Cancer: Lyon, France, 2024. Available online: https://gco.iarc.who.int/today (accessed on 26 September 2024).
- Prihantono Faruk, M. Breast cancer resistance to chemotherapy: When should we suspect it and how can we prevent it? Ann. Med. Surg. 2021, 70, 102793. [Google Scholar] [CrossRef] [PubMed]
- Senthebane, D.A.; Rowe, A.; Thomford, N.E.; Shipanga, H.; Munro, D.; Mazeedi, M.A.M.A.; Almazyadi, H.A.M.; Kallmeyer, K.; Dandara, C.; Pepper, M.S.; et al. The Role of Tumor Microenvironment in Chemoresistance: To Survive, Keep Your Enemies Closer. Int. J. Mol. Sci. 2017, 18, 1586. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, Y.; Tang, J.; Qin, S.; Shen, X.; He, S.; Ju, S. CAM-DR: Mechanisms, Roles and Clinical Application in Tumors. Front. Cell Dev. Biol. 2021, 9, 698047. [Google Scholar] [CrossRef]
- Shenoy, M.; Abdul, N.S.; Qamar, Z.; Bahri, B.M.A.; Al Ghalayini, K.Z.K.; Kakti, A. Collagen Structure, Synthesis, and Its Applications: A Systematic Review. Cureus 2022, 14, e24856. [Google Scholar] [CrossRef]
- Xu, S.; Xu, H.; Wang, W.; Li, S.; Li, H.; Li, T.; Zhang, W.; Yu, X.; Liu, L. The role of collagen in cancer: From bench to bedside. J. Transl. Med. 2019, 17, 309. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhou, J.; Li, J. Discoidin domain receptors orchestrate cancer progression: A focus on cancer therapies. Cancer Sci. 2021, 112, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Leitinger, B.; Hohenester, E. Mammalian collagen receptors. Matrix Biol. 2007, 26, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.; Chander, H.; Munshi, A. Complex roles of discoidin domain receptor tyrosine kinases in cancer. Clin. Transl. Oncol. 2021, 23, 1497–1510. [Google Scholar] [CrossRef]
- Ren, T.; Zhang, J.; Zhang, J.; Liu, X.; Yao, L. Increased expression of discoidin domain receptor 2 (DDR2): A novel independent prognostic marker of worse outcome in breast cancer patients. Med. Oncol. 2013, 30, 397. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Ying, J.; Dai, M.; Peng, J.; Zhang, D. Co-expression of DDR2 and IFITM1 promotes breast cancer cell proliferation, migration and invasion and inhibits apoptosis. J. Cancer Res. Clin. Oncol. 2022, 148, 3385–3398, Erratum in J. Cancer Res. Clin. Oncol. 2024, 150, 166. [Google Scholar] [CrossRef]
- Mitchell, A.V.; Wu, J.; Meng, F.; Dong, L.; Block, C.J.; Song, W.M.; Zhang, B.; Li, J.; Wu, G. DDR2 coordinates EMT and metabolic reprogramming as a shared effector of FOXQ1 and SNAI1. Cancer Res. Commun. 2022, 2, 1388–1403. [Google Scholar] [CrossRef] [PubMed]
- Bayer, S.V.; Grither, W.R.; Brenot, A.; Hwang, P.Y.; Barcus, C.E.; Ernst, M.; Pence, P.; Walter, C.; Pathak, A.; Longmore, G.D. DDR2 controls breast tumor stiffness and metastasis by regulating integrin mediated mechanotransduction in CAFs. Elife 2019, 8, e45508. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, W.; Zhang, F.; Quan, B.; Yao, F.; Chen, R.; Ren, Z.; Dong, L.; Yin, X. DDR2/STAT3 Positive Feedback Loop Mediates the Immunosuppressive Microenvironment by Upregulating PD-L1 and Recruiting MDSCs in Oxaliplatin-Resistant HCC. Cell Mol. Gastroenterol. Hepatol. 2024, 18, 101377. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Yan, Z.; Wu, C.; Zhou, X.; Bao, W. DNA methylation characteristics associated with chemotherapy resistance in epithelial ovarian cancer. Heliyon 2024, 10, e27212. [Google Scholar] [CrossRef]
- Beauchamp, E.M.; Woods, B.A.; Dulak, A.M.; Tan, L.; Xu, C.; Gray, N.S.; Bass, A.J.; Wong, K.K.; Meyerson, M.; Hammerman, P.S. Acquired resistance to dasatinib in lung cancer cell lines conferred by DDR2 gatekeeper mutation and NF1 loss. Mol. Cancer Ther. 2014, 13, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.Q.; Liu, Y.W.; Xie, Y.K.; Zhang, J.H.; Song, C.X.; Wang, J.Z.; Xie, B.H. Amplification of DDR2 mediates sorafenib resistance through NF-κB/c-Rel signaling in hepatocellular carcinoma. Cell Biol. Int. 2021, 45, 1906–1916. [Google Scholar] [CrossRef]
- Sala, M.; Allain, N.; Moreau, M.; Jabouille, A.; Henriet, E.; Abou-Hammoud, A.; Uguen, A.; Di-Tommaso, S.; Dourthe, C.; Raymond, A.A.; et al. Discoidin Domain Receptor 2 orchestrates melanoma resistance combining phenotype switching and proliferation. Oncogene 2022, 41, 2571–2586. [Google Scholar] [CrossRef]
- Loeffler, M.; Krüger, J.A.; Niethammer, A.G.; Reisfeld, R.A. Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. J. Clin. Investig. 2006, 116, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Jakubzig, B.; Baltes, F.; Henze, S.; Schlesinger, M.; Bendas, G. Mechanisms of Matrix-Induced Chemoresistance of Breast Cancer Cells-Deciphering Novel Potential Targets for a Cell Sensitization. Cancers 2018, 10, 495. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Yuan, J.; Peng, C.; Li, Y. Collagen as a double-edged sword in tumor progression. Tumour Biol. 2014, 35, 2871–2882. [Google Scholar] [CrossRef] [PubMed]
- Cross, V.L.; Zheng, Y.; Won Choi, N.; Verbridge, S.S.; Sutermaster, B.A.; Bonassar, L.J.; Fischbach, C.; Stroock, A.D. Dense type I collagen matrices that support cellular remodeling and microfabrication for studies of tumor angiogenesis and vasculogenesis in vitro. Biomaterials 2010, 31, 8596–8607. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, K.Y.; Rivera, L.B.; Hur, H.; Carbon, J.G.; Toombs, J.E.; Goldstein, C.D.; Dellinger, M.T.; Castrillon, D.H.; Brekken, R.A. Collagen signaling enhances tumor progression after anti-VEGF therapy in a murine model of pancreatic ductal adenocarcinoma. Cancer Res. 2014, 74, 1032–1044. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, J.; Wang, X.; Li, C.; Ma, Z.; Wan, Q.; Peng, F. New advances in the research of clinical treatment and novel anticancer agents in tumor angiogenesis. Biomed. Pharmacother. 2023, 163, 114806. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.A.; McInnes, K.J.; Takagi, K.; Ono, K.; Hunger, N.I.; Wang, L.; Sasano, H.; Simpson, E.R. LKB1 expression is inhibited by estradiol-17β in MCF-7 cells. J. Steroid Biochem. Mol. Biol. 2011, 127, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Toy, K.A.; Valiathan, R.R.; Núñez, F.; Kidwell, K.M.; Gonzalez, M.E.; Fridman, R.; Kleer, C.G. Tyrosine kinase discoidin domain receptors DDR1 and DDR2 are coordinately deregulated in triple-negative breast cancer. Breast Cancer Res. Treat. 2015, 150, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.H.; Ji, C.D.; Xiao, H.L.; Zhao, H.B.; Cui, Y.H.; Bian, X.W. Reorganized Collagen in the Tumor Microenvironment of Gastric Cancer and Its Association with Prognosis. J. Cancer 2017, 8, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- Hammond, M.E.; Hayes, D.F.; Dowsett, M.; Allred, D.C.; Hagerty, K.L.; Badve, S.; Fitzgibbons, P.L.; Francis, G.; Goldstein, N.S.; Hayes, M.; et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch. Pathol. Lab. Med. 2010, 134, e48–e72. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Takagi, K.; Narita, K.; Miki, Y.; Onodera, Y.; Miyashita, M.; Sasano, H.; Suzuki, T. Stromal CCL5 Promotes Breast Cancer Progression by Interacting with CCR3 in Tumor Cells. Int. J. Mol. Sci. 2021, 22, 1918. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, S.; Takagi, K.; Yamaguchi-Tanaka , M.; Sato, A.; Miki, Y.; Miyashita, M.; Tada, H.; Ishida, T.; Suzuki, T. Receptor for Hyaluronan Mediated Motility (RHAMM)/Hyaluronan Axis in Breast Cancer Chemoresistance. Cancers 2024, 16, 3600. [Google Scholar] [CrossRef]
- Grither, W.R.; Longmore, G.D. Inhibition of tumor-microenvironment interaction and tumor invasion by small-molecule allosteric inhibitor of DDR2 extracellular domain. Proc. Natl. Acad. Sci. USA 2018, 115, E7786–E7794. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Takagi, K.; Yoshimura, A.; Tsukamoto, W.; Yamaguchi-Tanaka, M.; Miki, Y.; Ebata, A.; Miyashita, M.; Suzuki, T. Kallikrein-Related Peptidase 12 (KLK12) in Breast Cancer as a Favorable Prognostic Marker. Int. J. Mol. Sci. 2023, 24, 8419. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi-Tanaka, M.; Takagi, K.; Miki, Y.; Sato, A.; Iwabuchi, E.; Miyashita, M.; Suzuki, T. The Pro-Tumorigenic Role of Chemotherapy-Induced Extracellular HSP70 from Breast Cancer Cells via Intratumoral Macrophages. Cancers 2023, 15, 1903. [Google Scholar] [CrossRef] [PubMed]
- Takagi, K.; Miki, Y.; Onodera, Y.; Ishida, T.; Watanabe, M.; Sasano, H.; Suzuki, T. ARHGAP15 in Human Breast Carcinoma: A Potent Tumor Suppressor Regulated by Androgens. Int. J. Mol. Sci. 2018, 19, 804. [Google Scholar] [CrossRef] [PubMed]
- Takagi, K.; Miki, Y.; Tanaka, S.; Hashimoto, C.; Watanabe, M.; Sasano, H.; Ito, K.; Suzuki, T. Nucleobindin 2 (NUCB2) in human endometrial carcinoma: A potent prognostic factor associated with cell proliferation and migration. Endocr. J. 2016, 63, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Insua-Rodríguez, J.; Oskarsson, T. The extracellular matrix in breast cancer. Adv. Drug Deliv. Rev. 2016, 97, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Salimian, N.; Peymani, M.; Ghaedi, K.; Hashemi, M.; Rahimi, E. Collagen 1A1 (COL1A1) and Collagen11A1(COL11A1) as diagnostic biomarkers in Breast, colorectal and gastric cancers. Gene 2024, 892, 147867. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Yang, W.H.; Lin, Y.T.; Tang, X.; Chen, P.H.; Ding, C.C.; Qu, D.C.; Alvarez, J.V.; Chi, J.T. DDR2 upregulation confers ferroptosis susceptibility of recurrent breast tumors through the Hippo pathway. Oncogene 2021, 40, 2018–2034. [Google Scholar] [CrossRef]
- Kurashige, J.; Hasegawa, T.; Niida, A.; Sugimachi, K.; Deng, N.; Mima, K.; Uchi, R.; Sawada, G.; Takahashi, Y.; Eguchi, H.; et al. Integrated Molecular Profiling of Human Gastric Cancer Identifies DDR2 as a Potential Regulator of Peritoneal Dissemination. Sci. Rep. 2016, 6, 22371. [Google Scholar] [CrossRef]
- Cechowska-Pasko, M.; Krętowski, R.; Bańkowski, E. Glucose deficiency reduces collagen synthesis in breast cancer MCF7 cells. Cell Biol. Int. 2011, 35, 141–145. [Google Scholar] [CrossRef]
- Audun Klingen, T.; Chen, Y.; Aas, H.; Akslen, L.A. DDR2 expression in breast cancer is associated with blood vessel invasion, basal-like tumors, tumor associated macrophages, regulatory T cells, detection mode and prognosis. Hum. Pathol. 2024, 150, 29–35. [Google Scholar] [CrossRef]
- Gonzalez, M.E.; Martin, E.E.; Anwar, T.; Arellano-Garcia, C.; Medhora, N.; Lama, A.; Chen, Y.C.; Tanager, K.S.; Yoon, E.; Kidwell, K.M.; et al. Mesenchymal Stem Cell-Induced DDR2 Mediates Stromal-Breast Cancer Interactions and Metastasis Growth. Cell Rep. 2017, 18, 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Corsa, C.A.; Ponik, S.M.; Prior, J.L.; Piwnica-Worms, D.; Eliceiri, K.W.; Keely, P.J.; Longmore, G.D. The collagen receptor discoidin domain receptor 2 stabilizes SNAIL1 to facilitate breast cancer metastasis. Nat. Cell Biol. 2013, 15, 677–687. [Google Scholar] [CrossRef]
- Kaur, B.; Mukhlis, Y.; Natesh, J.; Penta, D.; Musthapa Meeran, S. Identification of hub genes associated with EMT-induced chemoresistance in breast cancer using integrated bioinformatics analysis. Gene 2022, 809, 146016. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.; Arani, H.Z.; Orouei, S.; Fallah, S.; Ghorbani, A.; Khaledabadi, M.; Kakavand, A.; Tavakolpournegari, A.; Saebfar, H.; Heidari, H.; et al. EMT mechanism in breast cancer metastasis and drug resistance: Revisiting molecular interactions and biological functions. Biomed. Pharmacother. 2022, 155, 113774. [Google Scholar] [CrossRef] [PubMed]
- Baltes, F.; Pfeifer, V.; Silbermann, K.; Caspers, J.; Wantoch von Rekowski, K.; Schlesinger, M.; Bendas, G. β1-Integrin binding to collagen type 1 transmits breast cancer cells into chemoresistance by activating ABC efflux transporters. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118663. [Google Scholar] [CrossRef]

| DDR2 | Collagen Type I | |||||
|---|---|---|---|---|---|---|
| Negative (n = 149) | Positive (n = 75) | p | Negative (n = 86) | Positive (n = 138) | p | |
| Age * | 55 (31–88) | 56 (27–82) | 0.86 | 55 (29–88) | 55 (27–85) | 0.39 |
| pT | ||||||
| pT1 | 109 | 46 | 65 | 90 | ||
| pT2–4 | 40 | 29 | 0.071 | 21 | 48 | 0.10 |
| Lmph node metastasis | ||||||
| Negative | 100 | 51 | 65 | 86 | ||
| Positive | 49 | 24 | 0.89 | 21 | 52 | 0.039 |
| Stage | ||||||
| I | 90 | 38 | 56 | 72 | ||
| II | 38 | 23 | 21 | 40 | ||
| III | 21 | 14 | 0.37 | 9 | 26 | 0.11 |
| Histological grade | ||||||
| 1 | 67 | 15 | 27 | 55 | ||
| 2 | 56 | 42 | 37 | 61 | ||
| 3 | 26 | 18 | 0.0012 | 22 | 22 | 0.17 |
| ER | ||||||
| Negative | 25 | 16 | 20 | 21 | ||
| Positive | 124 | 59 | 0.41 | 66 | 117 | 0.13 |
| PR | ||||||
| Negative | 42 | 28 | 29 | 41 | ||
| Positive | 107 | 47 | 0.16 | 57 | 97 | 0.53 |
| HER2 | ||||||
| Negative | 128 | 61 | 70 | 119 | ||
| Positive | 21 | 14 | 0.37 | 17 | 19 | 0.33 |
| Ki67 LI (%) * | 10 (1–60) | 17 (1–72) | 0.0004 | 14 (2–53) | 12 (1–72) | 0.0076 |
| Neoadjuvant chemotherapy | ||||||
| Not received | 129 | 55 | 77 | 107 | ||
| Received | 19 | 20 | 0.010 | 8 | 31 | 0.013 |
| Collagen I | ||||||
| Negative | 51 | 35 | ||||
| Positive | 98 | 40 | 0.071 | |||
| Collagen I-Negative Group | Collagen I-Positive Group | |||||
|---|---|---|---|---|---|---|
| DDR2 | DDR2 | |||||
| Negative (n = 51)) | Positive (n = 35) | p | Negative (n = 124) | Positive (n = 59) | p | |
| Age * | 56 (31–88) | 58 (29–82) | 0.46 | 55 (33–85) | 55 (27–77) | 0.26 |
| pT | ||||||
| pT1 | 39 | 26 | 70 | 20 | ||
| pT2–4 | 12 | 9 | 0.82 | 28 | 20 | 0.017 |
| Lymph node metastasis | ||||||
| Negative | 38 | 27 | 62 | 24 | ||
| Positive | 13 | 8 | 0.78 | 36 | 16 | 0.72 |
| Stage | ||||||
| I | 34 | 22 | 56 | 16 | ||
| II | 14 | 7 | 24 | 16 | ||
| III | 3 | 6 | 0.22 | 18 | 8 | 0.14 |
| Histological grade | ||||||
| 1 | 21 | 6 | 46 | 9 | ||
| 2 | 16 | 21 | 40 | 21 | ||
| 3 | 14 | 8 | 0.019 | 12 | 10 | 0.018 |
| ER | ||||||
| Negative | 12 | 8 | 13 | 8 | ||
| Positive | 39 | 27 | 0.94 | 85 | 32 | 0.32 |
| PR | ||||||
| Negative | 19 | 10 | 23 | 18 | ||
| Positive | 32 | 25 | 0.40 | 75 | 22 | 0.012 |
| HER2 | ||||||
| Negative | 44 | 26 | 84 | 35 | ||
| Positive | 7 | 9 | 0.16 | 14 | 5 | 0.78 |
| Ki67 LI (%) * | 12 (2–52) | 16 (3–53) | 0.12 | 9 (1–60) | 18 (1–72) | 0.0033 |
| All Cases | Chemotherapy-Received Group | |||||
|---|---|---|---|---|---|---|
| Uni- | Multi- | Uni- | Multi- | |||
| p | p | CI | p | p | CI | |
| pT | <0.0001 * | 0.012 | 2.7 (1.2–5.7) | 0.063 | ||
| pT2–4/pT1 | ||||||
| Lymph node metastasis | 0.032 * | 0.31 | 1.5 (0.70–3.1) | 0.87 | ||
| Positive/Negative | ||||||
| Histological grade | 0.032 * | 0.26 | 0.60 (0.24–1.5) | 0.81 | ||
| 3/1+2 | ||||||
| ER | 0.012 * | 0.82 | 1.1 (0.42–3.0) | 0.38 | ||
| Negative/Positive | ||||||
| PR | 0.0006 * | 0.069 | 2.1 (0.94–4.9) | 0.049 * | 0.0004 | 3.7 (1.8–7.8) |
| Negative/Positive | ||||||
| HER2 | 0.13 | 0.032 * | 0.0036 | 0.16 (0.048–0.55) | ||
| Positive/Negative | ||||||
| Ki67 | 0.0002 * | 0.031 | 2.4 (1.1–5.3) | 0.072 | ||
| ≤20/<20 | ||||||
| DDR2 | 0.0019 * | 0.0095 | 2.4 (1.2–4.7) | 0.0038 * | 0.0033 | 2.8 (1.4–5.7) |
| Positive/Negative | ||||||
| Collagen type I | 0.94 | 0.75 | ||||
| Positive/Negative | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, A.; Takagi, K.; Yoshida, M.; Yamaguchi-Tanaka, M.; Sagehashi, M.; Miki, Y.; Miyashita, M.; Suzuki, T. Discoidin Domain Receptor 2 Contributes to Breast Cancer Progression and Chemoresistance by Interacting with Collagen Type I. Cancers 2024, 16, 4285. https://doi.org/10.3390/cancers16244285
Sato A, Takagi K, Yoshida M, Yamaguchi-Tanaka M, Sagehashi M, Miki Y, Miyashita M, Suzuki T. Discoidin Domain Receptor 2 Contributes to Breast Cancer Progression and Chemoresistance by Interacting with Collagen Type I. Cancers. 2024; 16(24):4285. https://doi.org/10.3390/cancers16244285
Chicago/Turabian StyleSato, Ai, Kiyoshi Takagi, Momoka Yoshida, Mio Yamaguchi-Tanaka, Mikoto Sagehashi, Yasuhiro Miki, Minoru Miyashita, and Takashi Suzuki. 2024. "Discoidin Domain Receptor 2 Contributes to Breast Cancer Progression and Chemoresistance by Interacting with Collagen Type I" Cancers 16, no. 24: 4285. https://doi.org/10.3390/cancers16244285
APA StyleSato, A., Takagi, K., Yoshida, M., Yamaguchi-Tanaka, M., Sagehashi, M., Miki, Y., Miyashita, M., & Suzuki, T. (2024). Discoidin Domain Receptor 2 Contributes to Breast Cancer Progression and Chemoresistance by Interacting with Collagen Type I. Cancers, 16(24), 4285. https://doi.org/10.3390/cancers16244285






