Metabolic Imaging in Electrochemotherapy: Insights from FDG-PET Analysis in Metastatic Melanoma—A Pilot Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Radiopharmaceutical and Imaging Protocol
2.3. Electrochemotherapy
2.4. Image Analysis
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
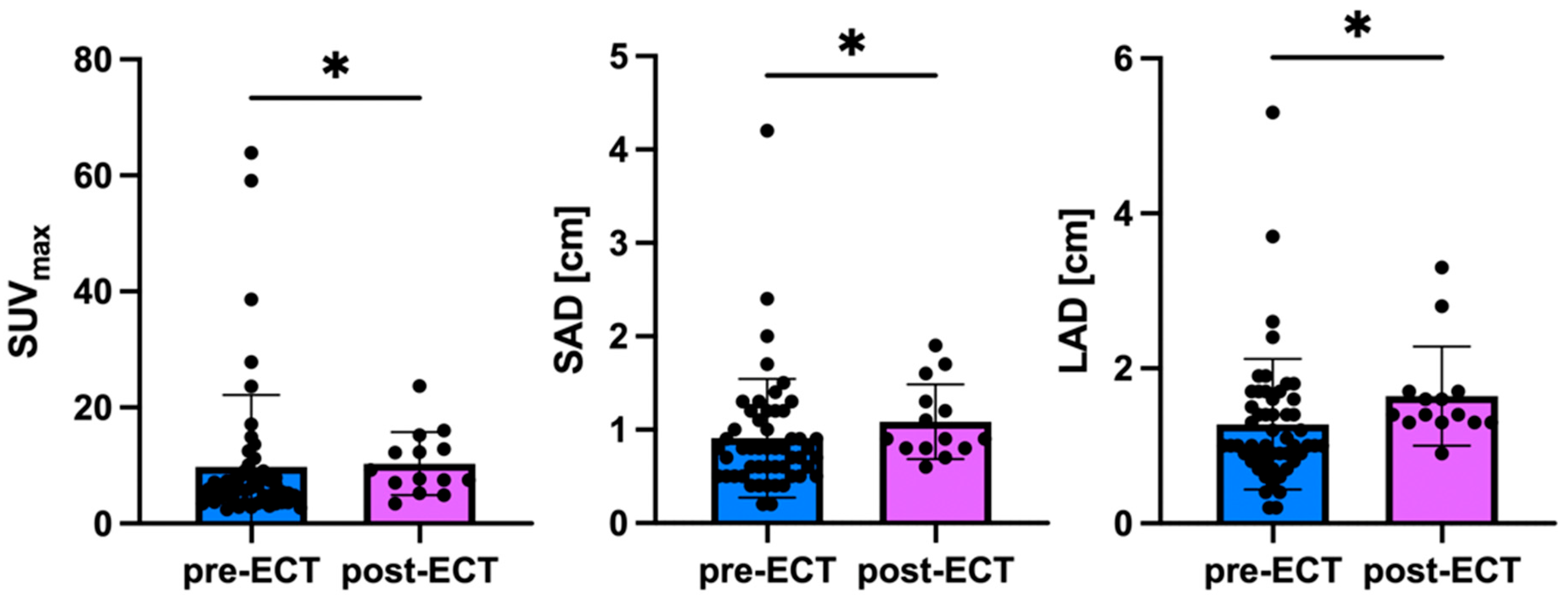
3.2. Lesion Detection and Imaging Characteristics
3.3. Impact of Concurrent Systemic Therapy
3.4. Follow-Up Imaging
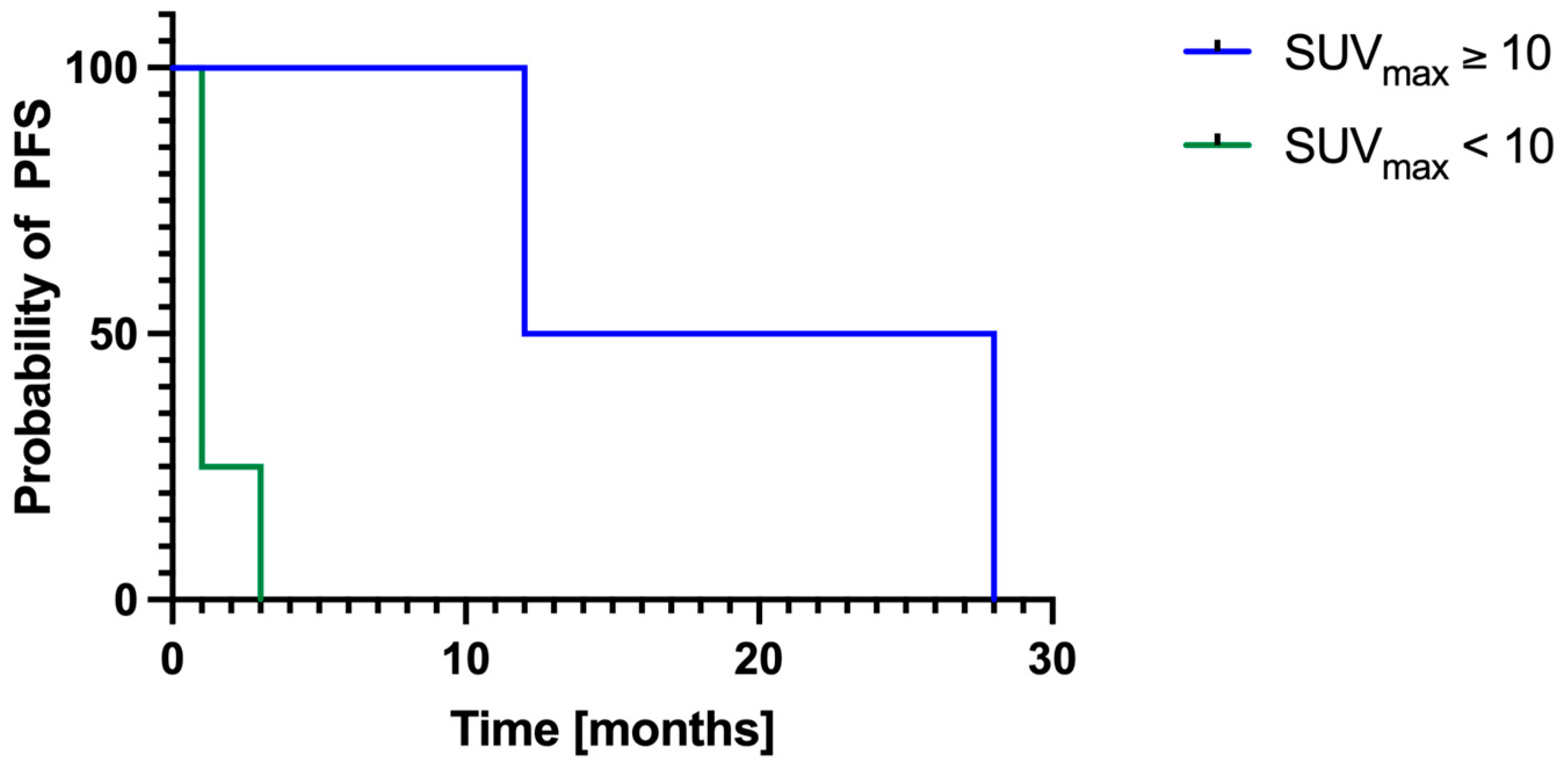
3.5. Local Progression-Free Survival
4. Discussion
4.1. Prognostic Value of Pre-ECT SUVmax
4.2. Impact of Concurrent Systemic Therapy on FDG Uptake
4.3. Interpretation of Post-ECT Findings
4.4. Complementarity of FDG PET/CT and Conventional CT
4.5. Study Limitations
4.6. Clinical Heterogeneity and Real-World Implications
4.7. Comparison of PET and CT in Response Assessment
4.8. Challenges in Long-Term Follow-Up
4.9. Technical Considerations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALM | acral lentiginous melanoma |
| CT | computed tomography |
| ECT | electrochemotherapy |
| F | female |
| FDG | [18F]2fluoro-2-deoxy-D-glucose |
| LAD | long axis diameter |
| M | male |
| n.a. | not available |
| NM | nodular melanoma |
| PET | positron emission tomography |
| PFS | progression-free survival |
| SAD | short axis diameter |
| SD | standard deviation |
| SSM | superficial spreading melanoma |
| SUVmax | maximum standardized uptake value |
References
- Guo, W.; Wang, H.; Li, C. Signal pathways of melanoma and targeted therapy. Signal Transduct. Target. Ther. 2021, 6, 424. [Google Scholar] [CrossRef]
- Rashid, S.; Shaughnessy, M.; Tsao, H. Melanoma classification and management in the era of molecular medicine. Dermatol. Clin. 2023, 41, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Scolyer, R.A.; Long, G.V.; Thompson, J.F. Evolving concepts in melanoma classification and their relevance to multidisciplinary melanoma patient care. Mol. Oncol. 2011, 5, 124–136. [Google Scholar] [CrossRef]
- Arozarena, I.; Wellbrock, C. Overcoming resistance to BRAF inhibitors. Ann. Transl. Med. 2017, 5, 387. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.C.; Tanabe, K.K.; Ariyan, C.E.; Miura, J.T.; Mutabdzic, D.; Farma, J.M.; Zager, J.S. Local and recurrent regional metastases of melanoma. In Cutaneous Melanoma; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–33. [Google Scholar]
- Savoia, P.; Fava, P.; Nardò, T.; Osella-Abate, S.; Quaglino, P.; Bernengo, M.G. Skin metastases of malignant melanoma: A clinical and prognostic survey. Melanoma Res. 2009, 19, 321–326. [Google Scholar] [CrossRef]
- Boutros, A.; Croce, E.; Ferrari, M.; Gili, R.; Massaro, G.; Marconcini, R.; Arecco, L.; Tanda, E.T.; Spagnolo, F. The treatment of advanced melanoma: Current approaches and new challenges. Crit. Rev. Oncol. Hematol. 2024, 196, 104276. [Google Scholar] [CrossRef] [PubMed]
- Rubegni, P.; Lamberti, A.; Mandato, F.; Perotti, R.; Fimiani, M. Dermoscopic patterns of cutaneous melanoma metastases. Int. J. Dermatol. 2014, 53, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Campana, L.G.; Miklavčič, D.; Bertino, G.; Marconato, R.; Valpione, S.; Imarisio, I.; Dieci, M.V.; Granziera, E.; Cemazar, M.; Alaibac, M.; et al. Electrochemotherapy of superficial tumors—Current status:: Basic principles, operating procedures, shared indications, and emerging applications. Semin. Oncol. 2019, 46, 173–191. [Google Scholar] [CrossRef] [PubMed]
- Heller, R.; Jaroszeski, M.J.; Reintgen, D.S.; Puleo, C.A.; DeConti, R.C.; Gilbert, R.A.; Glass, L.F. Treatment of cutaneous and subcutaneous tumors with electrochemotherapy using intralesional bleomycin. Cancer 1998, 83, 148–157. [Google Scholar] [CrossRef]
- Sersa, G.; Stabuc, B.; Cemazar, M.; Jancar, B.; Miklavcic, D.; Rudolf, Z. Electrochemotherapy with cisplatin: Potentiation of local cisplatin antitumour effectiveness by application of electric pulses in cancer patients. Eur. J. Cancer 1998, 34, 1213–1218. [Google Scholar] [CrossRef]
- Giri, P.; Mittal, L.; Camarillo, I.G.; Sundararajan, R. Analysis of pathways in triple-negative breast cancer cells treated with the combination of electrochemotherapy and cisplatin. Biointerface Res. Appl. Chem. 2021, 11, 13453–13464. [Google Scholar] [CrossRef]
- Kunte, C.; Letulé, V.; Gehl, J.; Dahlstroem, K.; Curatolo, P.; Rotunno, R.; Muir, T.; Occhini, A.; Bertino, G.; Powell, B.; et al. Electrochemotherapy in the treatment of metastatic malignant melanoma: A prospective cohort study by InspECT. Br. J. Dermatol. 2017, 176, 1475–1485. [Google Scholar] [CrossRef]
- Campana, L.G.; Quaglino, P.; de Terlizzi, F.; Mascherini, M.; Brizio, M.; Spina, R.; Bertino, G.; Kunte, C.; Odili, J.; Matteucci, P.; et al. Health-related quality of life trajectories in melanoma patients after electrochemotherapy: Real-world insights from the InspECT register. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 2352–2363. [Google Scholar] [CrossRef]
- Campana, L.G.; Peric, B.; Mascherini, M.; Spina, R.; Kunte, C.; Kis, E.; Rozsa, P.; Quaglino, P.; Jones, R.P.; Clover, A.J.P.; et al. Combination of Pembrolizumab with Electrochemotherapy in Cutaneous Metastases from Melanoma: A Comparative Retrospective Study from the InspECT and Slovenian Cancer Registry. Cancers 2021, 13, 4289. [Google Scholar] [CrossRef]
- Lopci, E.; Hicks, R.J.; Dimitrakopoulou-Strauss, A.; Dercle, L.; Iravani, A.; Seban, R.D.; Sachpekidis, C.; Humbert, O.; Gheysens, O.; Glaudemans, A.; et al. Joint EANM/SNMMI/ANZSNM practice guidelines/procedure standards on recommended use of [(18)F]FDG PET/CT imaging during immunomodulatory treatments in patients with solid tumors version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2323–2341. [Google Scholar] [CrossRef]
- Albano, D.; Familiari, D.; Fornito, M.C.; Scalisi, S.; Laudicella, R.; Galia, M.; Grassedonio, E.; Ruggeri, A.; Ganduscio, G.; Messina, M.; et al. Clinical and Prognostic Value of (18)F-FDG-PET/CT in the Restaging Process of Recurrent Cutaneous Melanoma. Curr. Radiopharm. 2020, 13, 42–47. [Google Scholar] [CrossRef]
- Annovazzi, A.; Ferraresi, V.; Rea, S.; Russillo, M.; Renna, D.; Carpano, S.; Sciuto, R. Prognostic value of total metabolic tumour volume and therapy-response assessment by [(18)F]FDG PET/CT in patients with metastatic melanoma treated with BRAF/MEK inhibitors. Eur. Radiol. 2022, 32, 3398–3407. [Google Scholar] [CrossRef]
- van Elmpt, W.; Ollers, M.; Dingemans, A.M.; Lambin, P.; De Ruysscher, D. Response assessment using 18F-FDG PET early in the course of radiotherapy correlates with survival in advanced-stage non-small cell lung cancer. J. Nucl. Med. 2012, 53, 1514–1520. [Google Scholar] [CrossRef] [PubMed]
- Riedl, C.C.; Pinker, K.; Ulaner, G.A.; Ong, L.T.; Baltzer, P.; Jochelson, M.S.; McArthur, H.L.; Gönen, M.; Dickler, M.; Weber, W.A. Comparison of FDG-PET/CT and contrast-enhanced CT for monitoring therapy response in patients with metastatic breast cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1428–1437. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Candil, A.; Rodríguez-Rey, C.; Cano-Carrizal, R.; Cala-Zuluaga, E.; González Larriba, J.L.; Jiménez-Ballvé, A.; Fuentes-Ferrer, M.E.; Cabrera-Martín, M.N.; Pérez-Castejón, M.J.; García García-Esquinas, M.; et al. Breslow thickness and (18)F-FDG PET-CT result in initial staging of cutaneous melanoma: Can a cut-off point be established? Rev. Esp. Med. Nucl. Imagen Mol. 2016, 35, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Soret, M.; Bacharach, S.L.; Buvat, I. Partial-volume effect in PET tumor imaging. J. Nucl. Med. 2007, 48, 932–945. [Google Scholar] [CrossRef]
- Matthiessen, L.W.; Johannesen, H.H.; Skougaard, K.; Gehl, J.; Hendel, H.W. Dual time point imaging fluorine-18 flourodeoxyglucose positron emission tomography for evaluation of large loco-regional recurrences of breast cancer treated with electrochemotherapy. Radiol. Oncol. 2013, 47, 358–365. [Google Scholar] [CrossRef][Green Version]
- Gehl, J.; Sersa, G.; Matthiessen, L.W.; Muir, T.; Soden, D.; Occhini, A.; Quaglino, P.; Curatolo, P.; Campana, L.G.; Kunte, C.; et al. Updated standard operating procedures for electrochemotherapy of cutaneous tumours and skin metastases. Acta Oncol. 2018, 57, 874–882. [Google Scholar] [CrossRef]
- Mir, L.M.; Gehl, J.; Sersa, G.; Collins, C.G.; Garbay, J.-R.; Billard, V.; Geertsen, P.F.; Rudolf, Z.; O’Sullivan, G.C.; Marty, M. Standard operating procedures of the electrochemotherapy: Instructions for the use of bleomycin or cisplatin administered either systemically or locally and electric pulses delivered by the CliniporatorTM by means of invasive or non-invasive electrodes. Eur. J. Cancer Suppl. 2006, 4, 14–25. [Google Scholar] [CrossRef]
- Marty, M.; Sersa, G.; Garbay, J.R.; Gehl, J.; Collins, C.G.; Snoj, M.; Billard, V.; Geertsen, P.F.; Larkin, J.O.; Miklavcic, D. Electrochemotherapy–An easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: Results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. Eur. J. Cancer Suppl. 2006, 4, 3–13. [Google Scholar] [CrossRef]
- Gandy, N.; Arshad, M.A.; Wallitt, K.L.; Dubash, S.; Khan, S.; Barwick, T.D. Immunotherapy-related adverse effects on (18)F-FDG PET/CT imaging. Br. J. Radiol. 2020, 93, 20190832. [Google Scholar] [CrossRef]
- Anderson, T.M.; Chang, B.H.; Huang, A.C.; Xu, X.; Yoon, D.; Shang, C.G.; Mick, R.; Schubert, E.; McGettigan, S.; Kreider, K.; et al. FDG PET/CT Imaging 1 Week after a Single Dose of Pembrolizumab Predicts Treatment Response in Patients with Advanced Melanoma. Clin. Cancer Res. 2024, 30, 1758–1767. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, D.; Pyka, T.; Scheidhauer, K.; Lütje, S.; Essler, M.; Bundschuh, R.A. Textural features in FDG-PET/CT can predict outcome in melanoma patients to treatment with Vemurafenib and Ipililumab. Nuklearmedizin 2020, 59, 228–234. [Google Scholar] [CrossRef]
- Sachpekidis, C.; Anwar, H.; Winkler, J.K.; Kopp-Schneider, A.; Larribere, L.; Haberkorn, U.; Hassel, J.C.; Dimitrakopoulou-Strauss, A. Longitudinal studies of the (18)F-FDG kinetics after ipilimumab treatment in metastatic melanoma patients based on dynamic FDG PET/CT. Cancer Immunol. Immunother. 2018, 67, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Martínez-Gómez, J.M.; Mangana, J.; Dummer, R.; Erlic, Z.; Nölting, S.; Beuschlein, F.; Maurer, A.; Messerli, M.; Huellner, M.W.; et al. 18 F-FDG PET/CT for Detection of Immunotherapy-Induced Hypophysitis-A Case-Control Study. Clin. Nucl. Med. 2024, 49, e656–e663. [Google Scholar] [CrossRef]
- Gilardi, L.; Colandrea, M.; Vassallo, S.; Travaini, L.L.; Paganelli, G. Ipilimumab-induced immunomediated adverse events: Possible pitfalls in (18)F-FDG PET/CT interpretation. Clin. Nucl. Med. 2014, 39, 472–474. [Google Scholar] [CrossRef]
- Anwar, H.; Sachpekidis, C.; Winkler, J.; Kopp-Schneider, A.; Haberkorn, U.; Hassel, J.C.; Dimitrakopoulou-Strauss, A. Absolute number of new lesions on (18)F-FDG PET/CT is more predictive of clinical response than SUV changes in metastatic melanoma patients receiving ipilimumab. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 376–383. [Google Scholar] [CrossRef]
- Ito, K.; Teng, R.; Schöder, H.; Humm, J.L.; Ni, A.; Michaud, L.; Nakajima, R.; Yamashita, R.; Wolchok, J.D.; Weber, W.A. (18)F-FDG PET/CT for Monitoring of Ipilimumab Therapy in Patients with Metastatic Melanoma. J. Nucl. Med. 2019, 60, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Coman, M.; Hîncu, M. Study of bone cells by confocal microscopy in fractures stimulated by ultrasound. Rom. J. Morphol. Embryol. 2013, 54, 357–360. [Google Scholar] [PubMed]
- Kohutek, Z.A.; Wu, A.J.; Zhang, Z.; Foster, A.; Din, S.U.; Yorke, E.D.; Downey, R.; Rosenzweig, K.E.; Weber, W.A.; Rimner, A. FDG-PET maximum standardized uptake value is prognostic for recurrence and survival after stereotactic body radiotherapy for non-small cell lung cancer. Lung Cancer 2015, 89, 115–120. [Google Scholar] [CrossRef] [PubMed]
- AbdElaal, A.A.; Zaher, A.M.; Abdelgawad, M.I.; Mekkawy, M.A.; Eloteify, L.M. Correlation of primary tumor metabolic parameters with clinical, histopathological and molecular characteristics in breast cancer patients at pre-operative staging FDG-PET/CT study. Egypt. J. Radiol. Nucl. Med. 2021, 52, 171. [Google Scholar] [CrossRef]
- Qi, C.; Sui, X.; Yu, H.; Wang, S.; Hu, Y.; Sun, H.; Yang, X.; Wang, Y.; Zhou, Y.; Shi, H. Phantom study and clinical application of total-body (18)F-FDG PET/CT imaging: How to use small voxel imaging better? EJNMMI Phys. 2024, 11, 17. [Google Scholar] [CrossRef]

| Pat. ID | Sex | Age | PET/CT | Systemic Treatment at ECT | Localization of ECT | Initial TNM Staging | Histological Subtype |
|---|---|---|---|---|---|---|---|
| 1 | F | 66 | prior ECT | gluteal | T1 N0 M0 | n.a. | |
| 2 | F | 65 | prior ECT | upper arm | T3 N1 M0 | NM | |
| 3 | F | 59 | after ECT | lower leg | TX N0 M0 | n.a. | |
| 4 | F | 79 | prior ECT | thigh, hip | T1 N0 M0 | SSM | |
| 5 | M | 58 | prior ECT | thigh | T3 N1 M0 | n.a. | |
| 6 | M | 59 | after ECT | parietal | T2a N2c M0 | SSM | |
| 7 | M | 52 | after ECT | INFalpha | thigh | T3b N3 M0 | NM |
| 8 | F | 82 | prior ECT | lower leg | T4 N1 M1 | ALM | |
| 9 | M | 77 | prior ECT | Ipilimumab | arm | T4b N3 M1c | NM |
| 10 | M | 65 | after ECT | Ipilimumab | trunk, arm, abdomen, thigh | T3 N0 M1c | n.a. |
| 11 | M | 76 | prior ECT | trunk | T3b N1a M0 | NM | |
| Mean | 67 | ||||||
| SD | 10 |
| SUVmax | SAD | LAD | |
|---|---|---|---|
| Mean ± SD all lesions | 9.9 ± 11.2 | 1.0 ± 0.6 | 1.4 ± 0.8 |
| Mean ± SD lesions prior ECT | 9.8 ± 12.3 | 0.9 ± 0.6 | 1.3 ± 0.8 |
| Mean ± SD lesions after ECT | 10.3 ± 5.5 | 1.1 ± 0.4 | 1.6 ± 1.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siegmund, S.C.; Deußing, M.; Werner, R.A.; Hartmann, D.; Kunte, C. Metabolic Imaging in Electrochemotherapy: Insights from FDG-PET Analysis in Metastatic Melanoma—A Pilot Study. Cancers 2025, 17, 2641. https://doi.org/10.3390/cancers17162641
Siegmund SC, Deußing M, Werner RA, Hartmann D, Kunte C. Metabolic Imaging in Electrochemotherapy: Insights from FDG-PET Analysis in Metastatic Melanoma—A Pilot Study. Cancers. 2025; 17(16):2641. https://doi.org/10.3390/cancers17162641
Chicago/Turabian StyleSiegmund, Sophie C., Maximilian Deußing, Rudolf A. Werner, Daniela Hartmann, and Christian Kunte. 2025. "Metabolic Imaging in Electrochemotherapy: Insights from FDG-PET Analysis in Metastatic Melanoma—A Pilot Study" Cancers 17, no. 16: 2641. https://doi.org/10.3390/cancers17162641
APA StyleSiegmund, S. C., Deußing, M., Werner, R. A., Hartmann, D., & Kunte, C. (2025). Metabolic Imaging in Electrochemotherapy: Insights from FDG-PET Analysis in Metastatic Melanoma—A Pilot Study. Cancers, 17(16), 2641. https://doi.org/10.3390/cancers17162641







