Lower Respiratory Tract Microbiome Signatures of Health and Lung Cancer Across Different Smoking Statuses
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Groups
2.2. Sputum Collection
2.3. DNA Extraction, Library Preparation, Sequencing, and Microbiome Analysis
2.4. Microbiome Taxonomy and Statistical Analysis
3. Results
3.1. Next-Generation Sequencing of Human Sputum Samples
3.2. Impact of Smoking on LRT Microbiota of Healthy Subjects
3.3. Impact of Smoking on LRT Microbiota of Lung Cancer Patients
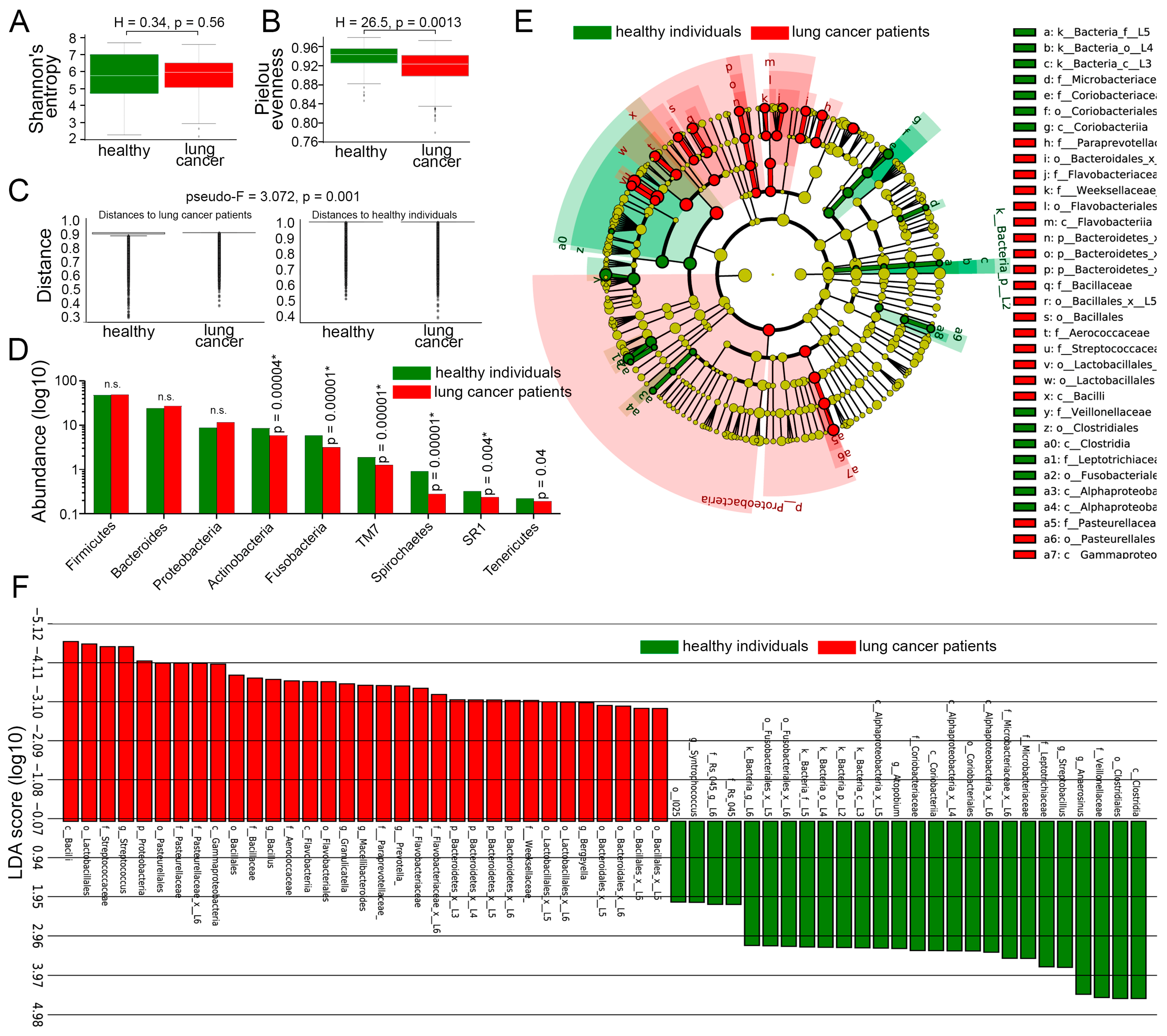
3.4. Comparison of LRT Microbiome Between Lung Cancer Patients and Healthy Individuals, Irrespective of Smoking Status
3.5. Comparison of LRT Microbiomes Between Lung Cancer Patients and Healthy Individuals, Dependent on Smoking Status
3.6. Abundance of Selenomonas in the LRT Correlates with Smoking Exposure in Lung Cancer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fu, M.; Mei, A.; Min, X.; Yang, H.; Wu, W.; Zhong, J.; Li, C.; Chen, J. Advancements in Cardiovascular Disease Research Affected by Smoking. Rev. Cardiovasc. Med. 2024, 25, 298. [Google Scholar] [CrossRef]
- Arcavi, L.; Benowitz, N.L. Cigarette smoking and infection. Arch. Intern. Med. 2004, 164, 2206–2216. [Google Scholar] [CrossRef]
- Bach, L.; Ram, A.; Ijaz, U.Z.; Evans, T.J.; Haydon, D.T.; Lindström, J. The Effects of Smoking on Human Pharynx Microbiota Composition and Stability. Microbiol. Spectr. 2023, 11, e0216621. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, R.F.; Zarrintan, S.; Brandenburg, S.M.; Kol, A.; de Bruin, H.G.; Jafari, S.; Dijk, F.; Kalicharan, D.; Kelders, M.; Gosker, H.R.; et al. Prolonged cigarette smoke exposure alters mitochondrial structure and function in airway epithelial cells. Respir. Res. 2013, 14, 97. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.A.; Goodson, W.H.; Hopf, H.W.; Hunt, T.K. Cigarette smoking decreases tissue oxygen. Arch. Surg. 1991, 126, 1131–1134. [Google Scholar] [CrossRef]
- Lugg, S.T.; Scott, A.; Parekh, D.; Naidu, B.; Thickett, D.R. Cigarette smoke exposure and alveolar macrophages: Mechanisms for lung disease. Thorax 2022, 77, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Prasetyo, A.; Sadhana, U.; Budiman, J. Nasal Mucociliary Clearance in Smokers: A Systematic Review. Int. Arch. Otorhinolaryngol. 2021, 25, e160–e169. [Google Scholar] [CrossRef]
- Sapkota, A.R.; Berger, S.; Vogel, T.M. Human pathogens abundant in the bacterial metagenome of cigarettes. Environ. Health Perspect. 2010, 118, 351–356. [Google Scholar] [CrossRef]
- Tyx, R.E.; Stanfill, S.B.; Keong, L.M.; Rivera, A.J.; Satten, G.A.; Watson, C.H. Characterization of Bacterial Communities in Selected Smokeless Tobacco Products Using 16S rDNA Analysis. PLoS ONE 2016, 11, e0146939. [Google Scholar] [CrossRef]
- Huang, C.; Shi, G. Smoking and microbiome in oral, airway, gut and some systemic diseases. J. Transl. Med. 2019, 17, 225. [Google Scholar] [CrossRef]
- Shapiro, H.; Goldenberg, K.; Ratiner, K.; Elinav, E. Smoking-induced microbial dysbiosis in health and disease. Clin. Sci. 2022, 136, 1371–1387. [Google Scholar] [CrossRef]
- Al-Zyoud, W.; Hajjo, R.; Abu-Siniyeh, A.; Hajjaj, S. Salivary Microbiome and Cigarette Smoking: A First of Its Kind Investigation in Jordan. Int. J. Environ. Res. Public Health 2019, 17, 256. [Google Scholar] [CrossRef]
- Brook, I.; Gober, A.E. Recovery of potential pathogens and interfering bacteria in the nasopharynx of smokers and nonsmokers. Chest 2005, 127, 2072–2075. [Google Scholar] [CrossRef]
- Galvin, S.; Anishchuk, S.; Healy, C.M.; Moran, G.P. Smoking, tooth loss and oral hygiene practices have significant and site-specific impacts on the microbiome of oral mucosal surfaces: A cross-sectional study. J. Oral Microbiol. 2023, 15, 2263971. [Google Scholar] [CrossRef]
- Mason, M.R.; Preshaw, P.M.; Nagaraja, H.N.; Dabdoub, S.M.; Rahman, A.; Kumar, P.S. The subgingival microbiome of clinically healthy current and never smokers. ISME J. 2015, 9, 268–272. [Google Scholar] [CrossRef]
- Turek, E.M.; Cox, M.J.; Hunter, M.; Hui, J.; James, P.; Willis-Owen, S.A.G.; Cuthbertson, L.; James, A.; Musk, A.; Moffatt, M.F.; et al. Airway microbial communities, smoking and asthma in a general population sample. EBioMedicine 2021, 71, 103538. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Peters, B.A.; Dominianni, C.; Zhang, Y.; Pei, Z.; Yang, L.; Ma, Y.; Purdue, M.P.; Jacobs, E.J.; Gapstur, S.M.; et al. Cigarette smoking and the oral microbiome in a large study of American adults. ISME J. 2016, 10, 2435–2446. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Phillips, S.; Gail, M.H.; Goedert, J.J.; Humphrys, M.S.; Ravel, J.; Ren, Y.; Caporaso, N.E. The effect of cigarette smoking on the oral and nasal microbiota. Microbiome 2017, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Erb-Downward, J.R.; Thompson, D.L.; Han, M.K.; Freeman, C.M.; McCloskey, L.; Schmidt, L.A.; Young, V.B.; Toews, G.B.; Curtis, J.L.; Sundaram, B.; et al. Analysis of the lung microbiome in the “healthy” smoker and in COPD. PLoS ONE 2011, 6, e16384. [Google Scholar] [CrossRef]
- Haldar, K.; George, L.; Wang, Z.; Mistry, V.; Ramsheh, M.Y.; Free, R.C.; John, C.; Reeve, N.F.; Miller, B.E.; Tal-Singer, R.; et al. The sputum microbiome is distinct between COPD and health, independent of smoking history. Respir. Res. 2020, 21, 183. [Google Scholar] [CrossRef]
- Morris, A.; Beck, J.M.; Schloss, P.D.; Campbell, T.B.; Crothers, K.; Curtis, J.L.; Flores, S.C.; Fontenot, A.P.; Ghedin, E.; Huang, L.; et al. Comparison of the respiratory microbiome in healthy nonsmokers and smokers. Am. J. Respir. Crit. Care Med. 2013, 187, 1067–1075. [Google Scholar] [CrossRef]
- Einarsson, G.G.; Comer, D.M.; McIlreavey, L.; Parkhill, J.; Ennis, M.; Tunney, M.M.; Elborn, J.S. Community dynamics and the lower airway microbiota in stable chronic obstructive pulmonary disease, smokers and healthy non-smokers. Thorax 2016, 71, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.Y.; Yoon, H.S.; Rho, M.; Sung, J.; Song, Y.-M.; Lee, K.; Ko, G. Analysis of the association between host genetics, smoking, and sputum microbiota in healthy humans. Sci. Rep. 2016, 6, 23745. [Google Scholar] [CrossRef]
- Pfeiffer, S.; Herzmann, C.; Gaede, K.I.; Kovacevic, D.; Krauss-Etschmann, S.; Schloter, M. Different responses of the oral, nasal and lung microbiomes to cigarette smoke. Thorax 2022, 77, 191–195. [Google Scholar] [CrossRef]
- Lin, L.; Yi, X.; Liu, H.; Meng, R.; Li, S.; Liu, X.; Yang, J.; Xu, Y.; Li, C.; Wang, Y.; et al. The airway microbiome mediates the interaction between environmental exposure and respiratory health in humans. Nat. Med. 2023, 29, 1750–1759. [Google Scholar] [CrossRef]
- Leiter, A.; Veluswamy, R.R.; Wisnivesky, J.P. The global burden of lung cancer: Current status and future trends. Nat. Rev. Clin. Oncol. 2023, 20, 624–639. [Google Scholar] [CrossRef]
- Czarnecka-Chrebelska, K.H.; Kordiak, J.; Brzeziańska-Lasota, E.; Pastuszak-Lewandoska, D. Respiratory Tract Oncobiome in Lung Carcinogenesis: Where Are We Now? Cancers 2023, 15, 4935. [Google Scholar] [CrossRef] [PubMed]
- Hosgood, H.D., III; Sapkota, A.R.; Rothman, N.; Rohan, T.; Hu, W.; Xu, J.; Vermeulen, R.; He, X.; White, J.R.; Wu, G.; et al. The potential role of lung microbiota in lung cancer attributed to household coal burning exposures. Environ. Mol. Mutagen. 2014, 55, 643–651. [Google Scholar] [CrossRef]
- Weinberg, F.; Dickson, R.P.; Nagrath, D.; Ramnath, N. The Lung Microbiome: A Central Mediator of Host Inflammation and Metabolism in Lung Cancer Patients? Cancers 2020, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Lozupone, C.; Knight, R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.R.; Pickle, L.W.; Zhu, L. Recent Spatiotemporal Patterns of US Lung Cancer by Histologic Type. Front. Public Health 2017, 5, 82. [Google Scholar] [CrossRef]
- Charlson, E.S.; Chen, J.; Custers-Allen, R.; Bittinger, K.; Li, H.; Sinha, R.; Hwang, J.; Bushman, F.D.; Collman, R.G.; Heimesaat, M.M. Disordered microbial communities in the upper respiratory tract of cigarette smokers. PLoS ONE 2010, 5, e15216. [Google Scholar] [CrossRef]
- Knight, T.M.; Forman, D.; Al-Dabbagh, S.A.; Doll, R. Estimation of dietary intake of nitrate and nitrate in Great Britain. Food Chem. Toxicol. 1987, 25, 277–285. [Google Scholar] [CrossRef]
- Rosier, B.T.; Moya-Gonzalvez, E.M.; Corell-Escuin, P.; Mira, A. Isolation and Characterization of Nitrate-Reducing Bacteria as Potential Probiotics for Oral and Systemic Health. Front. Microbiol. 2020, 11, 555465. [Google Scholar] [CrossRef] [PubMed]
- Leng, Q.; Holden, V.K.; Deepak, J.; Todd, N.W.; Jiang, F. Microbiota Biomarkers for Lung Cancer. Diagnostics 2021, 11, 407. [Google Scholar] [CrossRef] [PubMed]
- Cameron, S.J.S.; Lewis, K.E.; Huws, S.A.; Hegarty, M.J.; Lewis, P.D.; Pachebat, J.A.; Mur, L.A.J. A pilot study using metagenomic sequencing of the sputum microbiome suggests potential bacterial biomarkers for lung cancer. PLoS ONE 2017, 12, e0177062. [Google Scholar] [CrossRef]
- Liu, H.-X.; Tao, L.-L.; Zhang, J.; Zhu, Y.-G.; Zheng, Y.; Liu, D.; Zhou, M.; Ke, H.; Shi, M.-M.; Qu, J.-M. Difference of lower airway microbiome in bilateral protected specimen brush between lung cancer patients with unilateral lobar masses and control subjects. Int. J. Cancer 2018, 142, 769–778. [Google Scholar] [CrossRef]
- Bello, S.; Vengoechea, J.J.; Ponce-Alonso, M.; Figueredo, A.L.; Mincholé, E.; Rezusta, A.; Gambó, P.; Pastor, J.M.; Galeano, J.; del Campo, R. Core Microbiota in Central Lung Cancer With Streptococcal Enrichment as a Possible Diagnostic Marker. Arch. Bronconeumol. 2021, 57, 681–689. [Google Scholar] [CrossRef]
- Pu, C.Y.; Seshadri, M.; Manuballa, S.; Yendamuri, S. The Oral Microbiome and Lung Diseases. Curr. Oral Health Rep. 2020, 7, 79–86. [Google Scholar] [CrossRef]
- Ireland, A.S.; Micinski, A.M.; Kastner, D.W.; Guo, B.; Wait, S.J.; Spainhower, K.B.; Conley, C.C.; Chen, O.S.; Guthrie, M.R.; Soltero, D.; et al. MYC Drives Temporal Evolution of Small Cell Lung Cancer Subtypes by Reprogramming Neuroendocrine Fate. Cancer Cell 2020, 38, 60–78. [Google Scholar] [CrossRef] [PubMed]






| Baseline Characteristics | Lung Cancer Patients, n = 190 | Healthy Individuals, n = 107 | p-Value * | ||||
|---|---|---|---|---|---|---|---|
| Smokers, n = 113 | Nonsmokers, n = 65 | Former Smokers, n = 12 | Smokers, n = 48 | Nonsmokers, n = 39 | Former Smokers, n = 20 | ||
| Age, years | 61.3 | 61.9 | 62.7 | 53.3 | 55.8 | 56.2 | 0.416 |
| Gender (n/%): | |||||||
| Male | 101/89.6 | 36/55.4 | 12/100 | 45/93.7 | 26/66.7 | 17/82.2 | |
| Female | 12/10.6 | 29/44.6 | - | 3/6.3 | 13/33.3 | 3/15.0 | 2.53 × 10−8 |
| Chronic conditions (n/%): | |||||||
| Cardiovascular disease | 49/43.4 | 39/60.0 | 10/83.3 | 7/14.6 | 14/35.9 | 5/25.0 | 1.52 × 10−6 |
| Bronchitis | 22/19.5 | 10/15.4 | 3/25.0 | -/0 | -/0 | -/0 | 0.000266 |
| COPD | 27/23.9 | 5/7.7 | 2/16.7 | -/0 | -/0 | -/0 | 3.29 × 10−6 |
| Gastrointestinal disease | 12/10.6 | 9/13.9 | -/0 | 9/18.8 | 4/10.3 | 2/10.0 | 0.512 |
| Diabetes | 4/3.5 | 8/12.3 | -/0 | 1/2.1 | 2/5.1 | 1/5.0 | 0.127 |
| Asthma | -/0 | 3/4.6 | 2/16.7 | -/0 | 1/2.6 | -/0 | 0.00192 |
| Obesity | 6/5.3 | 7/10.8 | -/0 | -/0 | -/0 | -/0 | 0.0344 |
| Pack-years | 35.3 ± 15.7 | 27.2 ± 12.9 | 0.0018 | ||||
| Histological subtype of lung cancer (n/%): | |||||||
| Squamous cell carcinoma | 49/43.4 | 14/21.5 | 3/25.0 | ||||
| Adenocarcinoma | 38/33.6 | 33/50.8 | 4/33.3 | ||||
| Others | 26/23.0 | 18/27.7 | 5/41.7 | 0.0289 | |||
| TNM (n/%): | |||||||
| I, II | 43/38.1 | 30/46.2 | 3/25.0 | ||||
| III, IV | 70/61.9 | 35/53.8 | 9/75.0 | 0.3120 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Druzhinin, V.G.; Baranova, E.D.; Demenkov, P.S.; Matskova, L.V.; Larionov, A.V.; Yuzhalin, A.E. Lower Respiratory Tract Microbiome Signatures of Health and Lung Cancer Across Different Smoking Statuses. Cancers 2025, 17, 2643. https://doi.org/10.3390/cancers17162643
Druzhinin VG, Baranova ED, Demenkov PS, Matskova LV, Larionov AV, Yuzhalin AE. Lower Respiratory Tract Microbiome Signatures of Health and Lung Cancer Across Different Smoking Statuses. Cancers. 2025; 17(16):2643. https://doi.org/10.3390/cancers17162643
Chicago/Turabian StyleDruzhinin, Vladimir G., Elizaveta D. Baranova, Pavel S. Demenkov, Liudmila V. Matskova, Alexey V. Larionov, and Arseniy E. Yuzhalin. 2025. "Lower Respiratory Tract Microbiome Signatures of Health and Lung Cancer Across Different Smoking Statuses" Cancers 17, no. 16: 2643. https://doi.org/10.3390/cancers17162643
APA StyleDruzhinin, V. G., Baranova, E. D., Demenkov, P. S., Matskova, L. V., Larionov, A. V., & Yuzhalin, A. E. (2025). Lower Respiratory Tract Microbiome Signatures of Health and Lung Cancer Across Different Smoking Statuses. Cancers, 17(16), 2643. https://doi.org/10.3390/cancers17162643







