Neutrophils in Cancer: Phenotypic Heterogeneity Across Tumor Models and Significant Alteration of Splenic Neutrophil Phenotype in Lymphosarcoma RLS40 Model Following DNase I Treatment
Simple Summary
Abstract
1. Introduction
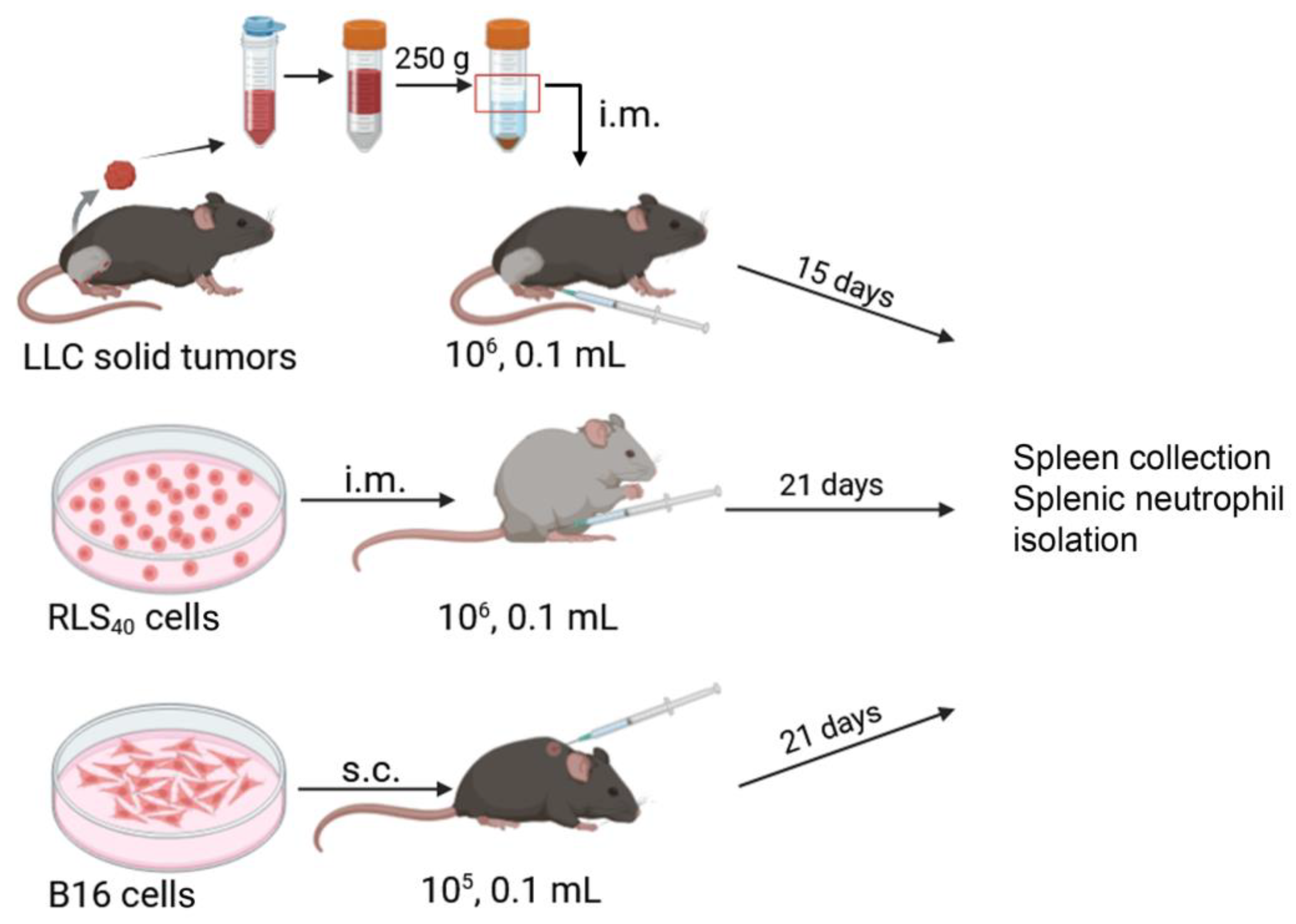
2. Materials and Methods
2.1. Cell Cultures
2.2. Tumor Strains
2.3. Mice
2.4. Tumor Implantation
2.5. Neutrophil Phenotype Experiment
2.6. Neutrophil Isolation
2.7. RNA Extraction
2.8. Primer Design
2.9. cDNA Preparation and RT-qPCR
2.10. Flowcytometry Analysis
2.11. NETosis Induction
2.12. Confocal Microscopy
2.13. Neutrophil Effect on Tumor Cell Viability
2.14. Peripheral Blood Analysis
2.15. Determination of Nuclear and Mitochondrial DNA Quantity in cfDNA Samples by qPCR
2.16. Histology and Immunohistochemistry
2.17. Morphometric Analysis
2.18. Data Analysis
3. Results
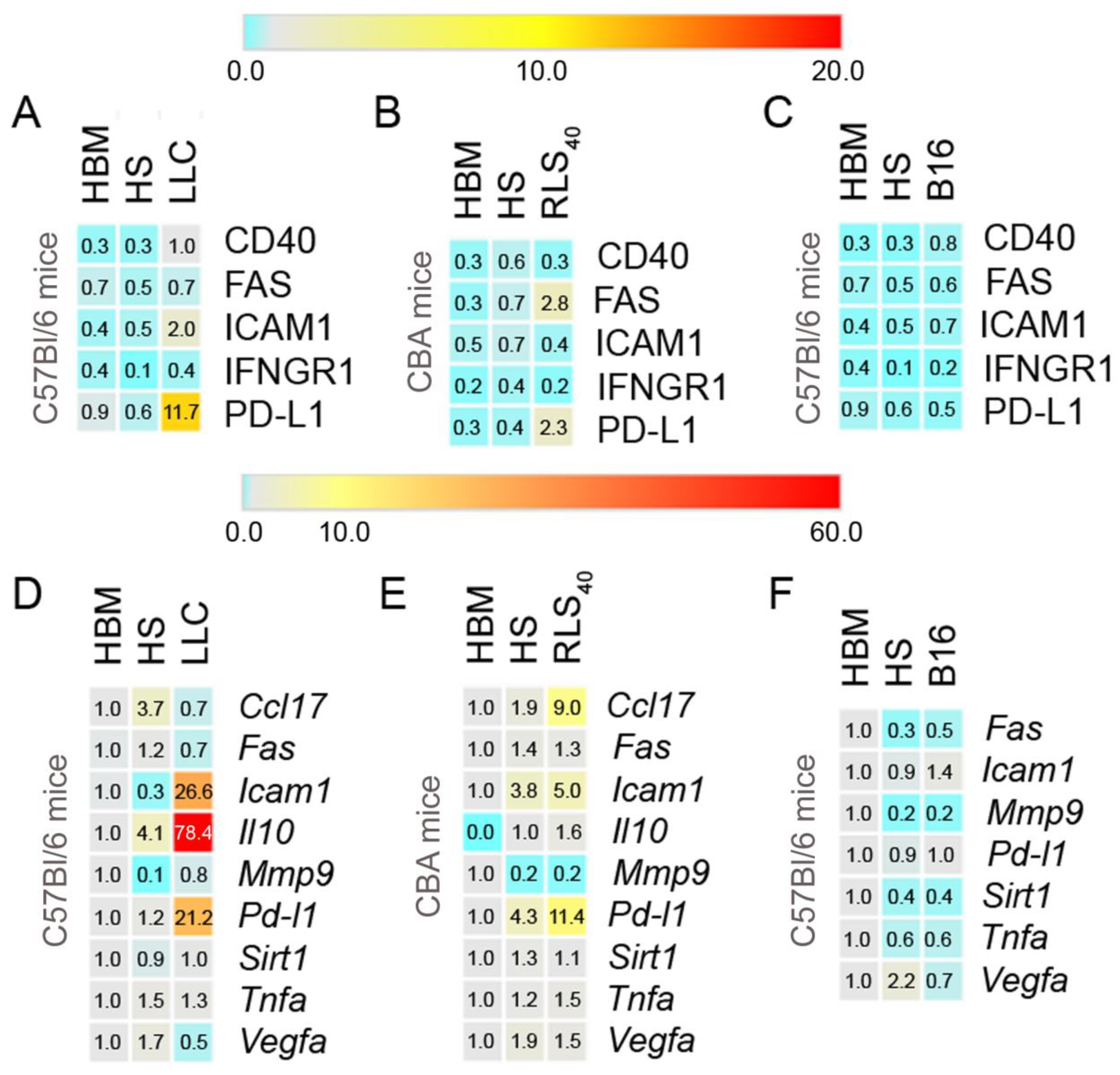
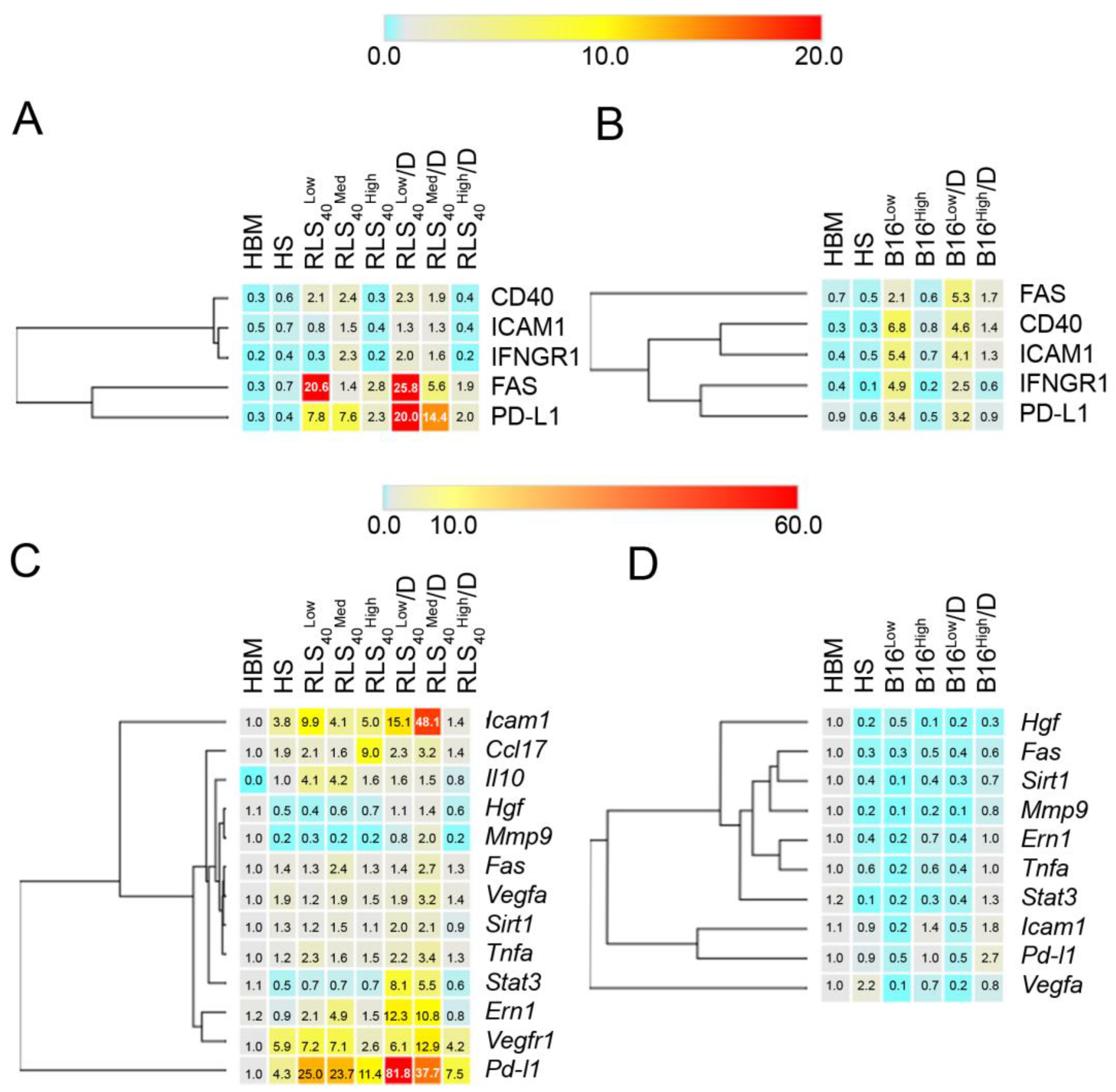
3.1. The Effect of Tumor Type on Neutrophil Phenotype
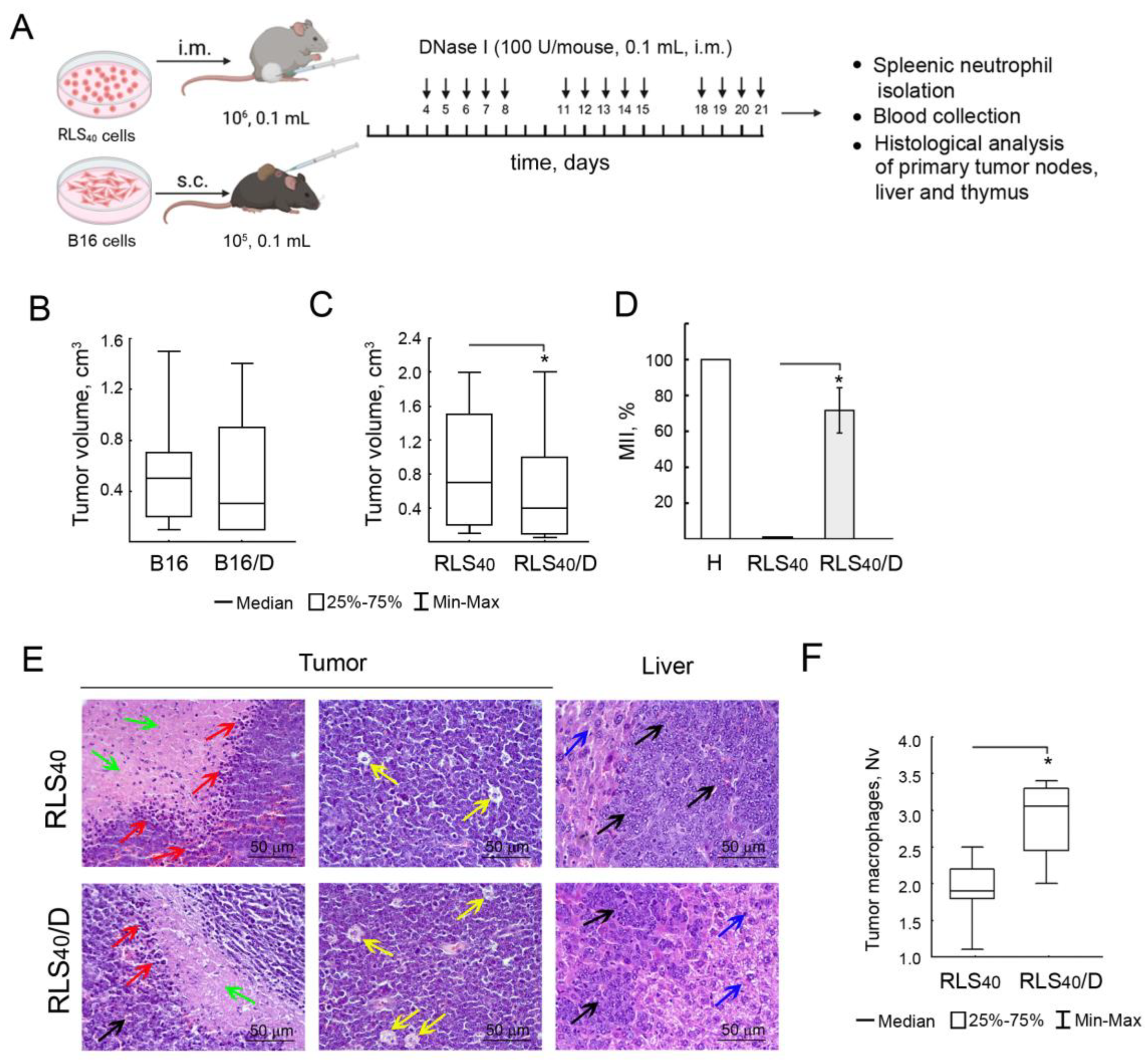
3.2. The Effect of DNase I on Tumor Progression, Immune Response and Neutrophil Phenotype of Mice with RLS40 and B16
3.2.1. The Effect of DNase I on Tumor Growth and Metastases Development in Mice with RLS40 and B16
3.2.2. Analysis of Concentration of Blood Serum cfDNA and Mitochondrial/Nuclear DNA Ratio
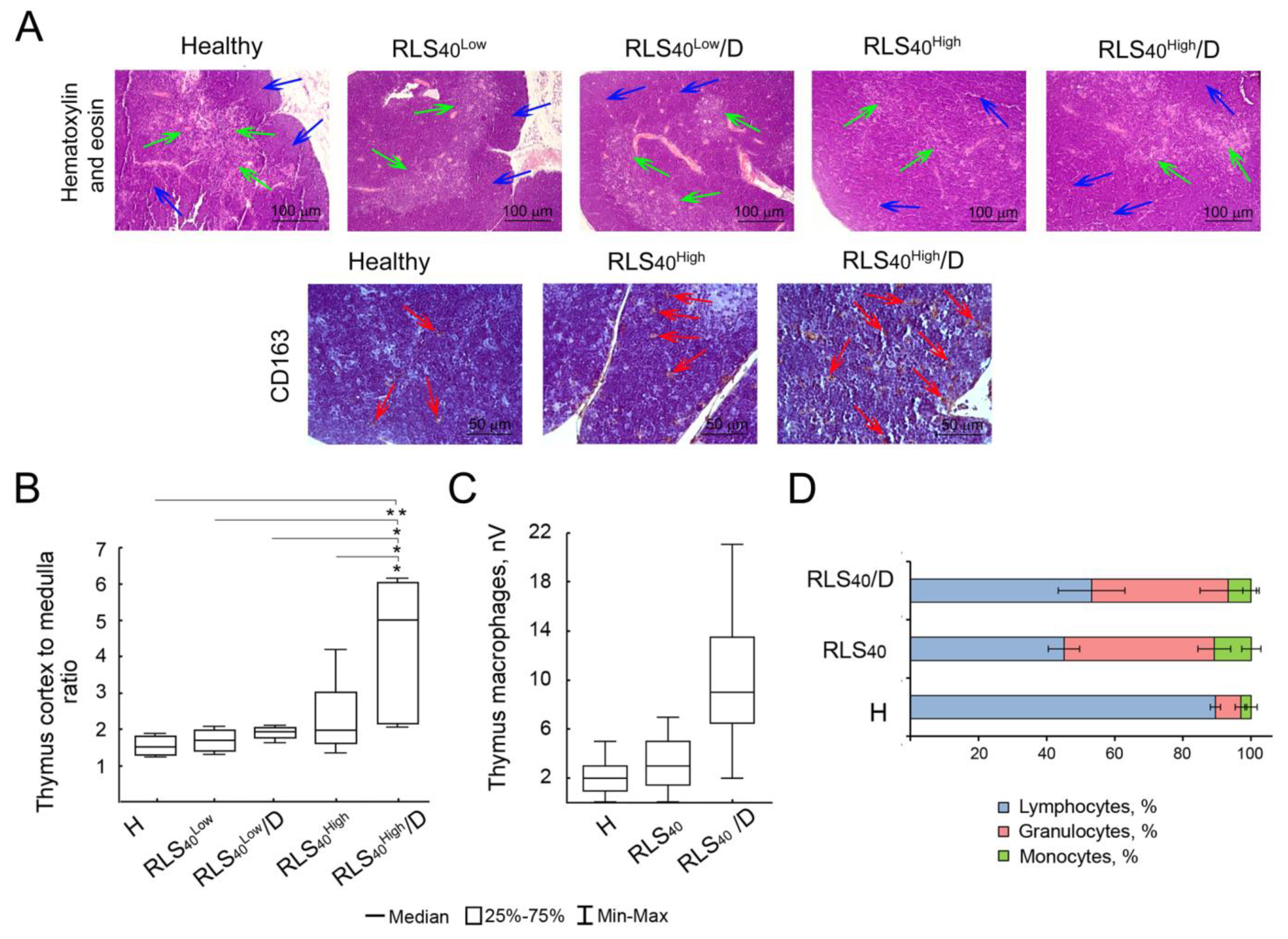
3.2.3. Alterations in Immunocompetent Organs
3.2.4. The Effect of DNase I on Neutrophil Phenotype of Mice with RLS40 and B16
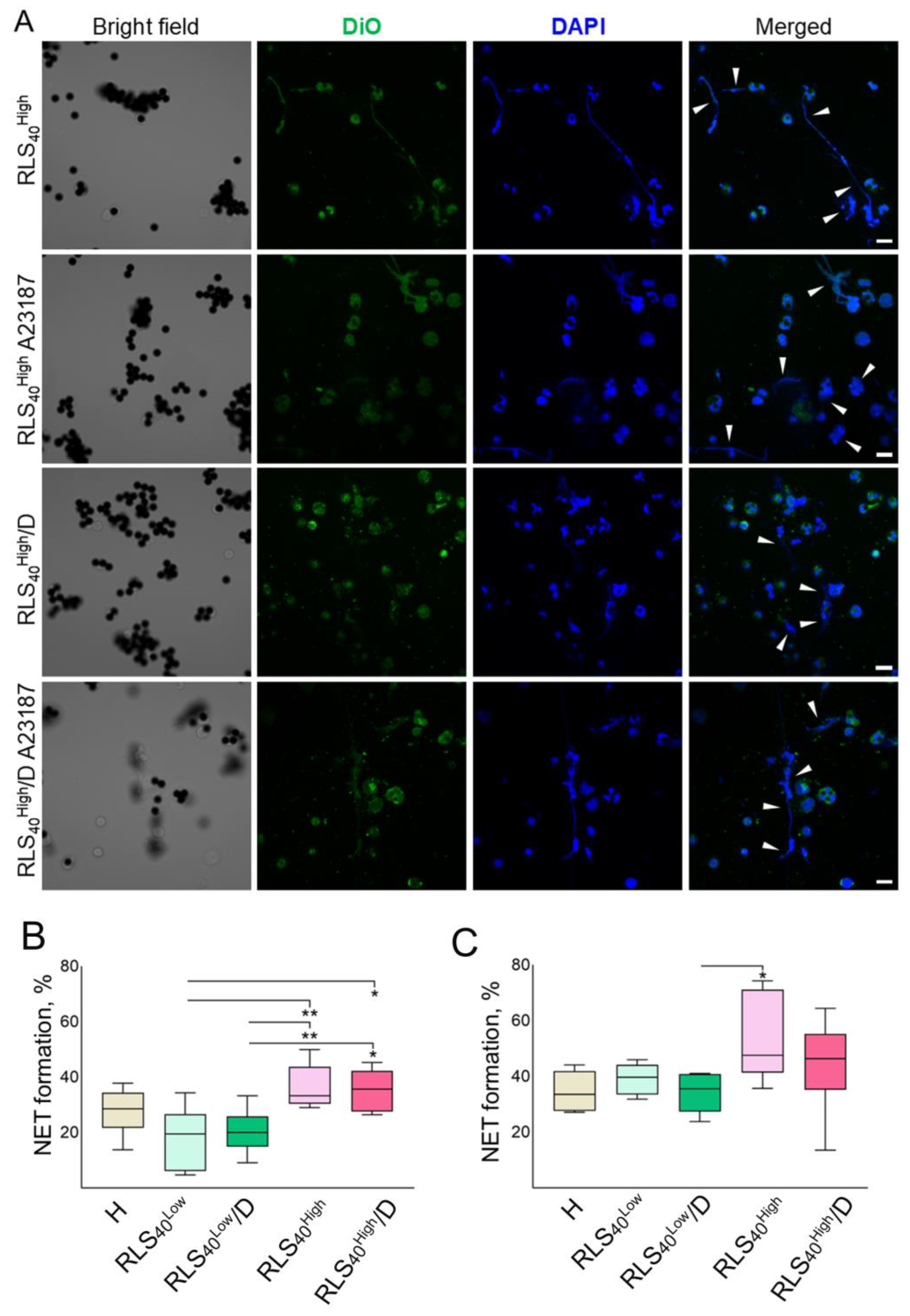
3.2.5. The Effect of DNase I on the Ability of Neutrophils to Generate NETs
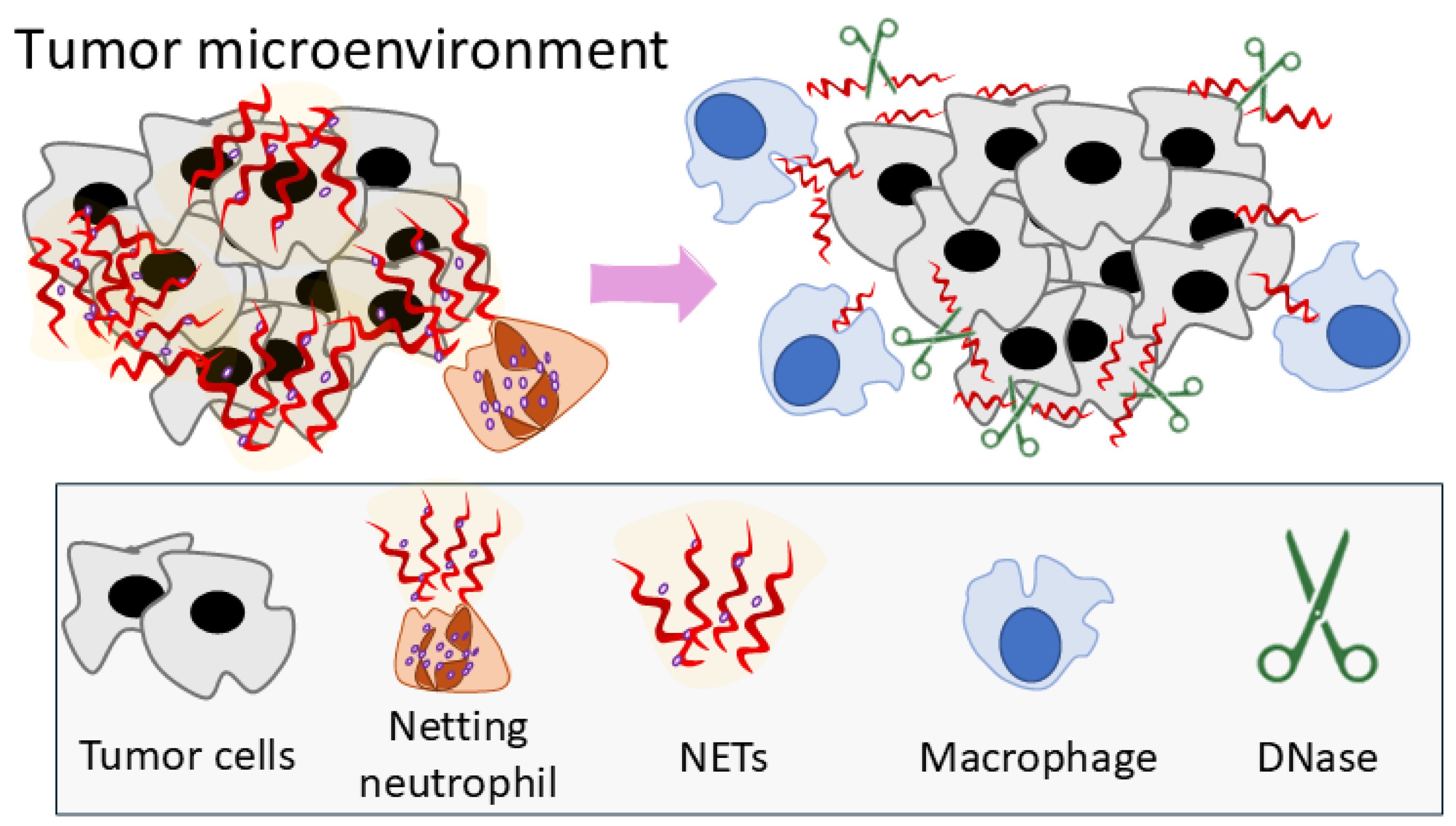
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Russo, M.; Nastasi, C. Targeting the Tumor Microenvironment: A Close Up of Tumor-Associated Macrophages and Neutrophils. Front. Oncol. 2022, 12, 871513. [Google Scholar] [CrossRef]
- Yu, X.; Li, C.; Wang, Z.; Xu, Y.; Shao, S.; Shao, F.; Wang, H.; Liu, J. Neutrophils in cancer: Dual roles through intercellular interactions. Oncogene 2024, 43, 1163–1177. [Google Scholar] [CrossRef]
- Sounbuli, K.; Mironova, N.; Alekseeva, L. Diverse Neutrophil Functions in Cancer and Promising Neutrophil-Based Cancer Therapies. Int. J. Mol. Sci. 2022, 23, 15827. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, L.; Zuo, J.; Feng, D. Tumor associated neutrophils governs tumor progression through an IL-10/STAT3/PD-L1 feedback signaling loop in lung cancer. Transl. Oncol. 2024, 40, 101866. [Google Scholar] [CrossRef] [PubMed]
- Luyang, H.; Zeng, F.; Lei, Y.; He, Q.; Zhou, Y.; Xu, J. Bidirectional role of neutrophils in tumor development. Mol. Cancer 2025, 24, 22. [Google Scholar] [CrossRef]
- Huang, S.; Shi, J.; Shen, J.; Fan, X. Metabolic reprogramming of neutrophils in the tumor microenvironment: Emerging therapeutic targets. Cancer Lett. 2025, 612, 217466. [Google Scholar] [CrossRef] [PubMed]
- Brunkhorst, H.; Schnellhardt, S.; Büttner-Herold, M.; Daniel, C.; Fietkau, R.; Distel, L.V. Neutrophils in HNSCC Can Be Associated with Both a Worse or Favorable Prognosis. Biomolecules 2024, 14, 205. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.; Distel, L.; Erber, R.; Büttner-Herold, M.; Rosahl, M.C.; Ott, O.J.; Strnad, V.; Hack, C.C.; Hartmann, A.; Hecht, M.; et al. Tumor-Associated Neutrophils Are a Negative Prognostic Factor in Early Luminal Breast Cancers Lacking Immunosuppressive Macrophage Recruitment. Cancers 2024, 16, 3160. [Google Scholar] [CrossRef] [PubMed]
- Pylaeva, E.; Korschunow, G.; Spyra, I.; Bordbari, S.; Siakaeva, E.; Ozel, I.; Domnich, M.; Squire, A.; Hasenberg, A.; Thangavelu, K.; et al. During early stages of cancer, neutrophils initiate anti-tumor immune responses in tumor-draining lymph nodes. Cell Rep. 2022, 40, 111171. [Google Scholar] [CrossRef]
- Pham, H.T.T.; Magez, S.; Choi, B.; Baatar, B.; Jung, J.; Radwanska, M. Neutrophil metalloproteinase driven spleen damage hampers infection control of trypanosomiasis. Nat. Commun. 2023, 14, 5418. [Google Scholar] [CrossRef]
- Wu, C.; Hua, Q.; Zheng, L. Generation of Myeloid Cells in Cancer: The Spleen Matters. Front. Immunol. 2020, 11, 1126. [Google Scholar] [CrossRef] [PubMed]
- Cortez-Retamozo, V.; Etzrodt, M.; Newton, A.; Rauch, P.J.; Chudnovskiy, A.; Berger, C.; Ryan, R.J.H.; Iwamoto, Y.; Marinelli, B.; Gorbatov, R.; et al. Origins of tumor-associated macrophages and neutrophils. Proc. Natl. Acad. Sci. USA 2012, 109, 2491–2496. [Google Scholar] [CrossRef]
- Alshetaiwi, H.; Pervolarakis, N.; McIntyre, L.L.; Ma, D.; Nguyen, Q.; Rath, J.A.; Nee, K.; Hernandez, G.; Evans, K.; Torosian, L.; et al. Defining the emergence of myeloid-derived suppressor cells in breast cancer using single-cell transcriptomics. Sci. Immunol. 2020, 5, eaay6017. [Google Scholar] [CrossRef]
- Gätjen, M.; Brand, F.; Grau, M.; Gerlach, K.; Kettritz, R.; Westermann, J.; Anagnostopoulos, I.; Lenz, P.; Lenz, G.; Höpken, U.E.; et al. Splenic marginal zone granulocytes acquire an accentuated neutrophil B-cell helper phenotype in chronic lymphocytic leukemia. Cancer Res. 2016, 76, 5253–5265. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, M.; Sun, J.; Li, X.; Shi, H.; Wang, X.; Liu, B.; Zhang, T.; Jiang, X.; Lin, L.; et al. Glycolytic neutrophils accrued in the spleen compromise anti-tumour T cell immunity in breast cancer. Nat. Metab. 2023, 5, 1408–1422. [Google Scholar] [CrossRef]
- Xie, X.; Shi, Q.; Wu, P.; Zhang, X.; Kambara, H.; Su, J.; Yu, H.; Park, S.Y.; Guo, R.; Ren, Q.; et al. Single-cell transcriptome profiling reveals neutrophil heterogeneity in homeostasis and infection. Nat. Immunol. 2020, 21, 1119–1133. [Google Scholar] [CrossRef]
- Sounbuli, K.; Alekseeva, L.A.; Markov, O.V.; Mironova, N.L. A Comparative Study of Different Protocols for Isolation of Murine Neutrophils from Bone Marrow and Spleen. Int. J. Mol. Sci. 2023, 24, 17273. [Google Scholar] [CrossRef] [PubMed]
- Maali, Y.; Molina, M.F.; Khedr, O.; Abdelnabi, M.N.; Dion, J.; Hassan, G.S.; Shoukry, N.H. Two transcriptionally and functionally distinct waves of neutrophils during mouse acute liver injury. Hepatol. Commun. 2024, 8, e0459. [Google Scholar] [CrossRef]
- He, X.Y.; Gao, Y.; Ng, D.; Michalopoulou, E.; George, S.; Adrover, J.M.; Sun, L.; Albrengues, J.; Daßler-Plenker, J.; Han, X.; et al. Chronic stress increases metastasis via neutrophil-mediated changes to the microenvironment. Cancer Cell 2024, 42, 474–486.e12. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Mesa, M.A.; Vasquez, G. NETosis. Autoimmune Dis. 2013, 2013, 65147. [Google Scholar] [CrossRef]
- Xu, W.; Liu, J.; Liu, Q.; Xu, J.; Zhou, L.; Liang, Z.; Huang, H.; Huang, B.; Xiao, G.G.; Guo, J. NFE2-driven neutrophil polarization promotes pancreatic cancer liver metastasis progression. Cell Rep. 2025, 44, 115226. [Google Scholar] [CrossRef]
- Kaltenmeier, C.; Simmons, R.L.; Tohme, S.; Yazdani, H.O. Neutrophil Extracellular Traps (NETs) in Cancer Metastasis. Cancers 2021, 13, 6131. [Google Scholar] [CrossRef]
- Lee, W.; Ko, S.Y.; Akasaka, H.; Weigert, M.; Lengyel, E.; Naora, H. Neutrophil extracellular traps promote pre-metastatic niche formation in the omentum by expanding innate-like B cells that express IL-10. Cancer Cell 2024, 43, 69–85.e11. [Google Scholar] [CrossRef] [PubMed]
- Zhai, R.; Gong, Z.; Wang, M.; Ni, Z.; Zhang, J.; Wang, M.; Zhang, Y.; Zeng, F.; Gu, Z.; Chen, X.; et al. Neutrophil extracellular traps promote invasion and metastasis via NLRP3-mediated oral squamous cell carcinoma pyroptosis inhibition. Cell Death Discov. 2024, 10, 214. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, Y.; Du, G.; Chen, X.; Xiao, L.; Jiang, L.; Jing, N.; Xu, P.; Zhao, C.; Liu, Y.; et al. 5-HT orchestrates histone serotonylation and citrullination to drive neutrophil extracellular traps and liver metastasis. J. Clin. Investig. 2025, 135, e183544. [Google Scholar] [CrossRef]
- Cools-Lartigue, J.; Spicer, J.; McDonald, B.; Gowing, S.; Chow, S.; Giannias, B.; Bourdeau, F.; Kubes, P.; Ferri, L. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J. Clin. Investig. 2013, 123, 3446–3458. [Google Scholar] [CrossRef]
- Xia, X.; Zhang, Z.; Zhu, C.; Ni, B.; Wang, S.; Yang, S.; Yu, F.; Zhao, E.; Li, Q.; Zhao, G. Neutrophil extracellular traps promote metastasis in gastric cancer patients with postoperative abdominal infectious complications. Nat. Commun. 2022, 13, 1017. [Google Scholar] [CrossRef]
- Ireland, A.S.; Oliver, T.G. Neutrophils Create an ImpeNETrable Shield between Tumor and Cytotoxic Immune Cells. Immunity 2020, 52, 729–731. [Google Scholar] [CrossRef] [PubMed]
- Ci, Y.; Zhang, Y.; Liu, Y.; Lu, S.; Cao, J.; Li, H.; Zhang, J.; Huang, Z.; Zhu, X.; Gao, J.; et al. Myricetin suppresses breast cancer metastasis through down-regulating the activity of matrix metalloproteinase (MMP)-2/9. Phytother. Res. 2018, 32, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- El Rayes, T.; Catena, R.; Lee, S.; Stawowczyk, M.; Joshi, N.; Fischbach, C.; Powell, C.A.; Dannenberg, A.J.; Altorki, N.K.; Gao, D.; et al. Lung inflammation promotes metastasis through neutrophil protease-mediated degradation of Tsp-1. Proc. Natl. Acad. Sci. USA 2015, 112, 16000–16005. [Google Scholar] [CrossRef] [PubMed]
- Oklu, R.; Sheth, R.A.; Wong, K.H.K.; Jahromi, A.H.; Albadawi, H. Neutrophil extracellular traps are increased in cancer patients but does not associate with venous thrombosis. Cardiovasc. Diagn. Ther. 2017, 7, S140–S149. [Google Scholar] [CrossRef]
- Tamkovich, S.N.; Cherepanova, A.V.; Kolesnikova, E.V.; Rykova, E.Y.; Pyshnyi, D.V.; Vlassov, V.V.; Laktionov, P.P. Circulating DNA and DNase Activity in Human Blood. Ann. N. Y. Acad. Sci. 2006, 1075, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Alekseeva, L.; Mironova, N. Role of Cell-Free DNA and Deoxyribonucleases in Tumor Progression. Int. J. Mol. Sci. 2021, 22, 12246. [Google Scholar] [CrossRef]
- Cherepanova, A.V.; Tamkovich, S.N.; Vlassov, V.V.; Laktionov, P.P. Blood deoxyribonuclease activity in health and diseases. Biochem. Moscow Suppl. Ser. B 2007, 1, 299–304. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, L.; Dai, Q.; Shang, B.; Xiao, T.; Di, X.; Zhang, K.; Feng, L.; Shou, J.; Wang, Y. A signature for pan-cancer prognosis based on neutrophil extracellular traps. J. Immunother. Cancer 2022, 10, e004210. [Google Scholar] [CrossRef]
- Stoetzer, O.J.; Fersching, D.M.I.; Salat, C.; Steinkohl, O.; Gabka, C.J.; Hamann, U.; Braun, M.; Feller, A.M.; Heinemann, V.; Siegele, B.; et al. Prediction of response to neoadjuvant chemotherapy in breast cancer patients by circulating apoptotic biomarkers nucleosomes, DNAse, cytokeratin-18 fragments and survivin. Cancer Lett. 2013, 336, 140–148. [Google Scholar] [CrossRef]
- Wittwer, C.; Boeck, S.; Heinemann, V.; Haas, M.; Stieber, P.; Nagel, D.; Holdenrieder, S. Circulating nucleosomes and immunogenic cell death markers HMGB1, sRAGE and DNAse in patients with advanced pancreatic cancer undergoing chemotherapy. Int. J. Cancer 2013, 133, 2619–2630. [Google Scholar] [CrossRef]
- Horaguchi, S.; Nakahara, Y.; Igarashi, Y.; Kouro, T.; Wei, F.; Murotani, K.; Udagawa, S.; Higashijima, N.; Matsuo, N.; Murakami, S.; et al. Prognostic Significance of Plasma Neutrophil Extracellular Trap Levels in Patients with Non-Small Cell Lung Cancer Treated with Immune Checkpoint Inhibitors. Biomedicines 2024, 12, 1831. [Google Scholar] [CrossRef] [PubMed]
- Kohles, N.; Nagel, D.; Jüngst, D.; Stieber, P.; Holdenrieder, S. DNase is a prognostic marker in liver cancer patients receiving transarterial chemoembolization therapy. Int. J. Clin. Pharmacol. Ther. 2013, 51, 80–83. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Golonka, R.M.; Yeoh, B.S.; Petrick, J.L.; Weinstein, S.J.; Albanes, D.; Gewirtz, A.T.; McGlynn, K.A.; Vijay-Kumar, M. Deoxyribonuclease I Activity, Cell-Free DNA, and Risk of Liver Cancer in a Prospective Cohort. JNCI Cancer Spectr. 2018, 2, pky083. [Google Scholar] [CrossRef]
- Alekseeva, L.; Sen’kova, A.; Savin, I.; Zenkova, M.; Mironova, N. Human Recombinant DNase I (Pulmozyme®) Inhibits Lung Metastases in Murine Metastatic B16 Melanoma Model That Correlates with Restoration of the DNase Activity and the Decrease SINE/LINE and c-Myc Fragments in Blood Cell-Free DNA. Int. J. Mol. Sci. 2021, 22, 12074. [Google Scholar] [CrossRef]
- Alexeeva, L.A.; Patutina, O.A.; Sen’kova, A.V.; Zenkova, M.A.; Mironova, N.L. Inhibition of Invasive Properties of Murine Melanoma by Bovine Pancreatic DNase I In Vitro and In Vivo. Mol. Biol. 2017, 51, 637–646. [Google Scholar] [CrossRef]
- Alekseeva, L.A.; Mironova, N.L.; Brenner, E.V.; Kurilshikov, A.M.; Patutina, O.A.; Zenkova, M.A. Alteration of the exDNA profile in blood serum of LLC-bearing mice under the decrease of tumour invasion potential by bovine pancreatic DNase I treatment. PLoS ONE 2017, 12, e0171988. [Google Scholar] [CrossRef]
- Patutina, O.A.; Mironova, N.L.; Ryabchikova, E.I.; Popova, N.A.; Nikolin, V.P.; Kaledin, V.I.; Vlassov, V.V.; Zenkova, M.A. Tumoricidal Activity of RNase A and DNase I. Acta Naturae 2010, 2, 88–93. [Google Scholar] [CrossRef]
- Shklyaeva, O.A.; Mironova, N.L.; Malkova, E.M.; Taranov, O.S.; Ryabchikova, E.I.; Zenkova, M.A.; Vlasov, V.V. Cancer-suppressive effect of RNase A and DNase I. Dokl. Biochem. Biophys. 2008, 420, 108–111. [Google Scholar] [CrossRef]
- Alekseeva, L.A.; Sen’kova, A.V.; Zenkova, M.A.; Mironova, N.L. Targeting Circulating SINEs and LINEs with DNase I Provides Metastases Inhibition in Experimental Tumor Models. Mol. Ther. Nucleic Acids 2020, 20, 50–61. [Google Scholar] [CrossRef]
- Rosner, K.; Kasprzak, M.F.; Horenstein, A.; Thurston, H.L.; Abrams, J.; Kerwin, L.Y.; Mehregan, D.A.; Mehregan, D.R. Engineering a waste management enzyme to overcome cancer resistance to apoptosis: Adding DNase1 to the anti-cancer toolbox. Cancer Gene Ther. 2011, 18, 346–357. [Google Scholar] [CrossRef][Green Version]
- Trejo-Becerril, C.; Pérez-Cardenas, E.; Gutiérrez-Díaz, B.; De La Cruz-Sigüenza, D.; Taja-Chayeb, L.; González-Ballesteros, M.; García-López, P.; Chanona, J.; Dueñas-González, A. Antitumor Effects of Systemic DNAse i and Proteases in an in Vivo Model. Integr. Cancer Ther. 2016, 15, NP35–NP43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Y.; Onuma, A.; He, J.; Wang, H.; Xia, Y.; Lal, R.; Cheng, X.; Kasumova, G.; Hu, Z.; et al. Neutrophils Extracellular Traps Inhibition Improves PD-1 Blockade Immunotherapy in Colorectal Cancer. Cancers 2021, 13, 5333. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, S.; Yamamoto, T.; Tanaka, H.; Kambara, T.; Hiraoka, T.; Miyauchi, Y. Deoxyribonuclease treatment prevents blood-borne liver metastasis of cutaneously transplanted tumour cells in mice. Br. J. Cancer 1993, 67, 66–70. [Google Scholar] [CrossRef]
- Wen, F.; Shen, A.; Choi, A.; Gerner, E.W.; Shi, J. Extracellular DNA in pancreatic cancer promotes cell invasion and metastasis. Cancer Res. 2013, 73, 4256–4266. [Google Scholar] [CrossRef]
- Rosner, K. DNase1: A new personalized therapy for cancer? Expert Rev. Anticancer Ther. 2011, 11, 983–986. [Google Scholar] [CrossRef]
- Volkov, D.V.; Tetz, G.V.; Rubtsov, Y.P.; Stepanov, A.V.; Gabibov, A.G. Neutrophil Extracellular Traps (NETs): Opportunities for Targeted Therapy. Acta Naturae 2021, 13, 15–23. [Google Scholar] [CrossRef]
- Hawes, M.C.; Wen, F.; Elquza, E. Extracellular DNA: A bridge to cancer. Cancer Res. 2015, 75, 4260–4264. [Google Scholar] [CrossRef]
- Li, W.; Nakano, H.; Fan, W.; Li, Y.; Sil, P.; Nakano, K.; Zhao, F.; Karmaus, P.W.; Grimm, S.A.; Shi, M.; et al. DNASE1L3 enhances antitumor immunity and suppresses tumor progression in colon cancer. JCI Insight 2023, 8, e168161. [Google Scholar] [CrossRef]
- Patutina, O.; Mironova, N.; Ryabchikova, E.; Popova, N.; Nikolin, V.; Kaledin, V.; Vlassov, V.; Zenkova, M. Inhibition of metastasis development by daily administration of ultralow doses of RNase A and DNase I. Biochimie 2011, 93, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef] [PubMed]
- Sounbuli, K.; Alekseeva, L.A.; Sen’kova, A.V.; Savin, I.A.; Zenkova, M.A.; Mironova, N.L. Tbp and Hprt1 Are Appropriate Reference Genes for Splenic Neutrophils Isolated from Healthy or Tumor-Bearing Mice. Biomedicines 2024, 12, 2571. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Neubert, E.; Senger-Sander, S.N.; Manzke, V.S.; Busse, J.; Polo, E.; Scheidmann, S.E.F.; Schön, M.P.; Kruss, S.; Erpenbeck, L. Serum and Serum Albumin Inhibit in vitro Formation of Neutrophil Extracellular Traps (NETs). Front. Immunol. 2019, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- von Köckritz-Blickwede, M.; Chow, O.A.; Nizet, V. Fetal calf serum contains heat-stable nucleases that degrade neutrophil extracellular traps. Blood 2009, 114, 5245–5246. [Google Scholar] [CrossRef]
- Filatova, A.A.; Alekseeva, L.A.; Sen’kova, A.V.; Savin, I.A.; Sounbuli, K.; Zenkova, M.A.; Mironova, N.L. Tumor- and Fibroblast-Derived Cell-Free DNAs Differently Affect the Progression of B16 Melanoma In Vitro and In Vivo. Int. J. Mol. Sci. 2024, 25, 5304. [Google Scholar] [CrossRef]
- Alekseeva, L.A.; Sen’kova, A.V.; Sounbuli, K.; Savin, I.A.; Zenkova, M.A.; Mironova, N.L. Pulmozyme Ameliorates LPS-Induced Lung Fibrosis but Provokes Residual Inflammation by Modulating Cell-Free DNA Composition and Controlling Neutrophil Phenotype. Biomolecules 2025, 15, 298. [Google Scholar] [CrossRef]
- Yang, Y.; Cai, H.; Yuan, X.; Xu, H.; Hu, Y.; Rui, X.; Wu, J.; Chen, J.; Li, J.; Gao, X.; et al. Efficient Targeting Drug Delivery System for Lewis Lung Carcinoma, Leading to Histomorphological Abnormalities Restoration, Physiological and Psychological Statuses Improvement, and Metastasis Inhibition. Mol. Pharm. 2018, 15, 2007–2016. [Google Scholar] [CrossRef]
- Mironova, N.; Shklyaeva, O.; Andreeva, E.; Popova, N.; Kaledin, V.; Nikolin, V.; Vlassov, V.; Zenkova, M. Animal Model of Drug-Resistant Tumor Progression. Ann. N. Y. Acad. Sci. 2006, 1091, 490–500. [Google Scholar] [CrossRef]
- Sen’kova, A.V.; Mironova, N.L.; Patutina, O.A.; Ageeva, T.A.; Zenkova, M.A. The Toxic Effects of Polychemotherapy onto the Liver Are Accelerated by the Upregulated MDR of Lymphosarcoma. ISRN Oncol. 2012, 2012, 721612. [Google Scholar] [CrossRef]
- Giavazzi, R.; Decio, A. Syngeneic murine metastasis models: B16 melanoma. Methods Mol. Biol. 2014, 1070, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Fidler, I.J. Biological Behavior of Malignant Melanoma Cells Correlated to Their Survival in Vivo. Cancer Res 1975, 35, 218–224. [Google Scholar] [PubMed]
- Overwijk, W.W.; Restifo, N.P. B16 as a Mouse Model for Human Melanoma. Curr. Protoc. Immunol. 2001, 39, 20.1.1–20.1.29. [Google Scholar] [CrossRef]
- Goncharova, E.P.; Ruzhenkova, J.S.; Petrov, I.S.; Shchelkunov, S.N.; Zenkova, M.A. Oncolytic virus efficiency inhibited growth of tumour cells with multiple drug resistant phenotype in vivo and in vitro. J. Transl. Med. 2016, 14, 241. [Google Scholar] [CrossRef]
- Mirlekar, B. Tumor promoting roles of IL-10, TGF-β, IL-4, and IL-35: Its implications in cancer immunotherapy. SAGE Open Med. 2022, 10, 20503121211069012. [Google Scholar] [CrossRef] [PubMed]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhou, Y.; Zhu, X.; Xu, L.; Bai, G.; Zhou, Y.; Zhang, N.; Liu, H.; Pan, Y.; Liu, Y.; et al. Enhancing PD-1 Inhibitor Efficacy in Cholangiocarcinoma by Reprogramming Tumor-Associated Neutrophils With CAF-Derived Extracellular Vesicles Loaded with Ruxolitinib. Adv. Funct. Mater. 2025, in press. [Google Scholar] [CrossRef]
- Zhou, S.L.; Zhou, Z.J.; Hu, Z.Q.; Huang, X.W.; Wang, Z.; Chen, E.B.; Fan, J.; Cao, Y.; Dai, Z.; Zhou, J. Tumor-Associated Neutrophils Recruit Macrophages and T-Regulatory Cells to Promote Progression of Hepatocellular Carcinoma and Resistance to Sorafenib. Gastroenterology 2016, 150, 1646–1658.e17. [Google Scholar] [CrossRef]
- Gregory, M.S.; Repp, A.C.; Holhbaum, A.M.; Saff, R.R.; Marshak-Rothstein, A.; Ksander, B.R. Membrane Fas ligand activates innate immunity and terminates ocular immune privilege. J. Immunol. 2002, 169, 2727–2735. [Google Scholar] [CrossRef]
- Sun, B.; Qin, W.; Song, M.; Liu, L.; Yu, Y.; Qi, X.; Sun, H. Neutrophil Suppresses Tumor Cell Proliferation via Fas /Fas Ligand Pathway Mediated Cell Cycle Arrested. Int. J. Biol. Sci. 2018, 14, 2103–2113. [Google Scholar] [CrossRef]
- Chung, J.Y.F.; Tang, P.C.T.; Chan, M.K.K.; Xue, V.W.; Huang, X.R.; Ng, C.S.H.; Zhang, D.; Leung, K.T.; Wong, C.K.; Lee, T.L.; et al. Smad3 is essential for polarization of tumor-associated neutrophils in non-small cell lung carcinoma. Nat. Commun. 2023, 14, 1794. [Google Scholar] [CrossRef]
- Gao, L.; Gülcüler, G.S.; Golbach, L.; Block, H.; Zarbock, A.; Martin-Villalba, A. Endothelial cell-derived CD95 ligand serves as a chemokine in induction of neutrophil slow rolling and adhesion. eLife 2016, 5, e18542. [Google Scholar] [CrossRef]
- Gungabeesoon, J.; Gort-Freitas, N.A.; Kiss, M.; Bolli, E.; Messemaker, M.; Siwicki, M.; Hicham, M.; Bill, R.; Koch, P.; Cianciaruso, C.; et al. A neutrophil response linked to tumor control in immunotherapy. Cell 2023, 186, 1448–1464.e20. [Google Scholar] [CrossRef]
- Ager, A. Cancer immunotherapy: T cells and neutrophils working together to attack cancers. Cell 2023, 186, 1304–1306. [Google Scholar] [CrossRef]
- Hirschhorn, D.; Budhu, S.; Kraehenbuehl, L.; Gigoux, M.; Schröder, D.; Chow, A.; Ricca, J.M.; Gasmi, B.; De Henau, O.; Mangarin, L.M.B.; et al. T cell immunotherapies engage neutrophils to eliminate tumor antigen escape variants. Cell 2023, 186, 1432–1447.e17. [Google Scholar] [CrossRef] [PubMed]
- Blankenstein, T.; Coulie, P.G.; Gilboa, E.; Jaffee, E.M. The determinants of tumour immunogenicity. Nat. Rev. Cancer 2012, 12, 307–313. [Google Scholar] [CrossRef]
- Alrhmoun, S.; Sennikov, S. The Role of Tumor-Associated Antigen HER2/neu in Tumor Development and the Different Approaches for Using It in Treatment: Many Choices and Future Directions. Cancers 2022, 14, 6173. [Google Scholar] [CrossRef] [PubMed]
- Yajuk, O.; Baron, M.; Toker, S.; Zelter, T.; Fainsod-Levi, T.; Granot, Z. The PD-L1/PD-1 Axis Blocks Neutrophil Cytotoxicity in Cancer. Cells 2021, 10, 1510. [Google Scholar] [CrossRef] [PubMed]
- Peshkova, A.D.; Saliakhutdinova, S.M.; Sounbuli, K.; Selivanova, Y.A.; Andrianova, I.A.; Khabirova, A.I.; Litvinov, R.I.; Weisel, J.W. The differential formation and composition of leukocyte-platelet aggregates induced by various cellular stimulants. Thromb. Res. 2024, 241, 109092. [Google Scholar] [CrossRef] [PubMed]
- Cognasse, F.; Duchez, A.C.; Audoux, E.; Ebermeyer, T.; Arthaud, C.A.; Prier, A.; Eyraud, M.A.; Mismetti, P.; Garraud, O.; Bertoletti, L.; et al. Platelets as Key Factors in Inflammation: Focus on CD40L/CD40. Front. Immunol. 2022, 13, 825892. [Google Scholar] [CrossRef]
- Vanichakarn, P.; Blair, P.; Wu, C.; Freedman, J.E.; Chakrabarti, S. Neutrophil CD40 enhances platelet-mediated inflammation. Thromb. Res. 2008, 122, 346–358. [Google Scholar] [CrossRef]
- Linde, I.L.; Prestwood, T.R.; Qiu, J.; Pilarowski, G.; Linde, M.H.; Zhang, X.; Shen, L.; Reticker-Flynn, N.E.; Chiu, D.K.C.; Sheu, L.Y.; et al. Neutrophil-activating therapy for the treatment of cancer. Cancer Cell 2023, 41, 356–372.e10. [Google Scholar] [CrossRef]
- Abuaita, B.H.; Sule, G.J.; Schultz, T.L.; Gao, F.; Knight, J.S.; O’Riordan, M.X. The IRE1α Stress Signaling Axis Is a Key Regulator of Neutrophil Antimicrobial Effector Function. J. Immunol. 2021, 207, 210–220. [Google Scholar] [CrossRef]
- Nguyen-Jackson, H.; Panopoulos, A.D.; Zhang, H.; Li, H.S.; Watowich, S.S. STAT3 controls the neutrophil migratory response to CXCR2 ligands by direct activation of G-CSF–induced CXCR2 expression and via modulation of CXCR2 signal transduction. Blood 2010, 115, 3354–3363. [Google Scholar] [CrossRef] [PubMed]
- Emmanuelli, A.; Salvagno, C.; Hwang, S.M.; Awasthi, D.; Sandoval, T.A.; Chae, C.S.; Cheong, J.G.; Tan, C.; Iwawaki, T.; Cubillos-Ruiz, J.R. High-grade serous ovarian cancer development and anti-PD-1 resistance is driven by IRE1α activity in neutrophils. Oncoimmunology 2024, 13, 2411070. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhang, X.; Zhang, L.; Li, W.; Wu, H.; Yuan, X.; Mao, F.; Wang, M.; Zhu, W.; Qian, H.; et al. The IL-6–STAT3 axis mediates a reciprocal crosstalk between cancer-derived mesenchymal stem cells and neutrophils to synergistically prompt gastric cancer progression. Cell Death Dis. 2014, 5, e1295. [Google Scholar] [CrossRef]
- Herath, T.D.K.; Larbi, A.; Teoh, S.H.; Kirkpatrick, C.J.; Goh, B.T. Neutrophil-mediated enhancement of angiogenesis and osteogenesis in a novel triple cell co-culture model with endothelial cells and osteoblasts. J. Tissue Eng. Regen. Med. 2018, 12, e1221–e1236. [Google Scholar] [CrossRef]
- Turner, T.C.; Sok, M.C.P.; Hymel, L.A.; Pittman, F.S.; York, W.Y.; Mac, Q.D.; Vyshnya, S.; Lim, H.S.; Kwong, G.A.; Qiu, P.; et al. Harnessing lipid signaling pathways to target specialized pro-angiogenic neutrophil subsets for regenerative immunotherapy. Sci. Adv. 2020, 6, eaba7702. [Google Scholar] [CrossRef]
- Braile, M.; Cristinziano, L.; Marcella, S.; Varricchi, G.; Marone, G.; Modestino, L.; Ferrara, A.L.; De Ciuceis, A.; Scala, S.; Galdiero, M.R.; et al. LPS-mediated neutrophil VEGF-A release is modulated by cannabinoid receptor activation. J. Leukoc. Biol. 2021, 109, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Nolan, E.; Malanchi, I. Connecting the dots: Neutrophils at the interface of tissue regeneration and cancer. Semin. Immunol. 2021, 57, 101598. [Google Scholar] [CrossRef]
- Laha, D.; Grant, R.; Mishra, P.; Nilubol, N. The Role of Tumor Necrosis Factor in Manipulating the Immunological Response of Tumor Microenvironment. Front. Immunol. 2021, 12, 656908. [Google Scholar] [CrossRef] [PubMed]
- Benguigui, M.; Cooper, T.J.; Kalkar, P.; Schif-Zuck, S.; Halaban, R.; Bacchiocchi, A.; Kamer, I.; Deo, A.; Manobla, B.; Menachem, R.; et al. Interferon-stimulated neutrophils as a predictor of immunotherapy response. Cancer Cell 2024, 42, 253–265.e12. [Google Scholar] [CrossRef]
- Teijeira, Á.; Garasa, S.; Gato, M.; Alfaro, C.; Migueliz, I.; Cirella, A.; de Andrea, C.; Ochoa, M.C.; Otano, I.; Etxeberria, I.; et al. CXCR1 and CXCR2 Chemokine Receptor Agonists Produced by Tumors Induce Neutrophil Extracellular Traps that Interfere with Immune Cytotoxicity. Immunity 2020, 52, 856–871.e8. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Chen, J.; Xu, T.; Cai, A.; Han, B.; Li, Y.; Fang, Z.; Yu, D.; Wang, S.; Zhou, J.; et al. VSIG4+ tumor-associated macrophages mediate neutrophil infiltration and impair antigen-specific immunity in aggressive cancers through epigenetic regulation of SPP1. J. Exp. Clin. Cancer Res. 2025, 44, 45. [Google Scholar] [CrossRef] [PubMed]
- Farrera, C.; Fadeel, B. Macrophage clearance of neutrophil extracellular traps is a silent process. J. Immunol. 2013, 191, 2647–2656. [Google Scholar] [CrossRef] [PubMed]
| cfDNA Concentration, ng/mL | mt DNA/nucDNA Ratio * | |
|---|---|---|
| RLS40 model | ||
| Healthy CBA mice | 420 ± 120 | 0.1 ± 0.2 |
| RLS40High | 3100 ± 745 ## | 1.0 ± 0.1 ## |
| RLS40Med | 1711 ± 480 # | 1.1 ± 0.1 ## |
| RLS40Low | 1558 ± 668 #§ | 1.7 ± 0.1 ##§ |
| RLS40High/D | 2033 ± 947 ## | 1.7 ± 0.1 ## |
| RLS40Med/D | 822 ± 39 $† | 2.1 ± 0.1 ## |
| RLS40Low/D | 722 ± 394 §† | 3.6 ± 0.2 ##§† |
| B16 model | ||
| Healthy C57Bl mice | 410 ± 120 | 0.1 ± 0.2 |
| B16High | 1320 ± 708 # | 1.0 ± 0.1 ## |
| B16Low | 806 ± 224 | 1.2 ± 0.2 ## |
| B16High/D | 1577 ± 819 # | 1.7 ± 0.3 ## |
| B16Low/D | 872 ± 433 | 0.8 ± 0.07 ## |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sounbuli, K.; Alekseeva, L.A.; Sen’kova, A.V.; Markov, O.V.; Savin, I.A.; Zenkova, M.A.; Mironova, N.L. Neutrophils in Cancer: Phenotypic Heterogeneity Across Tumor Models and Significant Alteration of Splenic Neutrophil Phenotype in Lymphosarcoma RLS40 Model Following DNase I Treatment. Cancers 2025, 17, 2631. https://doi.org/10.3390/cancers17162631
Sounbuli K, Alekseeva LA, Sen’kova AV, Markov OV, Savin IA, Zenkova MA, Mironova NL. Neutrophils in Cancer: Phenotypic Heterogeneity Across Tumor Models and Significant Alteration of Splenic Neutrophil Phenotype in Lymphosarcoma RLS40 Model Following DNase I Treatment. Cancers. 2025; 17(16):2631. https://doi.org/10.3390/cancers17162631
Chicago/Turabian StyleSounbuli, Khetam, Ludmila A. Alekseeva, Aleksandra V. Sen’kova, Oleg V. Markov, Innokenty A. Savin, Marina A. Zenkova, and Nadezhda L. Mironova. 2025. "Neutrophils in Cancer: Phenotypic Heterogeneity Across Tumor Models and Significant Alteration of Splenic Neutrophil Phenotype in Lymphosarcoma RLS40 Model Following DNase I Treatment" Cancers 17, no. 16: 2631. https://doi.org/10.3390/cancers17162631
APA StyleSounbuli, K., Alekseeva, L. A., Sen’kova, A. V., Markov, O. V., Savin, I. A., Zenkova, M. A., & Mironova, N. L. (2025). Neutrophils in Cancer: Phenotypic Heterogeneity Across Tumor Models and Significant Alteration of Splenic Neutrophil Phenotype in Lymphosarcoma RLS40 Model Following DNase I Treatment. Cancers, 17(16), 2631. https://doi.org/10.3390/cancers17162631








