Targeting of Mutant Isocitrate Dehydrogenase in Glioma: A Systematic Review
Simple Summary
Abstract
1. Introduction
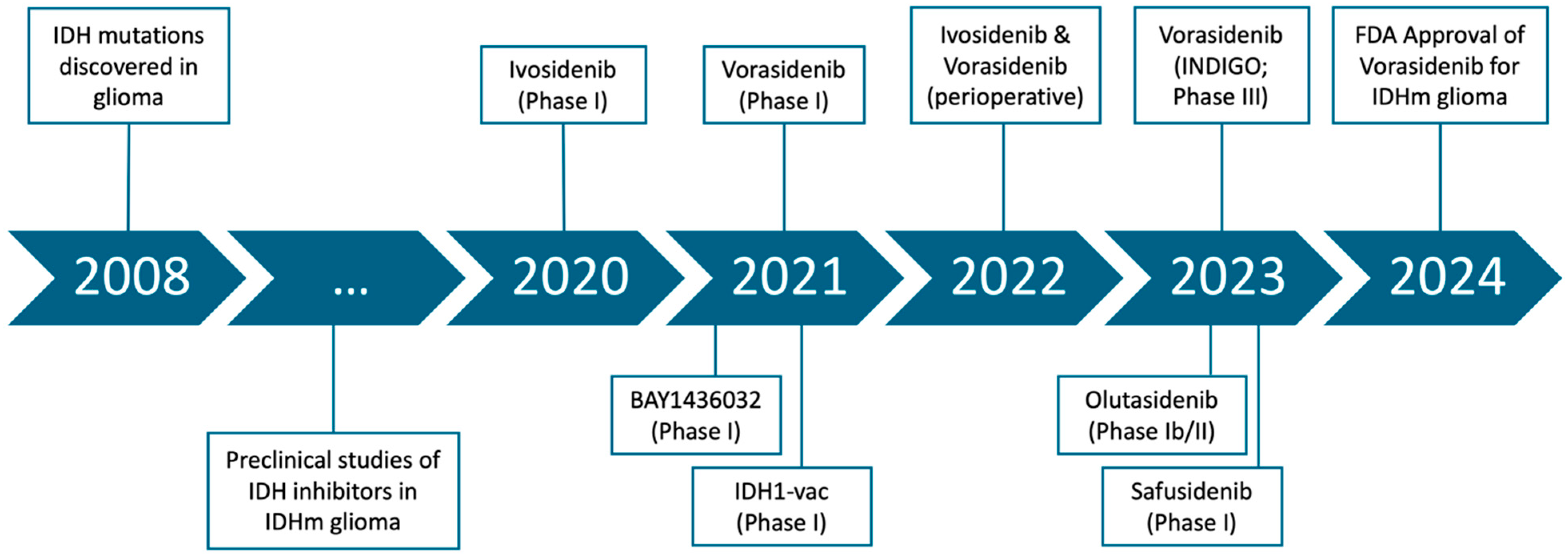
1.1. Discovery of IDH Mutations and Their Role in Gliomagenesis
1.2. Development of IDH Inhibitors
1.3. Initial Clinical Evaluation of IDH Inhibitors
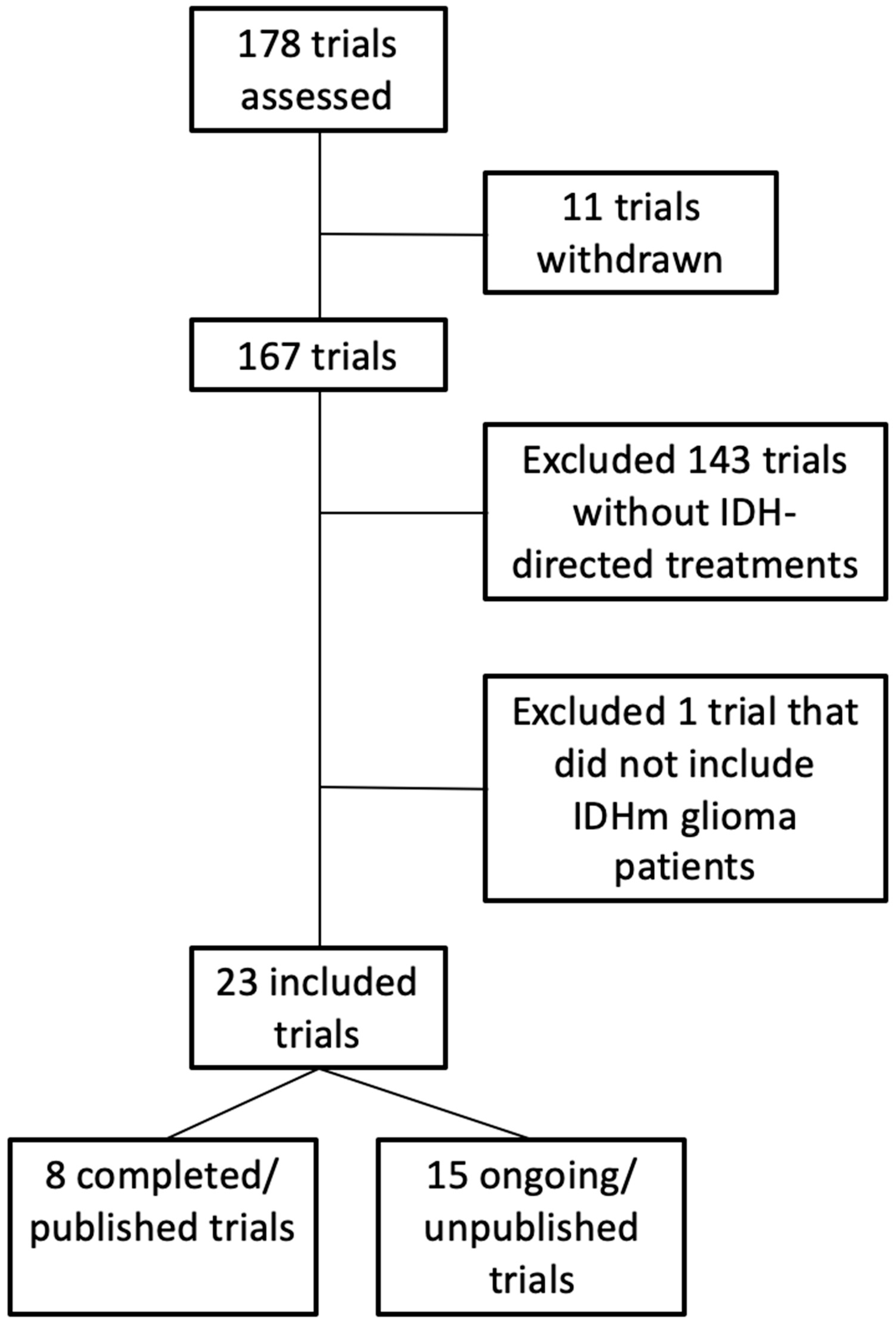
2. Methods
3. Results
3.1. Evaluation of IDH Inhibitors in Glioma
3.1.1. Ivosidenib in IDH1-Mutant Advanced Glioma
3.1.2. Safety and Therapeutic Activity of BAY1436032 in Patients with IDH1-Mutant Solid Tumors
3.1.3. Vorasidenib, a Dual Inhibitor of Mutant IDH1/2, in Recurrent or Progressive Glioma
3.1.4. A Vaccine Targeting Mutant IDH1 in Newly Diagnosed Glioma
3.1.5. Vorasidenib and Ivosidenib Perioperative Phase 1 Trial
3.1.6. Vorasidenib in IDH1- or IDH2-Mutant Low-Grade Glioma
3.1.7. Phase I Study of IDH1 Inhibitor DS-1001 in Recurrent or Progressive IDH1-Mutant Glioma
3.1.8. Olutasidenib (FT-2102) in Relapsed or Refractory IDH1-Mutant Glioma
3.2. Retrospective Studies
3.3. Updates on Tumor Volumetrics and Seizure Control with IDHi
4. Discussion
Future Directions and Ongoing Studies
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Miller, J.J.; Gonzalez Castro, L.N.; McBrayer, S.; Weller, M.; Cloughesy, T.; Portnow, J.; Andronesi, O.; Barnholtz-Sloan, J.S.; Baumert, B.G.; Berger, M.S.; et al. Isocitrate dehydrogenase (IDH) mutant gliomas: A Society for Neu-ro-Oncology (SNO) consensus review on diagnosis, management, and future directions. Neuro-Oncology 2023, 25, 4–25. [Google Scholar] [CrossRef]
- Xu, W.; Yang, H.; Liu, Y.; Yang, Y.; Wang, P.; Kim, S.H.; Ito, S.; Yang, C.; Wang, P.; Xiao, M.T.; et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of al-pha-ketoglutarate-dependent dioxygenases. Cancer Cell 2011, 19, 17–30. [Google Scholar] [CrossRef]
- Chowdhury, R.; Yeoh, K.K.; Tian, Y.-M.; Hillringhaus, L.; Bagg, E.A.; Rose, N.R.; Leung, I.K.H.; Li, X.S.; Woon, E.C.Y.; Yang, M.; et al. The oncometabolite 2-hydroxyglutarate inhibits histone lysine demethylases. EMBO Rep. 2011, 12, 463–469. [Google Scholar] [CrossRef]
- Flavahan, W.A.; Drier, Y.; Liau, B.B.; Gillespie, S.M.; Venteicher, A.S.; Stemmer-Rachamimov, A.O.; Suvà, M.L.; Bernstein, B.E. Insulator dysfunction and oncogene activation in IDH mutant gliomas. Nature 2016, 529, 110–114. [Google Scholar] [CrossRef]
- Martius, C.K.F. Der physiologische Abbau der Citronensaure. Z. Physiol. Chem. 1937, 246, 1–11. [Google Scholar] [CrossRef]
- Al-Khallaf, H. Isocitrate dehydrogenases in physiology and cancer: Biochemical and molecular insight. Cell Biosci. 2017, 7, 37. [Google Scholar] [CrossRef]
- Parsons, D.W.; Jones, S.; Zhang, X.; Lin, J.C.-H.; Leary, R.J.; Angenendt, P.; Mankoo, P.; Carter, H.; Siu, I.-M.; Gallia, G.L.; et al. An integrated genomic analysis of human glioblastoma multiforme. Science 2008, 321, 1807–1812. [Google Scholar] [CrossRef] [PubMed]
- Carosi, F.; Broseghini, E.; Fabbri, L.; Corradi, G.; Gili, R.; Forte, V.; Roncarati, R.; Filippini, D.M.; Ferracin, M. Targeting Isocitrate Dehydrogenase (IDH) in Solid Tumors: Current Evidence and Future Perspectives. Cancers 2024, 16, 2752. [Google Scholar] [CrossRef] [PubMed]
- Clark, O.; Yen, K.; Mellinghoff, I.K. Molecular Pathways: Isocitrate Dehydrogenase Mutations in Cancer. Clin. Cancer Res. 2016, 22, 1837–1842. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Parsons, D.W.; Jin, G.; McLendon, R.; Rasheed, B.A.; Yuan, W.; Kos, I.; Batinic-Haberle, I.; Jones, S.; Riggins, G.J.; et al. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 2009, 360, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Nobusawa, S.; Kleihues, P.; Ohgaki, H. IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am. J. Pathol. 2009, 174, 1149–1153. [Google Scholar] [CrossRef]
- Dang, L.; White, D.W.; Gross, S.; Bennett, B.D.; Bittinger, M.A.; Driggers, E.M.; Fantin, V.R.; Jang, H.G.; Jin, S.; Keenan, M.C.; et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 2009, 462, 739–744. [Google Scholar] [CrossRef]
- Turcan, S.; Rohle, D.; Goenka, A.; Walsh, L.A.; Fang, F.; Yilmaz, E.; Campos, C.; Fabius, A.W.M.; Lu, C.; Ward, P.S.; et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature 2012, 483, 479–483. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, Y. TET-mediated active DNA demethylation: Mechanism, function and beyond. Nat. Rev. Genet. 2017, 18, 517–534. [Google Scholar] [CrossRef]
- Lu, C.; Ward, P.S.; Kapoor, G.S.; Rohle, D.; Turcan, S.; Abdel-Wahab, O.; Edwards, C.R.; Khanin, R.; Figueroa, M.E.; Melnick, A.; et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature 2012, 483, 474–478. [Google Scholar] [CrossRef]
- Malta, T.M.; de Souza, C.F.; Sabedot, T.S.; Silva, T.C.; Mosella, M.S.; Kalkanis, S.N.; Snyder, J.; Castro, A.V.B.; Noushmehr, H. Glioma CpG island methylator phenotype (G-CIMP): Biological and clinical implications. Neuro-Oncology 2017, 20, 608–620. [Google Scholar] [CrossRef]
- Popovici-Muller, J.; Saunders, J.O.; Salituro, F.G.; Travins, J.M.; Yan, S.; Zhao, F.; Gross, S.; Dang, L.; Yen, K.E.; Yang, H.; et al. Discovery of the First Potent Inhibitors of Mutant IDH1 That Lower Tumor 2-HG in Vivo. ACS Med. Chem. Lett. 2012, 3, 850–855. [Google Scholar] [CrossRef]
- Rohle, D.; Popovici-Muller, J.; Palaskas, N.; Turcan, S.; Grommes, C.; Campos, C.; Tsoi, J.; Clark, O.; Oldrini, B.; Komisopoulou, E.; et al. An Inhibitor of Mutant IDH1 Delays Growth and Promotes Differentiation of Glioma Cells. Science 2013, 340, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Zhang, W.; Wang, Y.; Jin, R.; Wang, Y.; Guo, H.; Tang, Y.; Yao, X. Recent advances of IDH1 mutant inhibitor in cancer therapy. Front Pharmacol. 2022, 13, 982424. [Google Scholar] [CrossRef] [PubMed]
- Popovici-Muller, J.; Lemieux, R.M.; Artin, E.; Saunders, J.O.; Salituro, F.G.; Travins, J.; Cianchetta, G.; Cai, Z.; Zhou, D.; Cui, D.; et al. Discovery of AG-120 (Ivosidenib): A First-in-Class Mutant IDH1 In-hibitor for the Treatment of IDH1 Mutant Cancers. ACS Med. Chem. Lett. 2018, 9, 300–305. [Google Scholar] [CrossRef]
- Cazzola, M. IDH1 and IDH2 mutations in myeloid neoplasms—Novel paradigms and clinical implications. Haematologica 2010, 95, 1623–1627. [Google Scholar] [CrossRef]
- Yen, K.; Travins, J.; Wang, F.; David, M.D.; Artin, E.; Straley, K.; Padyana, A.; Gross, S.; DeLaBarre, B.; Tobin, E.; et al. AG-221, a First-in-Class Therapy Targeting Acute Myeloid Leukemia Harboring Oncogenic IDH2 Mutations. Cancer Discov. 2017, 7, 478–493. [Google Scholar] [CrossRef] [PubMed]
- Konteatis, Z.; Artin, E.; Nicolay, B.; Straley, K.; Padyana, A.K.; Jin, L.; Chen, Y.; Narayaraswamy, R.; Tong, S.; Wang, F.; et al. Vorasidenib (AG-881): A First-in-Class, Brain-Penetrant Dual Inhibitor of Mutant IDH1 and 2 for Treatment of Glioma. ACS Med. Chem. Lett. 2020, 11, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Pusch, S.; Krausert, S.; Fischer, V.; Balss, J.; Ott, M.; Schrimpf, D.; Capper, D.; Sahm, F.; Eisel, J.; Beck, A.-C.; et al. Pan-mutant IDH1 inhibitor BAY 1436032 for effective treatment of IDH1 mutant astrocytoma in vivo. Acta Neuropathol. 2017, 133, 629–644. [Google Scholar] [CrossRef]
- Caravella, J.A.; Lin, J.; Diebold, R.B.; Campbell, A.-M.; Ericsson, A.; Gustafson, G.; Wang, Z.; Castro, J.; Clarke, A.; Gotur, D.; et al. Structure-Based Design and Identification of FT-2102 (Olutasidenib), a Potent Mutant-Selective IDH1 Inhibitor. J. Med. Chem. 2020, 63, 1612–1623. [Google Scholar] [CrossRef]
- Cho, Y.S.; Levell, J.R.; Liu, G.; Caferro, T.; Sutton, J.; Shafer, C.M.; Costales, A.; Manning, J.R.; Zhao, Q.; Sendzik, M.; et al. Discovery and Evaluation of Clinical Candidate IDH305, a Brain Penetrant Mutant IDH1 Inhibitor. ACS Med. Chem. Lett. 2017, 8, 1116–1121. [Google Scholar] [CrossRef]
- Machida, Y.; Nakagawa, M.; Matsunaga, H.; Yamaguchi, M.; Ogawara, Y.; Shima, Y.; Yamagata, K.; Katsumoto, T.; Hattori, A.; Itoh, M.; et al. A Potent Blood-Brain Barrier-Permeable Mutant IDH1 Inhibitor Sup-presses the Growth of Glioblastoma with IDH1 Mutation in a Patient-Derived Orthotopic Xenograft Model. Mol. Cancer Ther. 2020, 19, 375–383. [Google Scholar] [CrossRef]
- Stein, E.M.; Dinardo, C.D.; Pollyea, D.A.; Fathi, A.T.; Roboz, G.J.; Altman, J.K.; Stone, R.M.; DeAngelo, D.J.; Levine, R.L.; Flinn, I.W.; et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood 2017, 130, 722–731. [Google Scholar] [CrossRef]
- Kim, E.S. Enasidenib: First Global Approval. Drugs 2017, 77, 1705–1711. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Stein, E.M.; de Botton, S.; Roboz, G.J.; Altman, J.K.; Mims, A.S.; Swords, R.; Collins, R.H.; Mannis, G.N.; Pollyea, D.A.; et al. Durable Remissions with Ivosidenib in IDH1-Mutated Relapsed or Refrac-tory AML. N. Engl. J. Med. 2018, 378, 2386–2398. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarci-noma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 796–807. [Google Scholar] [CrossRef]
- DiNardo, C.D.D.; Roboz, G.J.; Watts, J.M.; Madanat, Y.F.; Prince, G.T.; Baratam, P.; de Botton, S.; Stein, A.S.; Foran, J.M.; Arellano, M.L.; et al. Final phase 1 substudy results of ivosidenib for patients with mutant IDH1 relapsed/refractory myelodysplastic syndrome. Blood Adv. 2024, 8, 4209–4220. [Google Scholar] [CrossRef] [PubMed]
- de Botton, S.; Fenaux, P.; Yee, K.; Récher, C.; Wei, A.H.; Montesinos, P.; Taussig, D.C.; Pigneux, A.; Braun, T.; Curti, A.; et al. Olutasidenib (FT-2102) induces durable complete remissions in patients with re-lapsed or refractory IDH1-mutated AML. Blood Adv. 2023, 7, 3117–3127. [Google Scholar] [CrossRef]
- Mellinghoff, I.K.; Ellingson, B.M.; Touat, M.; Maher, E.; De La Fuente, M.I.; Holdhoff, M.; Cote, G.M.; Burris, H.; Janku, F.; Young, R.J.; et al. Ivosidenib in Isocitrate Dehydrogenase 1-Mutated Advanced Glioma. J. Clin. Oncol. 2020, 38, 3398–3406. [Google Scholar] [CrossRef]
- Pallud, J.; Taillandier, L.; Capelle, L.; Fontaine, D.; Peyre, M.; Ducray, F.; Duffau, H.; Mandonnet, E. Quantitative morphological magnetic resonance imaging follow-up of low-grade glioma: A plea for systematic measurement of growth rates. Neurosurgery 2012, 71, 729–739, discussion 739–740. [Google Scholar] [CrossRef]
- Gerstner, E.R. Volumetric measurements in low-grade glioma: Are we there yet? Neuro-Oncology 2022, 24, 779–780. [Google Scholar] [CrossRef] [PubMed]
- Wick, A.; Bähr, O.; Schuler, M.; Rohrberg, K.; Chawla, S.P.; Janku, F.; Schiff, D.; Heinemann, V.; Narita, Y.; Lenz, H.-J.; et al. Phase I Assessment of Safety and Therapeutic Activity of BAY1436032 in Patients with IDH1-Mutant Solid Tumors. Clin. Cancer Res. 2021, 27, 2723–2733. [Google Scholar] [CrossRef] [PubMed]
- Heuser, M.; Palmisiano, N.; Mantzaris, I.; Mims, A.; DiNardo, C.; Silverman, L.R.; Wang, E.S.; Fiedler, W.; Baldus, C.; Schwind, S.; et al. Safety and efficacy of BAY1436032 in IDH1-mutant AML: Phase I study results. Leukemia 2020, 34, 2903–2913. [Google Scholar] [CrossRef] [PubMed]
- Mellinghoff, I.K.; Penas-Prado, M.; Peters, K.B.; Burris, H.A.; Maher, E.A.; Janku, F.; Cote, G.M.; de la Fuente, M.I.; Clarke, J.L.; Ellingson, B.M.; et al. Vorasidenib, a Dual Inhibitor of Mutant IDH1/2, in Recurrent or Progressive Glioma; Results of a First-in-Human Phase I Trial. Clin. Cancer Res. 2021, 27, 4491–4499. [Google Scholar] [CrossRef]
- Platten, M.; Bunse, L.; Wick, A.; Bunse, T.; Le Cornet, L.; Harting, I.; Sahm, F.; Sanghvi, K.; Tan, C.L.; Poschke, I.; et al. A vaccine targeting mutant IDH1 in newly diagnosed glioma. Nature 2021, 592, 463–468. [Google Scholar] [CrossRef]
- Mellinghoff, I.K.; Lu, M.; Wen, P.Y.; Taylor, J.W.; Maher, E.A.; Arrillaga-Romany, I.; Peters, K.B.; Ellingson, B.M.; Rosenblum, M.K.; Chun, S.; et al. Vorasidenib and ivosidenib in IDH1-mutant low-grade glioma: A randomized, perioperative phase 1 trial. Nat. Med. 2023, 29, 615–622. [Google Scholar] [CrossRef]
- Khalili, N.; Kazerooni, A.F.; Familiar, A.; Haldar, D.; Kraya, A.; Foster, J.; Koptyra, M.; Storm, P.B.; Resnick, A.C.; Nabavizadeh, A. Radiomics for characterization of the glioma immune microenvironment. NPJ Precis. Oncol. 2023, 7, 59. [Google Scholar] [CrossRef]
- Mellinghoff, I.K.; Bent, M.J.v.D.; Blumenthal, D.T.; Touat, M.; Peters, K.B.; Clarke, J.; Mendez, J.; Yust-Katz, S.; Welsh, L.; Mason, W.P.; et al. Vorasidenib in IDH1- or IDH2-Mutant Low-Grade Glioma. N. Engl. J. Med. 2023, 389, 589–601. [Google Scholar] [CrossRef]
- Administration USFaD. Vorasidenib (Vorinigo) [Package Insert]. 2024. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/218784s000lbl.pdf (accessed on 16 March 2025).
- Natsume, A.; Arakawa, Y.; Narita, Y.; Sugiyama, K.; Hata, N.; Muragaki, Y.; Shinojima, N.; Kumabe, T.; Saito, R.; Motomura, K.; et al. The first-in-human phase I study of a brain-penetrant mutant IDH1 inhibitor DS-1001 in patients with recurrent or progressive IDH1-mutant gliomas. Neuro-Oncology 2022, 25, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.J.; Arrillaga-Romany, I. IDH-mutant glioma: A new IDH1 inhibitor moves forward. Neuro-Oncology 2023, 25, 337–338. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, M.I.; Colman, H.; Rosenthal, M.; Van Tine, B.A.; Levacic, D.; Walbert, T.; Gan, H.K.; Vieito, M.; Milhem, M.M.; Lipford, K.; et al. Olutasidenib (FT-2102) in patients with relapsed or refractory IDH1-mutant glioma: A multicenter, open-label, phase Ib/II trial. Neuro-Oncology 2023, 25, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.D.; Tsai, A.C.-Y.; Abdullah, K.G.; McBrayer, S.K.; Shi, D.D. Treatment of IDH-mutant glioma in the INDIGO era. NPJ Precis. Oncol. 2024, 8, 149. [Google Scholar] [CrossRef]
- Kinslow, C.J.; Brown, P.D.; Iwamoto, F.M.; Wu, C.-C.; Yu, J.B.; Cheng, S.K.; Wang, T.J. Where Do We (INDI)GO From Here? Int. J. Radiat. Oncol. Biol. Phys. 2024, 118, 330–333. [Google Scholar] [CrossRef]
- Lanman, T.A.; Cao, T.Q.; Miller, J.J.; Nagpal, S. Ready to INDIGO: Vorasidenib Ushers in the Era of Isocitrate Dehydrogenase Inhibition in Low-Grade Glioma. Int. J. Radiat. Oncol. 2024, 118, 334–336. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, M.I.; Touat, M.; van den Bent, M.J.; Preusser, M.; Peters, K.B.; Young, R.J.; Huang, R.Y.; Ellingson, B.M.; Capper, D.; Phillips, J.J.; et al. The role of vorasidenib in the treatment of isocitrate dehydrogen-ase-mutant glioma. Neuro-Oncology 2024, 5, 1135–1148. [Google Scholar]
- van den Bent, M.J.; French, P.J.; Brat, D.; Tonn, J.C.; Touat, M.; Ellingson, B.M.; Young, R.J.; Pallud, J.; von Deimling, A.; Sahm, F.; et al. The biological significance of tumor grade, age, enhancement, and extent of resection in IDH-mutant gliomas: How should they inform treatment decisions in the era of IDH inhibitors? Neuro-Oncology 2024, 26, 1805–1822. [Google Scholar] [CrossRef]
- Nakhate, V.; Lasica, A.B.; Wen, P.Y. The Role of Mutant IDH Inhibitors in the Treatment of Glioma. Curr. Neurol. Neurosci. Rep. 2024, 24, 631–643. [Google Scholar] [CrossRef]
- Peters, K.B.; Alford, C.; Heltemes, A.; Savelli, A.; Landi, D.B.; Broadwater, G.; Desjardins, A.; Johnson, M.O.; Low, J.T.; Khasraw, M.; et al. Use, access, and initial outcomes of off-label ivosidenib in patients with IDH1 mutant glioma. Neuro-Oncol. Pract. 2023, 11, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Kamson, D.O.; Puri, S.; Sang, Y.; Shi, M.J.; Blair, L.; Blakeley, J.O.; Laterra, J. Impact of Frontline Ivosidenib on Volumetric Growth Patterns in Isocitrate Dehy-drogenase-mutant Astrocytic and Oligodendroglial Tumors. Clin. Cancer Res. 2023, 29, 4863–4869. [Google Scholar] [CrossRef]
- Lanman, T.A.; Youssef, G.; Huang, R.; Rahman, R.; DeSalvo, M.; Flood, T.; Hassanzadeh, E.; Lang, M.; Lauer, J.; Potter, C.; et al. Ivosidenib for the treatment of IDH1-mutant glioma, grades 2-4: Tolerability, predictors of response, and outcomes. Neurooncol. Adv. 2025, 7, vdae227. [Google Scholar] [CrossRef] [PubMed]
- Ellingson, B.M.; Kim, G.H.J.; Brown, M.; Lee, J.; Salamon, N.; Steelman, L.; Hassan, I.; Pandya, S.S.; Chun, S.; Linetsky, M.; et al. Volumetric measurements are preferred in the evaluation of mutant IDH inhibition in non-enhancing diffuse gliomas: Evidence from a phase I trial of ivosidenib. Neuro-Oncology 2021, 24, 770–778. [Google Scholar] [CrossRef]
- Mellinghoff, I.; Wen, P.; Taylor, J.; Taylor, E.; Arrillaga-Romany, I.; Ellingson, B.; Mahboub-Johnson, P.; Baron, E.; Hassan, I.; Steelman, L.; et al. Ctni-47. A phase 1, randomized, perioperative trial of vorasidenib and ivosidenib in idh1-mutant diffuse glioma: Updated results. Neuro-Oncology 2024, 26, viii107. [Google Scholar] [CrossRef]
- Peters, K.; Mellinghoff, I.; Van Den Bent, M.; Blumenthal, D.; Touat, M.; Clarke, J.; Mendez, J.; Yust-Katz, S.; Mason, W.; Ducray, F.; et al. A Randomized, Double-blind, Phase 3 Study of Vorasidenib Versus Placebo in Patients with Mutant IDH1/2 Diffuse Glioma (INDIGO): Analysis of Health-related Quality of Life, Neurocognition and Seizures (PL5.003). Neurology 2024, 102, 5113. [Google Scholar] [CrossRef]
- Mellinghoff, I.K.; van den Bent, M.J.; Touat, M.; Blumenthal, D.T.; Peters, K.B.; Ellingson, B.M.; Clarke, J.L.; Mendez, J.; Yust-Katz, S.; Mason, W.P.; et al. Ctni-53. A global, randomized, double-blinded, phase 3 study of vorasidenib versus placebo in patients with adult-type diffuse glioma with an idh1/2 mutation (indigo): Updated efficacy results. Neuro-Oncology 2024, 26, viii108–viii109. [Google Scholar] [CrossRef]
- Drumm, M.R.; Wang, W.; Sears, T.K.; Bell-Burdett, K.; Javier, R.; Cotton, K.Y.; Webb, B.; Byrne, K.; Unruh, D.; Thirunavu, V.; et al. Postoperative risk of IDH mutant glioma–associated seizures and their potential management with IDH mutant inhibitors. J. Clin. Investig. 2023, 133, e168035. [Google Scholar] [CrossRef]
- Cloughesy, T.; Peters, K.; Colman, H.; Taylor, J.; Mellinghoff, I.; Shih, H.; Chacar, C.; Dewey, J.; Wen, P. Ctni-66. Idh-mutant glioma patients treated with vorasidenib: Ongoing data from the vorasidenib expanded access program. Neuro-Oncology 2024, 26, viii112. [Google Scholar] [CrossRef]
- Bunse, L.; Pusch, S.; Bunse, T.; Sahm, F.; Sanghvi, K.; Friedrich, M.; Alansary, D.; Sonner, J.K.; Green, E.; Deumelandt, K.; et al. Suppression of antitumor T cell immunity by the oncometabolite (R)-2-hydroxyglutarate. Nat. Med. 2018, 24, 1192–1203. [Google Scholar] [CrossRef]
- Wu, M.-J.; Kondo, H.; Kammula, A.V.; Shi, L.; Xiao, Y.; Dhiab, S.; Xu, Q.; Slater, C.J.; Avila, O.I.; Merritt, J.; et al. Mutant IDH1 inhibition induces dsDNA sensing to activate tumor immunity. Science 2024, 385, eadl6173. [Google Scholar] [CrossRef]
- Wu, J.; Castro, L.N.G.; Battaglia, S.; El Farran, C.A.; D’aNtonio, J.P.; Miller, T.E.; Suvà, M.L.; Bernstein, B.E. Evolving cell states and oncogenic drivers during the progression of IDH-mutant gliomas. Nat. Cancer 2024, 6, 145–157. [Google Scholar] [CrossRef]
- Gallus, M.; Kwok, D.; Lakshmanachetty, S.; Yamamichi, A.; Okada, H. Immunotherapy Approaches in Isocitrate-Dehydrogenase-Mutant Low-Grade Glioma. Cancers 2023, 15, 3726. [Google Scholar] [CrossRef]
- Schumacher, T.; Bunse, L.; Pusch, S.; Sahm, F.; Wiestler, B.; Quandt, J.; Menn, O.; Osswald, M.; Oezen, I.; Ott, M.; et al. A vaccine targeting mutant IDH1 induces antitumour immunity. Nature 2014, 512, 324–327. [Google Scholar] [CrossRef]
- Reardon, D.A.; Weller, M. Vaccination for IDH-mutant tumors: A novel therapeutic approach applied to glioma. Med 2021, 2, 450–452. [Google Scholar] [CrossRef]
- Lanman, T.A.; Wetzel, E.A.; Verma, K.; Gonzalez Castro, L.N. Reproductive health considerations for IDH-mutant glioma patients considering IDH inhibitor therapy: A retrospective cohort study. Neuro-Oncol. Pract. 2025; in press. [Google Scholar]
- Still, M.E.H.; Moor, R.S.F.; Ghiaseddin, A.P.; Leibetseder, A.; Hottinger, A.F.; Berghoff, A.; Leung, D. How do I prescribe and manage mIDH inhibitors in patients with IDH-mutant glioma? Neuro-Oncol. Pract. 2025, 12, i19–i25. [Google Scholar] [CrossRef] [PubMed]
- Peters, K.B.; Geurts, M. Practical management of patients with IDH-mutant glioma in the coming era of mIDH inhibitors: New drugs, new evidence, new guidelines, and new considerations. Neuro-Oncol. Pract. 2025, 12, i2–i5. [Google Scholar] [CrossRef] [PubMed]
| Treatment & Date Published | NCT Identifier | Study Design | Population | Sample Size and Timeline | Results | Safety * | Impact |
|---|---|---|---|---|---|---|---|
| Ivosidenib (2020) | NCT02073994 |
| G2–4 IDHm glioma, including NE and E tumors | n = 66 Median treatment duration: 18.4 mo (NE) and 1.9 mo (E) | NE: PFS 13.6 mo, DCR 91%, ORR 3% E: PFS 1.4 mo, DCR 45%, ORR 0% | Grade ≥ 3 TEAE: 19.7% Headache, fatigue, nausea |
|
| BAY1436032 (2021) | NCT02746081 |
| G2–4 IDHm glioma, some NE but predominantly E tumors | n = 49 Median treatment duration: 5.5 mo for those with SD or better | G2–3: 3 mo PFS 31%, DCR 54%, ORR 11% G4: 3 mo PFS 22%, DCR 29%, ORR 0% | Grade ≥ 3 TEAE: 72% Headache, diarrhea, nausea |
|
| Vorasidenib (2021) | NCT02481154 |
| G2–4 IDHm glioma, including NE and E tumors | n = 52 Median treatment duration: 26.8 mo (NE) and 3.3 (E) | NE: PFS 36.8 mo, DCR 91%, ORR 18.2% E: PFS 3.6 mo, DCR 57%, ORR 0% | Grade ≥ 3 TEAE: 19.2% Headache, transaminitis, fatigue |
|
| IDH1-vac (2021) | NCT02454634 |
| G3–4 IDHm astrocytoma including NE and E tumors | n = 33 94% of patients reached end of treatment Median follow-up time: 46.9 mo | 3 year progression-free rate: 63% 3 year death-free rate: 0.84% | Grade ≥ 3 TEAE: 0% Injection site reactions, skin/subcutaneous tissue disorders |
|
| Vorasidenib vs. Ivosidenib (2023) | NCT03343197 |
| NE G2–3 IDHm glioma (excluded E tumors) | n = 24 (vora); n = 25 (ivo) Median treatment duration: 14.3 mo (vora), 15.1 mo (ivo) | Tumor [2-HG] reduced by 93% (vora) and 91% (ivo) Vora: DCR 88%, ORR 29% Ivo: DCR 91%, ORR 27.3% | Grade ≥ 3 TEAE: 29.2% (vora) and 24% (ivo) Nausea, headache, diarrhea |
|
| Vorasidenib (2023) | NCT04164901 |
| NE G2 IDHm glioma (excluded E tumors) | n = 168 (vora); n = 163 (placebo) Median follow-up: 14.2 mo | Vora: PFS 27.7 mo, DCR 94%, ORR 10.7 Placebo: PFS 11.1 mo, DCR 91%, ORR 2.5% | Grade ≥ 3 TEAE: 22.8% Transaminitis, COVID-19, fatigue |
|
| Safusidenib (2023) | NCT05577416 |
| G2–4 IDHm glioma, including NE and E tumors | n = 47 Median treatment duration: 91.2 mo (NE) and 7.3 mo (E) | NE: PFS not reached; DCR 100%, ORR 33.3% E: PFS 10.4w, DCR 51%, ORR 17.1% | Grade ≥ 3 TEAE: 42.6% (all grade 3, none higher) Skin hyperpigmentation, pruritis, alopecia |
|
| Olutasidenib (2023) | NCT03684811 |
| G2–4 IDHm glioma, some NE but predominantly E tumors | n = 26 Median treatment duration: 4.2 mo | PFS 1.9 mo, DCR 48%, ORR 8% | Grade ≥ 3 TEAE: 42% Nausea, fatigue, transaminitis |
|
| Trial Name | NCT Identifier | Location | Study Design | Status * | Population | Objectives/Impact |
|---|---|---|---|---|---|---|
| Vorasidenib Maintenance for IDH Mutant Astrocytoma | NCT06809322 | Europe |
| Not yet recruiting |
| Assesses whether vorasidenib improves PFS immediately following first-line radiotherapy and chemotherapy (regimen determined by treating physicians) |
| ViCToRy: Vorasidenib in Combination With Tumor Specific Peptide Vaccine for Recurrent IDH1 Mutant Lower Grade Gliomas | NCT05609994 | Single institution (Duke) |
| Not yet recruiting |
| Assesses safety and efficacy of a novel IDH-targeted peptide vaccine in combination with an IDHi |
| Vorasidenib Maintenance for IDH Mutant Astrocytoma | NCT06780930 | China, Taiwan |
| Recruiting |
| Verifies results of INDIGO, but in a Chinese/Taiwanese population |
| Vorasidenib in Combination With Temozolomide (TMZ) in IDH-mutant Glioma | NCT06478212 | International (including US) |
| Recruiting |
| Assesses safety and efficacy of combining an IDHi with temozolomide chemotherapy after completion of radiation and/or chemotherapy or as therapy at first recurrence. |
| Study of Olutasidenib and Temozolomide in HGG | NCT06161974 | International (including US) |
| Recruiting |
| Assesses efficacy of combining this IDHi with chemotherapy after completion of radiotherapy in children and young adults |
| A Study of AB-218 (Safusidenib) in Patients With IDH1 Mutated Low Grade Glioma | NCT05577416 | Single institution (Royal Melbourne Hospital in Australia) |
| Recruiting |
| Assesses feasibility, PK and PD of safusidenib using a perioperative design |
| Study of Vorasidenib and Pembrolizumab Combination in Recurrent or Progressive IDH-1 Mutant Glioma | NCT05484622 | US |
| Recruiting |
| Ascertains recommended combination dose of an IDHi in combination with immunotherapy and then assesses safety and feasibility of this combination in those with enhancing disease |
| Safusidenib Phase 2 Study in IDH1 Mutant Glioma | NCT05303519 | US |
| Active, not recruiting |
| Assesses optimal dose for safusidenib and then assesses efficacy, safety, and PK of this drug |
| A Study of HMPL-306 in Advanced Solid Tumors With IDH Mutations | NCT04762602 | US, Spain |
| Active, not recruiting |
| Assesses safety, tolerability, PK, PD, and preliminary efficacy of a novel IDHi that targets both mutant IDH1 and IDH2 |
| Ivosidenib in Treating Patients With Advanced Solid Tumors, Lymphoma, or Histiocytic Disorders With IDH1 Mutations (A Pediatric MATCH Treatment Trial) | NCT04195555 | International (including US) |
| Active, not recruiting |
| Assesses long-term safety, tolerability, pharmacokinetics, and pharmacodynamics of ivosidenib in a pediatric/adolescent population |
| A Study of DS-1001b in Patients With Chemotherapy- and Radiotherapy-Naive IDH1 Mutated WHO Grade II Glioma | NCT04458272 | Japan |
| Active, not recruiting |
| Assesses safety and efficacy of safusidenib in the upfront (nontreated) setting. |
| Study of LY3410738 Administered to Patients With Advanced Solid Tumors With IDH1 or IDH2 Mutations | NCT04521686 | International (including US) |
| Active, not recruiting |
| Assesses safety and efficacy of a mutant IDH1/2 inhibitor either as monotherapy or in combination with chemotherapy or immunotherapy |
| Ivosidenib (AG-120) With Nivolumab in IDH1 Mutant Tumors | NCT04056910 | Single institution (UPMC Hillman Cancer Center in Pennsylvania) |
| Completed, not yet published |
| This will be the first trial evaluating the combination of IDH inhibition and immunotherapy |
| AMPLIFYing NEOepitope-specific VACcine Responses in Progressive Diffuse Glioma | NCT03893903 | Germany |
| Completed, not yet published |
| Assesses safety and immunogenicity of a novel IDHm-directed peptide vaccine, either alone or in combination with immunotherapy |
| IDH1 Peptide Vaccine for Recurrent Grade II Glioma | NCT02193347 | Single Institution (Duke) |
| Completed, not yet published |
| Assesses safety and immunogenicity of a novel IDHm-directed peptide combined with TMZ +/− RT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanman, T.A.; Gonzalez Castro, L.N. Targeting of Mutant Isocitrate Dehydrogenase in Glioma: A Systematic Review. Cancers 2025, 17, 2630. https://doi.org/10.3390/cancers17162630
Lanman TA, Gonzalez Castro LN. Targeting of Mutant Isocitrate Dehydrogenase in Glioma: A Systematic Review. Cancers. 2025; 17(16):2630. https://doi.org/10.3390/cancers17162630
Chicago/Turabian StyleLanman, Tyler A., and L. Nicolas Gonzalez Castro. 2025. "Targeting of Mutant Isocitrate Dehydrogenase in Glioma: A Systematic Review" Cancers 17, no. 16: 2630. https://doi.org/10.3390/cancers17162630
APA StyleLanman, T. A., & Gonzalez Castro, L. N. (2025). Targeting of Mutant Isocitrate Dehydrogenase in Glioma: A Systematic Review. Cancers, 17(16), 2630. https://doi.org/10.3390/cancers17162630






