Neutrophil Dynamics in Response to Cancer Therapies
Simple Summary
Abstract
1. Introduction
2. Polarization and Function Roles of TANs
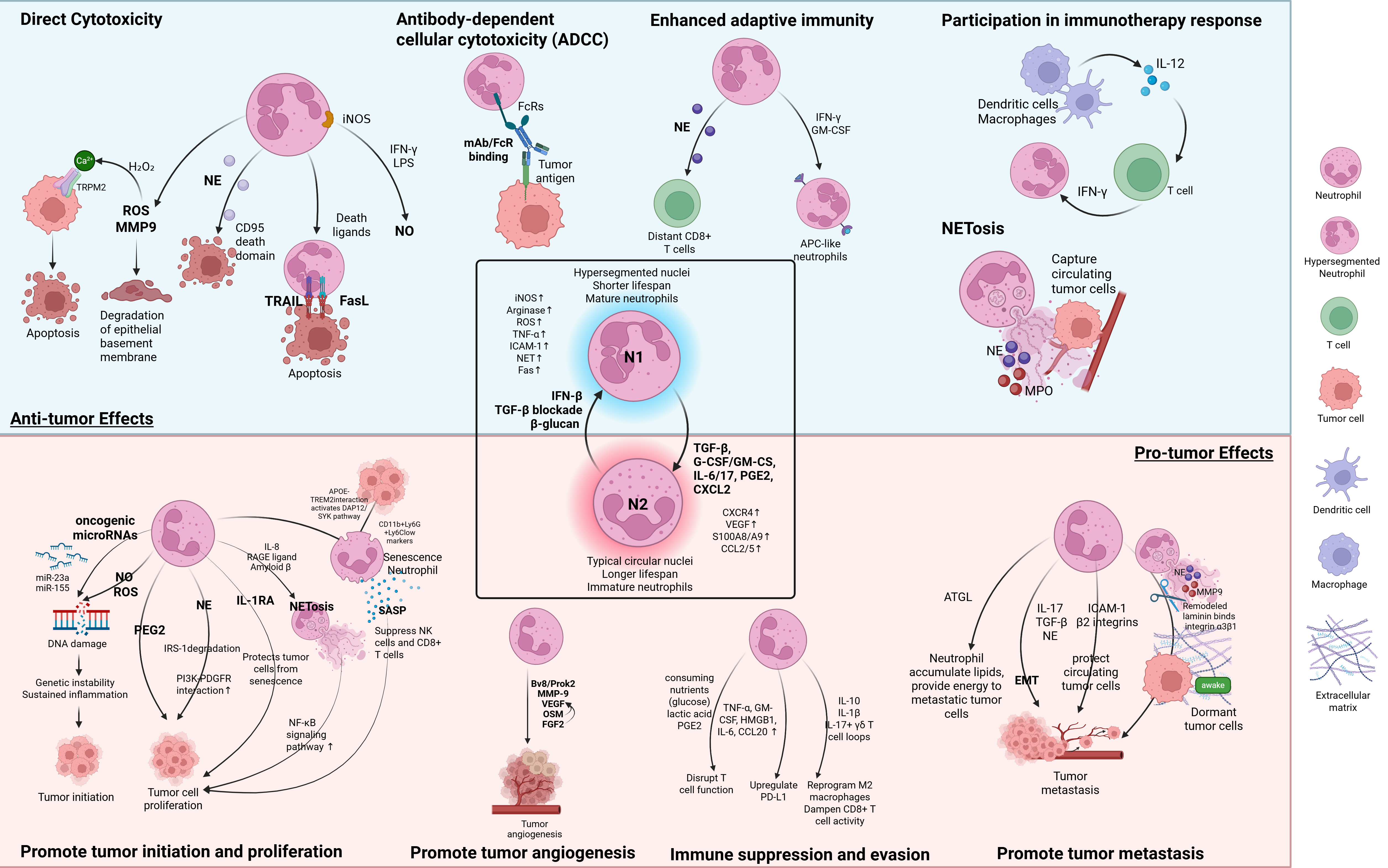
2.1. Anti-Tumor Functions of TANs
| Neutrophil Phenotype | Function/ Tumor State Categories | Effector | Mechanism | References |
|---|---|---|---|---|
| N1 | Direct Cytotoxicity | ROS, MMP9 | TANs release ROS and MMP-9, degrading the epithelial basement membrane and inducing apoptosis via H2O2-triggered Ca2+ influx via TRPM2 channels. | [31,43,44] |
| NE | NE secreted by TANs cleaves CD95 death domain, selectively killing tumor cells. | [45] | ||
| NO | HGF and TNF-α activate MET. MET-activated TANs produce NO to inhibit tumor proliferation and metastasis. | [46] | ||
| Death Ligands (TRAIL, FasL) | TANs expressing TRAIL and FasL induce apoptosis in tumor cells via death receptor signaling, enhanced by IL-17. | [20,40,47] | ||
| ADCC | FcRs | TANs bind the Fc region of mAbs via Fc receptors (FcRs), triggering ADCC-mediated killing | [48] | |
| Enhancing Adaptive Immunity | NE | NE enhances activation of CD8+ T cells at distant sites. | [45] | |
| T cell cooperation, iNOS | T cells detect tumor antigens and activate neutrophils to eliminate tumor escape via iNOS. | [49] | ||
| Antigen presentation | Immature neutrophils differentiate into antigen-presenting TANs under IFN-γ and GM-CSF, capturing tumor antigen, migrating to lymph nodes, and activating T cells. | [50,51] | ||
| Immunotherapy Response | Cytokine feedback loop | IL-12 released by Dendritic cells and macrophages stimulates T cells to produce IFN-γ, which activates IRF1 in neutrophils and thus amplifies antitumor activity and feedback to macrophages and T cells. | [52,53] | |
| NET-Mediated Tumor killing | NETs | NETs trap CTCs via β1-integrin interactions, limiting metastatic spread. NETs also carry cytotoxic proteins including NE and MPO that can damage tumor cells. | [55] | |
| N2 | Tumor Initiation | Genetic instability caused by NO, ROS, and Oncogenic miRNAs (miR-23a & miR-155) | Chronic NO/ROS cause DNA damage. Neutrophil-derived vesicles deliver miR-23a and miR-155, inducing DNA double-strand breaks and promoting carcinogenesis. | [56,57,58] |
| Tumor Proliferation | NE | NE degrades IRS-1, increasing PI3K-PDGFR interaction thus promoting cell proliferation. | [59] | |
| PGE2 | Neutrophil-secreted PGE2 promotes RAS-driven proliferation | [60] | ||
| Neutrophil senescence, APOE-TREM2 interaction, SASP, IL-1RA | APOE produced by tumor cells binds to TREM2, activating the downstream DAP12/SYK pathway and promoting neutrophil senescence. These senescent neutrophils adopt the SASP phenotype, secreting pro-inflammatory cytokines as well as IL-1RA, thus promoting tumor proliferation. | [61,62,63] | ||
| NET | NETosis is triggered by tumor-released IL-8, RAGE ligands, and Amyloid β and can promote tumor cell proliferation via the NF-κB signaling pathway. | [64,65,66,67] | ||
| Tumor Angiogenesis | Proangiogenic factors (Bv8/Prok2, VEGF, MMP-9, OSM, FGF2) | Neutrophils release VEGF, Bv8/Prok2, and FGF2; MMP-9 liberates ECM-bound VEGF; OSM activates JAK–STAT to upregulate VEGF in tumors. | [68,69,70,71,72] | |
| Tumor Metastasis | EMT Inducers (IL-17, TGF-β, and NE) | Neutrophil-released IL-17, TGF-β, and NE induce EMT, reduce adhesion, and enhance tumor invasion. | [73,74,75] | |
| NETs | NETs remodel ECM via NE and MMP-9, awaken dormant tumor cells, and trap CTCs. | [65,76] | ||
| Adhesion and Energy Transfer | Neutrophils bind to CTCs via β2 integrin–ICAM-1, protect from shear and immune attack, and transfer lipids to fuel metastasis. | [77,78,79] | ||
| Immune Suppression | Nutrient Depletion, cytokines, PD-L1 | Neutrophils consume glucose, produce lactic acid and PGE2, express PD-L1, and secrete IL-10/IL-1β, thereby suppressing T cells and promoting macrophages polarization. | [15,41,55,80,81,82,83] |
2.2. Pro-Tumor Functions of TANs
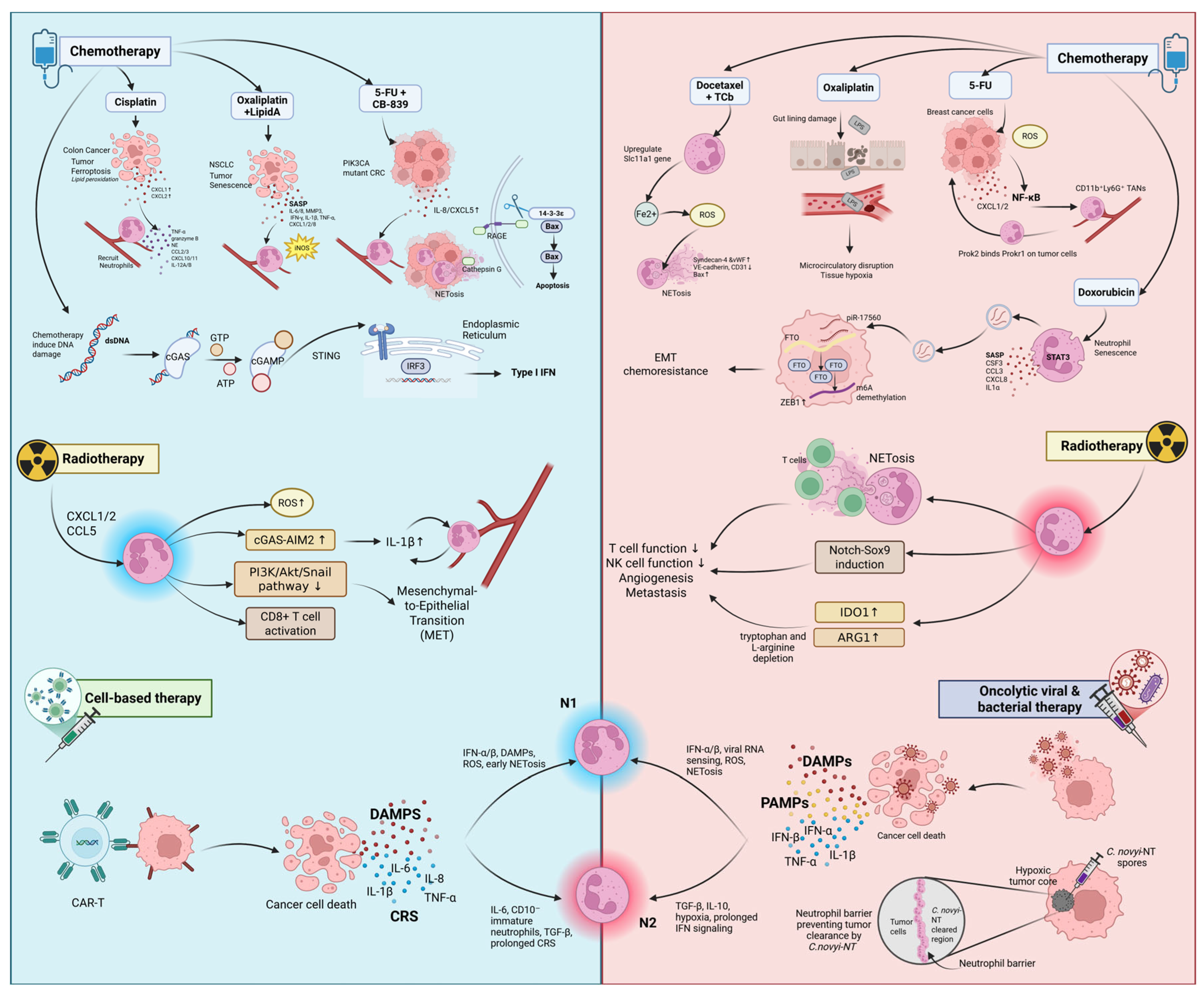
3. Neutrophils in Chemotherapy
| Chemo Agent/ Effector | Tumor Model (Species) | Polarization Mechanism | Phenotype | References |
|---|---|---|---|---|
| Oxaliplatin + Lipid A (OM-174) | Colorectal tumor; PROb (Rat), CT26 (mouse) | Oxaliplatin induces SASP and chemokines (CXCL1/2/8). Lipid A, a TLR4 agonist, promotes iNOS expression and N1 polarization, leading to over 95% tumor regression. | N1 | [102,103] |
| Cisplatin | NSCLC (A549, human) | Cisplatin-induced ferroptosis in tumor cells triggers the release of CXCL1/2 and DAMPs, recruiting neutrophils and polarizing TANs to N1 characterized by the upregulation of TNF-α, granzyme B, and NE. N1 TANs enhance T cell infiltration, CD4+ T cell differentiation, and CD8+ T cell activation and migration. | N1 | [45,104,105,106] |
| CB-839 + 5-FU/Capecitabine | PIK3CA-mutant CRC (mouse, human clinical phase II trial) | CB-839 and 5-FU/Capecitabine (oral form of 5-FU) combination treatment upregulate IL-8/CXCL5 (human/mice), leading to neutrophil recruitment. This increases ROS and induces NET formation, releasing CTSG that promotes tumor apoptosis via BAX activation. | N1 | [107] |
| DNA damage (cGAS-STING) | Murine tumors, melanoma patients (human) | Chemotherapy-induced DNA damage activates the cGAS-STING pathway and IFN-β signaling, promoting N1 polarization and enhancing cytotoxicity in tumors. | N1 | [26,41,108,109] |
| 5-FU | 4T1 (mouse, TNBC lung metastasis) | 5-FU induces ROS and activates NF-κB, upregulating CXCL1/2 thereby recruiting TANs that express Prok2. Promotes angiogenesis and metastasis. | N2 | [110] |
| Docetaxel + Carboplatin (TCb) | Human and mouse breast cancer | Docetaxel and TCb combination therapy upregulates the Slc11a1 gene in neutrophils, releases Fe2+ and ROS, promoting NET formation, which damages endothelium and supports metastasis. | N2 | [111] |
| Doxorubucin | Breast cancer MCF-7, MDA-MB-231 (human); xenograft (mouse) | Doxorubicin induces neutrophil senescence and exosome release via STAT3. Exosomal piR-17560 stabilized FTO and upregulated ZEB1 in tumor cells, promoting EMT and chemoresistance. | N2 | [84] |
| G-CSF (post-chemo) | 4T1 (mouse), human lung metastasis | G-CSF restores neutrophils but primes them for NET release and N2 polarization. This promotes metastasis in both human and mouse models. | N2 | [112,113,114,115,116] |
3.1. Chemotherapy-Induced Polarization Toward Antitumor N1 Neutrophils
3.2. Chemotherapy-Induced Polarization Toward Antitumor N2 Neutrophils
3.3. Therapeutic Strategies Targeting N2 Neutrophils to Enhance Chemotherapy Response
4. Neutrophils in Radiotherapy (RT)
| Tumor Model (Species) | Polarization Mechanism | Phenotype | References |
|---|---|---|---|
| LLC model (Mouse) | RT-induced DNA damage increases CXCL1, CXCL2, and CCL5 expression, recruiting ROS-producing neutrophils. G-CSF also enhances neutrophil recruitment. ROS generation in combination with RT suppresses PI3K/Akt/Snail signaling, inhibiting EMT and promoting MET. | N1 | [137] |
| RM-9 prostate, EG7 thymoma, 4T1 breast (Mouse) | RT rapidly recruits CD11b+Gr-1 high+ neutrophils, which produce ROS that triggers tumor apoptosis and initiate sterile inflammation, enhancing CTL activation. | N1 | [75] |
| In vitro (Human or Mouse); thymoma, breast, prostate, pancreatic (Mouse) | Higher RT doses enhance neutrophil ROS production, contributing to tumor regression; ROS inhibition reduces the antitumor effect. | N1 | [138,139,140] |
| MC38 colorectal and RM-9 prostate (Mouse) | RT activates cGAS and AIM2 pathways, increasing IL-1β expression, which in turn elevates CXCL chemokines and drives neutrophil infiltration. | N1 | [131] |
| Lung tissue pre-metastatic niche with breast cancer cells (Mouse) | RT recruits activated neutrophils to irradiated lung tissue, which promotes Notch–Sox9 signaling in infiltrating cancer cells, inducing stem-like, pro-metastatic traits. | N2 | [141] |
| Cervical cancer (Human) | High peripheral neutrophil counts during treatment correlate with poor local control and survival, suggesting a protumorigenic role for TANs in clinical settings. | N2 | [142] |
| Bladder cancer (Mouse and Human) | RT induces robust NET formation that physically blocks CD8+ T cells from accessing tumor cells and impairs cytotoxicity, contributing to immune evasion and treatment resistance. | N2 | [143] |
| In vitro colon carcinoma spheroids (Human) | Low RT dose (e.g., 0.25 Gy) stimulates NET formation that restricts immune cell-mediated tumor killing. | N2 | [144] |
| Prostate and pancreatic cancer (Mouse); Rectal cancer (Human) | RT increases expression of IDO1 and ARG1 in TANs, which deplete tryptophan and L-arginine, suppressing CD8+ T cells and NK cell functions and weakening antitumor immunity. | N2 | [136,145,146] |
4.1. RT-Induced Polarization Toward Antitumor N1 Neutrophils
4.2. RT-Induced Polarization Toward Antitumor N2 Neutrophils
4.3. Dose-Dependent Effects of RT on Neutrophil Function
5. Neutrophils in Cell-Based Therapies
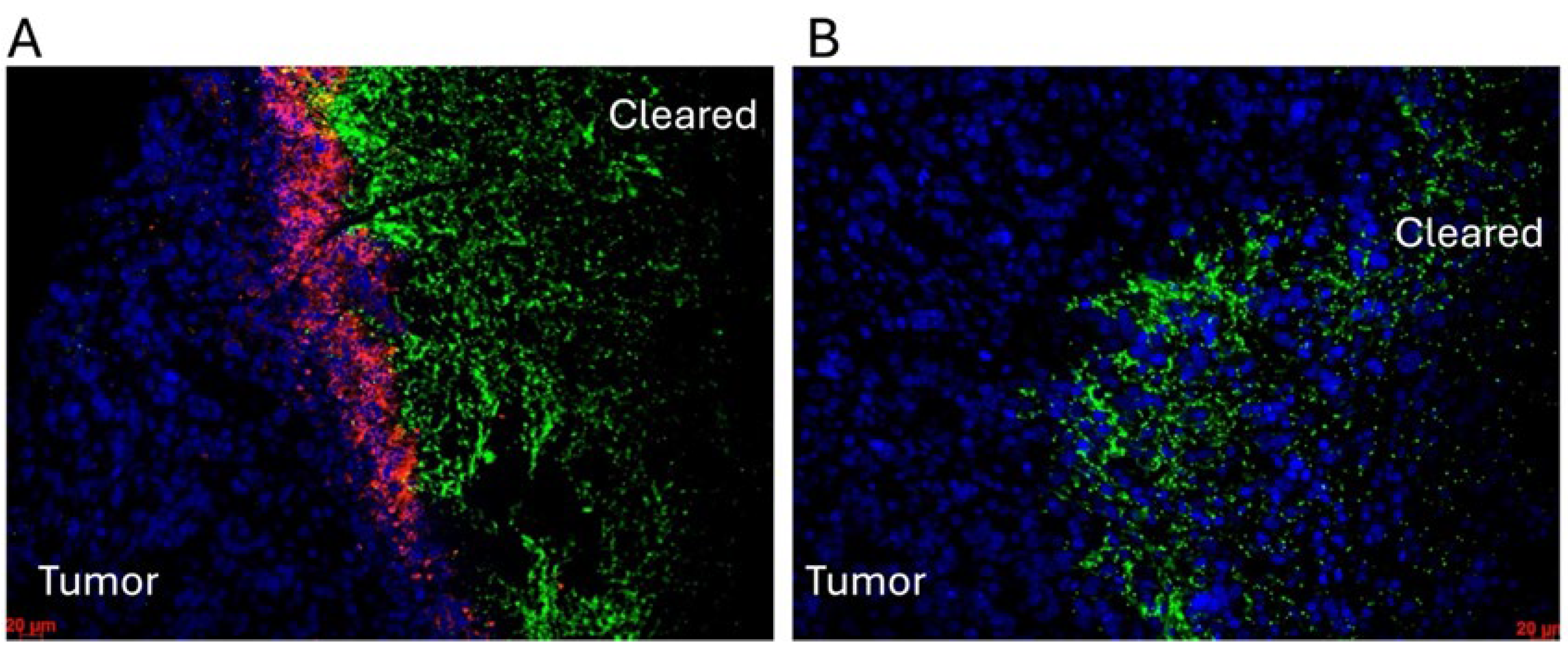
6. Neutrophils in Oncolytic Viral (OVT) and Bacterial Therapies
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| TME | tumor microenvironment |
| TANs | Tumor-associated neutrophils |
| VEGF | Vascular endothelial growth factor |
| NE | Neutrophil elastase |
| IL-6/8/10/12/17 | Interleukin 6/8/10/12/17 |
| IL-1β | Interleukin-1 beta |
| ARG1 | Arginase 1 |
| NETs | Neutrophil extracellular traps |
| NETosis | Neutrophil extracellular trap formation |
| TNF-α/β | Tumor necrosis factor alpha/beta |
| ROS | Reactive oxygen species |
| CTLs | Cytotoxic T lymphocytes |
| NK cells | Natural killer cells |
| TRAIL | TNF-Related Apoptosis-Inducing Ligand |
| TGF-β | Transforming growth factor-β |
| IFN-β/γ | Interferon beta/gamma |
| RT | Radiotherapy |
| OVT | Oncolytic virotherapy |
| NO | Nitric oxide |
| iNOS | Inducible nitric oxide synthase |
| ICAM-1 | Intercellular Adhesion Molecule 1 |
| CCL2/3/4/5/20 | C-C motif chemokine ligand 2/3/4/5/20 |
| CXCL1/2/5/8/9/10/11 | C-X-C motif chemokine ligand 1/2/5/8/9/10/11 |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| PGE2 | Prostaglandin E2 |
| G-CSF | Granulocyte colony-stimulating factor |
| HA | Hyaluronic acid |
| CXCR2/4 | C-X-C Motif Chemokine Receptor 2/4 |
| S100A8/A9 | S100 calcium-binding proteins A8/A9 |
| ADCC | Antibody-dependent cytotoxicity |
| MMP-3/9 | Matrix metalloproteinase-3/9 |
| H2O2 | Hydrogen peroxide |
| HGF | Ligand hepatocyte growth factor |
| mAbs | Monoclonal antibodies |
| IRF1/3 | Interferon regulatory factor 1/3 |
| CTCs | Circulating tumor cells |
| MPO | Myeloperoxidase |
| PI3K | Phosphatidylinositol 3-kinase |
| PDGFR | Potent mitogen platelet-derived growth factor receptor |
| APOE | Apolipoprotein E |
| TREM2 | Triggering receptor expressed on myeloid cells 2 |
| SASP | Senescence-Associated Secretory Phenotype |
| IL-1RA | Interleukin-1 receptor antagonist |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| Prok2 (Bv8) | Prokineticin-2 |
| FGF2 | Fibroblast growth factor 2 |
| OSM | Oncostatin M |
| EMT | Epithelial–mesenchymal transition |
| CAF | Cancer-associated fibroblast |
| ATGL | Activity of adipose triglyceride lipase |
| HMGB1 | High Mobility Group Box 1 |
| DAMPs | Damage-associated molecular patterns |
| ATP | Adenosine triphosphate |
| TLR4 | Toll-like receptor 4 |
| NSCLC | Non-small cell lung cancer |
| CCR5 | C-C Motif chemokine receptor 5 |
| 5-FU | 5-fluorouracil |
| CRCs | Colorectal cancer |
| CTSG | Cathepsin G |
| cGAS | Cyclic GMP-AMP synthase |
| STING | Stimulator of interferon genes |
| TBK1 | TANK-binding kinase 1 |
| ECM | Extracellular matrix |
| vWF | von Willebrand Factor |
| HUVECs | umbilical vein endothelial cells |
| PAD4 | Peptidyl Arginine Deiminase 4 |
| CSF3 | Colony stimulating factors 3 |
| BAX | Bcl-2-associated X protein |
| APC | Antigen presenting cells |
| IFNA2/B/G | Interferon alpha 2/beta/gamma |
| RAGE | Receptor for Advanced Glycation Endproducts |
| FTO | Obesity-associated protein |
| piR | piRNA |
| STAT3 | Signal transducer and activator of transcription 3 |
| TRPM2 | Transient receptor potential cation channel, subfamily M, member 2 |
| IRS-1 | Insulin receptor substrate-1 |
| DNase1 | Deoxyribonuclease 1 |
| FasL | Fas ligand |
| FcRs | Fc Receptors |
| Th1 | T helper type 1 |
| LLC | Lewis lung carcinoma |
| MET | Mesenchymal–epithelial transition |
| AIM2 | Absent in Melanoma 2 |
| Sox9 | SRY-Box Transcription Factor 9 |
| IDO1 | Indoleamine 2,3-dioxygenase 1 |
| MAPK | Mitogen-Activated Protein Kinase |
| CAR | Chimeric antigen receptor |
| CRS | Cytokine release syndrome |
| ANC | Absolute neutrophil count |
| hPSCs | Human pluripotent stem cells |
| HSV | Herpes simplex virus |
| ORFV | Oncolytic Orf virus |
| C. novyi-NT | Clostridium novyi-NT |
| NLR | Neutrophil-to-lymphocyte ratio |
References
- Tigner, A.; Ibrahim, S.A.; Murray, I.V. Histology, White Blood Cell. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Prinyakupt, J.; Pluempitiwiriyawej, C. Segmentation of White Blood Cells and Comparison of Cell Morphology by Linear and Naïve Bayes Classifiers. Biomed. Eng. Online 2015, 14, 63. [Google Scholar] [CrossRef]
- Beyrau, M.; Bodkin, J.V.; Nourshargh, S. Neutrophil Heterogeneity in Health and Disease: A Revitalized Avenue in Inflammation and Immunity. Open Biol. 2012, 2, 120134. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.G.; Ostuni, R.; Hidalgo, A. Heterogeneity of Neutrophils. Nat. Rev. Immunol. 2019, 19, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, Q.; Lu, L.; Xu, C.; Li, J.; Zha, J.; Ma, F.; Luo, H.R.; Hsu, A.Y. Heterogeneity of Neutrophils in Cancer: One Size Does Not Fit All. Cancer Biol. Med. 2022, 19, 1629–1648. [Google Scholar] [CrossRef] [PubMed]
- Jensen, H.K.; Donskov, F.; Marcussen, N.; Nordsmark, M.; Lundbeck, F.; Von Der Maase, H. Presence of Intratumoral Neutrophils Is an Independent Prognostic Factor in Localized Renal Cell Carcinoma. J. Clin. Oncol. 2009, 27, 4709–4717. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-H.; Avraham, H.; Lee, S.-H.; Avraham, S. Vascular Endothelial Growth Factor Modulates Neutrophil Transendothelial Migration via Up-Regulation of Interleukin-8 in Human Brain Microvascular Endothelial Cells. J. Biol. Chem. 2002, 277, 10445–10451. [Google Scholar] [CrossRef]
- Jablonska, E.; Piotrowski, L.; Jablonski, J.; Grabowska, Z. VEGF in the Culture of PMN and the Serum in Oral Cavity Cancer Patients. Oral Oncol. 2002, 38, 605–609. [Google Scholar] [CrossRef]
- Gong, Y.; Koh, D.-R. Neutrophils Promote Inflammatory Angiogenesis via Release of Preformed VEGF in an in Vivo Corneal Model. Cell Tissue Res. 2010, 339, 437–448. [Google Scholar] [CrossRef]
- Lerman, I.; Garcia-Hernandez, M.d.l.L.; Rangel-Moreno, J.; Chiriboga, L.; Pan, C.; Nastiuk, K.L.; Krolewski, J.J.; Sen, A.; Hammes, S.R. Infiltrating Myeloid Cells Exert Protumorigenic Actions via Neutrophil Elastase. Mol. Cancer Res. 2017, 15, 1138–1152. [Google Scholar] [CrossRef]
- Teijeira, A.; Garasa, S.; Ochoa, M.C.; Villalba, M.; Olivera, I.; Cirella, A.; Eguren-Santamaria, I.; Berraondo, P.; Schalper, K.A.; de Andrea, C.E.; et al. IL8, Neutrophils, and NETs in a Collusion against Cancer Immunity and Immunotherapy. Clin. Cancer Res. 2021, 27, 2383–2393. [Google Scholar] [CrossRef]
- García-Navas, R.; Gajate, C.; Mollinedo, F. Neutrophils Drive Endoplasmic Reticulum Stress-Mediated Apoptosis in Cancer Cells through Arginase-1 Release. Sci. Rep. 2021, 11, 12574. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, T.; Liang, Y.; Cui, W.; Li, D.; Zhang, G.; Deng, Z.; Chen, M.; Sha, K.; Xiao, W.; et al. N2-Polarized Neutrophils Reduce Inflammation in Rosacea by Regulating Vascular Factors and Proliferation of CD4+ T Cells. J. Investig. Dermatol. 2022, 142, 1835–1844.e2. [Google Scholar] [CrossRef]
- Griffin, G.K.; Newton, G.; Tarrio, M.L.; Bu, D.; Maganto-Garcia, E.; Azcutia, V.; Alcaide, P.; Grabie, N.; Luscinskas, F.W.; Croce, K.J.; et al. IL-17 and TNF-α Sustain Neutrophil Recruitment during Inflammation through Synergistic Effects on Endothelial Activation. J. Immunol. 2012, 188, 6287–6299. [Google Scholar] [CrossRef]
- Coffelt, S.B.; Kersten, K.; Doornebal, C.W.; Weiden, J.; Vrijland, K.; Hau, C.-S.; Verstegen, N.J.M.; Ciampricotti, M.; Hawinkels, L.J.A.C.; Jonkers, J.; et al. IL-17-Producing Γδ T Cells and Neutrophils Conspire to Promote Breast Cancer Metastasis. Nature 2015, 522, 345–348. [Google Scholar] [CrossRef]
- Beauvillain, C.; Delneste, Y.; Scotet, M.; Peres, A.; Gascan, H.; Guermonprez, P.; Barnaba, V.; Jeannin, P. Neutrophils Efficiently Cross-Prime Naive T Cells in Vivo. Blood 2007, 110, 2965–2973. [Google Scholar] [CrossRef]
- Eruslanov, E.B.; Bhojnagarwala, P.S.; Quatromoni, J.G.; Stephen, T.L.; Ranganathan, A.; Deshpande, C.; Akimova, T.; Vachani, A.; Litzky, L.; Hancock, W.W.; et al. Tumor-Associated Neutrophils Stimulate T Cell Responses in Early-Stage Human Lung Cancer. J. Clin. Investig. 2014, 124, 5466–5480. [Google Scholar] [CrossRef]
- Spörri, R.; Joller, N.; Hilbi, H.; Oxenius, A. A Novel Role for Neutrophils As Critical Activators of NK Cells. J. Immunol. 2008, 181, 7121–7130. [Google Scholar] [CrossRef]
- Ralph, S.J.; Reynolds, M.J. Intratumoral Pro-Oxidants Promote Cancer Immunotherapy by Recruiting and Reprogramming Neutrophils to Eliminate Tumors. Cancer Immunol. Immunother. CII 2023, 72, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Koga, Y.; Matsuzaki, A.; Suminoe, A.; Hattori, H.; Hara, T. Neutrophil-Derived TNF-Related Apoptosis-Inducing Ligand (TRAIL): A Novel Mechanism of Antitumor Effect by Neutrophils. Cancer Res. 2004, 64, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Kundu, M.; Greer, Y.E.; Lobanov, A.; Ridnour, L.; Donahue, R.N.; Ng, Y.; Ratnayake, S.; Voeller, D.; Weltz, S.; Chen, Q.; et al. TRAIL-Induced Cytokine Production via NFKB2 Pathway Promotes Neutrophil Chemotaxis and Immune Suppression in Triple Negative Breast Cancers. bioRxiv 2024, bioRxiv2024.07.19.604341. [Google Scholar] [CrossRef] [PubMed]
- Powell, D.R.; Huttenlocher, A. Neutrophils in the Tumor Microenvironment. Trends Immunol. 2016, 37, 41–52. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of Tumor-Associated Neutrophil Phenotype by TGF-β: “N1” versus “N2” TAN. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, X.; Zhang, X.; Lai, L.; Zhu, B.; Lin, P.; Kang, Z.; Yin, D.; Tian, D.; Chen, Z.; et al. The miR-941/FOXN4/TGF-β Feedback Loop Induces N2 Polarization of Neutrophils and Enhances Tumor Progression of Lung Adenocarcinoma. Front. Immunol. 2025, 16, 1561081. [Google Scholar] [CrossRef] [PubMed]
- Pylaeva, E.; Lang, S.; Jablonska, J. The Essential Role of Type I Interferons in Differentiation and Activation of Tumor-Associated Neutrophils. Front. Immunol. 2016, 7, 629. [Google Scholar] [CrossRef] [PubMed]
- Andzinski, L.; Kasnitz, N.; Stahnke, S.; Wu, C.-F.; Gereke, M.; Von Köckritz-Blickwede, M.; Schilling, B.; Brandau, S.; Weiss, S.; Jablonska, J. Type I IFN s Induce Anti-tumor Polarization of Tumor Associated Neutrophils in Mice and Human. Int. J. Cancer 2016, 138, 1982–1993. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, J.; Wang, X.; Ji, C.; Yu, D.; Wang, M.; Pan, J.; Santos, H.A.; Zhang, H.; Zhang, X. Engineering and Targeting Neutrophils for Cancer Therapy. Adv. Mater. 2024, 36, 2310318. [Google Scholar] [CrossRef]
- Paucek, R.D.; Baltimore, D.; Li, G. The Cellular Immunotherapy Revolution: Arming the Immune System for Precision Therapy. Trends Immunol. 2019, 40, 292–309. [Google Scholar] [CrossRef]
- Chen, X.; Chen, B.; Zhao, H. Role of Neutrophils in Anti-Tumor Activity: Characteristics and Mechanisms of Action. Cancers 2025, 17, 1298. [Google Scholar] [CrossRef]
- Antuamwine, B.B.; Bosnjakovic, R.; Hofmann-Vega, F.; Wang, X.; Theodosiou, T.; Iliopoulos, I.; Brandau, S. N1 versus N2 and PMN-MDSC: A Critical Appraisal of Current Concepts on Tumor-associated Neutrophils and New Directions for Human Oncology. Immunol. Rev. 2023, 314, 250–279. [Google Scholar] [CrossRef]
- Kalafati, L.; Kourtzelis, I.; Schulte-Schrepping, J.; Li, X.; Hatzioannou, A.; Grinenko, T.; Hagag, E.; Sinha, A.; Has, C.; Dietz, S.; et al. Innate Immune Training of Granulopoiesis Promotes Anti-Tumor Activity. Cell 2020, 183, 771–785.e12. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yin, H.; Liu, S.; Shoucair, S.; Ding, N.; Ji, Y.; Zhang, J.; Wang, D.; Kuang, T.; Xu, X.; et al. Prognostic Value of Tumor-Associated N1/N2 Neutrophil Plasticity in Patients Following Radical Resection of Pancreas Ductal Adenocarcinoma. J. Immunother. Cancer 2022, 10, e005798. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.S.F.; Kwok, I.; Tan, L.; Shi, C.; Cerezo-Wallis, D.; Tan, Y.; Leong, K.; Calvo, G.F.; Yang, K.; Zhang, Y.; et al. Deterministic Reprogramming of Neutrophils within Tumors. Science 2024, 383, eadf6493. [Google Scholar] [CrossRef]
- Mihaila, A.C.; Ciortan, L.; Macarie, R.D.; Vadana, M.; Cecoltan, S.; Preda, M.B.; Hudita, A.; Gan, A.-M.; Jakobsson, G.; Tucureanu, M.M.; et al. Transcriptional Profiling and Functional Analysis of N1/N2 Neutrophils Reveal an Immunomodulatory Effect of S100A9-Blockade on the Pro-Inflammatory N1 Subpopulation. Front. Immunol. 2021, 12, 708770. [Google Scholar] [CrossRef]
- Scapini, P.; Lapinet-Vera, J.A.; Gasperini, S.; Calzetti, F.; Bazzoni, F.; Cassatella, M.A. The Neutrophil as a Cellular Source of Chemokines. Immunol. Rev. 2000, 177, 195–203. [Google Scholar] [CrossRef]
- Veglia, F.; Tyurin, V.A.; Blasi, M.; De Leo, A.; Kossenkov, A.V.; Donthireddy, L.; To, T.K.J.; Schug, Z.; Basu, S.; Wang, F.; et al. Fatty Acid Transport Protein 2 Reprograms Neutrophils in Cancer. Nature 2019, 569, 73–78. [Google Scholar] [CrossRef]
- Hsieh, C.-C.; Hung, C.-H.; Chiang, M.; Tsai, Y.-C.; He, J.-T. Hepatic Stellate Cells Enhance Liver Cancer Progression by Inducing Myeloid-Derived Suppressor Cells through Interleukin-6 Signaling. Int. J. Mol. Sci. 2019, 20, 5079. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, Q.; Peng, C.; Sun, L.; Li, X.; Kuang, D. Neutrophils Promote Motility of Cancer Cells via a Hyaluronan-mediated TLR4/PI3K Activation Loop. J. Pathol. 2011, 225, 438–447. [Google Scholar] [CrossRef]
- He, K.; Liu, X.; Hoffman, R.D.; Shi, R.; Lv, G.; Gao, J. G-CSF/GM-CSF-induced Hematopoietic Dysregulation in the Progression of Solid Tumors. FEBS Open Bio 2022, 12, 1268–1285. [Google Scholar] [CrossRef]
- Chen, C.-L.; Wang, Y.; Huang, C.-Y.; Zhou, Z.-Q.; Zhao, J.-J.; Zhang, X.-F.; Pan, Q.-Z.; Wu, J.-X.; Weng, D.-S.; Tang, Y.; et al. IL-17 Induces Antitumor Immunity by Promoting Beneficial Neutrophil Recruitment and Activation in Esophageal Squamous Cell Carcinoma. OncoImmunology 2018, 7, e1373234. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Albelda, S.M. Tumor-Associated Neutrophils: Friend or Foe? Carcinogenesis 2012, 33, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Granot, Z.; Henke, E.; Comen, E.A.; King, T.A.; Norton, L.; Benezra, R. Tumor Entrained Neutrophils Inhibit Seeding in the Premetastatic Lung. Cancer Cell 2011, 20, 300–314. [Google Scholar] [CrossRef] [PubMed]
- Mahiddine, K.; Blaisdell, A.; Ma, S.; Créquer-Grandhomme, A.; Lowell, C.A.; Erlebacher, A. Relief of Tumor Hypoxia Unleashes the Tumoricidal Potential of Neutrophils. J. Clin. Investig. 2019, 130, 389–403. [Google Scholar] [CrossRef]
- Gershkovitz, M.; Caspi, Y.; Fainsod-Levi, T.; Katz, B.; Michaeli, J.; Khawaled, S.; Lev, S.; Polyansky, L.; Shaul, M.E.; Sionov, R.V.; et al. TRPM2 Mediates Neutrophil Killing of Disseminated Tumor Cells. Cancer Res. 2018, 78, 2680–2690. [Google Scholar] [CrossRef]
- Cui, C.; Chakraborty, K.; Tang, X.A.; Zhou, G.; Schoenfelt, K.Q.; Becker, K.M.; Hoffman, A.; Chang, Y.-F.; Blank, A.; Reardon, C.A.; et al. Neutrophil Elastase Selectively Kills Cancer Cells and Attenuates Tumorigenesis. Cell 2021, 184, 3163–3177.e21. [Google Scholar] [CrossRef]
- Finisguerra, V.; Di Conza, G.; Di Matteo, M.; Serneels, J.; Costa, S.; Thompson, A.A.R.; Wauters, E.; Walmsley, S.; Prenen, H.; Granot, Z.; et al. MET Is Required for the Recruitment of Anti-Tumoural Neutrophils. Nature 2015, 522, 349–353. [Google Scholar] [CrossRef]
- Sun, B.; Qin, W.; Song, M.; Liu, L.; Yu, Y.; Qi, X.; Sun, H. Neutrophil Suppresses Tumor Cell Proliferation via Fas /Fas Ligand Pathway Mediated Cell Cycle Arrested. Int. J. Biol. Sci. 2018, 14, 2103–2113. [Google Scholar] [CrossRef]
- Van Egmond, M.; Bakema, J.E. Neutrophils as Effector Cells for Antibody-Based Immunotherapy of Cancer. Semin. Cancer Biol. 2013, 23, 190–199. [Google Scholar] [CrossRef]
- Hirschhorn, D.; Budhu, S.; Kraehenbuehl, L.; Gigoux, M.; Schröder, D.; Chow, A.; Ricca, J.M.; Gasmi, B.; De Henau, O.; Mangarin, L.M.B.; et al. T Cell Immunotherapies Engage Neutrophils to Eliminate Tumor Antigen Escape Variants. Cell 2023, 186, 1432–1447.e17. [Google Scholar] [CrossRef]
- Singhal, S.; Bhojnagarwala, P.S.; O’Brien, S.; Moon, E.K.; Garfall, A.L.; Rao, A.S.; Quatromoni, J.G.; Stephen, T.L.; Litzky, L.; Deshpande, C.; et al. Origin and Role of a Subset of Tumor-Associated Neutrophils with Antigen-Presenting Cell Features in Early-Stage Human Lung Cancer. Cancer Cell 2016, 30, 120–135. [Google Scholar] [CrossRef]
- Pylaeva, E.; Korschunow, G.; Spyra, I.; Bordbari, S.; Siakaeva, E.; Ozel, I.; Domnich, M.; Squire, A.; Hasenberg, A.; Thangavelu, K.; et al. During Early Stages of Cancer, Neutrophils Initiate Anti-Tumor Immune Responses in Tumor-Draining Lymph Nodes. Cell Rep. 2022, 40, 111171. [Google Scholar] [CrossRef]
- Gungabeesoon, J.; Gort-Freitas, N.A.; Kiss, M.; Bolli, E.; Messemaker, M.; Siwicki, M.; Hicham, M.; Bill, R.; Koch, P.; Cianciaruso, C.; et al. A Neutrophil Response Linked to Tumor Control in Immunotherapy. Cell 2023, 186, 1448–1464.e20. [Google Scholar] [CrossRef] [PubMed]
- Ponzetta, A.; Carriero, R.; Carnevale, S.; Barbagallo, M.; Molgora, M.; Perucchini, C.; Magrini, E.; Gianni, F.; Kunderfranco, P.; Polentarutti, N.; et al. Neutrophils Driving Unconventional T Cells Mediate Resistance against Murine Sarcomas and Selected Human Tumors. Cell 2019, 178, 346–360.e24. [Google Scholar] [CrossRef] [PubMed]
- Najmeh, S.; Cools-Lartigue, J.; Rayes, R.F.; Gowing, S.; Vourtzoumis, P.; Bourdeau, F.; Giannias, B.; Berube, J.; Rousseau, S.; Ferri, L.E.; et al. Neutrophil Extracellular Traps Sequester Circulating Tumor Cells via Β1-Integrin Mediated Interactions: NETs Sequester CTCs via Integrin Β1. Int. J. Cancer 2017, 140, 2321–2330. [Google Scholar] [CrossRef] [PubMed]
- Sinha, P.; Clements, V.K.; Bunt, S.K.; Albelda, S.M.; Ostrand-Rosenberg, S. Cross-Talk between Myeloid-Derived Suppressor Cells and Macrophages Subverts Tumor Immunity toward a Type 2 Response. J. Immunol. 2007, 179, 977–983. [Google Scholar] [CrossRef]
- Erdman, S.E.; Rao, V.P.; Poutahidis, T.; Rogers, A.B.; Taylor, C.L.; Jackson, E.A.; Ge, Z.; Lee, C.W.; Schauer, D.B.; Wogan, G.N.; et al. Nitric Oxide and TNF-α Trigger Colonic Inflammation and Carcinogenesis in Helicobacter Hepaticus -Infected, Rag2 -Deficient Mice. Proc. Natl. Acad. Sci. USA 2009, 106, 1027–1032. [Google Scholar] [CrossRef]
- Canli, Ö.; Nicolas, A.M.; Gupta, J.; Finkelmeier, F.; Goncharova, O.; Pesic, M.; Neumann, T.; Horst, D.; Löwer, M.; Sahin, U.; et al. Myeloid Cell-Derived Reactive Oxygen Species Induce Epithelial Mutagenesis. Cancer Cell 2017, 32, 869–883.e5. [Google Scholar] [CrossRef]
- Butin-Israeli, V.; Bui, T.M.; Wiesolek, H.L.; Mascarenhas, L.; Lee, J.J.; Mehl, L.C.; Knutson, K.R.; Adam, S.A.; Goldman, R.D.; Beyder, A.; et al. Neutrophil-Induced Genomic Instability Impedes Resolution of Inflammation and Wound Healing. J. Clin. Investig. 2019, 129, 712–726. [Google Scholar] [CrossRef]
- Houghton, A.M.; Rzymkiewicz, D.M.; Ji, H.; Gregory, A.D.; Egea, E.E.; Metz, H.E.; Stolz, D.B.; Land, S.R.; Marconcini, L.A.; Kliment, C.R.; et al. Neutrophil Elastase–Mediated Degradation of IRS-1 Accelerates Lung Tumor Growth. Nat. Med. 2010, 16, 219–223. [Google Scholar] [CrossRef]
- Antonio, N.; Bønnelykke-Behrndtz, M.L.; Ward, L.C.; Collin, J.; Christensen, I.J.; Steiniche, T.; Schmidt, H.; Feng, Y.; Martin, P. The Wound Inflammatory Response Exacerbates Growth of Pre-neoplastic Cells and Progression to Cancer. EMBO J. 2015, 34, 2219–2236. [Google Scholar] [CrossRef]
- Bancaro, N.; Calì, B.; Troiani, M.; Elia, A.R.; Arzola, R.A.; Attanasio, G.; Lai, P.; Crespo, M.; Gurel, B.; Pereira, R.; et al. Apolipoprotein E Induces Pathogenic Senescent-like Myeloid Cells in Prostate Cancer. Cancer Cell 2023, 41, 602–619.e11. [Google Scholar] [CrossRef]
- Di Mitri, D.; Toso, A.; Chen, J.J.; Sarti, M.; Pinton, S.; Jost, T.R.; D’Antuono, R.; Montani, E.; Garcia-Escudero, R.; Guccini, I.; et al. Tumour-Infiltrating Gr-1+ Myeloid Cells Antagonize Senescence in Cancer. Nature 2014, 515, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Denk, D.; Greten, F.R. Inflammation: The Incubator of the Tumor Microenvironment. Trends Cancer 2022, 8, 901–914. [Google Scholar] [CrossRef] [PubMed]
- Boone, B.A.; Orlichenko, L.; Schapiro, N.E.; Loughran, P.; Gianfrate, G.C.; Ellis, J.T.; Singhi, A.D.; Kang, R.; Tang, D.; Lotze, M.T.; et al. The Receptor for Advanced Glycation End Products (RAGE) Enhances Autophagy and Neutrophil Extracellular Traps in Pancreatic Cancer. Cancer Gene Ther. 2015, 22, 326–334. [Google Scholar] [CrossRef]
- Munir, H.; Jones, J.O.; Janowitz, T.; Hoffmann, M.; Euler, M.; Martins, C.P.; Welsh, S.J.; Shields, J.D. Stromal-Driven and Amyloid β-Dependent Induction of Neutrophil Extracellular Traps Modulates Tumor Growth. Nat. Commun. 2021, 12, 683. [Google Scholar] [CrossRef]
- Wang, M.; Yu, F.; Zhang, Y.; Li, P. Novel Insights into Notch Signaling in Tumor Immunity: Potential Targets for Cancer Immunotherapy. Front. Immunol. 2024, 15, 1352484. [Google Scholar] [CrossRef] [PubMed]
- Zha, C.; Meng, X.; Li, L.; Mi, S.; Qian, D.; Li, Z.; Wu, P.; Hu, S.; Zhao, S.; Cai, J.; et al. Neutrophil Extracellular Traps Mediate the Crosstalk between Glioma Progression and the Tumor Microenvironment via the HMGB1/RAGE/IL-8 Axis. Cancer Biol. Med. 2020, 17, 154–168. [Google Scholar] [CrossRef]
- Shojaei, F.; Singh, M.; Thompson, J.D.; Ferrara, N. Role of Bv8 in Neutrophil-Dependent Angiogenesis in a Transgenic Model of Cancer Progression. Proc. Natl. Acad. Sci. USA 2008, 105, 2640–2645. [Google Scholar] [CrossRef]
- Kusumanto, Y.H.; Dam, W.A.; Hospers, G.A.P.; Meijer, C.; Mulder, N.H. Platelets and Granulocytes, in Particular the Neutrophils, Form Important Compartments for Circulating Vascular Endothelial Growth Factor. Angiogenesis 2003, 6, 283–287. [Google Scholar] [CrossRef]
- Gordon-Weeks, A.N.; Lim, S.Y.; Yuzhalin, A.E.; Jones, K.; Markelc, B.; Kim, K.J.; Buzzelli, J.N.; Fokas, E.; Cao, Y.; Smart, S.; et al. Neutrophils Promote Hepatic Metastasis Growth through Fibroblast Growth Factor 2–Dependent Angiogenesis in Mice. Hepatology 2017, 65, 1920–1935. [Google Scholar] [CrossRef]
- Yang, L.; DeBusk, L.M.; Fukuda, K.; Fingleton, B.; Green-Jarvis, B.; Shyr, Y.; Matrisian, L.M.; Carbone, D.P.; Lin, P.C. Expansion of Myeloid Immune Suppressor Gr+CD11b+ Cells in Tumor-Bearing Host Directly Promotes Tumor Angiogenesis. Cancer Cell 2004, 6, 409–421. [Google Scholar] [CrossRef]
- Queen, M.M.; Ryan, R.E.; Holzer, R.G.; Keller-Peck, C.R.; Jorcyk, C.L. Breast Cancer Cells Stimulate Neutrophils to Produce Oncostatin M: Potential Implications for Tumor Progression. Cancer Res. 2005, 65, 8896–8904. [Google Scholar] [CrossRef]
- Mittal, V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu. Rev. Pathol. Mech. Dis. 2018, 13, 395–412. [Google Scholar] [CrossRef]
- Li, S.; Cong, X.; Gao, H.; Lan, X.; Li, Z.; Wang, W.; Song, S.; Wang, Y.; Li, C.; Zhang, H.; et al. Tumor-Associated Neutrophils Induce EMT by IL-17a to Promote Migration and Invasion in Gastric Cancer Cells. J. Exp. Clin. Cancer Res. 2019, 38, 6. [Google Scholar] [CrossRef] [PubMed]
- Große-Steffen, T.; Giese, T.; Giese, N.; Longerich, T.; Schirmacher, P.; Hänsch, G.M.; Gaida, M.M. Epithelial-to-Mesenchymal Transition in Pancreatic Ductal Adenocarcinoma and Pancreatic Tumor Cell Lines: The Role of Neutrophils and Neutrophil-Derived Elastase. Clin. Dev. Immunol. 2012, 2012, 720768. [Google Scholar] [CrossRef]
- Albrengues, J.; Shields, M.A.; Ng, D.; Park, C.G.; Ambrico, A.; Poindexter, M.E.; Upadhyay, P.; Uyeminami, D.L.; Pommier, A.; Küttner, V.; et al. Neutrophil Extracellular Traps Produced during Inflammation Awaken Dormant Cancer Cells in Mice. Science 2018, 361, eaao4227. [Google Scholar] [CrossRef] [PubMed]
- Huh, S.J.; Liang, S.; Sharma, A.; Dong, C.; Robertson, G.P. Transiently Entrapped Circulating Tumor Cells Interact with Neutrophils to Facilitate Lung Metastasis Development. Cancer Res. 2010, 70, 6071–6082. [Google Scholar] [CrossRef]
- Teijeira, Á.; Garasa, S.; Gato, M.; Alfaro, C.; Migueliz, I.; Cirella, A.; De Andrea, C.; Ochoa, M.C.; Otano, I.; Etxeberria, I.; et al. CXCR1 and CXCR2 Chemokine Receptor Agonists Produced by Tumors Induce Neutrophil Extracellular Traps That Interfere with Immune Cytotoxicity. Immunity 2020, 52, 856–871.e8. [Google Scholar] [CrossRef]
- Li, P.; Lu, M.; Shi, J.; Gong, Z.; Hua, L.; Li, Q.; Lim, B.; Zhang, X.H.-F.; Chen, X.; Li, S.; et al. Lung Mesenchymal Cells Elicit Lipid Storage in Neutrophils That Fuel Breast Cancer Lung Metastasis. Nat. Immunol. 2020, 21, 1444–1455. [Google Scholar] [CrossRef]
- Sukumar, M.; Roychoudhuri, R.; Restifo, N.P. Nutrient Competition: A New Axis of Tumor Immunosuppression. Cell 2015, 162, 1206–1208. [Google Scholar] [CrossRef]
- Bohn, T.; Rapp, S.; Luther, N.; Klein, M.; Bruehl, T.-J.; Kojima, N.; Aranda Lopez, P.; Hahlbrock, J.; Muth, S.; Endo, S.; et al. Tumor Immunoevasion via Acidosis-Dependent Induction of Regulatory Tumor-Associated Macrophages. Nat. Immunol. 2018, 19, 1319–1329. [Google Scholar] [CrossRef]
- Kim, R.; Hashimoto, A.; Markosyan, N.; Tyurin, V.A.; Tyurina, Y.Y.; Kar, G.; Fu, S.; Sehgal, M.; Garcia-Gerique, L.; Kossenkov, A.; et al. Ferroptosis of Tumour Neutrophils Causes Immune Suppression in Cancer. Nature 2022, 612, 338–346. [Google Scholar] [CrossRef]
- Jin, C.; Lagoudas, G.K.; Zhao, C.; Bullman, S.; Bhutkar, A.; Hu, B.; Ameh, S.; Sandel, D.; Liang, X.S.; Mazzilli, S.; et al. Commensal Microbiota Promote Lung Cancer Development via Γδ T Cells. Cell 2019, 176, 998–1013.e16. [Google Scholar] [CrossRef]
- Ou, B.; Liu, Y.; Gao, Z.; Xu, J.; Yan, Y.; Li, Y.; Zhang, J. Senescent Neutrophils-Derived Exosomal piRNA-17560 Promotes Chemoresistance and EMT of Breast Cancer via FTO-Mediated m6A Demethylation. Cell Death Dis. 2022, 13, 905. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Zhang, X.; Zhang, L.; Li, W.; Wu, H.; Yuan, X.; Mao, F.; Wang, M.; Zhu, W.; Qian, H.; et al. The IL-6-STAT3 Axis Mediates a Reciprocal Crosstalk between Cancer-Derived Mesenchymal Stem Cells and Neutrophils to Synergistically Prompt Gastric Cancer Progression. Cell Death Dis. 2014, 5, e1295. [Google Scholar] [CrossRef]
- He, G.; Zhang, H.; Zhou, J.; Wang, B.; Chen, Y.; Kong, Y.; Xie, X.; Wang, X.; Fei, R.; Wei, L.; et al. Peritumoural Neutrophils Negatively Regulate Adaptive Immunity via the PD-L1/PD-1 Signalling Pathway in Hepatocellular Carcinoma. J. Exp. Clin. Cancer Res. 2015, 34, 141. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Li, H.; Deng, Y.; Tai, Y.; Zeng, K.; Zhang, Y.; Liu, W.; Zhang, Q.; Yang, Y. Cancer-Associated Fibroblasts Induce PDL1+ Neutrophils through the IL6-STAT3 Pathway That Foster Immune Suppression in Hepatocellular Carcinoma. Cell Death Dis. 2018, 9, 422. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, J.; Mao, Z.; Jiang, H.; Liu, W.; Shi, H.; Ji, R.; Xu, W.; Qian, H.; Zhang, X. Extracellular Vesicles From Gastric Cancer Cells Induce PD-L1 Expression on Neutrophils to Suppress T-Cell Immunity. Front. Oncol. 2020, 10, 629. [Google Scholar] [CrossRef] [PubMed]
- Noman, M.Z.; Desantis, G.; Janji, B.; Hasmim, M.; Karray, S.; Dessen, P.; Bronte, V.; Chouaib, S. PD-L1 Is a Novel Direct Target of HIF-1α, and Its Blockade under Hypoxia Enhanced MDSC-Mediated T Cell Activation. J. Exp. Med. 2014, 211, 781–790. [Google Scholar] [CrossRef]
- Kwantwi, L.B.; Wang, S.; Zhang, W.; Peng, W.; Cai, Z.; Sheng, Y.; Xiao, H.; Wang, X.; Wu, Q. Tumor-Associated Neutrophils Activated by Tumor-Derived CCL20 (C-C Motif Chemokine Ligand 20) Promote T Cell Immunosuppression via Programmed Death-Ligand 1 (PD-L1) in Breast Cancer. Bioengineered 2021, 12, 6996–7006. [Google Scholar] [CrossRef]
- Shang, A.; Wang, W.; Gu, C.; Chen, C.; Zeng, B.; Yang, Y.; Ji, P.; Sun, J.; Wu, J.; Lu, W.; et al. Long Non-Coding RNA HOTTIP Enhances IL-6 Expression to Potentiate Immune Escape of Ovarian Cancer Cells by Upregulating the Expression of PD-L1 in Neutrophils. J. Exp. Clin. Cancer Res. 2019, 38, 411. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, Y.; Peng, L.; Chen, N.; Chen, W.; Lv, Y.; Mao, F.; Zhang, J.; Cheng, P.; Teng, Y.; et al. Tumour-Activated Neutrophils in Gastric Cancer Foster Immune Suppression and Disease Progression through GM-CSF-PD-L1 Pathway. Gut 2017, 66, 1900–1911. [Google Scholar] [CrossRef]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer Chemotherapy and beyond: Current Status, Drug Candidates, Associated Risks and Progress in Targeted Therapeutics. Genes Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef]
- Amjad, M.T.; Chidharla, A.; Kasi, A. Cancer Chemotherapy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Chen, G.Y.; Nuñez, G. Sterile Inflammation: Sensing and Reacting to Damage. Nat. Rev. Immunol. 2010, 10, 826–837. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Tang, H.; Cai, X.; Lin, J.; Kang, R.; Tang, D.; Liu, J. DAMPs in Immunosenescence and Cancer. Semin. Cancer Biol. 2024, 106–107, 123–142. [Google Scholar] [CrossRef]
- Ozga, A.J.; Chow, M.T.; Luster, A.D. Chemokines and the Immune Response to Cancer. Immunity 2021, 54, 859–874. [Google Scholar] [CrossRef]
- Liu, S.; Wu, W.; Du, Y.; Yin, H.; Chen, Q.; Yu, W.; Wang, W.; Yu, J.; Liu, L.; Lou, W.; et al. The Evolution and Heterogeneity of Neutrophils in Cancers: Origins, Subsets, Functions, Orchestrations and Clinical Applications. Mol. Cancer 2023, 22, 148. [Google Scholar] [CrossRef]
- Zhang, M.; Qin, H.; Wu, Y.; Gao, Q. Complex Role of Neutrophils in the Tumor Microenvironment: An Avenue for Novel Immunotherapies. Cancer Biol. Med. 2024, 21, 849–863. [Google Scholar] [CrossRef]
- Onier, N.; Hilpert, S.; Reveneau, S.; Arnould, L.; Saint-Giorgio, V.; Exbrayat, J.M.; Jeannin, J.F. Expression of Inducible Nitric Oxide Synthase in Tumors in Relation with Their Regression Induced by Lipid A in Rats. Int. J. Cancer 1999, 81, 755–760. [Google Scholar] [CrossRef]
- Seignez, C.; Martin, A.; Rollet, C.-E.; Racoeur, C.; Scagliarini, A.; Jeannin, J.-F.; Bettaieb, A.; Paul, C. Senescence of Tumor Cells Induced by Oxaliplatin Increases the Efficiency of a Lipid A Immunotherapy via the Recruitment of Neutrophils. Oncotarget 2014, 5, 11442–11451. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhao, Y.; Chen, S.; Cui, G.; Fu, W.; Li, S.; Lin, X.; Hu, H. Cisplatin Promotes the Efficacy of Immune Checkpoint Inhibitor Therapy by Inducing Ferroptosis and Activating Neutrophils. Front. Pharmacol. 2022, 13, 870178. [Google Scholar] [CrossRef]
- Balkwill, F. Tumour Necrosis Factor and Cancer. Nat. Rev. Cancer 2009, 9, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Seignez, C.; Racoeur, C.; Isambert, N.; Mabrouk, N.; Scagliarini, A.; Reveneau, S.; Arnould, L.; Bettaieb, A.; Jeannin, J.-F.; et al. Tumor-Derived Granzyme B-Expressing Neutrophils Acquire Antitumor Potential after Lipid A Treatment. Oncotarget 2018, 9, 28364–28378. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, S.; Zhao, Y.; Dinh, T.; Jiang, D.; Selfridge, J.E.; Myers, G.; Wang, Y.; Zhao, X.; Tomchuck, S.; et al. Neutrophil Extracellular Traps Induced by Chemotherapy Inhibit Tumor Growth in Murine Models of Colorectal Cancer. J. Clin. Investig. 2024, 134, e175031. [Google Scholar] [CrossRef]
- Gan, Y.; Li, X.; Han, S.; Liang, Q.; Ma, X.; Rong, P.; Wang, W.; Li, W. The cGAS/STING Pathway: A Novel Target for Cancer Therapy. Front. Immunol. 2022, 12, 795401. [Google Scholar] [CrossRef]
- Jablonska, J.; Wu, C.-F.; Andzinski, L.; Leschner, S.; Weiss, S. CXCR2-mediated Tumor-associated Neutrophil Recruitment Is Regulated by IFN-β. Int. J. Cancer 2014, 134, 1346–1358. [Google Scholar] [CrossRef]
- Sasaki, S.; Baba, T.; Muranaka, H.; Tanabe, Y.; Takahashi, C.; Matsugo, S.; Mukaida, N. Involvement of Prokineticin 2–Expressing Neutrophil Infiltration in 5-Fluorouracil–Induced Aggravation of Breast Cancer Metastasis to Lung. Mol. Cancer Ther. 2018, 17, 1515–1525. [Google Scholar] [CrossRef]
- Kong, L.; Hu, S.; Zhao, Y.; Huang, Y.; Xiang, X.; Yu, Y.; Mao, X.; Xie, K.; Zhu, X.; Xu, P. Neutrophil Extracellular Traps Induced by Neoadjuvant Chemotherapy of Breast Cancer Promotes Vascular Endothelial Damage. Breast Cancer Res. 2025, 27, 61. [Google Scholar] [CrossRef]
- Martin, K.R.; Wong, H.L.; Witko-Sarsat, V.; Wicks, I.P. G-CSF—A Double Edge Sword in Neutrophil Mediated Immunity. Semin. Immunol. 2021, 54, 101516. [Google Scholar] [CrossRef]
- Mehta, H.M.; Malandra, M.; Corey, S.J. G-CSF and GM-CSF in Neutropenia. J. Immunol. 2015, 195, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Panopoulos, A.D.; Watowich, S.S. Granulocyte Colony-Stimulating Factor: Molecular Mechanisms of Action during Steady State and ‘Emergency’ Hematopoiesis. Cytokine 2008, 42, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Demers, M.; Krause, D.S.; Schatzberg, D.; Martinod, K.; Voorhees, J.R.; Fuchs, T.A.; Scadden, D.T.; Wagner, D.D. Cancers Predispose Neutrophils to Release Extracellular DNA Traps That Contribute to Cancer-Associated Thrombosis. Proc. Natl. Acad. Sci. USA 2012, 109, 13076–13081. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Wysocki, R.W.; Amoozgar, Z.; Maiorino, L.; Fein, M.R.; Jorns, J.; Schott, A.F.; Kinugasa-Katayama, Y.; Lee, Y.; Won, N.H.; et al. Cancer Cells Induce Metastasis-Supporting Neutrophil Extracellular DNA Traps. Sci. Transl. Med. 2016, 8, 361ra138. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, X.; Chen, Y.; Selfridge, J.E.; Gorityala, S.; Du, Z.; Wang, J.M.; Hao, Y.; Cioffi, G.; Conlon, R.A.; et al. 5-Fluorouracil Enhances the Antitumor Activity of the Glutaminase Inhibitor CB-839 against PIK3CA -Mutant Colorectal Cancers. Cancer Res. 2020, 80, 4815–4827. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Lin, T.-T.; Hu, L.; Xu, C.-J.; Hu, F.; Wan, L.; Yang, X.; Wu, X.-F.; Zhang, X.-T.; Li, Y.; et al. Neutrophil Extracellular Traps as a Unique Target in the Treatment of Chemotherapy-Induced Peripheral Neuropathy. eBioMedicine 2023, 90, 104499. [Google Scholar] [CrossRef]
- Rout, P.; Reynolds, S.B.; Zito, P.M. Neutropenia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Lustberg, M.B. Management of Neutropenia in Cancer Patients. Clin. Adv. Hematol. Oncol. HO 2012, 10, 825–826. [Google Scholar]
- Liu, X.; Arfman, T.; Wichapong, K.; Reutelingsperger, C.P.M.; Voorberg, J.; Nicolaes, G.A.F. PAD4 Takes Charge during Neutrophil Activation: Impact of PAD4 Mediated NET Formation on Immune-mediated Disease. J. Thromb. Haemost. 2021, 19, 1607–1617. [Google Scholar] [CrossRef]
- Vogel, B.; Shinagawa, H.; Hofmann, U.; Ertl, G.; Frantz, S. Acute DNase1 Treatment Improves Left Ventricular Remodeling after Myocardial Infarction by Disruption of Free Chromatin. Basic Res. Cardiol. 2015, 110, 15. [Google Scholar] [CrossRef]
- Fauvre, A.; Ursino, C.; Garambois, V.; Culerier, E.; Milazzo, L.-A.; Vezzio-Vié, N.; Jeanson, L.; Marchive, C.; Andrade, A.F.; Combes, E.; et al. Oxaliplatin, ATR Inhibitor and Anti-PD-1 Antibody Combination Therapy Controls Colon Carcinoma Growth, Induces Local and Systemic Changes in the Immune Compartment, and Protects against Tumor Rechallenge in Mice. J. Immunother. Cancer 2025, 13, e010791. [Google Scholar] [CrossRef]
- Wheldon, T.E.; O’Donoghue, J.A. The Radiobiology of Targeted Radiotherapy. Int. J. Radiat. Biol. 1990, 58, 1–21. [Google Scholar] [CrossRef]
- Jackson, S.P.; Bartek, J. The DNA-Damage Response in Human Biology and Disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef]
- Maier, P.; Hartmann, L.; Wenz, F.; Herskind, C. Cellular Pathways in Response to Ionizing Radiation and Their Targetability for Tumor Radiosensitization. Int. J. Mol. Sci. 2016, 17, 102. [Google Scholar] [CrossRef] [PubMed]
- Begg, A.C.; Stewart, F.A.; Vens, C. Strategies to Improve Radiotherapy with Targeted Drugs. Nat. Rev. Cancer 2011, 11, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and Radiation Therapy: Current Advances and Future Directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Maani, E.V.; Maani, C.V. Radiation Therapy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Raymakers, L.; Demmers, T.J.; Meijer, G.J.; Molenaar, I.Q.; Van Santvoort, H.C.; Intven, M.P.W.; Leusen, J.H.W.; Olofsen, P.A.; Daamen, L.A. The Effect of Radiation Treatment of Solid Tumors on Neutrophil Infiltration and Function: A Systematic Review. Int. J. Radiat. Oncol. 2024, 120, 845–861. [Google Scholar] [CrossRef]
- Zhang, F.; Mulvaney, O.; Salcedo, E.; Manna, S.; Zhu, J.Z.; Wang, T.; Ahn, C.; Pop, L.M.; Hannan, R. Radiation-Induced Innate Neutrophil Response in Tumor Is Mediated by the CXCLs/CXCR2 Axis. Cancers 2023, 15, 5686. [Google Scholar] [CrossRef]
- Chao, T.; Furth, E.E.; Vonderheide, R.H. CXCR2-Dependent Accumulation of Tumor-Associated Neutrophils Regulates T-Cell Immunity in Pancreatic Ductal Adenocarcinoma. Cancer Immunol. Res. 2016, 4, 968–982. [Google Scholar] [CrossRef]
- Guan, L.; Nambiar, D.K.; Cao, H.; Viswanathan, V.; Kwok, S.; Hui, A.B.; Hou, Y.; Hildebrand, R.; Von Eyben, R.; Holmes, B.J.; et al. NFE2L2 Mutations Enhance Radioresistance in Head and Neck Cancer by Modulating Intratumoral Myeloid Cells. Cancer Res. 2023, 83, 861–874. [Google Scholar] [CrossRef]
- Trappetti, V.; Potez, M.; Fernandez-Palomo, C.; Volarevic, V.; Shintani, N.; Pellicioli, P.; Ernst, A.; Haberthür, D.; Fazzari, J.M.; Krisch, M.; et al. Microbeam Radiation Therapy Controls Local Growth of Radioresistant Melanoma and Treats Out-of-Field Locoregional Metastasis. Int. J. Radiat. Oncol. 2022, 114, 478–493. [Google Scholar] [CrossRef]
- Yang, X.; Lu, Y.; Hang, J.; Zhang, J.; Zhang, T.; Huo, Y.; Liu, J.; Lai, S.; Luo, D.; Wang, L.; et al. Lactate-Modulated Immunosuppression of Myeloid-Derived Suppressor Cells Contributes to the Radioresistance of Pancreatic Cancer. Cancer Immunol. Res. 2020, 8, 1440–1451. [Google Scholar] [CrossRef]
- Oweida, A.J.; Mueller, A.C.; Piper, M.; Milner, D.; Van Court, B.; Bhatia, S.; Phan, A.; Bickett, T.; Jordan, K.; Proia, T.; et al. Response to Radiotherapy in Pancreatic Ductal Adenocarcinoma Is Enhanced by Inhibition of Myeloid-Derived Suppressor Cells Using STAT3 Anti-Sense Oligonucleotide. Cancer Immunol. Immunother. 2021, 70, 989–1000. [Google Scholar] [CrossRef]
- Liu, Q.; Hao, Y.; Du, R.; Hu, D.; Xie, J.; Zhang, J.; Deng, G.; Liang, N.; Tian, T.; Käsmann, L.; et al. Radiotherapy Programs Neutrophils to an Antitumor Phenotype by Inducing Mesenchymal-Epithelial Transition. Transl. Lung Cancer Res. 2021, 10, 1424–1443. [Google Scholar] [CrossRef] [PubMed]
- Takeshima, T.; Pop, L.M.; Laine, A.; Iyengar, P.; Vitetta, E.S.; Hannan, R. Key Role for Neutrophils in Radiation-Induced Antitumor Immune Responses: Potentiation with G-CSF. Proc. Natl. Acad. Sci. USA 2016, 113, 11300–11305. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.; Shi, L.; Kidder, K.; Zen, K.; Garnett-Benson, C.; Liu, Y. Intratumoral SIRPα-Deficient Macrophages Activate Tumor Antigen-Specific Cytotoxic T Cells under Radiotherapy. Nat. Commun. 2021, 12, 3229. [Google Scholar] [CrossRef]
- Wu, C.-T.; Chen, M.-F.; Chen, W.-C.; Hsieh, C.-C. The Role of IL-6 in the Radiation Response of Prostate Cancer. Radiat. Oncol. 2013, 8, 159. [Google Scholar] [CrossRef]
- Nolan, E.; Bridgeman, V.L.; Ombrato, L.; Karoutas, A.; Rabas, N.; Sewnath, C.A.N.; Vasquez, M.; Rodrigues, F.S.; Horswell, S.; Faull, P.; et al. Radiation Exposure Elicits a Neutrophil-Driven Response in Healthy Lung Tissue That Enhances Metastatic Colonization. Nat. Cancer 2022, 3, 173–187. [Google Scholar] [CrossRef]
- Wisdom, A.J.; Hong, C.S.; Lin, A.J.; Xiang, Y.; Cooper, D.E.; Zhang, J.; Xu, E.S.; Kuo, H.-C.; Mowery, Y.M.; Carpenter, D.J.; et al. Neutrophils Promote Tumor Resistance to Radiation Therapy. Proc. Natl. Acad. Sci. USA 2019, 116, 18584–18589. [Google Scholar] [CrossRef]
- Shinde-Jadhav, S.; Mansure, J.J.; Rayes, R.F.; Marcq, G.; Ayoub, M.; Skowronski, R.; Kool, R.; Bourdeau, F.; Brimo, F.; Spicer, J.; et al. Role of Neutrophil Extracellular Traps in Radiation Resistance of Invasive Bladder Cancer. Nat. Commun. 2021, 12, 2776. [Google Scholar] [CrossRef]
- Teijeira, A.; Garasa, S.; Ochoa, M.C.; Sanchez-Gregorio, S.; Gomis, G.; Luri-Rey, C.; Martinez-Monge, R.; Pinci, B.; Valencia, K.; Palencia, B.; et al. Low-Dose Ionizing γ-Radiation Elicits the Extrusion of Neutrophil Extracellular Traps. Clin. Cancer Res. 2024, 30, 4131–4142. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Yang, Y.; Liu, Q.; Ma, H.; Huang, A.; Zhao, Y.; Xia, Z.; Liu, T.; Wu, G. Polymorphonuclear-MDSCs Facilitate Tumor Regrowth After Radiation by Suppressing CD8+ T Cells. Int. J. Radiat. Oncol. 2021, 109, 1533–1546. [Google Scholar] [CrossRef] [PubMed]
- Leonard, W.; Dufait, I.; Schwarze, J.K.; Law, K.; Engels, B.; Jiang, H.; Van Den Berge, D.; Gevaert, T.; Storme, G.; Verovski, V.; et al. Myeloid-Derived Suppressor Cells Reveal Radioprotective Properties through Arginase-Induced l-Arginine Depletion. Radiother. Oncol. 2016, 119, 291–299. [Google Scholar] [CrossRef]
- Zheng, Z.; Su, J.; Bao, X.; Wang, H.; Bian, C.; Zhao, Q.; Jiang, X. Mechanisms and Applications of Radiation-Induced Oxidative Stress in Regulating Cancer Immunotherapy. Front. Immunol. 2023, 14, 1247268. [Google Scholar] [CrossRef] [PubMed]
- Haidenberger, A.; Hengster, P.; Kunc, M.; Micke, O.; Wolfgruber, T.; Auer, T.; Lukas, P.; DeVries, A. Influence of Fractionated Irradiation on Neutrophilic Granulocyte Function. Strahlenther. Onkol. 2003, 179, 45–49. [Google Scholar] [CrossRef]
- Schernberg, A.; Blanchard, P.; Chargari, C.; Deutsch, E. Neutrophils, a Candidate Biomarker and Target for Radiation Therapy? Acta Oncol. Stockh. Swed. 2017, 56, 1522–1530. [Google Scholar] [CrossRef]
- Fu, S.-Y.; Chen, F.-H.; Wang, C.-C.; Yu, C.-F.; Chiang, C.-S.; Hong, J.-H. Role of Myeloid-Derived Suppressor Cells in High-Dose-Irradiated TRAMP-C1 Tumors: A Therapeutic Target and an Index for Assessing Tumor Microenvironment. Int. J. Radiat. Oncol. 2021, 109, 1547–1558. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Cortegana, C.; Galassi, C.; Klapp, V.; Gabrilovich, D.I.; Galluzzi, L. Myeloid-Derived Suppressor Cells and Radiotherapy. Cancer Immunol. Res. 2022, 10, 545–557. [Google Scholar] [CrossRef]
- Yang, Y.; Li, C.; Liu, T.; Dai, X.; Bazhin, A.V. Myeloid-Derived Suppressor Cells in Tumors: From Mechanisms to Antigen Specificity and Microenvironmental Regulation. Front. Immunol. 2020, 11, 1371. [Google Scholar] [CrossRef]
- Liu, M.; Wang, X.; Wang, L.; Ma, X.; Gong, Z.; Zhang, S.; Li, Y. Targeting the IDO1 Pathway in Cancer: From Bench to Bedside. J. Hematol. Oncol. 2018, 11, 100. [Google Scholar] [CrossRef]
- Hu, J.; Pan, M.; Wang, Y.; Zhu, Y.; Wang, M. Functional Plasticity of Neutrophils after Low- or High-Dose Irradiation in Cancer Treatment—A Mini Review. Front. Immunol. 2023, 14, 1169670. [Google Scholar] [CrossRef]
- Kalos, M.; June, C.H. Adoptive T Cell Transfer for Cancer Immunotherapy in the Era of Synthetic Biology. Immunity 2013, 39, 49–60. [Google Scholar] [CrossRef]
- June, C.H.; O’Connor, R.S.; Kawalekar, O.U.; Ghassemi, S.; Milone, M.C. CAR T Cell Immunotherapy for Human Cancer. Science 2018, 359, 1361–1365. [Google Scholar] [CrossRef]
- Cheng, M.; Chen, Y.; Xiao, W.; Sun, R.; Tian, Z. NK Cell-Based Immunotherapy for Malignant Diseases. Cell. Mol. Immunol. 2013, 10, 230–252. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.R.; Minutolo, N.G.; Gill, S.; Klichinsky, M. Macrophage-Based Approaches for Cancer Immunotherapy. Cancer Res. 2021, 81, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Qiao, J.; Fu, Y.-X. Immunotherapy and Tumor Microenvironment. Cancer Lett. 2016, 370, 85–90. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, Z.; Xu, X.; Yu, Z.; Mi, J. The Influence of Microenvironment on Tumor Immunotherapy. FEBS J. 2019, 286, 4160–4175. [Google Scholar] [CrossRef]
- Yao, J.; Ji, L.; Wang, G.; Ding, J. Effect of Neutrophils on Tumor Immunity and Immunotherapy Resistance with Underlying Mechanisms. Cancer Commun. Lond. Engl. 2025, 45, 15–42. [Google Scholar] [CrossRef]
- Zhang, M.; Long, X.; Xiao, Y.; Jin, J.; Chen, C.; Meng, J.; Liu, W.; Liu, A.; Chen, L. Assessment and Predictive Ability of the Absolute Neutrophil Count in Peripheral Blood for in Vivo CAR T Cells Expansion and CRS. J. Immunother. Cancer 2023, 11, e007790. [Google Scholar] [CrossRef]
- Xiao, X.; Huang, S.; Chen, S.; Wang, Y.; Sun, Q.; Xu, X.; Li, Y. Mechanisms of Cytokine Release Syndrome and Neurotoxicity of CAR T-Cell Therapy and Associated Prevention and Management Strategies. J. Exp. Clin. Cancer Res. 2021, 40, 367. [Google Scholar] [CrossRef]
- Hao, Z.; Li, R.; Meng, L.; Han, Z.; Hong, Z. Macrophage, the Potential Key Mediator in CAR-T Related CRS. Exp. Hematol. Oncol. 2020, 9, 15. [Google Scholar] [CrossRef]
- Yang, S.; Xu, J.; Dai, Y.; Jin, S.; Sun, Y.; Li, J.; Liu, C.; Ma, X.; Chen, Z.; Chen, L.; et al. Neutrophil Activation and Clonal CAR-T Re-Expansion Underpinning Cytokine Release Syndrome during Ciltacabtagene Autoleucel Therapy in Multiple Myeloma. Nat. Commun. 2024, 15, 360. [Google Scholar] [CrossRef]
- Zhu, J.; Zhou, J.; Liang, X.; An, F.; Ding, Y.; Jiao, X.; Xiao, M.; Wu, F.; Li, Y.; Xiao, H.; et al. Elevated CD10- Neutrophils Correlate with Non-Response and Poor Prognosis of CD19 CAR T-Cell Therapy for B-Cell Acute Lymphoblastic Leukemia. BMC Med. 2025, 23, 138. [Google Scholar] [CrossRef]
- Harris, J.D.; Chang, Y.; Syahirah, R.; Lian, X.L.; Deng, Q.; Bao, X. Engineered Anti-Prostate Cancer CAR-Neutrophils from Human Pluripotent Stem Cells. J. Immunol. Regen. Med. 2023, 20, 100074. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Syahirah, R.; Wang, X.; Jin, G.; Torregrosa-Allen, S.; Elzey, B.D.; Hummel, S.N.; Wang, T.; Li, C.; Lian, X.; et al. Engineering Chimeric Antigen Receptor Neutrophils from Human Pluripotent Stem Cells for Targeted Cancer Immunotherapy. Cell Rep. 2022, 40, 111128. [Google Scholar] [CrossRef] [PubMed]
- Majumder, A.; Jung, H.S.; Zhang, J.; Suknuntha, K.; Thomson, J.; Slukvin, I. 356 Generation of GD2-CAR Neutrophils from hPSCs for Targeted Cancer Immunotherapy of Solid Tumors. J. Immunother. Cancer 2022, 10, A375. [Google Scholar]
- Chang, Y.; Cai, X.; Syahirah, R.; Yao, Y.; Xu, Y.; Jin, G.; Bhute, V.J.; Torregrosa-Allen, S.; Elzey, B.D.; Won, Y.-Y.; et al. CAR-Neutrophil Mediated Delivery of Tumor-Microenvironment Responsive Nanodrugs for Glioblastoma Chemo-Immunotherapy. Nat. Commun. 2023, 14, 2266. [Google Scholar] [CrossRef]
- Harrington, K.; Freeman, D.J.; Kelly, B.; Harper, J.; Soria, J.-C. Optimizing Oncolytic Virotherapy in Cancer Treatment. Nat. Rev. Drug Discov. 2019, 18, 689–706. [Google Scholar] [CrossRef]
- Varghese, S.; Rabkin, S.D. Oncolytic Herpes Simplex Virus Vectors for Cancer Virotherapy. Cancer Gene Ther. 2002, 9, 967–978. [Google Scholar] [CrossRef]
- Kirn, D. Oncolytic Virotherapy for Cancer with the Adenovirus Dl1520 (Onyx-015): Results of Phase I and II Trials. Expert Opin. Biol. Ther. 2001, 1, 525–538. [Google Scholar] [CrossRef]
- Deng, L.; Fan, J.; Ding, Y.; Zhang, J.; Zhou, B.; Zhang, Y.; Huang, B.; Hu, Z. Oncolytic Cancer Therapy with a Vaccinia Virus Strain. Oncol. Rep. 2019, 41, 686–692. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, J.; Lin, K. Immunogenic Cell Death-Based Oncolytic Virus Therapy: A Sharp Sword of Tumor Immunotherapy. Eur. J. Pharmacol. 2024, 981, 176913. [Google Scholar] [CrossRef]
- DePeaux, K.; Delgoffe, G.M. Integrating Innate and Adaptive Immunity in Oncolytic Virus Therapy. Trends Cancer 2024, 10, 135–146. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, C.; Sun, J.; Yuan, M. Neutrophils in Oncolytic Virus Immunotherapy. Front. Immunol. 2024, 15, 1490414. [Google Scholar] [CrossRef]
- Mealiea, D.; McCart, J.A. Cutting Both Ways: The Innate Immune Response to Oncolytic Virotherapy. Cancer Gene Ther. 2022, 29, 629–646. [Google Scholar] [CrossRef]
- Dai, W.; Tian, R.; Yu, L.; Bian, S.; Chen, Y.; Yin, B.; Luan, Y.; Chen, S.; Fan, Z.; Yan, R.; et al. Overcoming Therapeutic Resistance in Oncolytic Herpes Virotherapy by Targeting IGF2BP3-Induced NETosis in Malignant Glioma. Nat. Commun. 2024, 15, 131. [Google Scholar] [CrossRef]
- Zhou, D.; Xu, W.; Ding, X.; Guo, H.; Wang, J.; Zhao, G.; Zhang, C.; Zhang, Z.; Wang, Z.; Wang, P.; et al. Transient Inhibition of Neutrophil Functions Enhances the Antitumor Effect of Intravenously Delivered Oncolytic Vaccinia Virus. Cancer Sci. 2024, 115, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Minott, J.A.; van Vloten, J.P.; Chan, L.; Mehrani, Y.; Bridle, B.W.; Karimi, K. The Role of Neutrophils in Oncolytic Orf Virus-Mediated Cancer Immunotherapy. Cells 2022, 11, 2858. [Google Scholar] [CrossRef] [PubMed]
- Staedtke, V.; Sun, N.; Bai, R. Hypoxia-Targeting Bacteria in Cancer Therapy. Semin. Cancer Biol. 2024, 100, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Theys, J.; Patterson, A.V.; Mowday, A.M. Clostridium Bacteria: Harnessing Tumour Necrosis for Targeted Gene Delivery. Mol. Diagn. Ther. 2024, 28, 141–151. [Google Scholar] [CrossRef]
- Staedtke, V.; Roberts, N.J.; Bai, R.-Y.; Zhou, S. Clostridium Novyi-NT in Cancer Therapy. Genes Dis. 2016, 3, 144–152. [Google Scholar] [CrossRef]
- Staedtke, V.; Bai, R.-Y.; Sun, W.; Huang, J.; Kibler, K.K.; Tyler, B.M.; Gallia, G.L.; Kinzler, K.; Vogelstein, B.; Zhou, S.; et al. Clostridium Novyi -NT Can Cause Regression of Orthotopically Implanted Glioblastomas in Rats. Oncotarget 2015, 6, 5536–5546. [Google Scholar] [CrossRef]
- Janku, F.; Zhang, H.H.; Pezeshki, A.; Goel, S.; Murthy, R.; Wang-Gillam, A.; Shepard, D.R.; Helgason, T.; Masters, T.; Hong, D.S.; et al. Intratumoral Injection of Clostridium Novyi -NT Spores in Patients with Treatment-Refractory Advanced Solid Tumors. Clin. Cancer Res. 2021, 27, 96–106. [Google Scholar] [CrossRef]
- Bettegowda, C.; Huang, X.; Lin, J.; Cheong, I.; Kohli, M.; Szabo, S.A.; Zhang, X.; Diaz, L.A.; Velculescu, V.E.; Parmigiani, G.; et al. The Genome and Transcriptomes of the Anti-Tumor Agent Clostridium Novyi-NT. Nat. Biotechnol. 2006, 24, 1573–1580. [Google Scholar] [CrossRef]
- Zwagerman, N.T.; Friedlander, R.M.; Monaco, E.A. Intratumoral Clostridium Novyi as a Potential Treatment for Solid Necrotic Brain Tumors. Neurosurgery 2014, 75, N17–N18. [Google Scholar] [CrossRef] [PubMed]
- Staedtke, V.; Gray-Bethke, T.; Liu, G.; Liapi, E.; Riggins, G.J.; Bai, R.-Y. Neutrophil Depletion Enhanced the Clostridium Novyi-NT Therapy in Mouse and Rabbit Tumor Models. Neuro-Oncol. Adv. 2022, 4, vdab184. [Google Scholar] [CrossRef] [PubMed]
- Coffelt, S.B.; Wellenstein, M.D.; de Visser, K.E. Neutrophils in Cancer: Neutral No More. Nat. Rev. Cancer 2016, 16, 431–446. [Google Scholar] [CrossRef] [PubMed]
- Burnette, B.C.; Liang, H.; Lee, Y.; Chlewicki, L.; Khodarev, N.N.; Weichselbaum, R.R.; Fu, Y.-X.; Auh, S.L. The Efficacy of Radiotherapy Relies upon Induction of Type i Interferon-Dependent Innate and Adaptive Immunity. Cancer Res. 2011, 71, 2488–2496. [Google Scholar] [CrossRef]
- Xie, X.; Shi, Q.; Wu, P.; Zhang, X.; Kambara, H.; Su, J.; Yu, H.; Park, S.-Y.; Guo, R.; Ren, Q.; et al. Single-Cell Transcriptome Profiling Reveals Neutrophil Heterogeneity in Homeostasis and Infection. Nat. Immunol. 2020, 21, 1119–1133. [Google Scholar] [CrossRef]
- Wigerblad, G.; Cao, Q.; Brooks, S.; Naz, F.; Gadkari, M.; Jiang, K.; Gupta, S.; O’Neil, L.; Dell’Orso, S.; Kaplan, M.J.; et al. Single-Cell Analysis Reveals the Range of Transcriptional States of Circulating Human Neutrophils. J. Immunol. 2022, 209, 772–782. [Google Scholar] [CrossRef]
- LaSalle, T.J.; Gonye, A.L.K.; Freeman, S.S.; Kaplonek, P.; Gushterova, I.; Kays, K.R.; Manakongtreecheep, K.; Tantivit, J.; Rojas-Lopez, M.; Russo, B.C.; et al. Longitudinal Characterization of Circulating Neutrophils Uncovers Phenotypes Associated with Severity in Hospitalized COVID-19 Patients. Cell Rep. Med. 2022, 3, 100779. [Google Scholar] [CrossRef]
- Chen, Z.; Li, W.; Tang, Y.; Zhou, P.; He, Q.; Deng, Z. The Neutrophil-Lymphocyte Ratio Predicts All-Cause and Cardiovascular Mortality among United States Adults with COPD: Results from NHANES 1999–2018. Front. Med. 2024, 11, 1443749. [Google Scholar] [CrossRef]
- Xiao, M.; Zhou, J.; Zhang, W.; Ding, Y.; Guo, J.; Liang, X.; Zhu, J.; Jiao, X.; Zhai, Z.; Wang, H. Association of Immunosuppressive CD45+CD33+CD14−CD10−HLA-DR−/low Neutrophils with Poor Prognosis in Patients with Lymphoma and Their Expansion and Activation through STAT3/Arginase-1 Pathway in Vitro. Cytojournal 2024, 21, 69. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, K.; Chen, S.; Wang, X.; Yu, W. The Pretreatment Neutrophil-to-Lymphocyte Ratio as a Near-Term Prognostic Indicator in Patients with Locally Advanced Hepatocellular Carcinoma Treated with Hepatic Arterial Infusion Chemotherapy: A Propensity Score Matching Cohort Study. Br. J. Hosp. Med. 2024, 85, 1–16. [Google Scholar] [CrossRef]
- Mi, H.; Wei, W.; Zhang, D.; Liang, H.; Yue, C.; Xu, J. Neutrophil-to-Lymphocyte Ratio, Platelet-to-Lymphocyte Ratio, and Prognostic Nutritional Index as Prognostic Markers for Lung Carcinoma. Br. J. Hosp. Med. 2024, 85, 1–13. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Chen, X.; Lu, Y.; Sun, N.; Weisgerber, K.E.; Xu, M.; Bai, R.-Y. Neutrophil Dynamics in Response to Cancer Therapies. Cancers 2025, 17, 2593. https://doi.org/10.3390/cancers17152593
Xu H, Chen X, Lu Y, Sun N, Weisgerber KE, Xu M, Bai R-Y. Neutrophil Dynamics in Response to Cancer Therapies. Cancers. 2025; 17(15):2593. https://doi.org/10.3390/cancers17152593
Chicago/Turabian StyleXu, Huazhen, Xiaojun Chen, Yuqing Lu, Nihao Sun, Karis E. Weisgerber, Manzhu Xu, and Ren-Yuan Bai. 2025. "Neutrophil Dynamics in Response to Cancer Therapies" Cancers 17, no. 15: 2593. https://doi.org/10.3390/cancers17152593
APA StyleXu, H., Chen, X., Lu, Y., Sun, N., Weisgerber, K. E., Xu, M., & Bai, R.-Y. (2025). Neutrophil Dynamics in Response to Cancer Therapies. Cancers, 17(15), 2593. https://doi.org/10.3390/cancers17152593





