Validation of a Quantitative Ultrasound Texture Analysis Model for Early Prediction of Neoadjuvant Chemotherapy Response in Breast Cancer: A Prospective Serial Imaging Study
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Tumour Response Definition
2.3. Quantitative Ultrasound and Texture Parameters Estimation
2.4. Classification Model Algorithm
2.5. Statistical Analysis
3. Results
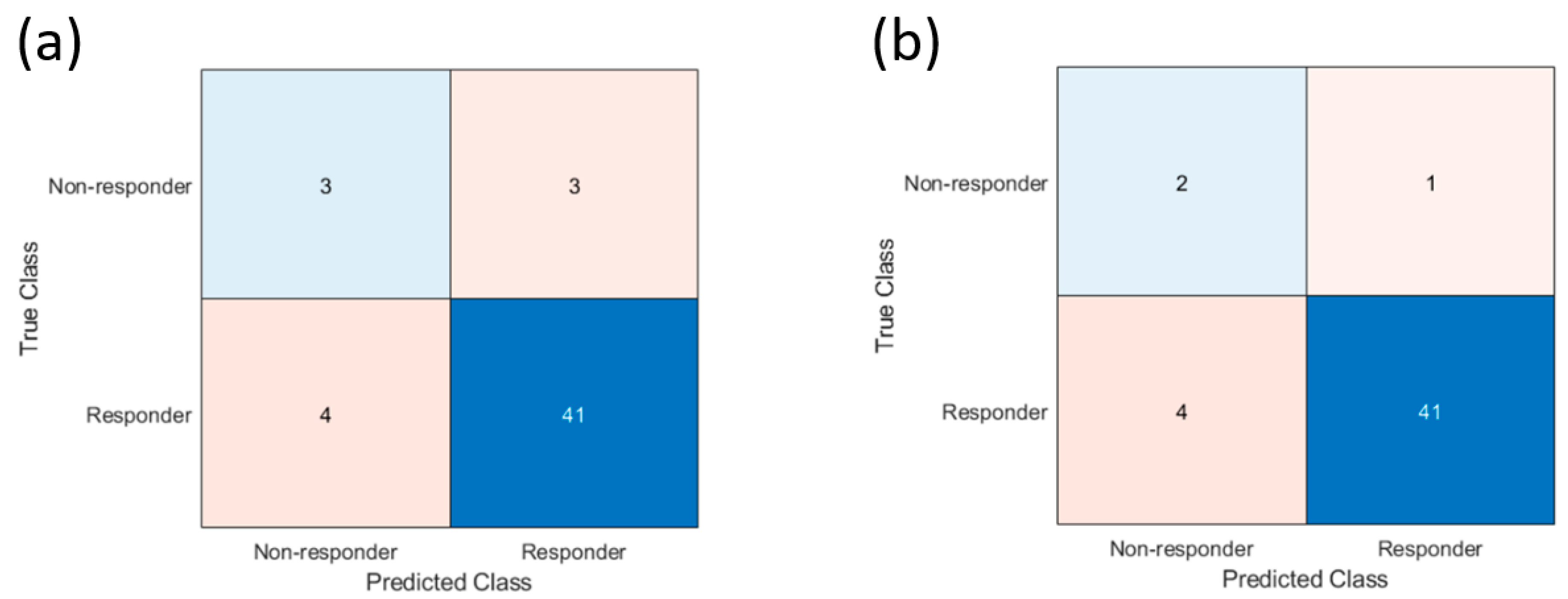
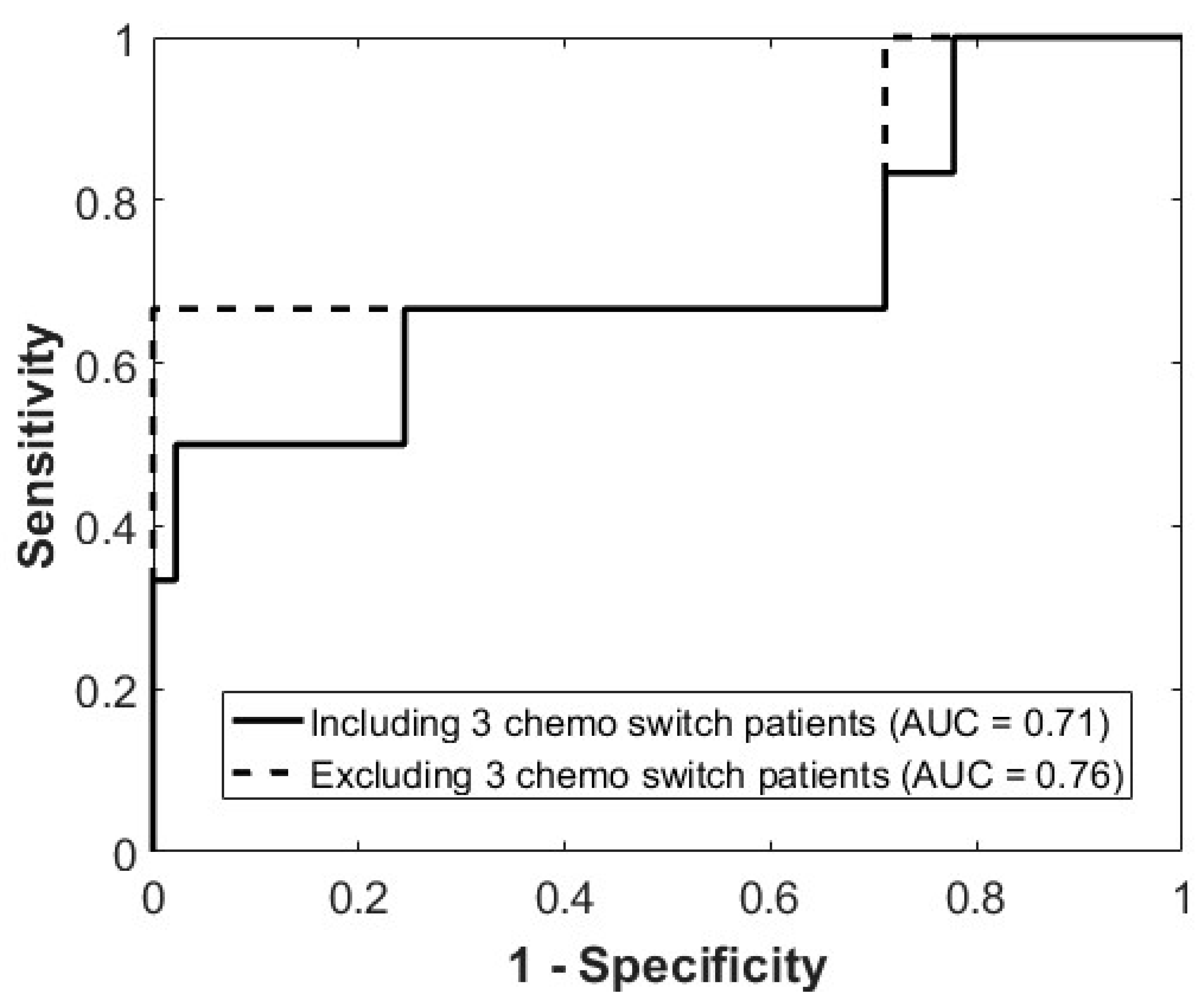
3.1. Accuracy of Prediction Model
3.2. Misclassified Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Burstein, H.J.; Chew, H.; Dang, C.; et al. Breast Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 691–722. [Google Scholar] [CrossRef]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Paluch-Shimon, S.; Senkus, E.; Curigliano, G.; Aapro, M.S.; André, F.; Barrios, C.H.; Bergh, J.; Bhattacharyya, G.S.; Biganzoli, L.; et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann. Oncol. 2020, 31, 1623–1649. [Google Scholar] [CrossRef] [PubMed]
- Von Minckwitz, G.; Huang, C.-S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef]
- Masuda, N.; Lee, S.-J.; Ohtani, S.; Im, Y.-H.; Lee, E.-S.; Yokota, I.; Kuroi, K.; Im, S.-A.; Park, B.-W.; Kim, S.-B.; et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N. Engl. J. Med. 2017, 376, 2147–2159. [Google Scholar] [CrossRef]
- Lejeune, M.; Reverté, L.; Sauras, E.; Gallardo, N.; Bosch, R.; Roso, A.; Petit, A.; Peg, V.; Riu, F.; García-Fontgivell, J.; et al. Prognostic Implications of the Residual Tumor Microenvironment after Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer Patients without Pathological Complete Response. Cancers 2023, 15, 597. [Google Scholar] [CrossRef]
- Van Der Voort, A.; Louis, F.M.; Van Ramshorst, M.S.; Kessels, R.; Mandjes, I.A.; Kemper, I.; Agterof, M.J.; Van Der Steeg, W.A.; Heijns, J.B.; Van Bekkum, M.L.; et al. MRI-guided optimisation of neoadjuvant chemotherapy duration in stage II–III HER2-positive breast cancer (TRAIN-3): A multicentre, single-arm, phase 2 study. Lancet Oncol. 2024, 25, 603–613. [Google Scholar] [CrossRef]
- Von Minckwitz, G.; Kummel, S.; Vogel, P.; Hanusch, C.; Eidtmann, H.; Hilfrich, J.; Gerber, B.; Huober, J.; Costa, S.D.; Jackisch, C.; et al. Neoadjuvant Vinorelbine-Capecitabine Versus Docetaxel-Doxorubicin-Cyclophosphamide in Early Nonresponsive Breast Cancer: Phase III Randomized GeparTrio Trial. JNCI J. Natl. Cancer Inst. 2008, 100, 542–551. [Google Scholar] [CrossRef]
- Pérez-García, J.M.; Cortés, J.; Ruiz-Borrego, M.; Colleoni, M.; Stradella, A.; Bermejo, B.; Dalenc, F.; Escrivá-de-Romaní, S.; Calvo Martínez, L.; Ribelles, N.; et al. 3-year invasive disease-free survival with chemotherapy de-escalation using an 18F-FDG-PET-based, pathological complete response-adapted strategy in HER2-positive early breast cancer (PHERGain): A randomised, open-label, phase 2 trial. Lancet 2024, 403, 1649–1659. [Google Scholar] [CrossRef] [PubMed]
- Lafci, O.; Resch, D.; Santonocito, A.; Clauser, P.; Helbich, T.; Baltzer, P.A.T. Role of imaging based response assesment for adapting neoadjuvant systemic therapy for breast cancer: A systematic review. Eur. J. Radiol. 2025, 187, 112105. [Google Scholar] [CrossRef] [PubMed]
- Dowling, G.P.; Toomey, S.; Bredin, P.; Parker, I.; Mulroe, E.; Marron, J.; McLoughlin, O.; Teiserskiene, A.; Power, C.; O’Shea, A.M.; et al. Neoadjuvant trastuzumab deruxtecan (T-DXd) with response-directed definitive therapy in early stage HER2-positive breast cancer: A phase II study protocol (SHAMROCK study). BMC Cancer 2024, 24, 91. [Google Scholar] [CrossRef]
- Spring, L.M.; Tolaney, S.M.; Fell, G.; Bossuyt, V.; Abelman, R.O.; Wu, B.; Maheswaran, S.; Trippa, L.; Comander, A.; Mulvey, T.; et al. Response-guided neoadjuvant sacituzumab govitecan for localized triple-negative breast cancer: Results from the NeoSTAR trial. Ann. Oncol. 2024, 35, 293–301. [Google Scholar] [CrossRef]
- Bhardwaj, D.; Dasgupta, A.; DiCenzo, D.; Brade, S.; Fatima, K.; Quiaoit, K.; Trudeau, M.; Gandhi, S.; Eisen, A.; Wright, F.; et al. Early Changes in Quantitative Ultrasound Imaging Parameters during Neoadjuvant Chemotherapy to Predict Recurrence in Patients with Locally Advanced Breast Cancer. Cancers 2022, 14, 1247. [Google Scholar] [CrossRef] [PubMed]
- Dobruch-Sobczak, K.S.; Piotrzkowska-Wróblewska, H.; Karwat, P.; Klimonda, Z.; Markiewicz-Grodzicka, E.; Litniewski, J. Quantitative Assessment of the Echogenicity of a Breast Tumor Predicts the Response to Neoadjuvant Chemotherapy. Cancers 2021, 13, 3546. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, A.; Brade, S.; Sannachi, L.; Quiaoit, K.; Fatima, K.; DiCenzo, D.; Osapoetra, L.O.; Saifuddin, M.; Trudeau, M.; Gandhi, S.; et al. Quantitative ultrasound radiomics using texture derivatives in prediction of treatment response to neo-adjuvant chemotherapy for locally advanced breast cancer. Oncotarget 2020, 11, 3782–3792. [Google Scholar] [CrossRef] [PubMed]
- Sannachi, L.; Gangeh, M.; Tadayyon, H.; Gandhi, S.; Wright, F.C.; Slodkowska, E.; Curpen, B.; Sadeghi-Naini, A.; Tran, W.; Czarnota, G.J. Breast Cancer Treatment Response Monitoring Using Quantitative Ultrasound and Texture Analysis: Comparative Analysis of Analytical Models. Transl. Oncol. 2019, 12, 1271–1281. [Google Scholar] [CrossRef]
- Sannachi, L.; Tadayyon, H.; Sadeghi-Naini, A.; Tran, W.; Gandhi, S.; Wright, F.; Oelze, M.; Czarnota, G. Non-invasive evaluation of breast cancer response to chemotherapy using quantitative ultrasonic backscatter parameters. Med. Image Anal. 2015, 20, 224–236. [Google Scholar] [CrossRef]
- Sadeghi-Naini, A.; Sannachi, L.; Pritchard, K.; Trudeau, M.; Gandhi, S.; Wright, F.C.; Zubovits, J.; Yaffe, M.J.; Kolios, M.C.; Czarnota, G.J. Early prediction of therapy responses and outcomes in breast cancer patients using quantitative ultrasound spectral texture. Oncotarget 2014, 5, 3497–3511. [Google Scholar] [CrossRef]
- Sadeghi-Naini, A.; Papanicolau, N.; Falou, O.; Zubovits, J.; Dent, R.; Verma, S.; Trudeau, M.; Boileau, J.F.; Spayne, J.; Iradji, S.; et al. Quantitative Ultrasound Evaluation of Tumor Cell Death Response in Locally Advanced Breast Cancer Patients Receiving Chemotherapy. Clin. Cancer Res. 2013, 19, 2163–2174. [Google Scholar] [CrossRef]
- Banihashemi, B.; Vlad, R.; Debeljevic, B.; Giles, A.; Kolios, M.C.; Czarnota, G.J. Ultrasound Imaging of Apoptosis in Tumor Response: Novel Preclinical Monitoring of Photodynamic Therapy Effects. Cancer Res. 2008, 68, 8590–8596. [Google Scholar] [CrossRef]
- Vlad, R.M.; Brand, S.; Giles, A.; Kolios, M.C.; Czarnota, G.J. Quantitative Ultrasound Characterization of Responses to Radiotherapy in Cancer Mouse Models. Clin. Cancer Res. 2009, 15, 2067–2075. [Google Scholar] [CrossRef]
- Czarnota, G.J.; Kolios, M.C.; Abraham, J.; Portnoy, M.; Ottensmeyer, F.P.; Hunt, J.W.; Sherar, M.D. Ultrasound imaging of apoptosis: High-resolution non-invasive monitoring of programmed cell death in vitro, in situ and in vivo. Br. J. Cancer 1999, 81, 520–527. [Google Scholar] [CrossRef]
- Groheux, D.; Ferrer, L.; Vargas, J.; Martineau, A.; Borgel, A.; Teixeira, L.; Menu, P.; Bertheau, P.; Gallinato, O.; Colin, T.; et al. FDG-PET/CT and Multimodal Machine Learning Model Prediction of Pathological Complete Response to Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer. Cancers 2025, 17, 1249. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, Z.; Son, J.B.; Feng, H.; Adrada, B.E.; Moseley, T.W.; Candelaria, R.P.; Guirguis, M.S.; Patel, M.M.; Whitman, G.J.; et al. Deep Learning Models Based on Pretreatment MRI and Clinicopathological Data to Predict Responses to Neoadjuvant Systemic Therapy in Triple-Negative Breast Cancer. Cancers 2025, 17, 966. [Google Scholar] [CrossRef]
- Jones, E.F.; Hathi, D.K.; Freimanis, R.; Mukhtar, R.A.; Chien, A.J.; Esserman, L.J.; Van’T Veer, L.J.; Joe, B.N.; Hylton, N.M. Current Landscape of Breast Cancer Imaging and Potential Quantitative Imaging Markers of Response in ER-Positive Breast Cancers Treated with Neoadjuvant Therapy. Cancers 2020, 12, 1511. [Google Scholar] [CrossRef]
- Lim, C.H.; Choi, J.Y.; Choi, J.H.; Lee, J.-H.; Lee, J.; Lim, C.W.; Kim, Z.; Woo, S.-K.; Park, S.B.; Park, J.M. Development and External Validation of 18F-FDG PET-Based Radiomic Model for Predicting Pathologic Complete Response after Neoadjuvant Chemotherapy in Breast Cancer. Cancers 2023, 15, 3842. [Google Scholar] [CrossRef] [PubMed]
- Saleh, G.A.; Batouty, N.M.; Gamal, A.; Elnakib, A.; Hamdy, O.; Sharafeldeen, A.; Mahmoud, A.; Ghazal, M.; Yousaf, J.; Alhalabi, M.; et al. Impact of Imaging Biomarkers and AI on Breast Cancer Management: A Brief Review. Cancers 2023, 15, 5216. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.-Y.; Tsai, T.-Y.; Wu, C.-H.; Chung, W.-S.; Wang, J.-C.; Hsu, J.-S.; Hou, M.-F.; Chou, M.-C. Integration of Clinical and CT-Based Radiomic Features for Pretreatment Prediction of Pathologic Complete Response to Neoadjuvant Systemic Therapy in Breast Cancer. Cancers 2022, 14, 6261. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.-S.; Chen, S.-C.; Ko, T.-M.; Lin, Y.-C.; Lin, S.-H.; Lo, Y.-F.; Tseng, S.-C.; Yu, C.-C. An Integrative Clinical Model for the Prediction of Pathological Complete Response in Patients with Operable Stage II and Stage III Triple-Negative Breast Cancer Receiving Neoadjuvant Chemotherapy. Cancers 2022, 14, 4170. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhu, M.; Xie, H.; Geng, X.; Wang, Y.; Wu, Z.; Lin, Y.; Xu, S.; Ye, Y.; Yin, W.; et al. Dynamic Evolution of Vascular Features Based on Magnetic Resonance Imaging to Predict Pathological Response, Patterns of Recurrence and Survival Outcomes in Breast Cancer Neoadjuvant Chemotherapy. Curr. Oncol. 2025, 32, 350. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, A.; DiCenzo, D.; Sannachi, L.; Gandhi, S.; Pezo, R.C.; Eisen, A.; Warner, E.; Wright, F.C.; Look-Hong, N.; Sadeghi-Naini, A.; et al. Quantitative ultrasound radiomics guided adaptive neoadjuvant chemotherapy in breast cancer: Early results from a randomized feasibility study. Front. Oncol. 2024, 14, 1273437. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | Development Cohort (n = 100) | Independent Validation Cohort (n = 51) | |
|---|---|---|---|
| Age (years) | 49 (29–84) | 49 (27–73) | |
| Primary tumour size (cm) | 5.3 (1.6–12.8) | 3.6 (1.7–12) | |
| Histological Type | |||
| IDC | 95 (95%) | 50 (98%) | |
| ILC | 5 (5%) | 1 (2%) | |
| Estrogen Receptor status | |||
| Positive | 63 (63%) | 33 (65%) | |
| Negative | 37 (37%) | 18 (35%) | |
| HER2 Status * | |||
| Positive | 24 (24%) | 17 (33%) | |
| Negative | 73 (73%) | 34 (67%) | |
| Planned Chemotherapy Regimen | |||
| AC-T ± Trastuzumab | 59 (59%) | 34 (67%) | |
| FEC-D ± Trastuzumab | 29 (29%) | 15 (29%) | |
| Others (i.e., TC or TH) | 12 (12%) | 2 (4%) | |
| Parameter | Development Cohort (95% CI) | Validation Cohort (95% CI) | |
|---|---|---|---|
| Entire Cohort | Exploratory Analysis | ||
| Sensitivity | 77% (75–78%) | 50% (50–50%) | 67% (65–69%) |
| Specificity | 80% (78–82%) | 91% (87–94%) | 91% (87–95%) |
| Positive predictive value | 52% (51–52%) | 43% (43–43%) | 33% (33–33%) |
| Negative predictive value | 93% (91–94%) | 93% (89–96%) | 98% (94–100%) |
| Accuracy | 78% (76–79%) | 86% (83–89%) | 90% (86–93%) |
| AUC | 0.79 (0.78–0.80) | 0.71 (0.68–0.73) | 0.76 (0.73–0.79) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moore-Palhares, D.; Sannachi, L.; Chan, A.W.; Dasgupta, A.; DiCenzo, D.; Gandhi, S.; Pezo, R.; Eisen, A.; Warner, E.; Wright, F.; et al. Validation of a Quantitative Ultrasound Texture Analysis Model for Early Prediction of Neoadjuvant Chemotherapy Response in Breast Cancer: A Prospective Serial Imaging Study. Cancers 2025, 17, 2594. https://doi.org/10.3390/cancers17152594
Moore-Palhares D, Sannachi L, Chan AW, Dasgupta A, DiCenzo D, Gandhi S, Pezo R, Eisen A, Warner E, Wright F, et al. Validation of a Quantitative Ultrasound Texture Analysis Model for Early Prediction of Neoadjuvant Chemotherapy Response in Breast Cancer: A Prospective Serial Imaging Study. Cancers. 2025; 17(15):2594. https://doi.org/10.3390/cancers17152594
Chicago/Turabian StyleMoore-Palhares, Daniel, Lakshmanan Sannachi, Adrian Wai Chan, Archya Dasgupta, Daniel DiCenzo, Sonal Gandhi, Rossanna Pezo, Andrea Eisen, Ellen Warner, Frances Wright, and et al. 2025. "Validation of a Quantitative Ultrasound Texture Analysis Model for Early Prediction of Neoadjuvant Chemotherapy Response in Breast Cancer: A Prospective Serial Imaging Study" Cancers 17, no. 15: 2594. https://doi.org/10.3390/cancers17152594
APA StyleMoore-Palhares, D., Sannachi, L., Chan, A. W., Dasgupta, A., DiCenzo, D., Gandhi, S., Pezo, R., Eisen, A., Warner, E., Wright, F., Look Hong, N., Sadeghi-Naini, A., Skarpathiotakis, M., Curpen, B., Betel, C., Kolios, M. C., Trudeau, M., & Czarnota, G. J. (2025). Validation of a Quantitative Ultrasound Texture Analysis Model for Early Prediction of Neoadjuvant Chemotherapy Response in Breast Cancer: A Prospective Serial Imaging Study. Cancers, 17(15), 2594. https://doi.org/10.3390/cancers17152594








