Contrast-Enhanced Harmonic Endoscopic Ultrasonography for Prediction of Aggressiveness and Treatment Response in Patients with Pancreatic Lesions
Simple Summary
Abstract
1. Introduction
2. EUS and CH-EUS
3. CH-EUS in Pancreatic Cancer: Tumor Aggressiveness and Response to Chemotherapy
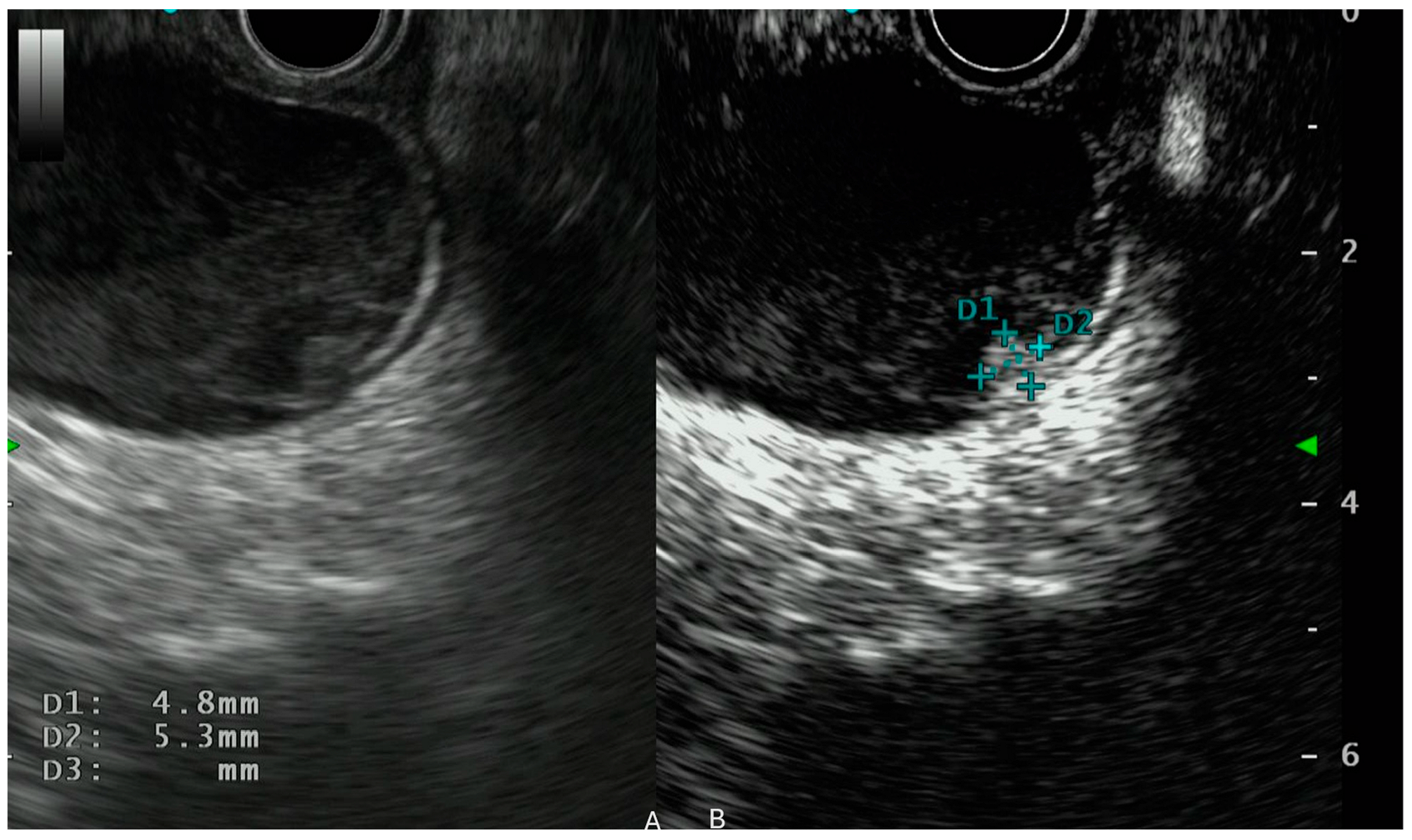
4. CH-EUS in Intraductal Papillary Mucinous Neoplasm (IPMN)
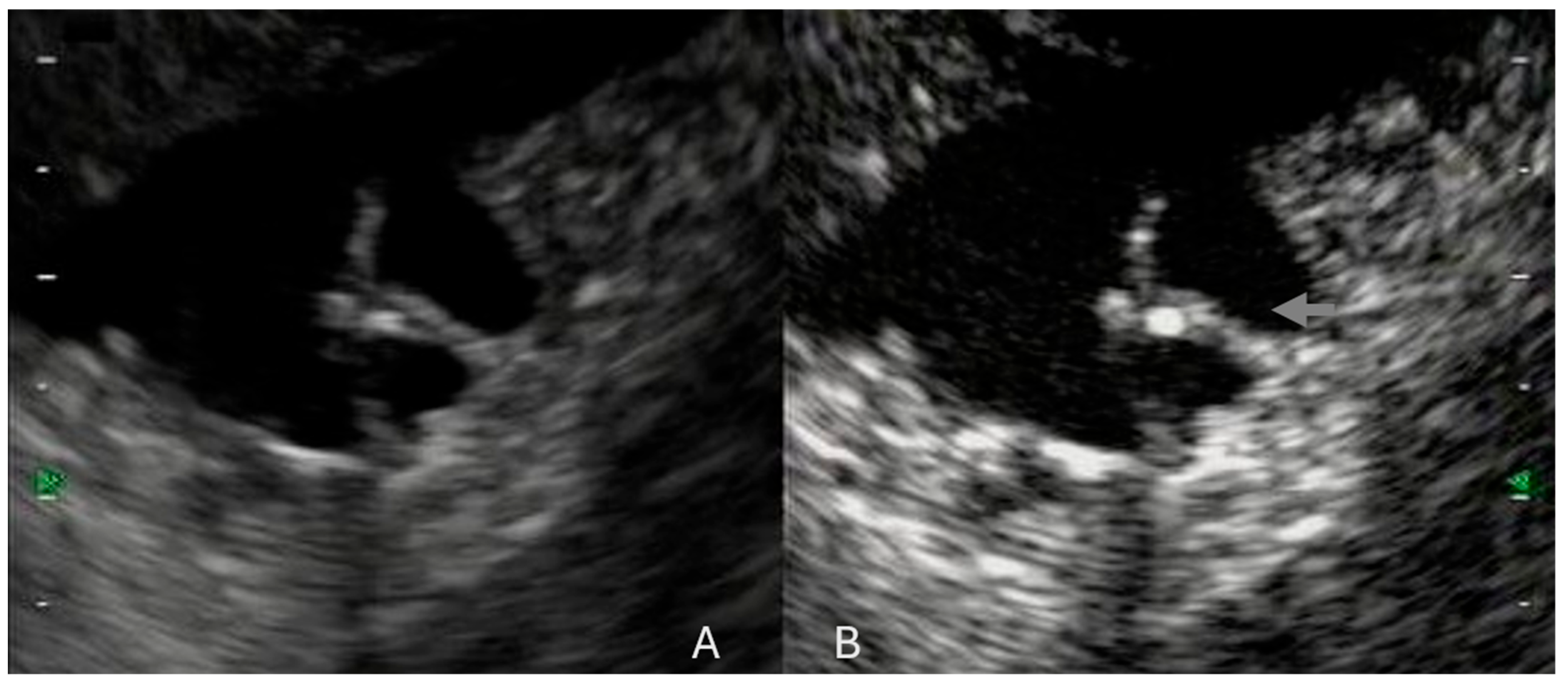
5. CH-EUS in Pancreatic NET (pNET)
6. Limitations
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
List of Abbreviations
References
- Yoshida, T.; Yamashita, Y.; Kitano, M. Endoscopic Ultrasound for Early Diagnosis of Pancreatic Cancer. Diagnostics 2019, 9, 81. [Google Scholar] [CrossRef]
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer Statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- DeWitt, J.; Devereaux, B.; Chriswell, M.; McGreevy, K.; Howard, T.; Imperiale, T.F.; Ciaccia, D.; Lane, K.A.; Maglinte, D.; Kopecky, K.; et al. Comparison of Endoscopic Ultrasonography and Multidetector Computed Tomography for Detecting and Staging Pancreatic Cancer. Ann. Intern. Med. 2004, 141, 753–763. [Google Scholar] [CrossRef]
- Khashab, M.A.; Yong, E.; Lennon, A.M.; Shin, E.J.; Amateau, S.; Hruban, R.H.; Olino, K.; Giday, S.; Fishman, E.K.; Wolfgang, C.L.; et al. EUS Is Still Superior to Multidetector Computerized Tomography for Detection of Pancreatic Neuroendocrine Tumors. Gastrointest. Endosc. 2011, 73, 691–696. [Google Scholar] [CrossRef] [PubMed]
- James, P.D.; Tsolakis, A.V.; Zhang, M.; Belletrutti, P.J.; Mohamed, R.; Roberts, D.J.; Heitman, S.J. Incremental Benefit of Preoperative EUS for the Detection of Pancreatic Neuroendocrine Tumors: A Meta-Analysis. Gastrointest. Endosc. 2015, 81, 848–856.e1. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Shimokawa, T.; Ashida, R.; Napoléon, B.; Lisotti, A.; Fusaroli, P.; Gincul, R.; Dietrich, C.F.; Omoto, S.; Kitano, M. Comparison of Endoscopic Ultrasonography with and without Contrast Enhancement for Characterization of Pancreatic Tumors: A Meta-Analysis. Endosc. Int. Open 2022, 10, E369–E377. [Google Scholar] [CrossRef] [PubMed]
- Nakai, A.; Kamata, K.; Hyodo, T.; Chikugo, T.; Hara, A.; Otsuka, Y.; Tanaka, H.; Yoshikawa, T.; Ishikawa, R.; Okamoto, A.; et al. Utility of Contrast-Enhanced Harmonic EUS for Diagnosis of Portal Vein Invasion by Pancreatic Cancer. Endosc. Ultrasound 2022, 11, 401–406. [Google Scholar] [CrossRef]
- Facciorusso, A.; Mohan, B.P.; Crinò, S.F.; Ofosu, A.; Ramai, D.; Lisotti, A.; Chandan, S.; Fusaroli, P. Contrast-Enhanced Harmonic Endoscopic Ultrasound-Guided Fine-Needle Aspiration versus Standard Fine-Needle Aspiration in Pancreatic Masses: A Meta-Analysis. Expert. Rev. Gastroenterol. Hepatol. 2021, 15, 821–828. [Google Scholar] [CrossRef]
- Crippa, S.; Marchegiani, G.; Belfiori, G.; Rancoita, P.V.M.; Pollini, T.; Burelli, A.; Apadula, L.; Scarale, M.G.; Socci, D.; Biancotto, M.; et al. Impact of Age, Comorbidities and Relevant Changes on Surveillance Strategy of Intraductal Papillary Mucinous Neoplasms: A Competing Risk Analysis. Gut 2024, 73, 1336–1342. [Google Scholar] [CrossRef]
- Ishikawa, R.; Kamata, K.; Hara, A.; Tanaka, H.; Okamoto, A.; Yamazaki, T.; Nakai, A.; Omoto, S.; Minaga, K.; Yamao, K.; et al. Utility of Contrast-Enhanced Harmonic Endoscopic Ultrasonography for Predicting the Prognosis of Pancreatic Neuroendocrine Neoplasms. Dig. Endosc. 2021, 33, 829–839. [Google Scholar] [CrossRef]
- Sofuni, A.; Itoi, T.; Itokawa, F.; Tsuchiya, T.; Kurihara, T.; Ishii, K.; Tsuji, S.; Ikeuchi, N.; Moriyasu, F. Usefulness of Contrast-Enhanced Ultrasonography in Determining Treatment Efficacy and Outcome after Pancreatic Cancer Chemotherapy. World J. Gastroenterol. 2008, 14, 7183–7191. [Google Scholar] [CrossRef]
- Kitano, M.; Kamata, K.; Imai, H.; Miyata, T.; Yasukawa, S.; Yanagisawa, A.; Kudo, M. Contrast-Enhanced Harmonic Endoscopic Ultrasonography for Pancreatobiliary Diseases. Dig. Endosc. 2015, 27 (Suppl. S1), 60–67. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Ignee, A.; Braden, B.; Barreiros, A.P.; Ott, M.; Hocke, M. Improved Differentiation of Pancreatic Tumors Using Contrast-Enhanced Endoscopic Ultrasound. Clin. Gastroenterol. Hepatol. 2008, 6, 590–597.e1. [Google Scholar] [CrossRef]
- Saftoiu, A.; Napoleon, B.; Arcidiacono, P.G.; Braden, B.; Burmeister, S.; Carrara, S.; Cui, X.W.; Fusaroli, P.; Gottschalk, U.; Hocke, M.; et al. Do We Need Contrast Agents for EUS? Endosc. Ultrasound 2020, 9, 361–368. [Google Scholar] [CrossRef]
- Săftoiu, A.; Dietrich, C.F.; Vilmann, P. Contrast-Enhanced Harmonic Endoscopic Ultrasound. Endoscopy 2012, 44, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Fusaroli, P.; Spada, A.; Mancino, M.G.; Caletti, G. Contrast Harmonic Echo-Endoscopic Ultrasound Improves Accuracy in Diagnosis of Solid Pancreatic Masses. Clin. Gastroenterol. Hepatol. 2010, 8, 629–634.e2. [Google Scholar] [CrossRef]
- Carrara, S.; Repici, A. Kupffer-Phase EUS: The Contrast Agent that Magnifies Liver Metastasis. Gastrointest. Endosc. 2021, 93, 442–443. [Google Scholar] [CrossRef] [PubMed]
- Minaga, K.; Kitano, M.; Nakai, A.; Omoto, S.; Kamata, K.; Yamao, K.; Takenaka, M.; Tsurusaki, M.; Chikugo, T.; Matsumoto, I.; et al. Improved Detection of Liver Metastasis Using Kupffer-Phase Imaging in Contrast-Enhanced Harmonic EUS in Patients with Pancreatic Cancer (with Video). Gastrointest. Endosc. 2021, 93, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Shimokawa, T.; Napoléon, B.; Fusaroli, P.; Gincul, R.; Kudo, M.; Kitano, M. Value of Contrast-Enhanced Harmonic Endoscopic Ultrasonography with Enhancement Pattern for Diagnosis of Pancreatic Cancer: A Meta-Analysis. Dig. Endosc. 2019, 31, 125–133. [Google Scholar] [CrossRef]
- Napoleon, B.; Alvarez-Sanchez, M.; Gincoul, R.; Pujol, B.; Lefort, C.; Lepilliez, V.; Labadie, M.; Souquet, J.; Queneau, P.; Scoazec, J.; et al. Contrast-Enhanced Harmonic Endoscopic Ultrasound in Solid Lesions of the Pancreas: Results of a Pilot Study. Endoscopy 2010, 42, 564–570. [Google Scholar] [CrossRef]
- Kitano, M.; Kudo, M.; Yamao, K.; Takagi, T.; Sakamoto, H.; Komaki, T.; Kamata, K.; Imai, H.; Chiba, Y.; Okada, M.; et al. Characterization of Small Solid Tumors in the Pancreas: The Value of Contrast-Enhanced Harmonic Endoscopic Ultrasonography. Am. J. Gastroenterol. 2012, 107, 303–310. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Correas, J.-M.; Cui, X.-W.; Dong, Y.; Havre, R.F.; Jenssen, C.; Jung, E.M.; Krix, M.; Lim, A.; Lassau, N.; et al. EFSUMB Technical Review—Update 2023: Dynamic Contrast-Enhanced Ultrasound (DCE-CEUS) for the Quantification of Tumor Perfusion. Ultraschall Med. 2024, 45, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yuan, C.; Dai, W.; Tang, L.; Shi, J.; Li, Z.; Chen, M. Evaluating Pathologic Response of Breast Cancer to Neoadjuvant Chemotherapy with Computer-Extracted Features from Contrast-Enhanced Ultrasound Videos. Phys. Med. 2017, 39, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Omoto, S.; Takenaka, M.; Kitano, M.; Miyata, T.; Kamata, K.; Minaga, K.; Arizumi, T.; Yamao, K.; Imai, H.; Sakamoto, H.; et al. Characterization of Pancreatic Tumors with Quantitative Perfusion Analysis in Contrast-Enhanced Harmonic Endoscopic Ultrasonography. Oncology 2017, 93 (Suppl. S1), 55–60. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Yamazaki, H.; Nakahata, A.; Shimokawa, T.; Tamura, T.; Kawaji, Y.; Tamura, T.; Hatamaru, K.; Itonaga, M.; Ashida, R.; et al. Endoscopic Ultrasonography for Microvascular Imaging without Contrast Enhancement in the Differential Diagnosis of Pancreatic Lesions. Dig. Endosc. 2025, 37, 192–198. [Google Scholar] [CrossRef]
- Hwang, J.S.; Seo, D.-W.; So, H.; Ko, S.W.; Joo, H.D.; Oh, D.; Song, T.J.; Park, D.H.; Lee, S.S.; Lee, S.K.; et al. Clinical Utility of Directional eFLOW Compared with Contrast-Enhanced Harmonic Endoscopic Ultrasound for Assessing the Vascularity of Pancreatic and Peripancreatic Masses. Pancreatology 2022, 22, 130–135. [Google Scholar] [CrossRef]
- Yamashita, Y.; Yoshikawa, T.; Yamazaki, H.; Kawaji, Y.; Tamura, T.; Hatamaru, K.; Itonaga, M.; Ashida, R.; Ida, Y.; Maekita, T.; et al. A Novel Endoscopic Ultrasonography Imaging Technique for Depicting Microcirculation in Pancreatobiliary Lesions without the Need for Contrast-Enhancement: A Prospective Exploratory Study. Diagnostics 2021, 11, 2018. [Google Scholar] [CrossRef]
- Mulqui, M.V.; Caillol, F.; Ratone, J.P.; Hoibian, S.; Dahel, Y.; Meunier, É.; Archimbaud, C.; Giovannini, M. Detective Flow Imaging versus Contrast-Enhanced EUS in Solid Pancreatic Lesions. Endosc. Ultrasound 2024, 13, 248–252. [Google Scholar] [CrossRef]
- Couvelard, A.; O’Toole, D.; Leek, R.; Turley, H.; Sauvanet, A.; Degott, C.; Ruszniewski, P.; Belghiti, J.; Harris, A.L.; Gatter, K.; et al. Expression of Hypoxia-Inducible Factors Is Correlated with the Presence of a Fibrotic Focus and Angiogenesis in Pancreatic Ductal Adenocarcinomas. Histopathology 2005, 46, 668–676. [Google Scholar] [CrossRef]
- Hata, H.; Mori, H.; Matsumoto, S.; Yamada, Y.; Kiyosue, H.; Tanoue, S.; Hongo, N.; Kashima, K. Fibrous Stroma and Vascularity of Pancreatic Carcinoma: Correlation with Enhancement Patterns on CT. Abdom. Imaging 2010, 35, 172–180. [Google Scholar] [CrossRef]
- Kitano, M.; Sakamoto, H.; Matsui, U.; Ito, Y.; Maekawa, K.; von Schrenck, T.; Kudo, M. A Novel Perfusion Imaging Technique of the Pancreas: Contrast-Enhanced Harmonic EUS (with Video). Gastrointest. Endosc. 2008, 67, 141–150. [Google Scholar] [CrossRef]
- Hesse, F.; Ritter, J.; Hapfelmeier, A.; Braren, R.; Phillip, V. Comparison of Magnetic Resonance Imaging and Endoscopic Ultrasound in the Sizing of Intraductal Papillary Mucinous Neoplasia of the Pancreas. Pancreas 2023, 52, e315–e320. [Google Scholar] [CrossRef] [PubMed]
- Masaki, T.; Ohkawa, S.; Amano, A.; Ueno, M.; Miyakawa, K.; Tarao, K. Noninvasive Assessment of Tumor Vascularity by Contrast-Enhanced Ultrasonography and the Prognosis of Patients with Nonresectable Pancreatic Carcinoma. Cancer 2005, 103, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Ueda, K.; Itonaga, M.; Yoshida, T.; Maeda, H.; Maekita, T.; Iguchi, M.; Tamai, H.; Ichinose, M.; Kato, J. Tumor Vessel Depiction with Contrast-Enhanced Endoscopic Ultrasonography Predicts Efficacy of Chemotherapy in Pancreatic Cancer. Pancreas 2013, 42, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Emori, T.; Ashida, R.; Tamura, T.; Kawaji, Y.; Hatamaru, K.; Itonaga, M.; Yamashita, Y.; Shimokawa, T.; Higashino, N.; Ikoma, A.; et al. Contrast-Enhanced Harmonic Endoscopic Ultrasonography for Predicting the Efficacy of First-Line Gemcitabine and Nab-Paclitaxel Chemotherapy in Pancreatic Cancer. Pancreatology 2022, 22, 525–533. [Google Scholar] [CrossRef]
- Tanaka, H.; Kamata, K.; Takenaka, M.; Yoshikawa, T.; Ishikawa, R.; Okamoto, A.; Yamazaki, T.; Nakai, A.; Omoto, S.; Minaga, K.; et al. Contrast-Enhanced Harmonic Endoscopic Ultrasonography for Evaluating the Response to Chemotherapy in Pancreatic Cancer. Dig. Liver Dis. 2019, 51, 1130–1134. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Fernandez-Del Castillo, C.; Furukawa, T.; Hijioka, S.; Jang, J.-Y.; Lennon, A.M.; Miyasaka, Y.; Ohno, E.; Salvia, R.; Wolfgang, C.L.; et al. International Evidence-Based Kyoto Guidelines for the Management of Intraductal Papillary Mucinous Neoplasm of the Pancreas. Pancreatology 2024, 24, 255–270. [Google Scholar] [CrossRef]
- European Study Group on Cystic Tumours of the Pancreas European Evidence-Based Guidelines on Pancreatic Cystic Neoplasms. Gut 2018, 67, 789–804. [CrossRef]
- Ohno, E.; Kuzuya, T.; Kawabe, N.; Nakaoka, K.; Tanaka, H.; Nakano, T.; Funasaka, K.; Miyahara, R.; Hashimoto, S.; Hirooka, Y. Current Status of Endoscopic Ultrasound in the Diagnosis of Intraductal Papillary Mucinous Neoplasms. DEN Open 2025, 5, e413. [Google Scholar] [CrossRef]
- Kamata, K.; Kitano, M.; Kudo, M.; Sakamoto, H.; Kadosaka, K.; Miyata, T.; Imai, H.; Maekawa, K.; Chikugo, T.; Kumano, M.; et al. Value of EUS in Early Detection of Pancreatic Ductal Adenocarcinomas in Patients with Intraductal Papillary Mucinous Neoplasms. Endoscopy 2014, 46, 22–29. [Google Scholar] [CrossRef]
- Tada, M.; Kawabe, T.; Arizumi, M.; Togawa, O.; Matsubara, S.; Yamamoto, N.; Nakai, Y.; Sasahira, N.; Hirano, K.; Tsujino, T.; et al. Pancreatic Cancer in Patients with Pancreatic Cystic Lesions: A Prospective Study in 197 Patients. Clin. Gastroenterol. Hepatol. 2006, 4, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Ohno, E.; Itoh, A.; Kawashima, H.; Ishikawa, T.; Matsubara, H.; Itoh, Y.; Nakamura, Y.; Hiramatsu, T.; Nakamura, M.; Miyahara, R.; et al. Malignant Transformation of Branch Duct-Type Intraductal Papillary Mucinous Neoplasms of the Pancreas Based on Contrast-Enhanced Endoscopic Ultrasonography Morphological Changes: Focus on Malignant Transformation of Intraductal Papillary Mucinous Neoplasm Itself. Pancreas 2012, 41, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Song, T.J.; Hwang, J.-H.; Yoo, K.-S.; Lee, W.-J.; Lee, K.-H.; Dong, S.-H.; Park, C.-H.; Park, E.-T.; Moon, J.-H.; et al. Predictors of Malignancy in Pure Branch Duct Type Intraductal Papillary Mucinous Neoplasm of the Pancreas: A Nationwide Multicenter Study. Pancreatology 2015, 15, 405–410. [Google Scholar] [CrossRef]
- Du, C.; Chai, N.-L.; Linghu, E.-Q.; Li, H.-K.; Sun, L.-H.; Jiang, L.; Wang, X.-D.; Tang, P.; Yang, J. Comparison of Endoscopic Ultrasound, Computed Tomography and Magnetic Resonance Imaging in Assessment of Detailed Structures of Pancreatic Cystic Neoplasms. World J. Gastroenterol. 2017, 23, 3184–3192. [Google Scholar] [CrossRef]
- Kin, T.; Shimizu, Y.; Hijioka, S.; Hara, K.; Katanuma, A.; Nakamura, M.; Yamada, R.; Itoi, T.; Ueki, T.; Masamune, A.; et al. A Comparative Study between Computed Tomography and Endoscopic Ultrasound in the Detection of a Mural Nodule in Intraductal Papillary Mucinous Neoplasm -Multicenter Observational Study in Japan. Pancreatology 2023, 23, 550–555. [Google Scholar] [CrossRef]
- Huynh, T.; Ali, K.; Vyas, S.; Dezsi, K.; Strickland, D.; Basinski, T.; Chen, D.-T.; Jiang, K.; Centeno, B.; Malafa, M.; et al. Comparison of Imaging Modalities for Measuring the Diameter of Intraductal Papillary Mucinous Neoplasms of the Pancreas. Pancreatology 2020, 20, 448–453. [Google Scholar] [CrossRef]
- Hocke, M.; Cui, X.-W.; Domagk, D.; Ignee, A.; Dietrich, C.F. Pancreatic Cystic Lesions: The Value of Contrast-Enhanced Endoscopic Ultrasound to Influence the Clinical Pathway. Endosc. Ultrasound 2014, 3, 123–130. [Google Scholar] [CrossRef]
- Iwaya, H.; Hijioka, S.; Mizuno, N.; Kuwahara, T.; Okuno, N.; Tajika, M.; Tanaka, T.; Ishihara, M.; Hirayama, Y.; Onishi, S.; et al. Usefulness of Septal Thickness Measurement on Endoscopic Ultrasound as a Predictor of Malignancy of Branched-Duct and Mixed-Type Intraductal Papillary Mucinous Neoplasm of the Pancreas. Dig. Endosc. 2019, 31, 672–681. [Google Scholar] [CrossRef]
- Ugbarugba, E.E.; Grieco, C.; Hart, P.A.; Li, F.; Sklaw, B.; Cronley, K.; Oza, V.M.; Swanson, B.J.; Walker, J.P.; El-Dika, S.; et al. Diagnostic Accuracy of Preoperative Imaging for Differentiation of Branch Duct Versus Mixed Duct Intraductal Papillary Mucinous Neoplasms. Pancreas 2018, 47, 556–560. [Google Scholar] [CrossRef]
- Kobayashi, N.; Sugimori, K.; Shimamura, T.; Hosono, K.; Watanabe, S.; Kato, S.; Ueda, M.; Endo, I.; Inayama, Y.; Maeda, S.; et al. Endoscopic Ultrasonographic Findings Predict the Risk of Carcinoma in Branch Duct Intraductal Papillary Mucinous Neoplasms of the Pancreas. Pancreatology 2012, 12, 141–145. [Google Scholar] [CrossRef]
- Ohno, E.; Balduzzi, A.; Hijioka, S.; De Pastena, M.; Marchegiani, G.; Kato, H.; Takenaka, M.; Haba, S.; Salvia, R. Association of High-Risk Stigmata and Worrisome Features with Advanced Neoplasia in Intraductal Papillary Mucinous Neoplasms (IPMN): A Systematic Review. Pancreatology 2024, 24, 48–61. [Google Scholar] [CrossRef]
- Ridtitid, W.; DeWitt, J.M.; Schmidt, C.M.; Roch, A.; Stuart, J.S.; Sherman, S.; Al-Haddad, M.A. Management of Branch-Duct Intraductal Papillary Mucinous Neoplasms: A Large Single-Center Study to Assess Predictors of Malignancy and Long-Term Outcomes. Gastrointest. Endosc. 2016, 84, 436–445. [Google Scholar] [CrossRef]
- Lee, L.S. Updates in Diagnosis and Management of Pancreatic Cysts. World J. Gastroenterol. 2021, 27, 5700–5714. [Google Scholar] [CrossRef]
- Fujita, M.; Itoi, T.; Ikeuchi, N.; Sofuni, A.; Tsuchiya, T.; Ishii, K.; Kamada, K.; Umeda, J.; Tanaka, R.; Tonozuka, R.; et al. Effectiveness of Contrast-Enhanced Endoscopic Ultrasound for Detecting Mural Nodules in Intraductal Papillary Mucinous Neoplasm of the Pancreas and for Making Therapeutic Decisions. Endosc. Ultrasound 2016, 5, 377–383. [Google Scholar] [CrossRef]
- Wang, L.; Vatsavayi, P.; Majumder, S.; Gleeson, F.C.; Rajan, E.; Abu Dayyeh, B.K.; Storm, A.C.; Umar, S.; Velaga, S.T.; Harmsen, W.S.; et al. Trends and Impact of Endoscopic Ultrasound Utilization for Suspected Intraductal Papillary Mucinous Neoplasms. Pancreatology 2025, 25, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Kamata, K.; Kitano, M.; Omoto, S.; Kadosaka, K.; Miyata, T.; Yamao, K.; Imai, H.; Sakamoto, H.; Harwani, Y.; Chikugo, T.; et al. Contrast-Enhanced Harmonic Endoscopic Ultrasonography for Differential Diagnosis of Pancreatic Cysts. Endoscopy 2016, 48, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Kawaji, Y.; Shimokawa, T.; Yamazaki, H.; Tamura, T.; Hatamaru, K.; Itonaga, M.; Ashida, R.; Kawai, M.; Kitano, M. Usefulness of Contrast-Enhanced Harmonic Endoscopic Ultrasonography for Diagnosis of Malignancy in Intraductal Papillary Mucinous Neoplasm. Diagnostics 2022, 12, 2141. [Google Scholar] [CrossRef]
- Intraductal Papillary Mucinous Neoplasms of the Pancreas: Differentiation of Malignant and Benign Tumors by Endoscopic Ultrasound Findings of Mural Nodules—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/19300203/ (accessed on 20 February 2025).
- Ohno, E.; Kawashima, H.; Ishikawa, T.; Iida, T.; Suzuki, H.; Uetsuki, K.; Yashika, J.; Yamada, K.; Yoshikawa, M.; Gibo, N.; et al. Can Contrast-Enhanced Harmonic Endoscopic Ultrasonography Accurately Diagnose Main Pancreatic Duct Involvement in Intraductal Papillary Mucinous Neoplasms? Pancreatology 2020, 20, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Barron, M.R.; Roch, A.M.; Waters, J.A.; Parikh, J.A.; DeWitt, J.M.; Al-Haddad, M.A.; Ceppa, E.P.; House, M.G.; Zyromski, N.J.; Nakeeb, A.; et al. Does Preoperative Cross-Sectional Imaging Accurately Predict Main Duct Involvement in Intraductal Papillary Mucinous Neoplasm? J. Gastrointest. Surg. 2014, 18, 447–455. [Google Scholar] [CrossRef]
- Crippa, S.; Pergolini, I.; Rubini, C.; Castelli, P.; Partelli, S.; Zardini, C.; Marchesini, G.; Zamboni, G.; Falconi, M. Risk of Misdiagnosis and Overtreatment in Patients with Main Pancreatic Duct Dilatation and Suspected Combined/Main-Duct Intraductal Papillary Mucinous Neoplasms. Surgery 2016, 159, 1041–1049. [Google Scholar] [CrossRef]
- Correa-Gallego, C.; Ferrone, C.R.; Thayer, S.P.; Wargo, J.A.; Warshaw, A.L.; Fernández-Del Castillo, C. Incidental Pancreatic Cysts: Do We Really Know What We Are Watching? Pancreatology 2010, 10, 144–150. [Google Scholar] [CrossRef]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, L.; Dai, S.; Chen, M.; Li, F.; Sun, J.; Luo, F. Epidemiologic Trends of and Factors Associated With Overall Survival for Patients With Gastroenteropancreatic Neuroendocrine Tumors in the United States. JAMA Netw. Open 2021, 4, e2124750. [Google Scholar] [CrossRef]
- Stensbøl, A.B.; Krogh, J.; Holmager, P.; Klose, M.; Oturai, P.; Kjaer, A.; Hansen, C.P.; Federspiel, B.; Langer, S.W.; Knigge, U.; et al. Incidence, Clinical Presentation and Trends in Indication for Diagnostic Work-Up of Small Intestinal and Pancreatic Neuroendocrine Tumors. Diagnostics 2021, 11, 2030. [Google Scholar] [CrossRef]
- Rindi, G.; Klimstra, D.S.; Abedi-Ardekani, B.; Asa, S.L.; Bosman, F.T.; Brambilla, E.; Busam, K.J.; de Krijger, R.R.; Dietel, M.; El-Naggar, A.K.; et al. A Common Classification Framework for Neuroendocrine Neoplasms: An International Agency for Research on Cancer (IARC) and World Health Organization (WHO) Expert Consensus Proposal. Mod. Pathol. 2018, 31, 1770–1786. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Gangi, A. The Evolving Landscape of Neuroendocrine Tumors. Surg. Oncol. Clin. N. Am. 2023, 32, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Martin-Perez, E.; Capdevila, J.; Castellano, D.; Jimenez-Fonseca, P.; Salazar, R.; Beguiristain-Gomez, A.; Alonso-Orduña, V.; Martinez Del Prado, P.; Villabona-Artero, C.; Diaz-Perez, J.A.; et al. Prognostic Factors and Long-Term Outcome of Pancreatic Neuroendocrine Neoplasms: Ki-67 Index Shows a Greater Impact on Survival than Disease Stage. The Large Experience of the Spanish National Tumor Registry (RGETNE). Neuroendocrinology 2013, 98, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Fulp, W.J.; Strosberg, J.R.; Kvols, L.K.; Centeno, B.A.; Hodul, P.J. Predictors of Lymph Node Metastases and Impact on Survival in Resected Pancreatic Neuroendocrine Tumors: A Single-Center Experience. Am. J. Surg. 2014, 208, 775–780. [Google Scholar] [CrossRef]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A.; ESMO Guidelines Committee. Electronic address: Clinicalguidelines@esmo.org Gastroenteropancreatic Neuroendocrine Neoplasms: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef]
- Kos-Kudła, B.; Castaño, J.P.; Denecke, T.; Grande, E.; Kjaer, A.; Koumarianou, A.; de Mestier, L.; Partelli, S.; Perren, A.; Stättner, S.; et al. European Neuroendocrine Tumour Society (ENETS) 2023 Guidance Paper for Nonfunctioning Pancreatic Neuroendocrine Tumours. J. Neuroendocr. 2023, 35, e13343. [Google Scholar] [CrossRef]
- Rossi, R.E.; Elvevi, A.; Gallo, C.; Palermo, A.; Invernizzi, P.; Massironi, S. Endoscopic Techniques for Diagnosis and Treatment of Gastro-Entero-Pancreatic Neuroendocrine Neoplasms: Where We Are. World J. Gastroenterol. 2022, 28, 3258–3273. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Itoh, A.; Kawashima, H.; Ohno, E.; Matsubara, H.; Itoh, Y.; Nakamura, Y.; Nakamura, M.; Miyahara, R.; Hayashi, K.; et al. Usefulness of EUS Combined with Contrast-Enhancement in the Differential Diagnosis of Malignant versus Benign and Preoperative Localization of Pancreatic Endocrine Tumors. Gastrointest. Endosc. 2010, 71, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Pais, S.A.; Al-Haddad, M.; Mohamadnejad, M.; Leblanc, J.K.; Sherman, S.; McHenry, L.; DeWitt, J.M. EUS for Pancreatic Neuroendocrine Tumors: A Single-Center, 11-Year Experience. Gastrointest. Endosc. 2010, 71, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Fujimori, N.; Osoegawa, T.; Lee, L.; Tachibana, Y.; Aso, A.; Kubo, H.; Kawabe, K.; Igarashi, H.; Nakamura, K.; Oda, Y.; et al. Efficacy of Endoscopic Ultrasonography and Endoscopic Ultrasonography-Guided Fine-Needle Aspiration for the Diagnosis and Grading of Pancreatic Neuroendocrine Tumors. Scand. J. Gastroenterol. 2016, 51, 245–252. [Google Scholar] [CrossRef]
- Lai, T.-Y.; Chiang, T.-C.; Lee, C.-Y.; Kuo, T.-C.; Wu, C.-H.; Chen, Y.-I.; Hu, C.-M.; Maskey, M.; Tang, S.-C.; Jeng, Y.-M.; et al. Unraveling the Impact of Cancer-Associated Fibroblasts on Hypovascular Pancreatic Neuroendocrine Tumors. Br. J. Cancer 2024, 130, 1096–1108. [Google Scholar] [CrossRef]
- Kubo, H.; Ohgi, K.; Ohike, N.; Norose, T.; Ashida, R.; Yamada, M.; Otsuka, S.; Uesaka, K.; Sugiura, T. Tumor Vascularity on Contrast-Enhanced Computed Tomography as a Predictive Marker of Metastatic Potential for Small Nonfunctioning Pancreatic Neuroendocrine Tumors. Surgery 2024, 175, 484–490. [Google Scholar] [CrossRef]
- Notake, T.; Shimizu, A.; Kubota, K.; Sugenoya, S.; Umemura, K.; Goto, T.; Yamada, A.; Fujinaga, Y.; Soejima, Y. Usefulness of Intratumoral Perfusion Analysis for Assessing Biological Features of Non-Functional Pancreatic Neuroendocrine Neoplasm. Langenbecks Arch. Surg. 2024, 409, 38. [Google Scholar] [CrossRef]
- Meng, F.-S.; Zhang, Z.-H.; Ji, F. New Endoscopic Ultrasound Techniques for Digestive Tract Diseases: A Comprehensive Review. World J. Gastroenterol. 2015, 21, 4809–4816. [Google Scholar] [CrossRef]
- Braden, B.; Jenssen, C.; D’Onofrio, M.; Hocke, M.; Will, U.; Möller, K.; Ignee, A.; Dong, Y.; Cui, X.-W.; Sãftoiu, A.; et al. B-Mode and Contrast-Enhancement Characteristics of Small Nonincidental Neuroendocrine Pancreatic Tumors. Endosc. Ultrasound 2017, 6, 49–54. [Google Scholar] [CrossRef]
- Săftoiu, A.; Vilmann, P. Differential Diagnosis of Focal Pancreatic Masses by Semiquantitative EUS Elastography: Between Strain Ratios and Strain Histograms. Gastrointest. Endosc. 2013, 78, 188–189. [Google Scholar] [CrossRef]
- Liao, S. Sa1473 CONTRAST-ENHANCEMENT ENDOSCOPIC ULTRASOUND FOR PANCREATIC NEUROENDOCRINE TUMORS: EXPERIENCE FROM ONE SINGLE INSTITOTION OF TAIWAN. Gastrointest. Endosc. 2020, 91, AB206. [Google Scholar] [CrossRef]
- Battistella, A.; Partelli, S.; Andreasi, V.; Marinoni, I.; Palumbo, D.; Tacelli, M.; Lena, M.S.; Muffatti, F.; Mushtaq, J.; Capurso, G.; et al. Preoperative Assessment of Microvessel Density in Nonfunctioning Pancreatic Neuroendocrine Tumors (NF-PanNETs). Surgery 2022, 172, 1236–1244. [Google Scholar] [CrossRef]
- Palazzo, M.; Napoléon, B.; Gincul, R.; Pioche, M.; Pujol, B.; Lefort, C.; Fumex, F.; Hautefeuille, V.; Fabre, M.; Cros, J.; et al. Contrast Harmonic EUS for the Prediction of Pancreatic Neuroendocrine Tumor Aggressiveness (with Videos). Gastrointest. Endosc. 2018, 87, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Sugihara, Y.; Yamazaki, H.; Koutani, H.; Tamura, T.; Tsuda, I.; Emori, T.; Kawaji, Y.; Hatamaru, K.; Yamashita, Y.; et al. Contrast-Enhanced Harmonic Endoscopic Ultrasound for Diagnosis of the Aggressiveness of Pancreatic Neuroendocrine Neoplasm. Diagnostics 2022, 12, 2988. [Google Scholar] [CrossRef] [PubMed]
- Takada, S.; Kato, H.; Saragai, Y.; Muro, S.; Uchida, D.; Tomoda, T.; Matsumoto, K.; Horiguchi, S.; Tanaka, N.; Okada, H. Contrast-Enhanced Harmonic Endoscopic Ultrasound Using Time-Intensity Curve Analysis Predicts Pathological Grade of Pancreatic Neuroendocrine Neoplasm. J. Med. Ultrason 2019, 46, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Constantin, A.L.; Cazacu, I.; Burtea, D.E.; Harbiyeli, I.C.; Bejinariu, N.; Popescu, C.; Serbanescu, M.; Tabacelia, D.; Copaescu, C.; Bhutani, M.; et al. Quantitative Contrast-Enhanced Endoscopic Ultrasound in Pancreatic Ductal Adenocarcinoma and Pancreatic Neuroendocrine Tumors: Can We Predict Survival Using Perfusion Parameters? A Pilot Study. Med. Ultrason. 2022, 24, 393–398. [Google Scholar] [CrossRef]
- Yang, D.-H.; Cheng, J.; Tian, X.-F.; Zhang, Q.; Yu, L.-Y.; Qiu, Y.-J.; Lu, X.-Y.; Lou, W.-H.; Dong, Y.; Wang, W.-P. Prediction of Pathological Grades of Pancreatic Neuroendocrine Tumors Based on Dynamic Contrast-Enhanced Ultrasound Quantitative Analysis. Diagnostics 2023, 13, 238. [Google Scholar] [CrossRef]
- Tacelli, M.; Lauri, G.; Tabacelia, D.; Tieranu, C.G.; Arcidiacono, P.G.; Săftoiu, A. Integrating Artificial Intelligence with Endoscopic Ultrasound in the Early Detection of Bilio-Pancreatic Lesions: Current Advances and Future Prospects. Best. Pr. Res. Clin. Gastroenterol. 2025, 74, 101975. [Google Scholar] [CrossRef]
- Mo, S.; Wang, Y.; Huang, C.; Wu, W.; Qin, S. A Novel Endoscopic Ultrasomics-Based Machine Learning Model and Nomogram to Predict the Pathological Grading of Pancreatic Neuroendocrine Tumors. Heliyon 2024, 10, e34344. [Google Scholar] [CrossRef]
- Huang, J.; Xie, X.; Wu, H.; Zhang, X.; Zheng, Y.; Xie, X.; Wang, Y.; Xu, M. Development and Validation of a Combined Nomogram Model Based on Deep Learning Contrast-Enhanced Ultrasound and Clinical Factors to Predict Preoperative Aggressiveness in Pancreatic Neuroendocrine Neoplasms. Eur. Radiol. 2022, 32, 7965–7975. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spadaccini, M.; Franchellucci, G.; Andreozzi, M.; Terrin, M.; Tacelli, M.; Zaccari, P.; Petrone, M.C.; Lauri, G.; Colombo, M.; Poletti, V.; et al. Contrast-Enhanced Harmonic Endoscopic Ultrasonography for Prediction of Aggressiveness and Treatment Response in Patients with Pancreatic Lesions. Cancers 2025, 17, 2545. https://doi.org/10.3390/cancers17152545
Spadaccini M, Franchellucci G, Andreozzi M, Terrin M, Tacelli M, Zaccari P, Petrone MC, Lauri G, Colombo M, Poletti V, et al. Contrast-Enhanced Harmonic Endoscopic Ultrasonography for Prediction of Aggressiveness and Treatment Response in Patients with Pancreatic Lesions. Cancers. 2025; 17(15):2545. https://doi.org/10.3390/cancers17152545
Chicago/Turabian StyleSpadaccini, Marco, Gianluca Franchellucci, Marta Andreozzi, Maria Terrin, Matteo Tacelli, Piera Zaccari, Maria Chiara Petrone, Gaetano Lauri, Matteo Colombo, Valeria Poletti, and et al. 2025. "Contrast-Enhanced Harmonic Endoscopic Ultrasonography for Prediction of Aggressiveness and Treatment Response in Patients with Pancreatic Lesions" Cancers 17, no. 15: 2545. https://doi.org/10.3390/cancers17152545
APA StyleSpadaccini, M., Franchellucci, G., Andreozzi, M., Terrin, M., Tacelli, M., Zaccari, P., Petrone, M. C., Lauri, G., Colombo, M., Poletti, V., Marcozzi, G., Durante, A., Leone, R., Massaro, M. M., Facciorusso, A., Fugazza, A., Repici, A., Arcidiacono, P. G., & Carrara, S. (2025). Contrast-Enhanced Harmonic Endoscopic Ultrasonography for Prediction of Aggressiveness and Treatment Response in Patients with Pancreatic Lesions. Cancers, 17(15), 2545. https://doi.org/10.3390/cancers17152545








