Overall Survival Associated with Real-World Treatment Sequences in Patients with CLL/SLL in the United States
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
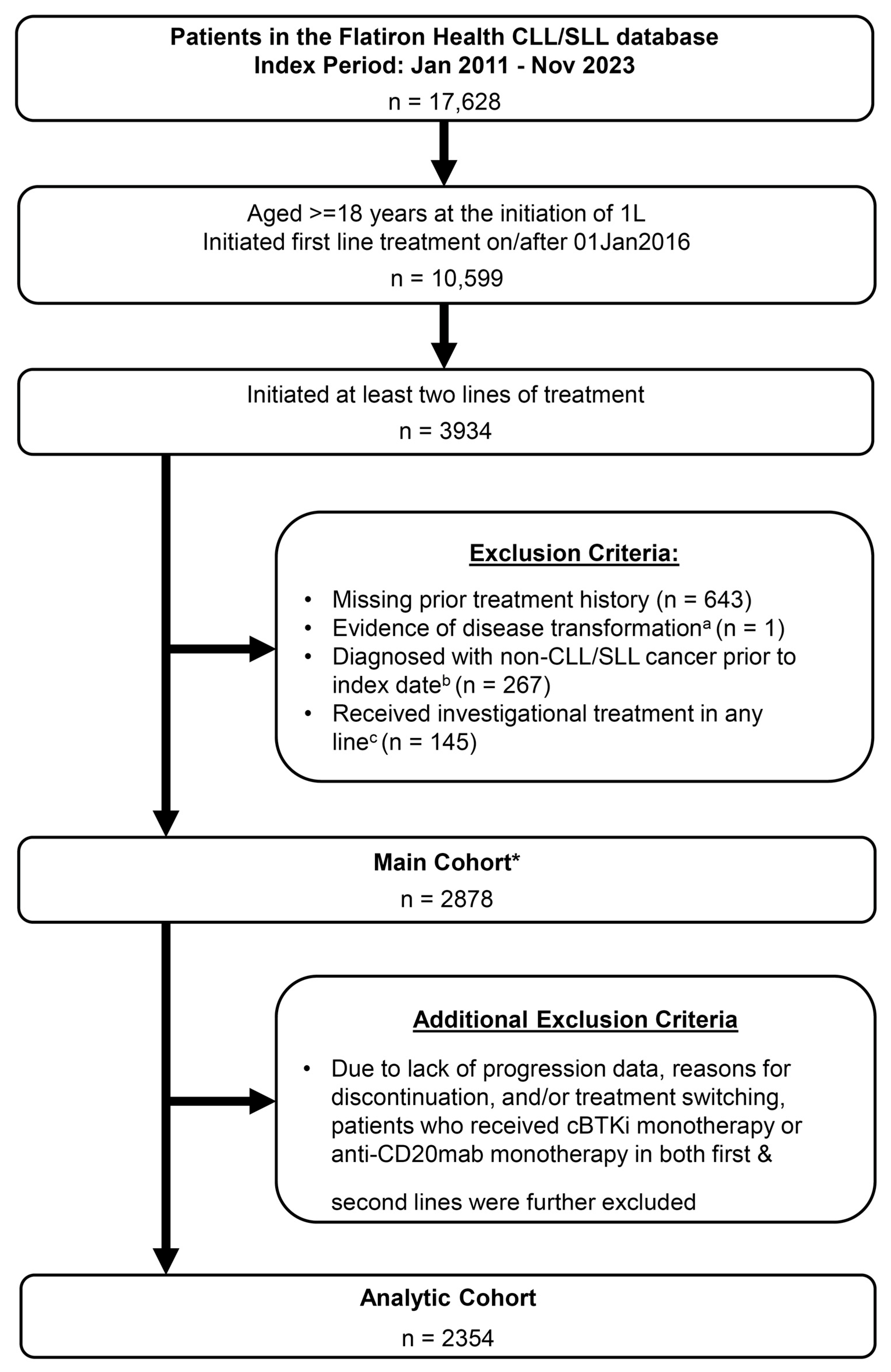
2.1. Study Design and Data Source
2.2. Study Population
2.3. Outcome
2.4. Statistical Analyses
3. Results
3.1. Baseline Patient Characteristics
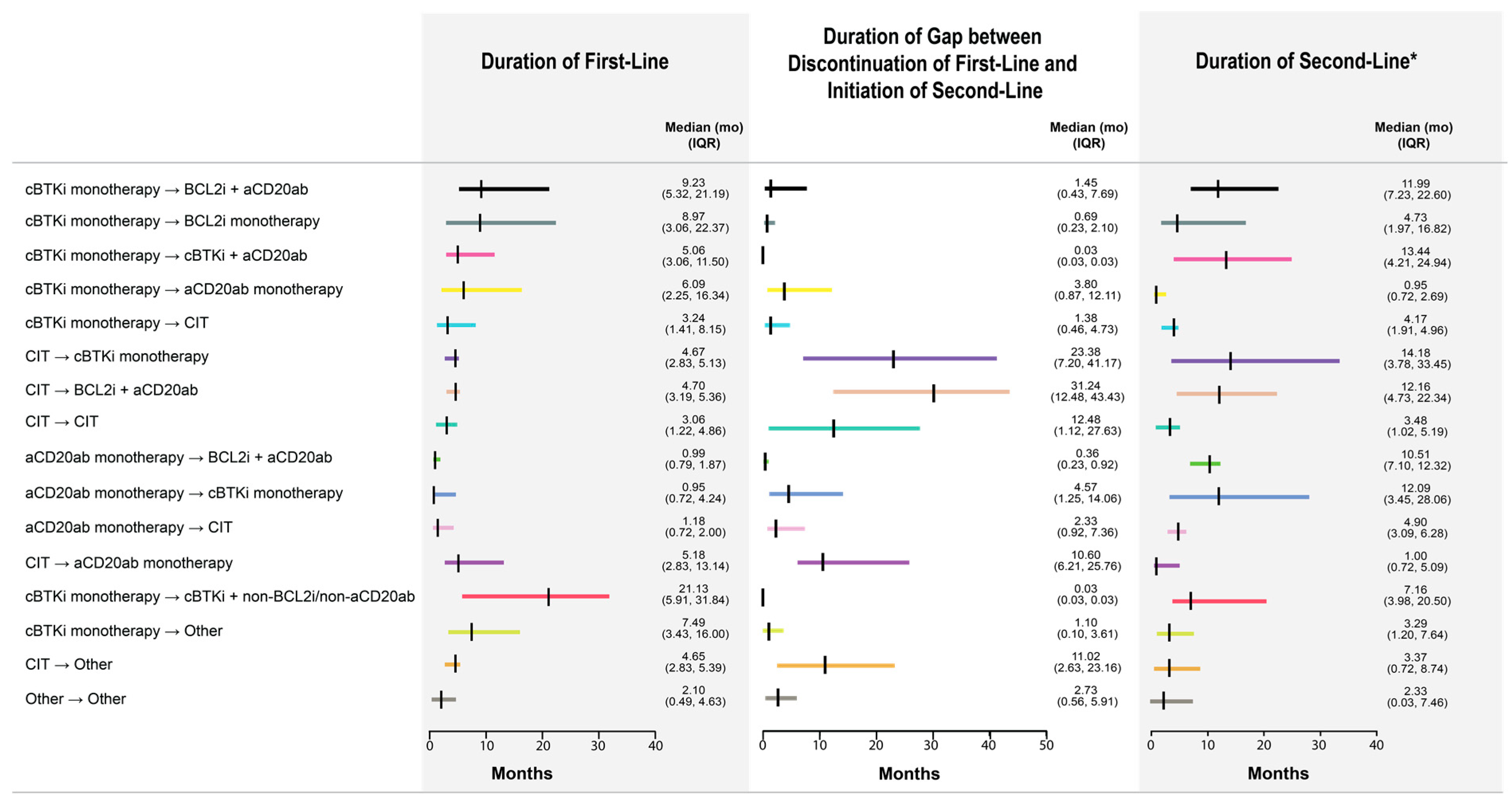
3.2. Frequently Observed Treatment Sequences
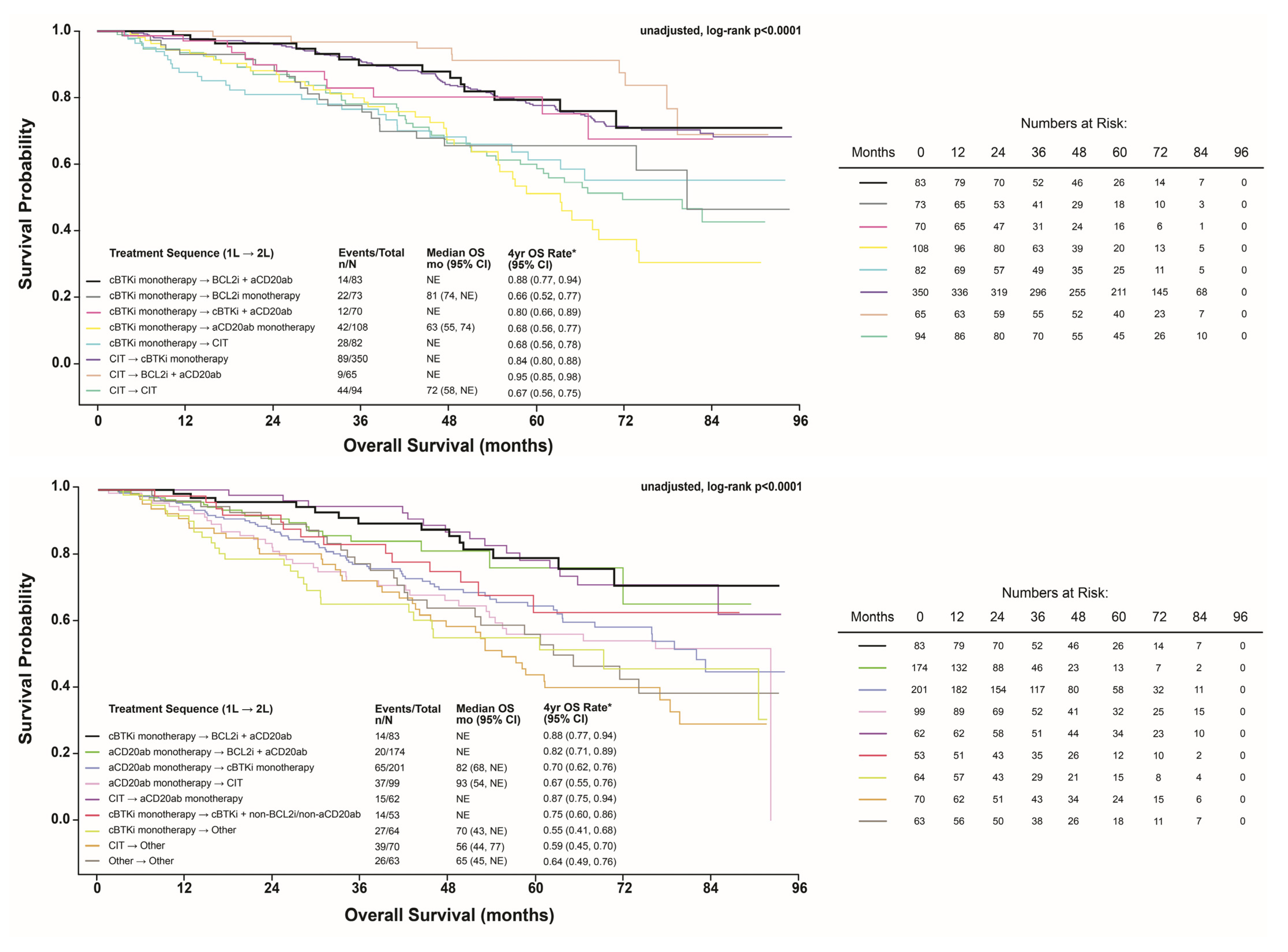
3.3. Unadjusted Comparison of OS
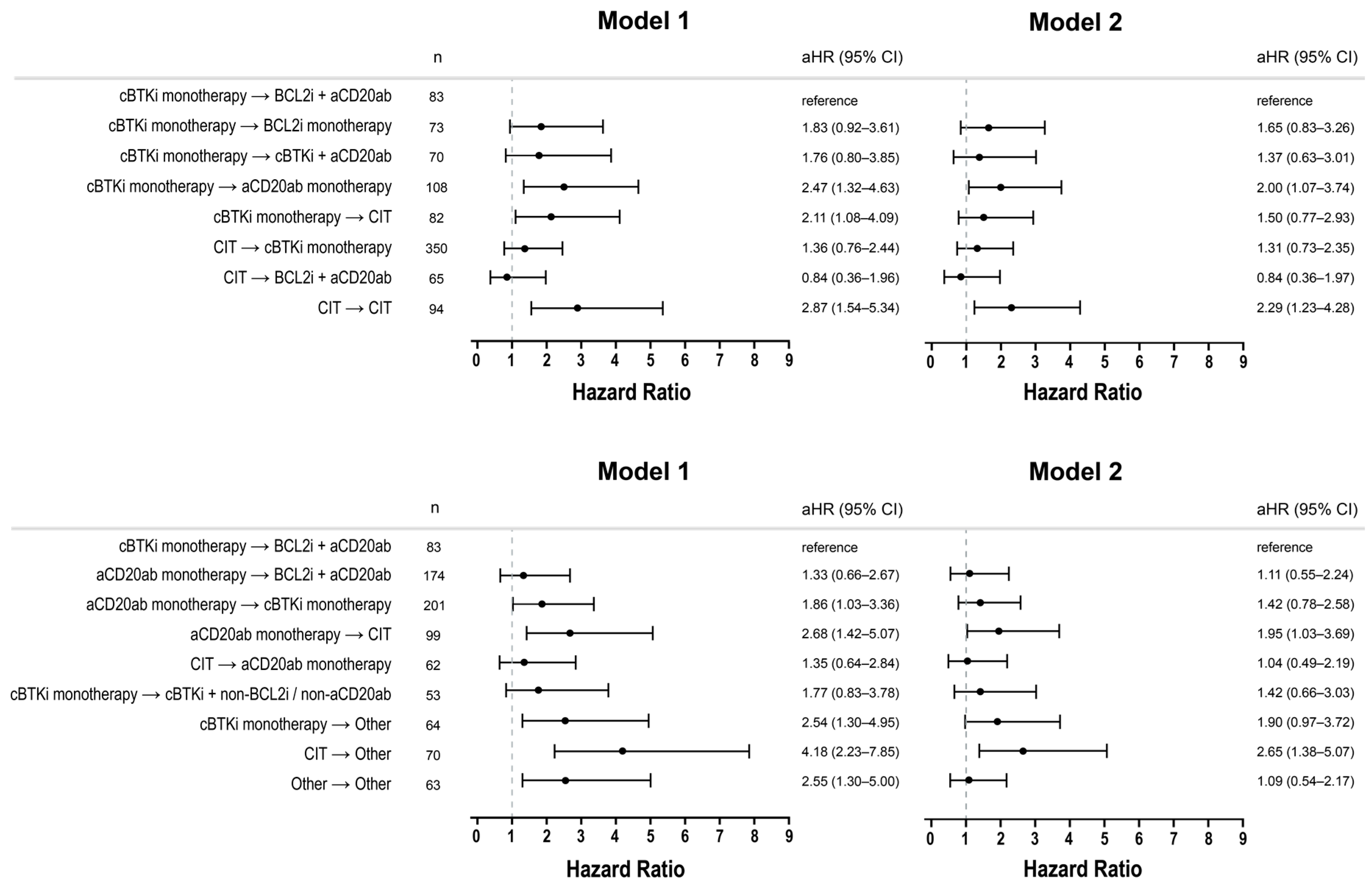
3.4. Adjusted Comparison of OS
3.5. Post Hoc Subgroups
3.6. Outcomes by Age
3.7. Outcomes by Race/Ethnicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| cBTKi | covalent Bruton tyrosine kinase inhibitors |
| BCL2i | B-cell lymphoma 2 inhibitors |
| anti-CD20mab | anti-CD20 monoclonal antibody |
| CLL/SLL | chronic lymphocytic leukemia/small lymphocytic lymphoma |
| CIT | ChemoImmunoTherapy (anti-CD20 monoclonal antibody + Chemotherapy) |
| M1 | Model 1 |
| M2 | Model 2 |
References
- Jain, N.; Wierda, W.G.; O’Brien, S. Chronic lymphocytic leukaemia. Lancet 2024, 404, 694–706. [Google Scholar] [CrossRef] [PubMed]
- Bewarder, M.; Stilgenbauer, S.; Thurner, L.; Kaddu-Mulindwa, D. Current Treatment Options in CLL. Cancers 2021, 13, 2468. [Google Scholar] [CrossRef] [PubMed]
- Islam, P. Current Treatment Options in Relapsed and Refractory Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma: A Review. Curr. Treat. Options Oncol. 2023, 24, 1259–1273. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.; Umoru, G.; Abboud, K.; Hobaugh, E. Sequencing and combination of current small-molecule inhibitors for chronic lymphocytic leukemia: Where is the evidence? Eur. J. Haematol. 2023, 111, 15–28. [Google Scholar] [CrossRef]
- Duchesneau, E.D.; McNeill, A.M.; Schary, W.; Pate, V.; Lund, J.L. Prognosis of older adults with chronic lymphocytic leukemia: A Surveillance, Epidemiology, and End Results-Medicare cohort study. J. Geriatr. Oncol. 2023, 14, 101602. [Google Scholar] [CrossRef]
- Soumerai, J.D.; Barrientos, J.; Ahn, I.; Coombs, C.; Gladstone, D.; Hoffman, M.; Kittai, A.; Jacobs, R.; Lipsky, A.; Patel, K.; et al. Consensus recommendations from the 2024 Lymphoma Research Foundation workshop on treatment selection and sequencing in CLL or SLL. Blood Adv. 2025, 9, 1213–1229. [Google Scholar] [CrossRef]
- Gladstone, D.E.; Blackford, A.; Cho, E.; Swinnen, L.; Kasamon, Y.; Gocke, C.D.; Griffin, C.A.; Bolaños-Meade, J.; Jones, R.J. The importance of IGHV mutational status in del(11q) and del(17p) chronic lymphocytic leukemia. Clin. Lymphoma Myeloma Leuk. 2012, 12, 132–137. [Google Scholar] [CrossRef]
- Jain, N.; O’Brien, S. Initial treatment of CLL: Integrating biology and functional status. Blood 2015, 126, 463–470. [Google Scholar] [CrossRef]
- Bomben, R.; Rossi, F.M.; Vit, F.; Bittolo, T.; Zucchetto, A.; Papotti, R.; Tissino, E.; Pozzo, F.; Degan, M.; Polesel, J.; et al. Clinical impact of TP53 disruption in chronic lymphocytic leukemia patients treated with ibrutinib: A campus CLL study. Leukemia 2023, 37, 914–918. [Google Scholar] [CrossRef]
- Campo, E.; Cymbalista, F.; Ghia, P.; Jager, U.; Pospisilova, S.; Rosenquist, R.; Schuh, A.; Stilgenbauer, S. TP53 aberrations in chronic lymphocytic leukemia: An overview of the clinical implications of improved diagnostics. Haematologica 2018, 103, 1956–1968. [Google Scholar] [CrossRef]
- Ahn, I.E.; Brown, J.R. Selecting initial therapy in CLL. Hematology 2022, 2022, 323–328. [Google Scholar] [CrossRef]
- Patel, K.; Pagel, J.M. Current and future treatment strategies in chronic lymphocytic leukemia. J. Hematol. Oncol. 2021, 14, 69. [Google Scholar] [CrossRef] [PubMed]
- Simon, F.; Bohn, J.P. Next-Generation Sequencing-Optimal Sequencing of Therapies in Relapsed/Refractory Chronic Lymphocytic Leukemia (CLL). Curr. Oncol. Rep. 2023, 25, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Friends of Cancer Research. Enhancing Study Designs and Interpretation of Interim Overall Survival Data in Oncology Trials. Available online: https://friendsofcancerresearch.org/wp-content/uploads/Enhancing-Study-Designs-and-Interpretation-of-Interim-Overall-Survival-Data-in-Oncology-Trials-.pdf (accessed on 16 June 2024).
- Varghese, A.M.; Wright, L.; Munir, T.; Patmore, R.; Parbutt, C.; Smith, A. Population-Based Registry Data over 15 Years Suggests Comparable Overall Survival for CLL Patients Treated with Chemoimmunotherapy and Targeted Therapies As First-Line Treatments. Blood 2024, 144, 5122. [Google Scholar] [CrossRef]
- Therneau, T.; Crowson, C.; Clinic, M. Using Time Dependent Covariates and Time Dependent Coefficients in the Cox Model. Surviv. Vignettes 2014, 2, 1–25. [Google Scholar]
- Zhang, Z.; Reinikainen, J.; Adeleke, K.A.; Pieterse, M.E.; Groothuis-Oudshoorn, C.G.M. Time-varying covariates and coefficients in Cox regression models. Ann. Transl. Med. 2018, 6, 121. [Google Scholar] [CrossRef]
- Abernethy, A.P.; Gippetti, J.; Parulkar, R.; Revol, C. Use of Electronic Health Record Data for Quality Reporting. J. Oncol. Prac. 2017, 13, 530–534. [Google Scholar] [CrossRef]
- Ma, X.; Long, L.; Moon, S.; Adamson, B.J.S.; Baxi, S.S. Comparison of Population Characteristics in Real-World Clinical Oncology Databases in the US: Flatiron Health, SEER, and NPCR. medRxiv 2020. [Google Scholar] [CrossRef]
- Birnbaum, B.; Nussbaum, N.; Seidl-Rathkopf, K.; Agrawal, M.; Estevez, M.; Estola, E.; Haimson, J.; He, L.; Larson, P.; Richardson, P. Model-assisted cohort selection with bias analysis for generating large-scale cohorts from the EHR for oncology research. arXiv 2020, arXiv:2001.09765. [Google Scholar] [CrossRef]
- VanderWeele, T.J.; Ding, P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann. Intern. Med. 2017, 167, 268–274. [Google Scholar] [CrossRef]
- Linden, A.; Mathur, M.B.; VanderWeele, T.J. Conducting sensitivity analysis for unmeasured confounding in observational studies using E-values: The evalue package. Stata J. 2020, 20, 162–175. [Google Scholar] [CrossRef]
- Yost, K.; Perkins, C.; Cohen, R.; Morris, C.; Wright, W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control 2001, 12, 703–711. [Google Scholar] [CrossRef]
- Guadamuz, J.S.; Wang, X.; Ryals, C.A.; Miksad, R.A.; Snider, J.; Walters, J.; Calip, G.S. Socioeconomic status and inequities in treatment initiation and survival among patients with cancer, 2011–2022. JNCI Cancer Spectr. 2023, 7, pkad058. [Google Scholar] [CrossRef]
- Sharman, J.P.; Munir, T.; Grosicki, S.; Roeker, L.; Burke, J.M.; Chen, C.I.; Grzasko, N.; Follows, G.; Mátrai, Z.; Sanna, A.; et al. BRUIN CLL-321: Randomized Phase III Trial of Pirtobrutinib Versus Idelalisib Plus Rituximab (IdelaR) or Bendamustine Plus Rituximab (BR) in BTK Inhibitor Pretreated Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma. Blood 2024, 144, 886. [Google Scholar] [CrossRef]
- Robak, T.; Michael, D.; Emmanuelle, F.; Joris, D.; Liva, A.; Sabine, W.; Healy, N.C.; Lynne, N.; van Sanden, S. Overall survival of patients with CLL treated with ibrutinib in the first line compared to second-line ibrutinib after chemotherapy/chemoimmunotherapy. Curr. Med. Res. Opin. 2024, 40, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, N.; Sharman, J.P.; Gutierrez, M.; Khan, W.; Qureshi, Z.P.; Raz, A.; Girardi, V.; Krigsfeld, G.S.; Barrientos, J.C. Real-World Treatment Patterns and Outcomes by Line of Therapy and Race in Patients With Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma Treated in the United States: Results From the Final Analysis of the Prospective, Observational, informCLL Registry. Clin. Lymphoma Myeloma Leuk. 2024, 24, e301–e313. [Google Scholar] [CrossRef] [PubMed]
- Huntington, S.F.; Cheng, W.Y.; Sarpong, E.M.; Siyang, L.; Farooqui, M.Z.H.; Samuel, A.U.; Maryaline, C.; Dominique, L.; Nathaniel, D.; Lisa, M.; et al. Real-world patterns and sequences of targeted therapy use in chronic lymphocytic leukemia and small lymphocytic lymphoma in the United States: A longitudinal study. Leuk. Lymphoma 2024, 65, 932–942. [Google Scholar] [CrossRef]
- Roeker, L.E.; Burke, J.M.; Rhodes, J.M.; Emechebe, N.; Jawaid, D.; Manzoor, B.S.; Jensen, C.E.; Ryland, L.; Liu, Y.; Marx, S.E.; et al. Real-World Effectiveness of Frontline Treatments Among Patients with Chronic Lymphocytic Leukemia: Results from ConcertAI. Cancers 2025, 17, 799. [Google Scholar] [CrossRef]
- Lansigan, F.; Eyre, T.A.; Ghosh, N.; Manzoor, B.S.; Coombs, C.C.; Lamanna, N.; Tuncer, H.H.; Emechebe, D.; Brown, J.R.; Roeker, L.E.; et al. P648: Real-World Treatment and Outcomes of Patients With Chronic Lymphocytic Leukemia (CLL) Receiving First-Line (1L) Therapy In The Novel Agent Era: An International Study. HemaSphere 2023, 7, e6855326. [Google Scholar] [CrossRef]
- Mato, A.R.; Sail, K.; Yazdy, M.S.; Hill, B.T.; Shadman, M.; Manzoor, B.S.; Tuncer, H.H.; Allan, J.N.; Ujjani, C.S.; Sharmokh, S.; et al. Treatment Sequences and Outcomes of Patients with CLL Treated with Venetoclax and Other Novel Agents Post Introduction of Novel Therapies. Blood 2019, 134, 1756. [Google Scholar] [CrossRef]
- Mato, A.R.; Hess, L.M.; Chen, Y.; Abada, P.B.; Konig, H.; Pagel, J.M.; Walgren, R.A. Outcomes for Patients With Chronic Lymphocytic Leukemia (CLL) Previously Treated With Both a Covalent BTK and BCL2 Inhibitor in the United States: A Real-World Database Study. Clin. Lymphoma Myeloma Leuk. 2023, 23, 57–67. [Google Scholar] [CrossRef]
- Mato, A.R.; Sarraf Yazdy, M.; Hill, B.T.; Shadman, M.; Tuncer, H.; Winter, A.M.; Kennard, K.; Allan, J.N.; Ujjani, C.S.; Brander, D.M.; et al. Treatment Patterns and Outcomes of Patients with CLL Treated with Chemoimmuno- and Novel Agent-Based Therapy: A Multicenter Study. Blood 2018, 132, 4759. [Google Scholar] [CrossRef]
- Chanan-Khan, A.A.; Challagulla, S.; Kaushiva, A.; Miller, V.; Wang, Y.; Smith, H.; Yang, K. Impact of Real-World Treatment Sequencing Patterns on Time to Next Treatment among Patients with Chronic Lymphocytic Leukemia in the United States. Blood 2023, 142, 5143. [Google Scholar] [CrossRef]
- Davids, M.S.; Ambrose, J.; de Nigris, E.; Prescott, J.; Leng, S.; Farooqui, M.Z.H.; Gandra, S.R.; Zettler, C.M.; Fernandes, L.L.; Wang, C.K.; et al. Real-world characteristics, treatment patterns, and outcomes of patients with 2 or more LOTs for CLL/SLL in the United States. Blood Neoplasia 2025, 2, 100047. [Google Scholar] [CrossRef] [PubMed]
- Bruno, D.S.; Khanal, M.; Li, X.I.; Escalon, M.P.; Winfree, K.B.; Hess, L.M. Racial and Ethnic Characteristics and Outcomes of Patients Diagnosed with CLL/SLL in the USA. Acta Haematol. 2024, 148, 148–162. [Google Scholar] [CrossRef]
- Shenoy, P.J.; Malik, N.; Sinha, R.; Nooka, A.; Nastoupil, L.J.; Smith, M.; Flowers, C.R. Racial Differences in the Presentation and Outcomes of Chronic Lymphocytic Leukemia and Variants in the United States. Clin. Lymphoma Myeloma Leuk. 2011, 11, 498–506. [Google Scholar] [CrossRef]
- Coombs, C.C.; Lorenzo, F.; Brice, W.J.; Alessandra, F.; Lanasa, M.C. Chronic lymphocytic leukemia in African Americans. Leuk. Lymphoma 2012, 53, 2326–2329. [Google Scholar] [CrossRef]
- Falchi, L.; Keating, M.J.; Wang, X.; Coombs, C.C.; Lanasa, M.C.; Strom, S.; Wierda, W.G.; Ferrajoli, A. Clinical characteristics, response to therapy, and survival of African American patients diagnosed with chronic lymphocytic leukemia. Cancer 2013, 119, 3177–3185. [Google Scholar] [CrossRef]
| Characteristics—Analytic Cohort | Overall N = 2354 | Patients Included in Comparative Analyses N = 1711 a |
|---|---|---|
| Age, median (IQR) | 71 (64, 78) | 71 (63, 78) |
| Age subgroups, n (%) | ||
| ≤75 years | 1547 (65.7) | 1129 (66.0) |
| >75 years | 807 (34.3) | 582 (34.0) |
| Sex, n (%) | ||
| Male | 1496 (63.6) | 1083 (63.3) |
| Female | 858 (36.4) | 628 (36.7) |
| Combined Ethnicity and Race, n (%) | ||
| Non-Hispanic White | 1489 (63.3) | 1078 (63.0) |
| Non-Hispanic Black/African American | 163 (6.9) | 126 (7.4) |
| Hispanic | 107 (4.5) | 72 (4.2) |
| Other b | 595 (25.2) | 435 (25.4) |
| Socioeconomic status c, n (%) | ||
| 1 | 290 (12.3) | 216 (12.6) |
| 2 | 358 (15.2) | 259 (15.1) |
| 3 | 444 (18.9) | 325 (19.0) |
| 4 | 554 (23.5) | 417 (24.4) |
| 5 | 532 (22.6) | 371 (21.7) |
| Missing | 176 (7.5) | 123 (7.2) |
| Practice type, n (%) | ||
| Academic | 308 (13.1) | 215 (12.6) |
| Community | 2046 (86.9) | 1496 (87.4) |
| Disease subtype, n (%) | ||
| CLL | 2153 (91.5) | 1555 (90.9) |
| SLL | 201 (8.5) | 156 (9.1) |
| Year of initiation of first LoT, n (%) | ||
| 2016 | 491 (20.9) | 384 (22.4) |
| 2017 | 418 (17.8) | 339 (19.8) |
| 2018 | 368 (15.6) | 274 (16.0) |
| 2019 | 327 (13.9) | 220 (12.9) |
| 2020 | 274 (11.6) | 181 (10.6) |
| 2021 | 248 (10.5) | 154 (9.0) |
| 2022 | 164 (7.0) | 111 (6.5) |
| 2023 | 64 (2.7) | 48 (2.8) |
| Total Number of LoT received, n (%) | ||
| 2 | 1539 (65.4) | 1153 (67.4) |
| 3 | 512 (21.8) | 353 (20.6) |
| 4+ | 303 (12.9) | 205 (12.0) |
| Patients with data available, n (%) d | ||
| ECOG PS | n = 1670 | n =1224 |
| 0–1 | 1534 (91.9) | 1119 (91.4) |
| 2–4 | 136 (8.1) | 105 (8.6) |
| Rai Stage | n = 1410 | n = 1039 |
| 0–I | 904 (64.1) | 651 (62.7) |
| II–IV | 506 (35.9) | 388 (37.3) |
| IGHV | n = 884 | n = 653 |
| Mutated | 339 (38.3) | 247 (37.8) |
| Unmutated | 545 (61.7) | 406 (62.2) |
| del(11q) | n = 1836 | n = 1355 |
| No | 1512 (82.4) | 1122 (82.8) |
| Yes | 324 (17.6) | 233 (17.2) |
| del(13q) | n = 1852 | n = 1371 |
| No | 1015 (54.8) | 749 (54.6) |
| Yes | 837 (45.2) | 622 (45.4) |
| del(17p)/TP53 | n = 1848 | n = 1365 |
| No | 1631 (88.3) | 1217 (89.2) |
| Yes | 217 (11.7) | 148 (10.8) |
| Trisomy 12 | n = 1829 | n = 1355 |
| No | 1342 (73.4) | 995 (73.4) |
| Yes | 487 (26.6) | 360 (26.6) |
| Frequently Observed Treatment Sequence (n = 1711) | n (%) Estimated 4-Year Rate (95% CI) of OS | ||||
|---|---|---|---|---|---|
| Age at Initiation of First-Line Treatment, Years | Race/Ethnicity * | ||||
| ≤75 | >75 | Non-Hispanic White | Non-Hispanic Black | Hispanic | |
| 1129/1711 (66%) | 582/1711 (34%) | 1078/1711 (63%) | 126/1711 (7%) | 72/1711 (4%) | |
| CIT → cBTKi monotherapy | 274 (24.3%) 0.86 (0.81, 0.90) | 76 (13.1%) 0.77 (0.66, 0.86) | 228 (21.2%) 0.84 (0.79, 0.89) | 39 (31.0%) 0.86 (0.69, 0.94) | 15 (20.8%) 0.86 (0.54, 0.96) |
| Anti-CD20mab monotherapy → cBTKi monotherapy | 102 (9%) 0.88 (0.78, 0.93) | 99 (17.0%) 0.51 (0.39, 0.62) | 127 (11.8%) 0.72 (0.62, 0.80) | 8 (6.3%) 0.45 (0.11, 0.75) | 9 (12.5%) 0.86 (0.33, 0.98) |
| Anti-CD20mab monotherapy → BCL2i + Anti-CD20mab only | 129 (11.4%) 0.85 (0.69, 0.93) | 45 (7.7%) 0.71 (0.50, 0.84) | 103 (9.6%) 0.78 (0.63, 0.87) | 9 (7.1%) 1.00 (1.00, 1.00) | 12 (16.7%) NE (NE, NE) |
| cBTKi monotherapy → Anti-CD20mab monotherapy | 55 (4.9%) 0.86 (0.71, 0.94) | 53 (9.1%) 0.47 (0.30, 0.63) | 73 (6.8%) 0.65 (0.50, 0.76) | 6 (4.8%) 0.67 (0.05, 0.95) | 6 (8.3%) 0.83 (0.27, 0.98) |
| Anti-CD20mab monotherapy → CIT | 58 (5.1%) 0.88 (0.74, 0.94) | 41 (7.0%) 0.36 (0.20, 0.52) | 60 (5.6%) 0.68 (0.53, 0.78) | 6 (4.8%) 0.50 (0.06, 0.85) | NR |
| CIT → CIT | 59 (5.2%) 0.76 (0.63, 0.86) | 35 (6.0%) 0.51 (0.33, 0.66) | 48 (4.5%) 0.64 (0.48, 0.76) | 8 (6.3%) 0.75 (0.32, 0.93) | NR |
| cBTKi monotherapy → BCL2i + Anti-CD20mab only | 53 (4.7%) 0.93 (0.79, 0.98) | 30 (5.2%) 0.80 (0.57, 0.91) | 61 (5.7%) 0.90 (0.78, 0.96) | NR | NR |
| cBTKi monotherapy → CIT | 48 (4.3%) 0.84 (0.70, 0.92) | 34 (5.8%) 0.47 (0.28, 0.64) | 54 (5.0%) 0.65 (0.50, 0.77) | NR | NR |
| cBTKi monotherapy → BCL2i mono | 47 (4.2%) 0.74 (0.57, 0.85) | 26 (4.5%) 0.49 (0.25, 0.69) | 40 (3.7%) 0.58 (0.39, 0.74) | NR | NR |
| CIT → Other | 50 (4.4%) 0.64 (0.48, 0.76) | 20 (3.4%) 0.44 (0.20, 0.67) | 46 (4.3%) 0.62 (0.45, 0.75) | 6 (4.8%) 0.67 (0.20, 0.90) | NR |
| cBTKi monotherapy → cBTKi + Anti-CD20mab only | 50 (4.4%) 0.94 (0.78, 0.99) | 20 (3.4%) 0.35 (0.10, 0.63) | 39 (3.6%) 0.75 (0.54, 0.88) | 6 (4.8%) 0.50 (0.06, 0.85) | NR |
| CIT → BCL2i + Anti-CD20mab only | 51 (4.5%) 0.96 (0.85, 0.99) | 14 (2.4%) 0.92 (0.54, 0.99) | 45 (4.2%) 0.98 (0.84, 1.00) | NR | NR |
| cBTKi monotherapy → Other | 33 (2.9%) 0.70 (0.48, 0.84) | 31 (5.3%) 0.39 (0.21, 0.58) | 40 (3.7%) 0.62 (0.44, 0.76) | 10 (7.9%) 0.25 (0.04, 0.56) | NR |
| Other → Other | 37 (3.3%) 0.73 (0.54, 0.86) | 26 (4.5%) 0.51 (0.28, 0.71) | 44 (4.1%) 0.63 (0.46, 0.77) | NR | NR |
| CIT → Anti-CD20mab monotherapy | 48 (4.3%) 0.91 (0.78, 0.97) | 14 (2.4%) 0.71 (0.34, 0.90) | 37 (3.4%) 0.89 (0.72, 0.96) | NR | NR |
| cBTKi monotherapy → cBTKi + non-BCL2i/non-Anti-CD20mab | 35 (3.1%) 0.87 (0.68, 0.95) | 18 (3.1%) 0.52 (0.24, 0.75) | 33 (3.1%) 0.79 (0.58, 0.90) | 7 (5.6%) 0.80 (0.20, 0.97) | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rhodes, J.M.; Bhandari, N.R.; Khanal, M.; He, D.; Abhyankar, S.; Pagel, J.M.; Hess, L.M.; Skarbnik, A.Z. Overall Survival Associated with Real-World Treatment Sequences in Patients with CLL/SLL in the United States. Cancers 2025, 17, 2592. https://doi.org/10.3390/cancers17152592
Rhodes JM, Bhandari NR, Khanal M, He D, Abhyankar S, Pagel JM, Hess LM, Skarbnik AZ. Overall Survival Associated with Real-World Treatment Sequences in Patients with CLL/SLL in the United States. Cancers. 2025; 17(15):2592. https://doi.org/10.3390/cancers17152592
Chicago/Turabian StyleRhodes, Joanna M., Naleen Raj Bhandari, Manoj Khanal, Dan He, Sarang Abhyankar, John M. Pagel, Lisa M. Hess, and Alan Z. Skarbnik. 2025. "Overall Survival Associated with Real-World Treatment Sequences in Patients with CLL/SLL in the United States" Cancers 17, no. 15: 2592. https://doi.org/10.3390/cancers17152592
APA StyleRhodes, J. M., Bhandari, N. R., Khanal, M., He, D., Abhyankar, S., Pagel, J. M., Hess, L. M., & Skarbnik, A. Z. (2025). Overall Survival Associated with Real-World Treatment Sequences in Patients with CLL/SLL in the United States. Cancers, 17(15), 2592. https://doi.org/10.3390/cancers17152592






