Auxiliary Value of [18F]F-Fluorocholine PET/CT in Evaluating Post-Stereotactic Radiosurgery Recurrence of Lung Cancer Brain Metastases: A Comparative Analysis with Contrast-Enhanced MRI
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Brain CE-MRI Imaging and Analysis
2.3. [18F]F-FCH Synthesis
2.4. Brain PET/CT Imaging
2.5. PET/CT Image Analysis
2.6. Follow-Up and Outcome of Patients
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics
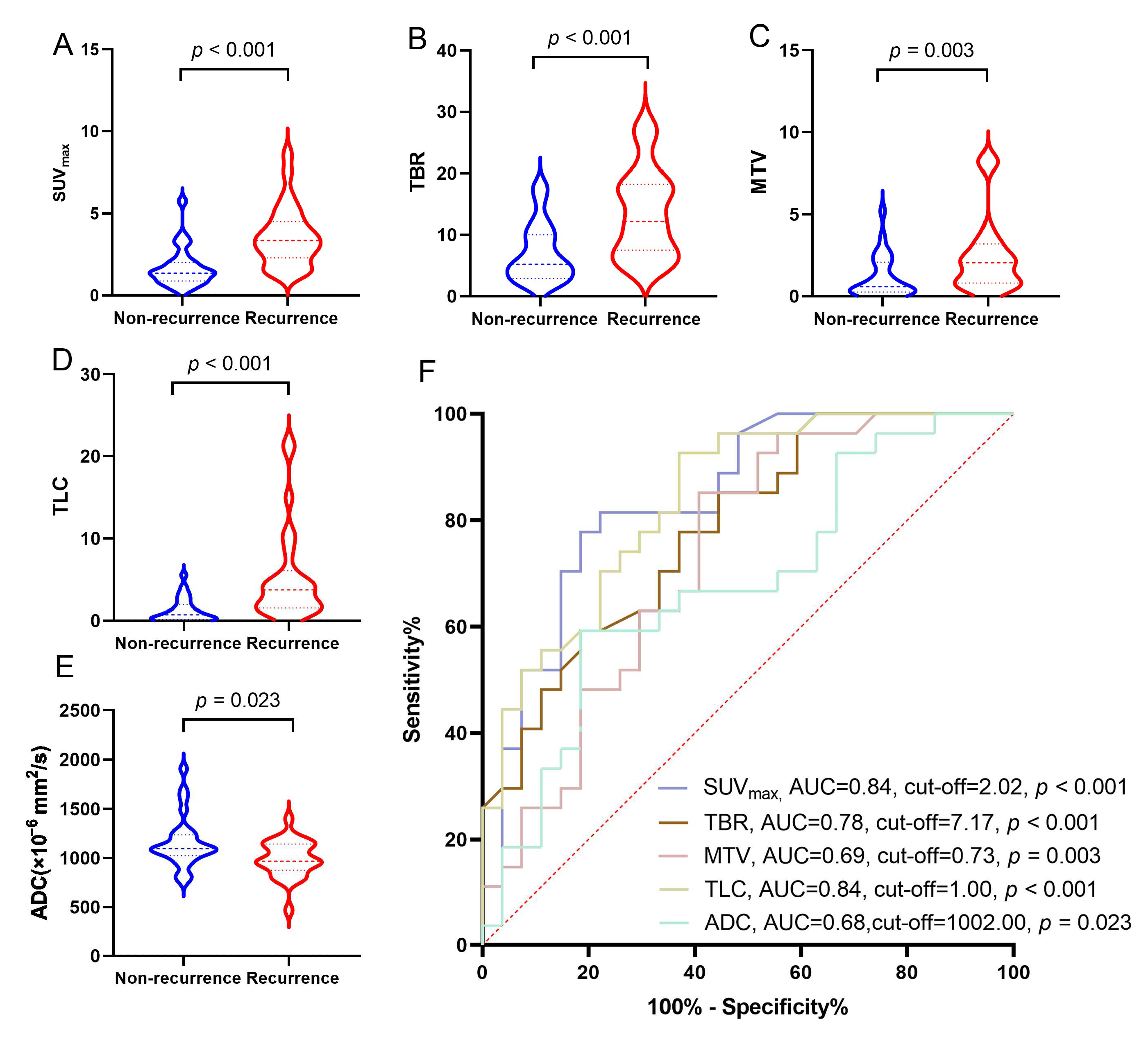
3.2. Diagnostic Performance of [18F]F-FCH PET/CT and CE-MRI in Detecting Recurrence of BM in LCBM After SRS
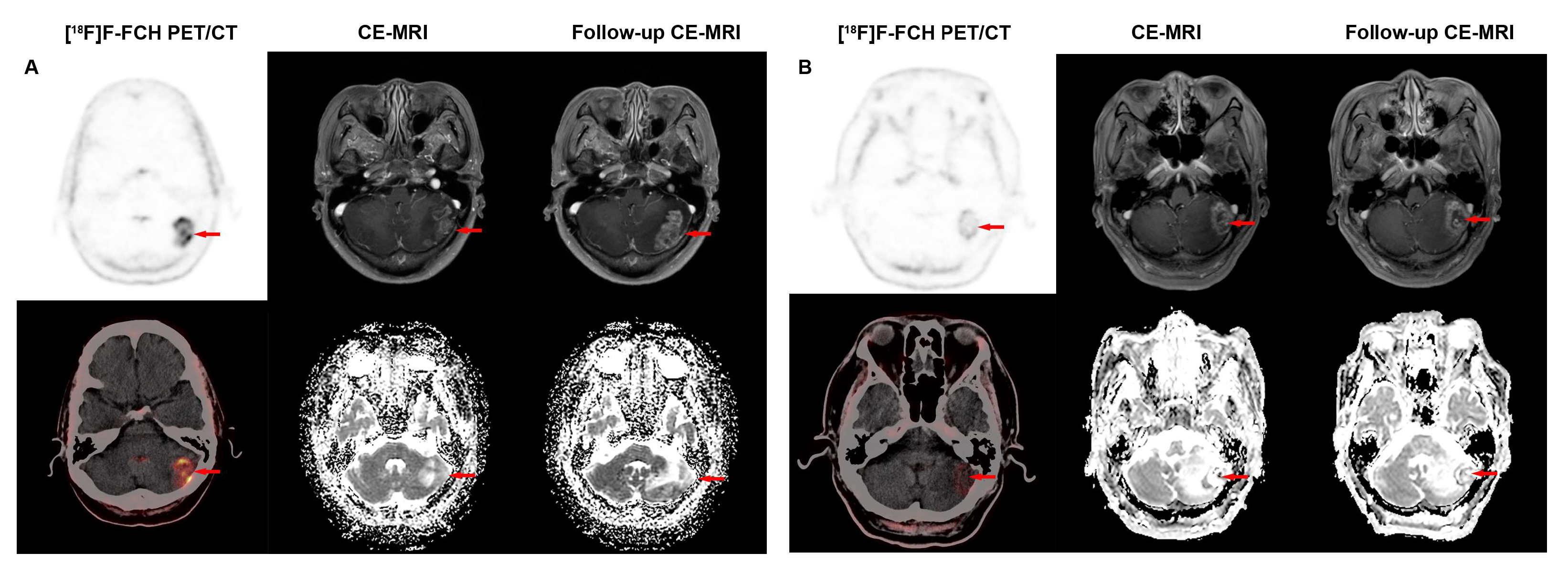
3.3. Comparison of Diagnostic Performance of [18F]F-FCH PET/CT and CE-MRI in Detecting Lesion Distributions and Sizes of Recurrent BM
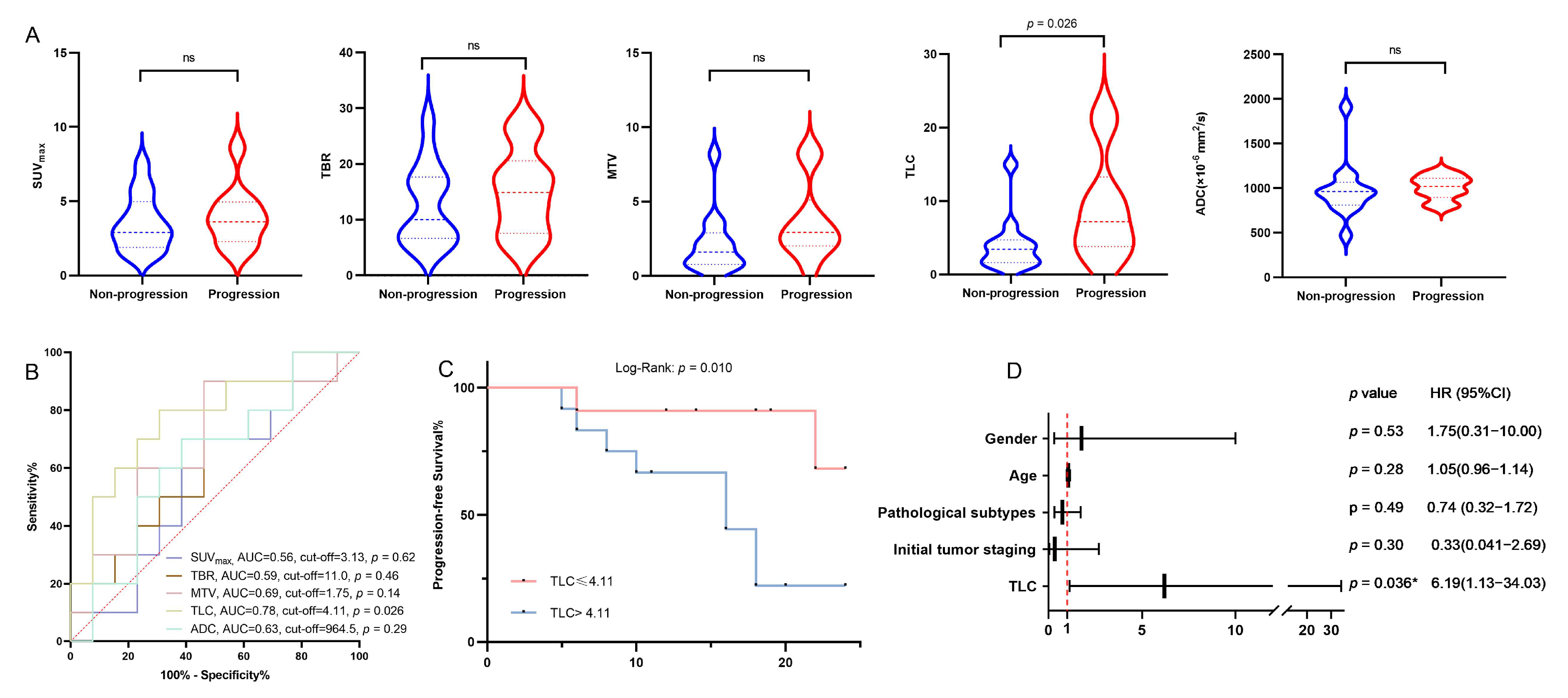
3.4. Correlation Between Clinical Features, Parameters of [18F]F-FCH PET/CT, CE-MRI, and iPFS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PET/CT | Positron emission tomography/computed tomography |
| FCH | Fluorocholine |
| CE | Contrast-enhanced |
| MRI | Magnetic resonance imaging |
| iPFS | Intracranial progression-free survival |
| BM | Brain metastasis |
| SRS | Stereotactic radiosurgery |
| BBB | Blood–brain barrier |
| RN | Radiation necrosis |
| LCBM | Lung cancer brain metastasis |
| SUVmax | Standardized uptake value |
| MTV | Metabolic tumor volume |
| TLC | Total lesion of choline uptake |
| ADC | Apparent diffusion coefficient |
| DWI | Diffusion-weighted imaging |
| ROC | Receiver operating characteristic |
| ROI | Region of interest |
| AUC | Area under the ROC curve |
References
- Achrol, A.S.; Rennert, R.C.; Anders, C.; Soffietti, R.; Ahluwalia, M.S.; Nayak, L.; Peters, S.; Arvold, N.D.; Harsh, G.R.; Steeg, P.S.; et al. Brain metastases. Nat. Rev. Dis. Primers 2019, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.; Jiang, K.; Rincon-Torroella, J.; Materi, J.; Azad, T.D.; OKamson, D.; Kleinberg, L.R.; Bettegowda, C. Epidemiological trends, prognostic factors, and survival outcomes of synchronous brain metastases from 2015 to 2019: A population-based study. Neurooncol. Adv. 2023, 5, vdad015. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.H.; Kotecha, R.; Chao, S.T.; Ahluwalia, M.S.; Sahgal, A.; Chang, E.L. Current approaches to the management of brain metastases. Nat. Rev. Clin. Oncol. 2020, 17, 279–299. [Google Scholar] [CrossRef] [PubMed]
- Nayak, L.; Lee, E.Q.; Wen, P.Y. Epidemiology of brain metastases. Curr. Oncol. Rep. 2012, 14, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Boire, A.; Brastianos, P.K.; Garzia, L.; Valiente, M. Brain metastasis. Nat. Rev. Cancer 2020, 20, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Nieder, C.; Grosu, A.L.; Gaspar, L.E. Stereotactic radiosurgery (SRS) for brain metastases: A systematic review. Radiat. Oncol. 2014, 9, 155. [Google Scholar] [CrossRef] [PubMed]
- Tsao, M.N.; Rades, D.; Wirth, A.; Lo, S.S.; Danielson, B.L.; Gaspar, L.E.; Sperduto, P.W.; Vogelbaum, M.A.; Radawski, J.D.; Wang, J.Z.; et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): An American Society for Radiation Oncology evidence-based guideline. Pract. Radiat. Oncol. 2012, 2, 210–225. [Google Scholar] [CrossRef] [PubMed]
- Chao, S.T.; De Salles, A.; Hayashi, M.; Levivier, M.; Ma, L.; Martinez, R.; Paddick, I.; Régis, J.; Ryu, S.; Slotman, B.J.; et al. Stereotactic Radiosurgery in the Management of Limited (1-4) Brain Metasteses: Systematic Review and International Stereotactic Radiosurgery Society Practice Guideline. Neurosurgery 2018, 83, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Boström, J.P.; Kinfe, T.; Meyer, A.; Pintea, B.; Gerlach, R.; Surber, G.; Lammering, G.; Hamm, K. Treatment of acromegaly patients with risk-adapted single or fractionated stereotactic high-precision radiotherapy: High local control and low toxicity in a pooled series. Strahlenther. Onkol. 2015, 191, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Pearce, J.B.; Hsu, F.-C.; Lanier, C.M.; Cramer, C.K.; Ruiz, J.; Lo, H.-W.; Xing, F.; Smith, M.; Li, W.; Whitlow, C.; et al. Five-year survivors from brain metastases treated with stereotactic radiosurgery: Biology, improving treatments, or just plain luck? Neurooncol. Pract. 2023, 10, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Narloch, J.L.; Farber, S.H.; Sammons, S.; McSherry, F.; EHerndon, J.; Hoang, J.K.; Yin, F.-F.; Sampson, J.H.; EFecci, P.; Blackwell, K.L.; et al. Biopsy of enlarging lesions after stereotactic radiosurgery for brain metastases frequently reveals radiation necrosis. Neuro Oncol. 2017, 19, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
- Chen, W. Clinical applications of PET in brain tumors. J. Nucl. Med. 2007, 48, 1468–1481. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, P.; Stoffels, G.; Ceccon, G.; Rapp, M.; Sabel, M.; Filss, C.P.; Kamp, M.A.; Stegmayr, C.; Neumaier, B.; Shah, N.J.; et al. Radiation injury vs. recurrent brain metastasis: Combining textural feature radiomics analysis and standard parameters may increase (18)F-FET PET accuracy without dynamic scans. Eur. Radiol. 2017, 27, 2916–2927. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Cowperthwaite, M.C.; Burnett, M.G.; Markey, M.K. Differentiating tumor recurrence from treatment necrosis: A review of neuro-oncologic imaging strategies. Neuro Oncol. 2013, 15, 515–534. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Moltoni, G.; Blandino, A.; Palizzi, S.; Romano, A.; de Rosa, G.; Palma, L.D.B.; Monopoli, C.; Guarnera, A.; Minniti, G.; et al. Radiosurgery for Brain Metastases: Challenges in Imaging Interpretation after Treatment. Cancers 2023, 15, 5092. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.K.; Kim, M.S.; Park, H.-J.; Park, N.H.; Park, S.I.; Lee, Y.S. Intracranial metastases: Spectrum of MR imaging findings. Acta Radiol. 2012, 53, 1173–1185. [Google Scholar] [CrossRef] [PubMed]
- Hirano, H.; Yokoyama, S.; Yunoue, S.; Yonezawa, H.; Yatsushiro, K.; Yoshioka, T.; Hanaya, R.; Tokimura, H.; Arita, K. MRI T2 hypointensity of metastatic brain tumors from gastric and colonic cancers. Int. J. Clin. Oncol. 2014, 19, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-Meyer, J.E.; Mabray, M.C.; Cha, S. Current Clinical Brain Tumor Imaging. Neurosurgery 2017, 81, 397–415. [Google Scholar] [CrossRef] [PubMed]
- Overcast, W.B.; Davis, K.M.; Ho, C.Y.; Hutchins, G.D.; Green, M.A.; Graner, B.D.; Veronesi, M.C. Advanced imaging techniques for neuro-oncologic tumor diagnosis, with an emphasis on PET-MRI imaging of malignant brain tumors. Curr. Oncol. Rep. 2021, 23, 34. [Google Scholar] [CrossRef] [PubMed]
- Kerkhof, M.; Ganeff, I.; Wiggenraad, R.G.J.; Nijeholt, G.J.L.À.; Hammer, S.; Taphoorn, M.J.B.; Dirven, L.; Vos, M.J. Clinical applicability of and changes in perfusion MR imaging in brain metastases after stereotactic radiotherapy. J. Neurooncol. 2018, 138, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Parvez, K.; Parvez, A.; Zadeh, G. The diagnosis and treatment of pseudoprogression, radiation necrosis and brain tumor recurrence. Int. J. Mol. Sci. 2014, 15, 11832–11846. [Google Scholar] [CrossRef] [PubMed]
- Cicone, F.; Minniti, G.; Romano, A.; Papa, A.; Scaringi, C.; Tavanti, F.; Bozzao, A.; Enrici, R.M.; Scopinaro, F. Accuracy of F-DOPA PET and perfusion-MRI for differentiating radionecrotic from progressive brain metastases after radiosurgery. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Lizarraga, K.J.; Allen-Auerbach, M.; Czernin, J.; DeSalles, A.A.; Yong, W.H.; Phelps, M.E.; Chen, W. (18)F-FDOPA PET for differentiating recurrent or progressive brain metastatic tumors from late or delayed radiation injury after radiation treatment. J. Nucl. Med. 2014, 55, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Terakawa, Y.; Tsuyuguchi, N.; Iwai, Y.; Yamanaka, K.; Higashiyama, S.; Takami, T.; Ohata, K. Diagnostic accuracy of 11C-methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy. J. Nucl. Med. 2008, 49, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Spaeth, N.; Wyss, M.T.; Weber, B.; Scheidegger, S.; Lutz, A.; Verwey, J.; Radovanovic, I.; Pahnke, J.; Wild, D.; Westera, G.; et al. Uptake of 18F-fluorocholine, 18F-fluoroethyl-L-tyrosine, and 18F-FDG in acute cerebral radiation injury in the rat: Implications for separation of radiation necrosis from tumor recurrence. J. Nucl. Med. 2004, 45, 1931–1938. [Google Scholar] [PubMed]
- Bell, J.B.; Jin, W.; Goryawala, M.Z.; Azzam, G.A.; Abramowitz, M.C.; Diwanji, T.; Ivan, M.E.; Eibl, M.d.P.G.P.; de la Fuente, M.I.; Mellon, E.A. Delineation of recurrent glioblastoma by whole brain spectroscopic magnetic resonance imaging. Radiat. Oncol. 2023, 18, 37. [Google Scholar] [CrossRef] [PubMed]
- Kapplinger, J.D.; Lima, L.M.F.; Packard, A.T.; Nathan, M.A.; Young, J.R.; Stish, B.J.; Hough, D.M. Safety and Efficacy of CT-Guided Percutaneous Biopsy of Suspicious Subcentimeter Pelvic and Retroperitoneal Lymph Nodes Detected by (11)C-Choline PET in Patients With Prostate Cancer. AJR Am. J. Roentgenol. 2023, 220, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Ghidaglia, J.; Laurent, V.; Sebagh, M.; Pascale, A.; Durand, E.; Golse, N.; Besson, F.L. Influence of key histological characteristics on 18F-fluorodeoxyglucose /18F-choline positron emission tomography positivity in hepatocellular carcinoma: A machine learning study. Front. Med. 2023, 10, 1087957. [Google Scholar] [CrossRef] [PubMed]
- Liberini, V.; Morand, G.B.; Rupp, N.J.; Orita, E.; Deandreis, D.; Däppen, M.B.; Hofbauer, M.; Maurer, A.; Husmann, L.; Mader, C.E.; et al. Histopathological Features of Parathyroid Adenoma and 18F-Choline Uptake in PET/MR of Primary Hyperparathyroidism. Clin. Nucl. Med. 2022, 47, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Grkovski, M.; Kohutek, Z.A.; Schöder, H.; Brennan, C.W.; Tabar, V.S.; Gutin, P.H.; Zhang, Z.; Young, R.J.; Beattie, B.J.; Zanzonico, P.B.; et al. (18)F-Fluorocholine PET uptake correlates with pathologic evidence of recurrent tumor after stereotactic radiosurgery for brain metastases. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1446–1457. [Google Scholar] [CrossRef] [PubMed]
- Vicente, A.M.G.; Pérez-Beteta, J.; Amo-Salas, M.; Bosque, J.J.; Noriega-Álvarez, E.; Castrejon, Á.M.S.; Pérez-García, V.M. A Head-to-Head Comparison of (18)F-Fluorocholine PET/CT and Conventional MRI as Predictors of Outcome in IDH Wild-Type High-Grade Gliomas. J. Clin. Med. 2022, 11, 6065. [Google Scholar] [CrossRef]
- DeGrado, T.R.; Baldwin, S.W.; Wang, S.; Orr, M.D.; Liao, R.P.; Friedman, H.S.; Reiman, R.; Price, D.T.; Coleman, R.E. Synthesis and evaluation of (18)F-labeled choline analogs as oncologic PET tracers. J. Nucl. Med. 2001, 42, 1805–1814. [Google Scholar] [PubMed]
- DeGrado, T.R.; EColeman, R.; Wang, S.; Baldwin, S.W.; Orr, M.D.; Robertson, C.N.; Polascik, T.J.; Price, D.T. Synthesis and evaluation of 18F-labeled choline as an oncologic tracer for positron emission tomography: Initial findings in prostate cancer. Cancer Res. 2001, 61, 110–117. [Google Scholar] [PubMed]
- Lin, N.U.; Lee, E.Q.; Aoyama, H.; Barani, I.J.; Barboriak, D.P.; Baumert, B.G.; Brown, P.D.; Camidge, D.R.; Chang, S.M.; Dancey, J.; et al. Response assessment criteria for brain metastases: Proposal from the RANO group. Lancet Oncol. 2015, 16, e270–e278. [Google Scholar] [CrossRef] [PubMed]
- Albert, N.L.; Galldiks, N.; Ellingson, B.M.; Bent, M.J.v.D.; Chang, S.M.; Cicone, F.; Koh, E.-S.; Law, I.; Le Rhun, E.; Mair, M.J.; et al. RANO criteria for response assessment of brain metastases based on amino acid PET imaging. Nat. Med. 2025, 31, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Dhermain, F.G.; Hau, P.; Lanfermann, H.; Jacobs, A.H.; van den Bent, M.J. Advanced MRI and PET imaging for assessment of treatment response in patients with gliomas. Lancet Neurol. 2010, 9, 906–920. [Google Scholar] [CrossRef] [PubMed]
- Higano, S.; Yun, X.; Kumabe, T.; Watanabe, M.; Mugikura, S.; Umetsu, A.; Sato, A.; Yamada, T.; Takahashi, S. Malignant astrocytic tumors: Clinical importance of apparent diffusion coefficient in prediction of grade and prognosis. Radiology 2006, 241, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Bozdag, M.; Er, A.; Cinkooglu, A. Histogram Analysis of ADC Maps for Differentiating Brain Metastases From Different Histological Types of Lung Cancers. Can. Assoc. Radiol. J. 2021, 72, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Hoefnagels, F.W.A.; Lagerwaard, F.J.; Sanchez, E.; Haasbeek, C.J.A.; Knol, D.L.; Slotman, B.J.; Vandertop, W.P. Radiological progression of cerebral metastases after radiosurgery: Assessment of perfusion MRI for differentiating between necrosis and recurrence. J. Neurol. 2009, 256, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Barajas, R.F.; Chang, J.S.; Sneed, P.K.; Segal, M.R.; McDermott, M.W.; Cha, S. Distinguishing recurrent intra-axial metastatic tumor from radiation necrosis following gamma knife radiosurgery using dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. AJNR Am. J. Neuroradiol. 2009, 30, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Mitsuya, K.; Nakasu, Y.; Horiguchi, S.; Harada, H.; Nishimura, T.; Bando, E.; Okawa, H.; Furukawa, Y.; Hirai, T.; Endo, M. Perfusion weighted magnetic resonance imaging to distinguish the recurrence of metastatic brain tumors from radiation necrosis after stereotactic radiosurgery. J. Neurooncol. 2010, 99, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Hainc, N.; Alsafwani, N.; Gao, A.; O’hAlloran, P.J.; Kongkham, P.; Zadeh, G.; Gutierrez, E.; Shultz, D.; Krings, T.; Alcaide-Leon, P. The centrally restricted diffusion sign on MRI for assessment of radiation necrosis in metastases treated with stereotactic radiosurgery. J. Neurooncol. 2021, 155, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Fischl, B.; Dale, A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. USA 2000, 97, 11050–11055. [Google Scholar] [CrossRef] [PubMed]
- Säll, C.; Spotorno, N.; Sundgren, P.C.; van Westen, D.; Westin, C.; Szczepankiewicz, F.; Nilsson, M. Diffusion MRI in the cortex of the brain: Reducing partial volume effects from CSF and white matter in the mean diffusivity using high b-values and spherical b-tensor encoding. Magn. Reson. Med. 2025, 94, 1166–1181. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, S.; Shiga, T.; Hattori, N.; Kubo, N.; Takei, T.; Katoh, N.; Sawamura, Y.; Nishijima, K.; Kuge, Y.; Tamaki, N. Semiquantitative analysis of C-11 methionine PET may distinguish brain tumor recurrence from radiation necrosis even in small lesions. Ann. Nucl. Med. 2011, 25, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Enslow, M.S.; Zollinger, L.V.; Morton, K.A.; Butterfield, R.I.; Kadrmas, D.J.; Christian, P.E.; Boucher, K.M.; Heilbrun, M.E.; Jensen, R.L.; Hoffman, J.M. Comparison of 18F-fluorodeoxyglucose and 18F-fluorothymidine PET in differentiating radiation necrosis from recurrent glioma. Clin. Nucl. Med. 2012, 37, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Rullmann, M.; Dukart, J.; Hoffmann, K.-T.; Luthardt, J.; Tiepolt, S.; Patt, M.; Gertz, H.-J.; Schroeter, M.L.; Seibyl, J.; Schulz-Schaeffer, W.J.; et al. Partial-Volume Effect Correction Improves Quantitative Analysis of 18F-Florbetaben beta-Amyloid PET Scans. J. Nucl. Med. 2016, 57, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhu, Y.; Bilgel, M.; Ashrafinia, S.; Lu, L.; Rahmim, A. Voxel-based partial volume correction of PET images via subtle MRI guided non-local means regularization. Phys. Med. 2021, 89, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Park, I.; Kim, S.; Pucciarelli, D.; Song, J.; Choi, J.M.; Lee, K.-H.; Kim, Y.H.; Jung, S.; Yoon, W.; Nakamura, J.L. Differentiating Radiation Necrosis from Brain Tumor Using Hyperpolarized Carbon-13 MR Metabolic Imaging. Mol. Imaging Biol. 2021, 23, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Vicente, A.M.G.; Pardo, F.J.P.; Amo-Salas, M.; Martín, M.V.; Menéndez, C.L.; Castrejón, Á.M.S.; Pérez-Beteta, J. Prognostic Potential of Postoperative 18F-Fluorocholine PET/CT in Patients with High-Grade Glioma. Clinical Validation of FuMeGA Postoperative PET Criteria. Clin. Nucl. Med. 2022, 47, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Caroli, P.; De Giorgi, U.; Scarpi, E.; Fantini, L.; Moretti, A.; Galassi, R.; Celli, M.; Conteduca, V.; Rossi, L.; Bianchi, E.; et al. Prognostic value of 18F-choline PET/CT metabolic parameters in patients with metastatic castration-resistant prostate cancer treated with abiraterone or enzalutamide. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Conteduca, V.; Scarpi, E.; Caroli, P.; Lolli, C.; Gurioli, G.; Brighi, N.; Poti, G.; Farolfi, A.; Altavilla, A.; Schepisi, G.; et al. Combining liquid biopsy and functional imaging analysis in metastatic castration-resistant prostate cancer helps predict treatment outcome. Mol. Oncol. 2022, 16, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Mollica, V.; Marchetti, A.; Fraccascia, N.; Nanni, C.; Tabacchi, E.; Malizia, C.; Argalia, G.; Rosellini, M.; Tassinari, E.; Paccapelo, A.; et al. A prospective study on the early evaluation of response to androgen receptor-targeted agents with (11)C-Choline, (68)Ga-PSMA, and (18)F-FACBC PET in metastatic castration-resistant prostate cancer: A single-center experience. ESMO Open 2024, 9, 103448. [Google Scholar] [CrossRef] [PubMed]
- Meignan, M. Quantitative FDG-PET: A new biomarker in PMBCL. Blood 2015, 126, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Fagerland, M.W. t-tests, non-parametric tests, and large studies—A paradox of statistical practice? BMC Med. Res. Methodol. 2012, 12, 78. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Values (n = 31) |
|---|---|
| Sex | |
| M | 17 (54.84%) |
| F | 14 (45.16%) |
| Age (year) | 54 (35–77) |
| <60 | 13 (41.94%) |
| ≥60 | 18 (58.06%) |
| Pathological subtypes | |
| Non-small-cell lung cancer | 25 (80.66%) |
| Small-cell lung cancer | 6 (19.35%) |
| Initial tumor staging (ITS) | |
| I | 1 (3.22%) |
| II | 6 (19.35%) |
| III | 13 (41.94%) |
| IV | 11 (35.48%) |
| Previous systemic treatment | |
| Targeted therapy | 5 (16.13%) |
| Chemotherapy | 12 (38.71%) |
| Chemotherapy + targeted therapy | 14 (45.16%) |
| Neural symptoms | |
| Yes | 16 (51.61%) |
| Headaches | 8 (25.80%) |
| Seizures | 4 (12.90%) |
| Nausea and vomiting | 4 (12.90%) |
| No | 15 (48.39%) |
| Method of confirmation | |
| Pathological results | 9 (29.03%) |
| Follow-up results | 22 (70.97%) |
| Parameter (n) | 18F-FCH PET/CT n, %, [95% CI] | CE-MRI n, %, [95% CI] | p-Value |
|---|---|---|---|
| Sensitivity (n = 27) | 24 (88.89%) [76.22~101.56] | 23 (85.19%) [70.86~99.51] | 0.338 |
| Specificity (n = 27) | 22 (81.48%) [65.82~97.14] | 11 (40.74%) [20.93~60.55] | 0.003 ** |
| Accuracy (n = 54) | 46 (85.19%) [75.40~94.97] | 34 (62.96%) [49.66~76.27] | 0.008 ** |
| Short-Diameter Lesions (cm) | No. of Lesions | [18F]F-FCH PET/CT | CE-MRI | p-Value |
|---|---|---|---|---|
| Accurate Lesions | Accurate Lesions | |||
| Total | 54 | 46 (85.19%) | 34 (62.96%) | 0.008 * |
| Φ ≥ 3 | 8 | 6 (75.00%) | 7 (87.50%) | 0.38 |
| 2 ≤ Φ <3 | 12 | 9 (75.00%) | 6 (50.00%) | 0.38 |
| 1 ≤ Φ <2 | 15 | 14 (93.33%) | 7 (46.67%) | 0.016 * |
| Φ < 1 | 19 | 17 (89.47%) | 14 (73.68%) | 0.42 |
| Variable | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Clinical parameters | ||||||
| Gender | 0.65 | 0.74–0.21 | 0.64 | 1.75 | 0.31–10.00 | 0.53 |
| Age | 1.03 | 0.97–1.09 | 0.38 | 1.05 | 0.96–1.14 | 0.28 |
| Pathological subtypes | 1.04 | 0.48–2.24 | 0.93 | 0.74 | 0.32–1.72 | 0.49 |
| Initial tumor staging | 2.89 | 0.36–22.78 | 0.32 | 0.33 | 0.04–2.69 | 0.30 |
| [18F]F-FCH PET/CT parameter | ||||||
| TLC | 5.50 | 1.13–26.76 | 0.035 * | 6.19 | 1.13–34.03 | 0.036 * |
| CE-MRI parameter | ||||||
| ADC | 0.27 | 0.55–8.48 | 2.16 | / | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Xu, M.; Yang, S.; Lin, L.; Wang, H.; Zhao, K.; Yang, H.; Su, X. Auxiliary Value of [18F]F-Fluorocholine PET/CT in Evaluating Post-Stereotactic Radiosurgery Recurrence of Lung Cancer Brain Metastases: A Comparative Analysis with Contrast-Enhanced MRI. Cancers 2025, 17, 2591. https://doi.org/10.3390/cancers17152591
Zhang Y, Xu M, Yang S, Lin L, Wang H, Zhao K, Yang H, Su X. Auxiliary Value of [18F]F-Fluorocholine PET/CT in Evaluating Post-Stereotactic Radiosurgery Recurrence of Lung Cancer Brain Metastases: A Comparative Analysis with Contrast-Enhanced MRI. Cancers. 2025; 17(15):2591. https://doi.org/10.3390/cancers17152591
Chicago/Turabian StyleZhang, Yafei, Mimi Xu, Shuye Yang, Lili Lin, Huatao Wang, Kui Zhao, Hong Yang, and Xinhui Su. 2025. "Auxiliary Value of [18F]F-Fluorocholine PET/CT in Evaluating Post-Stereotactic Radiosurgery Recurrence of Lung Cancer Brain Metastases: A Comparative Analysis with Contrast-Enhanced MRI" Cancers 17, no. 15: 2591. https://doi.org/10.3390/cancers17152591
APA StyleZhang, Y., Xu, M., Yang, S., Lin, L., Wang, H., Zhao, K., Yang, H., & Su, X. (2025). Auxiliary Value of [18F]F-Fluorocholine PET/CT in Evaluating Post-Stereotactic Radiosurgery Recurrence of Lung Cancer Brain Metastases: A Comparative Analysis with Contrast-Enhanced MRI. Cancers, 17(15), 2591. https://doi.org/10.3390/cancers17152591





