FLOT Versus CROSS—What Is the Optimal Therapeutic Approach for Locally Advanced Adenocarcinoma of the Esophagus and the Esophagogastric Junction?
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Methods
2.2. Treatment and Follow-Up Strategy
2.3. CROSS
2.4. FLOT
2.5. Surgery
2.6. Endpoints
2.7. Statistics
3. Results
3.1. Patient Baseline, Radiochemotherapy, Perioperative Chemotherapy, Surgical, and Histopathological Characteristics
3.2. Toxicity, Treatment Compliance, and Surgical Outcomes
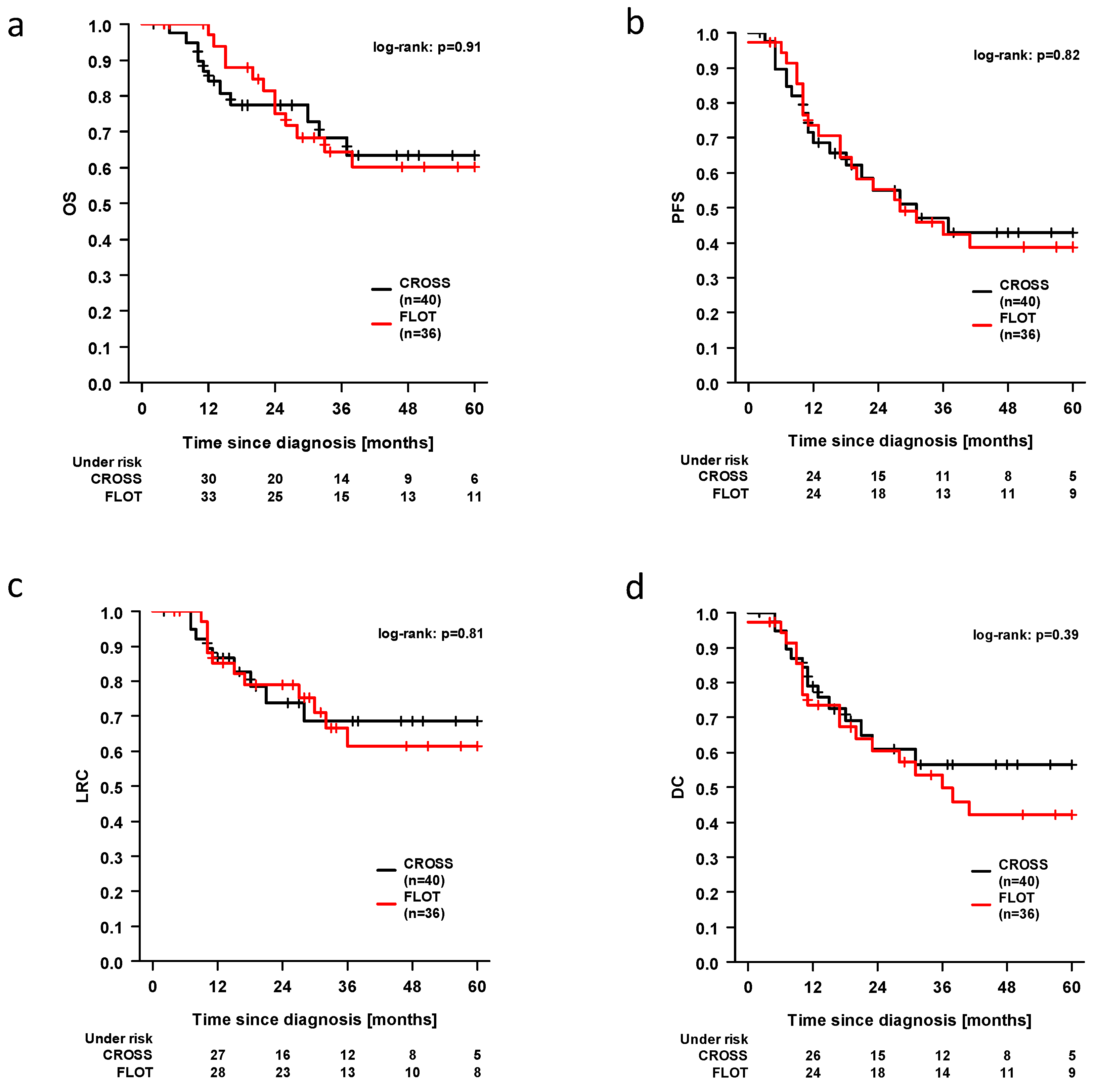
3.3. Survival Outcomes
3.4. Uni- and Multivariable Analysis for Factors Affecting OS, PFS, and LRC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EAC | adenocarcinoma of the esophagus |
| AEGJ | adenocarcinoma of the esophagogastric junction |
| LRC | loco-regional control |
| PFS | progression-free survival |
| OS | overall survival |
| DC | distant control |
| AC | adenocarcinoma |
| CRT | chemoradiation |
| CTCAE | Common Terminology Criteria for Adverse Events |
| 3DCRT | three-dimensional conformal radiotherapy |
| IMRT | intensity modulated radiotherapy |
| VMAT | volume modulated radiotherapy |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Bollschweiler, E.; Hölscher, A.H.; Markar, S.R.; Alakus, H.; Drebber, U.; Mönig, S.P.; Plum, P.S. Premature mortality for patients after completely resected early adenocarcinoma of the esophagus or stomach. Cancer Med. 2024, 13, e7223. [Google Scholar] [CrossRef] [PubMed]
- Obermannová, R.; Alsina, M.; Cervantes, A.; Leong, T.; Lordick, F.; Nilsson, M.; van Grieken, N.C.T.; Vogel, A.; Smyth, E.C. Oesophageal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 992–1004. [Google Scholar] [CrossRef] [PubMed]
- Langer, T. S3-Leitlinie Diagnostik und Therapie der Plattenepithelkarzinome und Adenokarzinome des Ösophagus. Available online: https://www.leitlinienprogramm-onkologie.de/fileadmin/user_upload/Downloads/Leitlinien/Oesophaguskarzinom/Version_4/LL_%C3%96sophaguskarzinom_Langversion_4.0.pdf (accessed on 20 March 2025).
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.C.; Enzler, T.; Farjah, F.; Gerdes, H.; et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc Netw. 2023, 21, 393–422. [Google Scholar] [CrossRef] [PubMed]
- van Hagen, P.; Hulshof, M.C.C.M.; van Lanschot, J.J.B.; Steyerberg, E.W.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; Richel, D.J.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 2012, 366, 2074–2084. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.-E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.-G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 2019, 393, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; van de Velde, C.J.H.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Schuhmacher, C.; Gretschel, S.; Lordick, F.; Reichardt, P.; Hohenberger, W.; Eisenberger, C.F.; Haag, C.; Mauer, M.E.; Hasan, B.; Welch, J.; et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. JCO 2010, 28, 5210–5218. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.V.; Preston, S.R.; O’Neill, B.; Lowery, M.A.; Baeksgaard, L.; Crosby, T.; Cunningham, M.; Cuffe, S.; Griffiths, G.O.; Parker, I.; et al. Trimodality therapy versus perioperative chemotherapy in the management of locally advanced adenocarcinoma of the oesophagus and oesophagogastric junction (Neo-AEGIS): An open-label, randomised, phase 3 trial. Lancet Gastroenterol. Hepatol. 2023, 8, 1015–1027. [Google Scholar] [CrossRef] [PubMed]
- Hoeppner, J.; Brunner, T.; Schmoor, C.; Bronsert, P.; Kulemann, B.; Claus, R.; Utzolino, S.; Izbicki, J.R.; Gockel, I.; Gerdes, B.; et al. Perioperative Chemotherapy or Preoperative Chemoradiotherapy in Esophageal Cancer. N. Engl. J. Med. 2025, 392, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Common Terminology Criteria for Adverse Events (CTCAE)|Protocol Development|CTEP, 2024. Available online: https://dctd.cancer.gov/research/ctep-trials/trial-development#ctcae-and-ctep-codes (accessed on 26 August 2024).
- Dröge, L.H.; Karras, P.J.; Guhlich, M.; Schirmer, M.A.; Ghadimi, M.; Rieken, S.; Conradi, L.-C.; Leu, M. Preoperative Radiochemotherapy in Esophageal Squamous Cell Cancer with 5-Fluorouracil/Cisplatin or Carboplatin/Paclitaxel: Treatment Practice over a 20-Year Period and Implications for the Individual Treatment Modalities. Cancers 2021, 13, 1834. [Google Scholar] [CrossRef] [PubMed]
- Mandard, A.-M.; Dalibard, F.; Mandard, J.-C.; Marnay, J.; Henry-Amar, M.; Petiot, J.-F.; Roussel, A.; Jacob, J.-H.; Segol, P.; Samama, G.; et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 1994, 73, 2680–2686. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.; Ziepert, M.; Scholz, M. KMWin—A convenient tool for graphical presentation of results from Kaplan-Meier survival time analysis. PLoS ONE 2012, 7, e38960. [Google Scholar] [CrossRef] [PubMed]
- Ychou, M.; Boige, V.; Pignon, J.-P.; Conroy, T.; Bouché, O.; Lebreton, G.; Ducourtieux, M.; Bedenne, L.; Fabre, J.-M.; Saint-Aubert, B.; et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: An FNCLCC and FFCD multicenter phase III trial. JCO 2011, 29, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Lindenmann, J.; Fediuk, M.; Fink-Neuboeck, N.; Porubsky, C.; Pichler, M.; Brcic, L.; Anegg, U.; Balic, M.; Dandachi, N.; Maier, A.; et al. Hazard Curves for Tumor Recurrence and Tumor-Related Death Following Esophagectomy for Esophageal Cancer. Cancers 2020, 12, 2066. [Google Scholar] [CrossRef] [PubMed]
- Elshaer, A.M.; Wijeyaratne, M.; Higgs, S.M.; Hornby, S.T.; Dwerryhouse, S.J. Peri-operative chemotherapy versus preoperative chemoradiotherapy in treatment of gastro-oesophageal junctional adenocarcinomas: A 10-year cohort study. Eur. J. Surg. Oncol. 2023, 49, 107016. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, R.; Bachmann, J.; Hungbauer, A.; Schmehl, J.; Sitzmann, G.; Königsrainer, A.; Ladurner, R. Impact of response evaluation for resectable esophageal adenocarcinoma—A retrospective cohort study. Int. J. Surg. 2014, 12, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.J.; Ajani, J.A.; Kuzdzal, J.; Zander, T.; van Cutsem, E.; Piessen, G.; Mendez, G.; Feliciano, J.; Motoyama, S.; Lièvre, A.; et al. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N. Engl. J. Med. 2021, 384, 1191–1203. [Google Scholar] [CrossRef] [PubMed]
| Baseline Patient and Disease Characteristics | |||
|---|---|---|---|
| Patient and Disease Characteristics, N (%) | FLOT (n = 36) | CROSS (n = 40) | p-Value * |
| Follow-up in months, median (min, max) | 32.0 (4–90) | 22 (2–101) | 0.08 |
| Age in years, median (min, max) | 61 (38–79) | 66 (31–77) | 0.11 |
| Female | 6 (16.7) | 6 (15.0) | 0.92 |
| Behavioral factors | |||
| Smoking w/o regular alcohol | 18 (50.0) | 21 (52.5) | 0.64 |
| Smoking and alcohol abuse | 3 (8.3) | 2 (5.0) | 0.60 |
| Neither smoking nor regular alcohol | 14 (38.9) | 12 (30.0) | 0.46 |
| Undetermined | 1 (2.8) | 5 (12.5) | |
| BMI | 0.33 | ||
| <25 | 12 (33.3) | 18 (45.0) | |
| ≥25 | 24 (66.7) | 22 (55.0) | |
| Tumor localization | <0.001 | ||
| AEG I | 10 (27.8) | 26 (65.0) | |
| AEG II | 13 (36.1) | 13 (32.5) | |
| AEG III | 13 (36.1) | 1 (2.5) | |
| Initial T category clinical/ultrasound | 0.02 | ||
| cT1 | 1 (2.8) | 2 (5.0) | |
| cT2 | 4 (11.1) | 11 (27.5) | |
| cT3 | 27 (75.0) | 27 (67.5) | |
| cT4 | 4 (11.1) | 0 | |
| Initial nodal status clinical/ultrasound | 0.43 | ||
| cN0 | 4 (10.1) | 7 (16.7) | |
| cN+ | 32 (88.9) | 33 (82.5) | |
| Radiotherapy | |||
| Dose administered, median (min, max) | n.a. | 41.4 (41.4–41.4) | |
| Dose planned, median (min, max) | n.a. | 41.4 (41.4–41.4) | |
| Incomplete | n.a. | 0 | |
| Technique | |||
| 3D | n.a. | 24 (60.0) | |
| IMRT/VMAT | n.a. | 16 (40.0) | |
| CCI | 0.11 | ||
| 0–3 | 13 (36.1) | 6 (15.0) | |
| 4–7 | 22 (61.1) | 34 (85.0) | |
| 8–10 | 1 (5.6) | 0 | |
| ECOG | 0.07 | ||
| 0 | 14 (36.1) | 22 (55.0) | |
| 1 | 22 (61.1) | 18 (45.0) | |
| 2 | 2 (5.6) | 0 | |
| Adjuvant immunotherapy | |||
| Yes | n.a. | 5 (12.5) | |
| Completion Rate and Acute Toxicity | |||
|---|---|---|---|
| Hematologic Toxicity, N (%) | FLOT (n = 36) | CROSS (n = 40) | p-Value * |
| Anemia | |||
| Anemia ≥ III° | 4 (11.1) | 1 (2.5) | 0.13 |
| 0 | 0 | 13 (32.5) | <0.001 |
| I° | 18 (50.0) | 24 (60.0) | |
| II° | 14 (38.8) | 2 (5.0) | |
| III° | 4 (11.1) | 1 (2.5) | |
| IV° | 4 (11.1) | 0 | |
| Leukopenia | |||
| Leukopenia ≥ III° | 3 (7.9) | 10 (25.0) | 0.05 |
| 0 | 27 (75.0) | 13 (32.5) | 0.003 |
| I° | 2 (5.6) | 9 (22.5) | |
| II° | 4 (11.1) | 8 (20.0) | |
| III° | 3 (8.3) | 10 (25.0) | |
| IV° | 0 | 0 | |
| Thrombopenia | |||
| Thrombopenia ≥ III° | 0 | 0 | 0.59 |
| 0 | 22 (61.1) | 20 (50.0) | 0.49 |
| I° | 13 (36.1) | 17 (42.5) | |
| II° | 1 (2.8) | 3 (7.5) | |
| III° | 0 | 0 | |
| IV° | 0 | 0 | |
| Chemotherapy, N (%) | |||
| Incomplete | 18 (50.0) | 7 (17.5) | 0.003 |
| Acute non-hematologic toxicity | |||
| Fatigue ≥ II° | 2 (5.6) | 3 (7.5) | 0.27 |
| Nausea ≥ II° | 3 (8.3) | 2 (5.0) | 0.67 |
| Odynophagia ≥ II° | 1 (2.8) | 5 (12.5) | <0.001 |
| Post-Surgical Tumor Status and Outcome | |||
|---|---|---|---|
| Surgical Outcome, N (%) | FLOT (n = 36) | CROSS (n = 40) | p-Value * |
| Pathological complete response | |||
| Yes | 5 (13.9) | 7 (17.5) | 0.53 |
| Tumor regression grade | 0.08 | ||
| 1 | 13 (36.1) | 20 (50.0) | |
| 2 | 11 (30.6) | 11 (27.5) | |
| 3 | 9 (25.0) | 4 (10.0) | |
| Undetermined | 3 (8.3) | 5 (12.5) | |
| Post-surgery T category | 0.65 | ||
| ypT0 | 8 (22.2) | 7 (17.5) | |
| ypT1 | 2 (5.6) | 5 (12.5) | |
| ypT2 | 5 (13.9) | 5 (12.5) | |
| ypT3 | 13 (36.1) | 21 (52.5) | |
| ypT4 | 7 (19.4) | 0 | |
| Undetermined | 1 (2.8) | 2 (5.0) | |
| Post-surgery nodal status + | 0.64 | ||
| ypN0 | 21 (58.3) | 24 (60.0) | |
| ypN+ | 15 (41.7) | 14 (35.0) | |
| Pathological lymph node response + | 0.99 | ||
| Yes | 19 (52.8) | 20 (50.0) | |
| No | 17 (47.2) | 18 (45.0) | |
| Resection status + | 0.33 | ||
| R0 | 36 (100) | 37 (92.5) | |
| R1 | 0 | 1 (2.5) | |
| Anastomotic leakage | |||
| Yes | 4 (10.0) | 1 (2.6) | 0.13 |
| Death within 30 days post-surgery | 1.00 | ||
| Yes | 0 | 0 | |
| Hospital stay surgery | 0.52 | ||
| Median (min, max) | 15 (9, 94) | 14 (9, 76) | |
| Peritoneal carcinomatosis during follow-up | |||
| Yes | 7 (18.4) | 2 (5.0) | 0.10 |
| Operation details | <0.001 | ||
| Esophagostomy | 3 (8.6) | 35 (92.1) | |
| Extended Gastrectomy | 32 (91.4) | 3 (7.9) | |
| Clavien-Dindo | 0.31 | ||
| 3 or higher | 6 (17.1) | 3 (7.9) | |
| Variable | PFS | OS | LRC | |||
|---|---|---|---|---|---|---|
| Hazard Ratio | p Value | Hazard Ratio | p Value | Hazard Ratio | p Value | |
| (95% CI) | (95% CI) | (95% CI) | ||||
| Age (per year) | 1.02 (0.99–1.05) | 0.25 | 1.01 (0.96–1.05) | 0.79 | 0.99 (0.95–1.03) | 0.63 |
| Sex | ||||||
| Female (12) vs. male (64) | 0.62 (0.24–1.58) | 0.32 | 0.43 (0.10–1.82) | 0.25 | 1.17 (0.39–3.52) | 0.78 |
| ECOG | ||||||
| 0 (34) vs. 1–2 (41) | 0.89 (0.47–1.71) | 0.73 | 0.64 (0.28–1.47) | 0.29 | 0.49 (0.20–1.19) | 0.12 |
| BMI | ||||||
| ≥25 (46) vs. <25 (30) | 0.54 (0.29–1.02) | 0.06 | 0.72 (0.31–1.66) | 0.44 | 0.44 (0.18–1.07) | 0.07 |
| Grading | ||||||
| G3 (20) vs. G1–G2 (49) | 2.13 (1.08–4.21) | 0.03 | 3.27 (1.38–7.69) | 0.007 | 1.46 (0.55–3.84) | 0.45 |
| Charlson Index | ||||||
| >4 (36) vs. ≤4 (40) | 1.01 (0.54–1.88) | 0.97 | 1.02 (0.45–2.34) | 0.95 | 0.41 (0.15–1.13) | 0.09 |
| Pathological complete response | ||||||
| Yes (12) vs. no (62) | 0.20 (0.05–0.83) | 0.03 | 0.42 (0.10–1.78) | 0.24 | 0.22 (0.03–1.61) | 0.21 |
| Post-surgery TNM | ||||||
| ypT3–4 (41) vs. 1–2 (33) | 2.31 (1.18–4.50) | 0.01 | 1.30 (0.57–2.96) | 0.54 | 4.22 (1.41–12.68) | 0.01 |
| ypN+ (29) vs. ypN0 (45) | 5.65 (2.89–11.03) | <0.001 | 5.18 (2.11–12.68) | <0.001 | 3.73 (1.51–9.24) | 0.004 |
| Variable | PFS | OS | LRC | |||
|---|---|---|---|---|---|---|
| Hazard Ratio | p Value | Hazard Ratio | p Value | Hazard Ratio | p Value | |
| (95% CI) | (95% CI) | (95% CI) | ||||
| Grading | ||||||
| G3 (20) vs. G1–G2 (49) | 1.51 (0.75–3.05) | 0.25 | 2.53 (1.05–6.05) | 0.04 | n.a. | |
| Pathological complete response | ||||||
| Yes (12) vs. no (62) | 0.53 (0.11–2.63) | 0.44 | n.a. | n.a. | ||
| Post-surgery TNM | ||||||
| ypT3–4 (41) vs. 1–2 (33) | 1.55 (0.73–3.33) | 0.26 | n.a. | 3.19 (1.03–9.89) | 0.04 | |
| ypN+ (29) vs. ypN0 (45) | 5.42 (2.70–10.87) | <0.001 | 4.19 (1.67–10.51) | 0.002 | 2.76 (1.01–7.01) | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leu, M.; Mahler, H.; Reinecke, J.; König, U.M.; Dröge, L.H.; Guhlich, M.; Steuber, B.; Grade, M.; Ghadimi, M.; Ellenrieder, V.; et al. FLOT Versus CROSS—What Is the Optimal Therapeutic Approach for Locally Advanced Adenocarcinoma of the Esophagus and the Esophagogastric Junction? Cancers 2025, 17, 2587. https://doi.org/10.3390/cancers17152587
Leu M, Mahler H, Reinecke J, König UM, Dröge LH, Guhlich M, Steuber B, Grade M, Ghadimi M, Ellenrieder V, et al. FLOT Versus CROSS—What Is the Optimal Therapeutic Approach for Locally Advanced Adenocarcinoma of the Esophagus and the Esophagogastric Junction? Cancers. 2025; 17(15):2587. https://doi.org/10.3390/cancers17152587
Chicago/Turabian StyleLeu, Martin, Hannes Mahler, Johanna Reinecke, Ute Margarethe König, Leif Hendrik Dröge, Manuel Guhlich, Benjamin Steuber, Marian Grade, Michael Ghadimi, Volker Ellenrieder, and et al. 2025. "FLOT Versus CROSS—What Is the Optimal Therapeutic Approach for Locally Advanced Adenocarcinoma of the Esophagus and the Esophagogastric Junction?" Cancers 17, no. 15: 2587. https://doi.org/10.3390/cancers17152587
APA StyleLeu, M., Mahler, H., Reinecke, J., König, U. M., Dröge, L. H., Guhlich, M., Steuber, B., Grade, M., Ghadimi, M., Ellenrieder, V., Rieken, S., & König, A. O. (2025). FLOT Versus CROSS—What Is the Optimal Therapeutic Approach for Locally Advanced Adenocarcinoma of the Esophagus and the Esophagogastric Junction? Cancers, 17(15), 2587. https://doi.org/10.3390/cancers17152587







