An Overview of the Treatment Strategy of Esophagogastric Junction Cancer
Simple Summary
Abstract
1. Introductions
2. Surgical Treatment
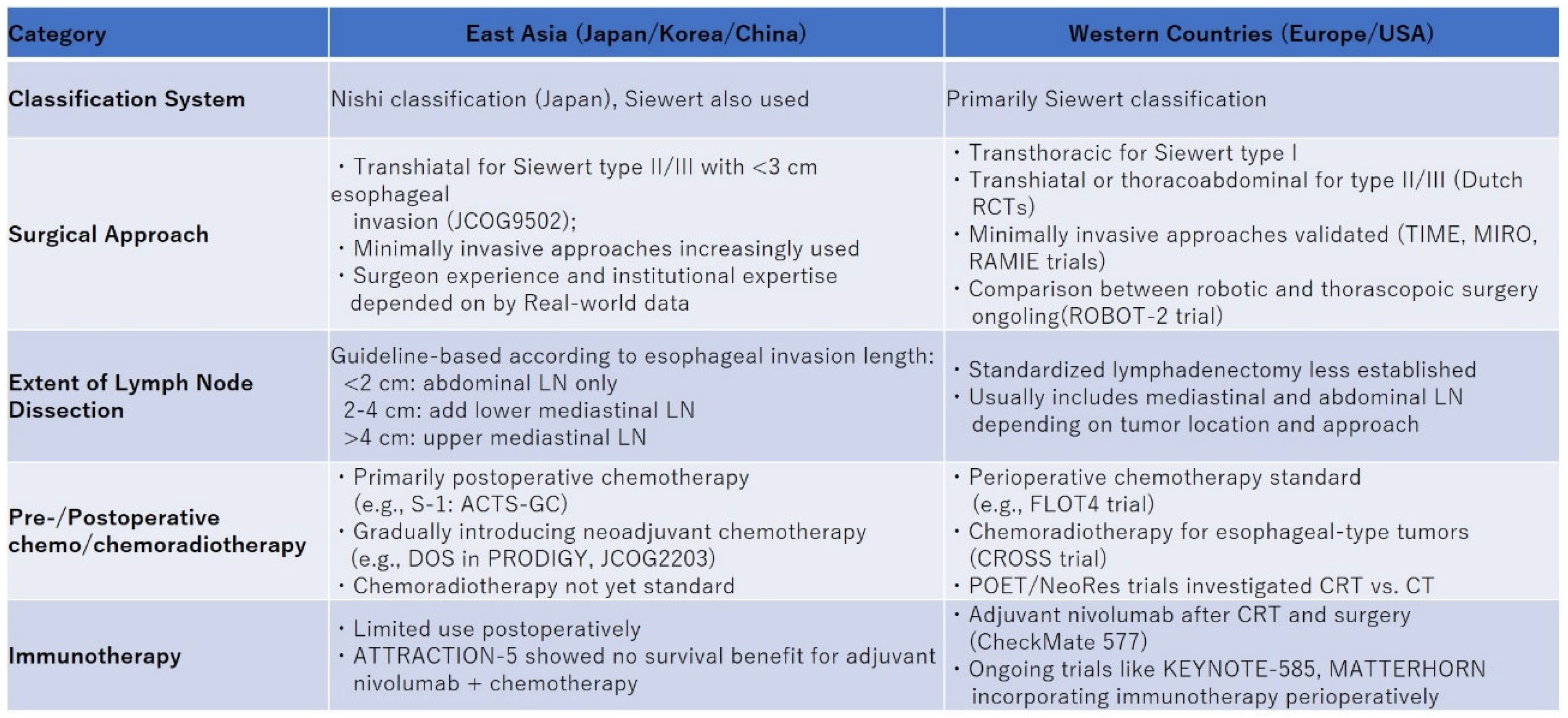
2.1. Surgical Approach
2.2. Optimal Extent of Lymph Node Dissection
3. Pre- and Postoperative Treatment
3.1. Pre- and Postoperative Chemotherapy
3.2. Pre- and Postoperative Chemoradiotherapy
3.3. Pre- and Postoperative Immunotherapy
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mariette, C.; Piessen, G.; Briez, N.; Gronnier, C.; Triboulet, J.P. Oesophagogastric junction adenocarcinoma: Which therapeutic approach? Lancet Oncol. 2011, 12, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Siewert, J.R.; Holscher, A.H.; Becker, K.; Gossner, W. Cardia cancer: Attempt at a therapeutically relevant classification. Chirurg 1987, 58, 25–32. [Google Scholar] [PubMed]
- Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer 2023, 26, 1–25. [Google Scholar] [CrossRef]
- Sakuramoto, S.; Sasako, M.; Yamaguchi, T.; Kinoshita, T.; Fujii, M.; Nashimoto, A.; Furukawa, H.; Nakajima, T.; Ohashi, Y.; Imamura, H.; et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N. Engl. J. Med. 2007, 357, 1810–1820. [Google Scholar] [CrossRef]
- Yoshida, K.; Kodera, Y.; Kochi, M.; Ichikawa, W.; Kakeji, Y.; Sano, T.; Nagao, N.; Takahashi, M.; Takagane, A.; Watanabe, T.; et al. Addition of Docetaxel to Oral Fluoropyrimidine Improves Efficacy in Patients With Stage III Gastric Cancer: Interim Analysis of JACCRO GC-07, a Randomized Controlled Trial. J. Clin. Oncol. 2019, 37, 1296–1304. [Google Scholar] [CrossRef]
- Bang, Y.J.; Kim, Y.W.; Yang, H.K.; Chung, H.C.; Park, Y.K.; Lee, K.H.; Lee, K.W.; Kim, Y.H.; Noh, S.I.; Cho, J.Y.; et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): A phase 3 open-label, randomised controlled trial. Lancet 2012, 379, 315–321. [Google Scholar] [CrossRef]
- Al-Batran, S.E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 2019, 393, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- van Hagen, P.; Hulshof, M.C.; van Lanschot, J.J.; Steyerberg, E.W.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.; Richel, D.J.; Nieuwenhuijzen, G.A.; Hospers, G.A.; Bonenkamp, J.J.; et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 2012, 366, 2074–2084. [Google Scholar] [CrossRef]
- Hulscher, J.B.; van Sandick, J.W.; de Boer, A.G.; Wijnhoven, B.P.; Tijssen, J.G.; Fockens, P.; Stalmeier, P.F.; ten Kate, F.J.; van Dekken, H.; Obertop, H.; et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N. Engl. J. Med. 2002, 347, 1662–1669. [Google Scholar] [CrossRef]
- Sasako, M.; Sano, T.; Yamamoto, S.; Sairenji, M.; Arai, K.; Kinoshita, T.; Nashimoto, A.; Hiratsuka, M.; Japan Clinical Oncology, G. Left thoracoabdominal approach versus abdominal-transhiatal approach for gastric cancer of the cardia or subcardia: A randomised controlled trial. Lancet Oncol. 2006, 7, 644–651. [Google Scholar] [CrossRef]
- Mariette, C.; Markar, S.R.; Dabakuyo-Yonli, T.S.; Meunier, B.; Pezet, D.; Collet, D.; D’Journo, X.B.; Brigand, C.; Perniceni, T.; Carrere, N.; et al. Hybrid Minimally Invasive Esophagectomy for Esophageal Cancer. N. Engl. J. Med. 2019, 380, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Straatman, J.; van der Wielen, N.; Cuesta, M.A.; Daams, F.; Roig Garcia, J.; Bonavina, L.; Rosman, C.; van Berge Henegouwen, M.I.; Gisbertz, S.S.; van der Peet, D.L. Minimally Invasive Versus Open Esophageal Resection: Three-year Follow-up of the Previously Reported Randomized Controlled Trial: The TIME Trial. Ann. Surg. 2017, 266, 232–236. [Google Scholar] [CrossRef] [PubMed]
- van der Sluis, P.C.; van der Horst, S.; May, A.M.; Schippers, C.; Brosens, L.A.A.; Joore, H.C.A.; Kroese, C.C.; Haj Mohammad, N.; Mook, S.; Vleggaar, F.P.; et al. Robot-assisted Minimally Invasive Thoracolaparoscopic Esophagectomy Versus Open Transthoracic Esophagectomy for Resectable Esophageal Cancer: A Randomized Controlled Trial. Ann. Surg. 2019, 269, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Tagkalos, E.; van der Sluis, P.C.; Berlth, F.; Poplawski, A.; Hadzijusufovic, E.; Lang, H.; van Berge Henegouwen, M.I.; Gisbertz, S.S.; Muller-Stich, B.P.; Ruurda, J.P.; et al. Robot-assisted minimally invasive thoraco-laparoscopic esophagectomy versus minimally invasive esophagectomy for resectable esophageal adenocarcinoma, a randomized controlled trial (ROBOT-2 trial). BMC Cancer 2021, 21, 1060. [Google Scholar] [CrossRef]
- Mine, S.; Kurokawa, Y.; Takeuchi, H.; Terashima, M.; Yasuda, T.; Yoshida, K.; Yabusaki, H.; Shirakawa, Y.; Fujitani, K.; Sano, T.; et al. Postoperative complications after a transthoracic esophagectomy or a transhiatal gastrectomy in patients with esophagogastric junctional cancers: A prospective nationwide multicenter study. Gastric Cancer 2022, 25, 430–437. [Google Scholar] [CrossRef]
- Rudiger Siewert, J.; Feith, M.; Werner, M.; Stein, H.J. Adenocarcinoma of the esophagogastric junction: Results of surgical therapy based on anatomical/topographic classification in 1,002 consecutive patients. Ann. Surg. 2000, 232, 353–361. [Google Scholar] [CrossRef]
- Pedrazzani, C.; de Manzoni, G.; Marrelli, D.; Giacopuzzi, S.; Corso, G.; Minicozzi, A.M.; Rampone, B.; Roviello, F. Lymph node involvement in advanced gastroesophageal junction adenocarcinoma. J. Thorac. Cardiovasc. Surg. 2007, 134, 378–385. [Google Scholar] [CrossRef]
- Kurokawa, Y.; Hiki, N.; Yoshikawa, T.; Kishi, K.; Ito, Y.; Ohi, M.; Wada, N.; Takiguchi, S.; Mine, S.; Hasegawa, S.; et al. Mediastinal lymph node metastasis and recurrence in adenocarcinoma of the esophagogastric junction. Surgery 2015, 157, 551–555. [Google Scholar] [CrossRef]
- Yamashita, H.; Seto, Y.; Sano, T.; Makuuchi, H.; Ando, N.; Sasako, M.; Japanese Gastric Cancer, A.; the Japan Esophageal, S. Results of a nation-wide retrospective study of lymphadenectomy for esophagogastric junction carcinoma. Gastric Cancer 2017, 20, 69–83. [Google Scholar] [CrossRef]
- Kurokawa, Y.; Takeuchi, H.; Doki, Y.; Mine, S.; Terashima, M.; Yasuda, T.; Yoshida, K.; Daiko, H.; Sakuramoto, S.; Yoshikawa, T.; et al. Mapping of Lymph Node Metastasis From Esophagogastric Junction Tumors: A Prospective Nationwide Multicenter Study. Ann. Surg. 2021, 274, 120–127. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Takeuchi, H.; Hasegawa, S.; Nozaki, I.; Kishi, K.; Ito, S.; Ohi, M.; Mine, S.; Hara, J.; Matsuda, T.; et al. Theoretical therapeutic impact of lymph node dissection on adenocarcinoma and squamous cell carcinoma of the esophagogastric junction. Gastric Cancer 2016, 19, 143–149. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, H.; Li, Z.; Xue, Y.; Wang, Y.; Zhou, Z.; Yu, J.; Bu, Z.; Chen, L.; Du, Y.; et al. Perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy (RESOLVE): An open-label, superiority and non-inferiority, phase 3 randomised controlled trial. Lancet Oncol. 2021, 22, 1081–1092. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.K.; Yook, J.H.; Park, Y.K.; Lee, J.S.; Kim, Y.W.; Kim, J.Y.; Ryu, M.H.; Rha, S.Y.; Chung, I.J.; Kim, I.H.; et al. PRODIGY: A Phase III Study of Neoadjuvant Docetaxel, Oxaliplatin, and S-1 Plus Surgery and Adjuvant S-1 Versus Surgery and Adjuvant S-1 for Resectable Advanced Gastric Cancer. J. Clin. Oncol. 2021, 39, 2903–2913. [Google Scholar] [CrossRef] [PubMed]
- Kita, R.; Yanagimoto, Y.; Imazeki, H.; Booka, E.; Tsushima, T.; Mizusawa, J.; Sasaki, K.; Fukuda, H.; Kurokawa, Y.; Takeuchi, H.; et al. Protocol digest of a randomized controlled adaptive Phase II/III trial of neoadjuvant chemotherapy for Japanese patients with oesophagogastric junction adenocarcinoma: Japan Clinical Oncology Group Study JCOG2203 (NEO-JPEG). Jpn. J. Clin. Oncol. 2024, 54, 206–211. [Google Scholar] [CrossRef]
- Stahl, M.; Walz, M.K.; Stuschke, M.; Lehmann, N.; Meyer, H.J.; Riera-Knorrenschild, J.; Langer, P.; Engenhart-Cabillic, R.; Bitzer, M.; Konigsrainer, A.; et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J. Clin. Oncol. 2009, 27, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Klevebro, F.; Alexandersson von Dobeln, G.; Wang, N.; Johnsen, G.; Jacobsen, A.B.; Friesland, S.; Hatlevoll, I.; Glenjen, N.I.; Lind, P.; Tsai, J.A.; et al. A randomized clinical trial of neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the oesophagus or gastro-oesophageal junction. Ann. Oncol. 2016, 27, 660–667. [Google Scholar] [CrossRef]
- Kelly, R.J.; Ajani, J.A.; Kuzdzal, J.; Zander, T.; Van Cutsem, E.; Piessen, G.; Mendez, G.; Feliciano, J.; Motoyama, S.; Lievre, A.; et al. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N. Engl. J. Med. 2021, 384, 1191–1203. [Google Scholar] [CrossRef]
- Kang, Y.K.; Terashima, M.; Kim, Y.W.; Boku, N.; Chung, H.C.; Chen, J.S.; Ji, J.; Yeh, T.S.; Chen, L.T.; Ryu, M.H.; et al. Adjuvant nivolumab plus chemotherapy versus placebo plus chemotherapy for stage III gastric or gastro-oesophageal junction cancer after gastrectomy with D2 or more extensive lymph-node dissection (ATTRACTION-5): A randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Gastroenterol. Hepatol. 2024, 9, 705–717. [Google Scholar] [CrossRef]
- Shitara, K.; Rha, S.Y.; Wyrwicz, L.S.; Oshima, T.; Karaseva, N.; Osipov, M.; Yasui, H.; Yabusaki, H.; Afanasyev, S.; Park, Y.K.; et al. Neoadjuvant and adjuvant pembrolizumab plus chemotherapy in locally advanced gastric or gastro-oesophageal cancer (KEYNOTE-585): An interim analysis of the multicentre, double-blind, randomised phase 3 study. Lancet Oncol. 2024, 25, 212–224. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Van Cutsem, E.; Muro, K.; Wainberg, Z.; Al-Batran, S.E.; Hyung, W.J.; Molena, D.; Marcovitz, M.; Ruscica, D.; Robbins, S.H.; et al. MATTERHORN: Phase III study of durvalumab plus FLOT chemotherapy in resectable gastric/gastroesophageal junction cancer. Future Oncol. 2022, 18, 2465–2473. [Google Scholar] [CrossRef]
- Joshi, S.S.; Badgwell, B.D. Current treatment and recent progress in gastric cancer. CA Cancer J. Clin. 2021, 71, 264–279. [Google Scholar] [CrossRef] [PubMed]
- Janjigian, Y.Y.; Ajani, J.A.; Moehler, M.; Shen, L.; Garrido, M.; Gallardo, C.; Wyrwicz, L.; Yamaguchi, K.; Cleary, J.M.; Elimova, E.; et al. First-Line Nivolumab Plus Chemotherapy for Advanced Gastric, Gastroesophageal Junction, and Esophageal Adenocarcinoma: 3-Year Follow-Up of the Phase III CheckMate 649 Trial. J. Clin. Oncol. 2024, 42, 2012–2020. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.M.; Shen, L.; Shah, M.A.; Enzinger, P.; Adenis, A.; Doi, T.; Kojima, T.; Metges, J.P.; Li, Z.; Kim, S.B.; et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): A randomised, placebo-controlled, phase 3 study. Lancet 2021, 398, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Janjigian, Y.Y.; Kawazoe, A.; Bai, Y.; Xu, J.; Lonardi, S.; Metges, J.P.; Yanez, P.; Wyrwicz, L.S.; Shen, L.; Ostapenko, Y.; et al. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: Interim analyses from the phase 3 KEYNOTE-811 randomised placebo-controlled trial. Lancet 2023, 402, 2197–2208. [Google Scholar] [CrossRef]
| Author | Hulscher | Sasako | Biere | Mariette | van der Sluise |
|---|---|---|---|---|---|
| Country | Netherlands | Japan | Netherlands | France | Netherlands |
| Year | 2007 | 2006 | 2017 | 2019 | 2019 |
| Number of patients | 220 | 167 | 115 | 207 | 112 |
| Surgical approach ① | Transhiatal | Transhiatal | Thoracoscopy/Laparoscopy | Thoracotomy/Laparoscopy | RAMIE/Laparoscopy |
| Surgical approach ② | Right transthoracic | Left thoracoabdominal | Thoracotomy/Laparotomy | Thoracotomy/Laparotomy | Thoracotomy/Laparoscopy |
| Tumor location | |||||
| Upper/middle esophagus | 0 | 0 | 45.2% | 30.9% | 12.8% |
| Lower esophagus/EGJC | 100% | 95.8% | 54.8% | 69.1% | 87.2% |
| Siewert type I | 43.9% | 0 | NA | NA | NA |
| Siewert type II | 56.1% | 57.6% | NA | NA | NA |
| Siewert type III | 0 | 38.2% | NA | NA | NA |
| Stomach | 0 | 4.2% | 0 | 0 | 0 |
| Histological type | |||||
| AC | 96.1% | 100% | 61.7% | 59.4% | 77.1% |
| SCC | 3.9% | 0 | 37.4% | 40.6% | 22.9% |
| Other | 0 | 0 | 0.90% | 0 | 0 |
| Neoadjuvant treatment | |||||
| Chemoradiotherapy | 0 | 0 | 92.2% | 31.9% | 79.5% |
| Chemotherapy | 0 | 0 | 7.8% | 41.5% | 8.9% |
| None | 100% | 100% | 0 | 26.6% | 11.6% |
| R0 resection | 71.6% vs. 71.8% (p = 0.28) | 92.7% vs. 88.2% | 91.5% vs. 83.9% | 95.1% vs 98.1% | 92.5% vs. 96.4% (p = 0.35) |
| Operation time (min) | 210 vs. 360 (p < 0.001) | 305 vs. 338 | 329 vs. 299 min (p = 0.002) | 327 vs 330 min | 349 vs. 296 min (p < 0.001) |
| Blood loss (mL) | 1000 vs. 1900 (p < 0.001) | 673 vs. 655 | 200 vs. 475 mL (p < 0.001) | NA | 400 vs. 568 mL (p < 0.001) |
| Open conversion rate | NA | NA | 13.6% | 2.9% | 5.4% |
| Complication | NA | 34.1% vs. 49.4% (p = 0.06) | NA | 35.9% vs 64.4% | 59.2 vs. 80.0% (p = 0.02) |
| Anastomotic leakage | 14.1% vs. 15.8% (p = 0.85) | 6.1% vs. 8.2% (p = 0.77) | 11.9% vs. 7.1% (p = 0.390) | 10.8% vs. 6.8% | 24.0% vs. 20.0% (p = 0.57) |
| Pulmonary complications | 27.3% vs. 57.0% (p < 0.001) | 3.7% vs. 12.9% (p = 0.05) | 11.9% vs. 33.9% (p = 0.005) | 17.6% vs. 30.1% | 31.5% vs. 58.2% (p = 0.005) |
| Cardiac | 16.0% vs. 26.3% (p = 0.10) | NA | NA | 11.8% vs. 13.4% | 22.2% vs. 47.3% (p = 0.006) |
| Vocal cord paralysis | 13.2% vs. 21.1% (p = 0.15) | NA | 1.7% vs. 14.3% (p = 0.012) | NA | 9.3% vs. 10.9% (p = 0.78) |
| Chylous leakage | 1.9% vs. 9.6% (p = 0.02) | NA | NA | 4.9% vs. 6.8% | 31.5% vs. 21.8% (p = 0.69) |
| Pancreatic fistula | NA | 12.1% vs. 16.5% (p = 0.51) | NA | NA | NA |
| Abdominal Abscess | NA | 8.5% vs. 14.1% (p = 0.33) | NA | NA | NA |
| Pyothorax | NA | 1.2% vs. 4.7% (p = 0.37) | NA | NA | NA |
| Mediastinitis | NA | 0 vs. 4.7% (p = 0.12) | NA | NA | NA |
| Reoperation | NA | NA | 13.6% vs. 10.7% (p = 0.641) | NA | 24.0% vs. 32.7% (p = 0.32) |
| Mortality | 1.9% vs. 4.4% (p = 0.45) | 0% vs. 5.9% (p = 0.25) | 1.7% vs. 0 (p = 0.590) | 1.0% vs. 1.9% | 1.8% vs. 0 (p = 0.62) |
| Survival | 5-year OS Siewert type I 37% vs. 51% (p = 0.33) Siewert type II 31% vs. 27% (p = 0.81) | 5-year OS Siewert type II 50% vs. 42% (p = 0.496) Siewert type III 59% vs. 36% (p = 0.102) | 3-year OS 42.9% vs. 41.2% (p = 0.633) 3-year DFS 37.3% vs. 42.9% (p = 0.602) | 5-year OS 60% vs. 40% (HR 0.67, 95%CI 0.44–1.01) 5-year DFS 53% vs. 43% (HR 0.76, 95%CI 0.52–1.11) | Median DFS 26 vs. 28 months (p = 0.983) |
| Author | Siewert | Pedrazzani | Kurokawa | Yoshikawa | Yamashita | Kurokawa |
|---|---|---|---|---|---|---|
| Country | Germany | Italy | Japan | Japan | Japan | Japan |
| Year | 2000 | 2007 | 2015 | 2016 | 2017 | 2021 |
| Study design | Retrospective | Retrospective | Retrospective | Retrospective | Retrospective | Prospective |
| Number of patients | 271 | 62 | 315 | 381 | 2807 | 363 |
| Definition of EGJC | Siewert type II | Siewert type II | Siewert type II | Siewert type II | Nishi classification | Nishi classification |
| Eligibility | pT1-4 | pT2-4 | pT2-4 | pT1-4 | pT1-4 tumor size ≤ 4 cm | cT2-4 |
| Histological type | ||||||
| SCC | 0 | 0 | 0 | 0 | 13.2 | 8.5 |
| AC | 100 | 100 | 100 | 100 | 84.9 | 91.5 |
| Other | 0 | 0 | 0 | 0 | 1.9 | 0 |
| Tumor size, mm * | NA | NA | 55 (8–100) | 50 (10–180) | 25 (16–39) | 46 (10–150) |
| Preoperative treatment, % | 22.6 | 0 | 14.0 | 10.8 | 0 | 33.3 |
| pT status | ||||||
| T0 | 0 | 0 | 0 | 0 | 0 | 4.4 |
| T1 | 14.0 | 0 | 0 | 20.7 | 56.6 | 13.2 |
| T2 | 57.2 | 51.6 | 18.1 | 14.7 | 19.2 | 16.5 |
| T3 | 20.3 | 46.8 | 45.1 | 36.0 | 24.1 | 48.2 |
| T4 | 8.5 | 1.6 | 36.8 | 28.6 | (T3 and T4) | 16.3 |
| pN status | ||||||
| N0 | 31.4 | 29.0 | 23.8 | 35.7 | 69.5 | 30.6 |
| N1 | 29.5 | 71.0 (N positive) | 21.6 | 20.7 | 16.7 | 25.1 |
| N2 | 22.5 | 27.6 | 22.6 | 9.0 | 20.9 | |
| N3 | 16.6 | 27.0 | 21.0 | 4.8 | 22.0 | |
| pM status | ||||||
| M0 | 83.8 | 100 | 100 | 93.2 | 100 | 96.1 |
| M1 | 16.2 | 0 | 0 | 6.8 | 0 | 3.9 |
| Esophagectomy | ||||||
| Total/subtotal | NA | NA | 7.0 | 7.1 | NA | 35.5 |
| Lower/abdominal | predominated | NA | 93.0 | 92.9 | NA | 64.5 |
| Gastrectomy | ||||||
| Total | NA | NA | 77.1 | 69.3 | NA | 49.0 |
| Proximal/upper | NA | NA | 22.9 | 30.7 | NA | 51.0 |
| Metastatic lymph nodes, % | ||||||
| Upper mediastinal nodes | NA | NA | 3.8 | NA | 0.0–5.1 | 6.1 |
| No. 105 | NA | NA | NA | NA | 0.0–1.1 | 1 |
| No. 106recL | NA | NA | NA | NA | NA | 1 |
| No. 106recR | NA | NA | NA | NA | 0.0–5.1 | 5.1 |
| No. 106tb | NA | NA | NA | NA | 0 | NA |
| Middle mediastinal nodes | NA | NA | 7.0 | NA | 0.0–4.0 | 7.1 |
| No. 107 | NA | 1.6 | NA | NA | 0.0–1.7 | 3.1 |
| No. 108 | NA | <5.0 | NA | NA | 0.8–4.0 | 5.1 |
| No. 109 | NA | NA | NA | NA | 0.0–2.8 | NA |
| No. 109L | NA | NA | NA | NA | NA | 3.1 |
| No. 109R | NA | NA | NA | NA | NA | 2.0 |
| Lower mediastinal nodes | 15.6 | NA | 11.4 | NA | 0.3–11.9 | 13.3 |
| No. 110 | NA | 12.9 | NA | NA | 0.5–11.9 | 9.3 |
| No. 111 | NA | 5.0–10.0 | NA | NA | 0.3–3.4 | 3.4 |
| No. 112 | NA | 5.0–10.0 | NA | NA | 0.0–2.3 | 2.0 |
| Abdominal nodes | NA | NA | NA | NA | NA | NA |
| No. 1 | 56.9 | 50 | NA | 39.8 | 4.0–34.6 | 35.2 |
| No. 2 | 67.8 | 30–35 | NA | 30.8 | 1.6–16.5 | 27.1 |
| No. 3 | 67.8 | 50–55 | NA | 41.5 | 3.9–39.5 | 38 |
| No. 4 | 16.1 | NA | NA | NA | NA | NA |
| No. 4sa | NA | 0–5.0 | NA | 4.3 | 0.1–0.3 | 4.2 |
| No. 4sb | NA | NA | NA | 2.7 | 0.0–1.3 | 0.8 |
| No. 4d | NA | 5.0–10.0 | NA | 2.9 | 0.0–0.8 | 2.2 |
| No. 5 | 1.6 | 0–5.0 | NA | 1.7 | 0.0–0.5 | 1.1 |
| No. 6 | NA | 0–5.0 | NA | 0.8 | 0.0–0.9 | 1.7 |
| No. 7 | 15.1 | 30.6 | NA | 26.7 | 1.1–17.7 | 23.5 |
| No. 8a | NA | 15–20 | NA | 4.9 | 0.2–3.8 | 7.1 |
| No. 9 | 7 | 15–20 | NA | 11.7 | 0.3–6.8 | 12.4 |
| No. 10 | NA | 0–5.0 | NA | 9.5 | 0.1–0.9 | NA |
| No. 11 | 4.8 | 5.0–10.0 | NA | NA | NA | NA |
| No. 11p | NA | NA | NA | 17.2 | 0.3–4.5 | 13.6 |
| No. 11d | NA | NA | NA | 6.3 | 0.0–2.1 | 4.3 |
| No. 12 | 4.8 | 0 | NA | 1.4 | NA | NA |
| No. 16a1 | NA | 0–5.0 | NA | NA | 0.0–0.3 | NA |
| No. 16a2 | NA | NA | NA | 14.4 | 0.0–0.6 | 4.7 |
| No. 19 | NA | NA | NA | 6.3 | 0.0–0.8 | 5.4 |
| No. 20 | NA | NA | NA | 0.0 | 0.0–0.8 | 4.8 |
| Types of Treatment | Chemotherapy | Chemoradiotherapy | Immunotherapy | ||||
|---|---|---|---|---|---|---|---|
| Study name | FLOT4 | PRODIGY | RESOLVE | CROSS | POET | NeoRes | Checkmate577 |
| Country | Germany | South Korea | China | Netherlands | Germany | Norway, Sweden | 29 countries |
| Phase | II/III | III | III | III | III | II | III |
| Eligibility | cT2–4 or cN(+) | cT2–3 cN(+) or cT4 | cT4a cN(+) or cT4b | cT1N1M0 or cT2–3N0–1M0 | cT3–4NXM0 | cT1N(+)M0 or cT2–3NXM0 | ypStage II-III after pre-CRT plus surgery |
| Experimental arm | Pre- and Post-FLOT | Pre-DOS and Post S-1 | Pre- and post-SOX | Pre-(CBDCA+PTX+RT) | Pre-(FP+RT) | Pre-(PLF+RT) | Post-nivolumab |
| Control arm | Pre- and Post-ECF/ECX | Post S-1 | Post CAPEOX | Surgery alone | Pre-FP | Pre-PLF | None |
| Number of patients | 716 | 484 | 1022 | 368 | 119 | 181 | 794 |
| Histological type | |||||||
| AC | 100% | 100% | NA | 75% | 100% | 72% | 71% |
| SCC | 0 | 0 | NA | 25% | 0 | 28% | 29% |
| Unknown | 0 | 0 | NA | 0 | 0 | 0 | 0.10% |
| Tumor location | |||||||
| Esophagus | 0 | 0 | 0 | 73% | 0 | 83% | 58% |
| EGJ | 56% | 6% | 36% | 24% | 100% | 17% | 42% |
| Stomach | 44% | 94% | 64% | 0 | 0 | 0 | 0 |
| Unknown | 0 | 0 | 0 | 3% | 0 | 0 | 0 |
| cT status | |||||||
| T0 | 0 | 0 | 3% (T1–T3) | 0 | 0 | 0 | 6% |
| T1 | 1% | 0 | 1% | 0 | 1% | 39% | |
| T2 | 15% | 5% | 17% | 0 | 34% | 0 | |
| T3 | 73% | 24% | 81% | 92% | 65% | 55% | |
| T4 | 9% | 71% | 97% | 0.3% | 8% | 0 | 0 |
| Unknown | 3% | 0 | NA | 1% | 0 | 0 | 0.4% |
| cN status | |||||||
| N(−) | 21% | 2% | NA | 32% | NA | 37% | 42% |
| N(+) | 79% | 98% | NA | 64% | NA | 63% | 58% |
| Other | 0 | 0 | NA | 0 | NA | 0 | 0.1% |
| Survival | Median OS 50 vs. 35 months 3-year OS rate 57% vs. 48% 5-year OS rate 45% vs. 36% (HR 0.77, 95% CI 0.63–0.94) | 3-year PFS 66% vs. 60% 5-year PFS 60% vs. 56% (HR 0.70, 95% CI 0.52–0.95, p = 0.02) | 3-year OS 59.4% vs. 51.1% (HR 0.77, 95% CI 0.61–0.97) | Median OS 49 vs. 24 months (p = 0.003, HR 0.657, 95% CI 0.495–0.871) 3-year OS 58% vs. 44% 5-year OS 47% vs. 33% (HR 0.68, 95% CI 0.53–0.88) 3-year PFS 51% vs. 35% 5-year PFS rate 44% vs. 27% | Median OS 33 vs. 21 months 3-year OS 47% vs. 28% (HR 0.67, 95% CI 0.41–1.07) | 3-year OS 47% vs. 49% (HR 1.09, 95% CI 0.73–1.64) 3-year PFS 44% vs. 44% | Median DFS 22.4 vs. 11.0 months (HR 0.69 96.4% CI 0.56–0.86, p < 0.001) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakagawa, M.; Nakajima, M.; Yoshimatsu, M.; Ueta, Y.; Inoue, N.; Ochiai, T.; Takise, S.; Fujita, J.; Morita, S.; Kojima, K. An Overview of the Treatment Strategy of Esophagogastric Junction Cancer. Cancers 2025, 17, 1961. https://doi.org/10.3390/cancers17121961
Nakagawa M, Nakajima M, Yoshimatsu M, Ueta Y, Inoue N, Ochiai T, Takise S, Fujita J, Morita S, Kojima K. An Overview of the Treatment Strategy of Esophagogastric Junction Cancer. Cancers. 2025; 17(12):1961. https://doi.org/10.3390/cancers17121961
Chicago/Turabian StyleNakagawa, Masatoshi, Masanobu Nakajima, Masaki Yoshimatsu, Yu Ueta, Noboru Inoue, Takahiro Ochiai, Shuhei Takise, Junki Fujita, Shinji Morita, and Kazuyuki Kojima. 2025. "An Overview of the Treatment Strategy of Esophagogastric Junction Cancer" Cancers 17, no. 12: 1961. https://doi.org/10.3390/cancers17121961
APA StyleNakagawa, M., Nakajima, M., Yoshimatsu, M., Ueta, Y., Inoue, N., Ochiai, T., Takise, S., Fujita, J., Morita, S., & Kojima, K. (2025). An Overview of the Treatment Strategy of Esophagogastric Junction Cancer. Cancers, 17(12), 1961. https://doi.org/10.3390/cancers17121961





