Efficacy of Radiotherapy for Oligometastatic Lung Cancer and Irradiation Methods Based on Metastatic Site
Simple Summary
Abstract
1. Introduction
2. Clinical Trials for Oligometastases of Lung Cancer
3. Pulmonary Oligometastasis of Lung Cancer
4. Liver Oligometastases of Lung Cancer
5. Adrenal Oligometastases of Lung Cancer
6. Bone Oligometastases of Lung Cancer
7. Stereotactic Body Radiotherapy for Oligometastases of Lymph Node
8. Metastatic Brain Tumors of Lung Cancer
9. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| EORTC | European Organization for Research and Treatment of Cancer |
| SBRT | Stereotactic body radiotherapy |
| NSCLC | Non-small cell lung cancer |
| PFS | Progression-free survival |
| HR | Hazard ratio |
| CI | Confidence interval |
| OS | Overall survival |
| TKI | Tyrosine kinase inhibitor |
| EGFR | Epidermal growth factor receptor |
| BED | Biologically effective dose |
| PTV | Planning target volume |
| SCLC | Small cell lung cancer |
| ECOG | Eastern Cooperative Oncology Group |
| CT | Computed tomography |
| MRI | Magnetic resonance imaging |
| FDG-PET | 18-fluoro-deoxyglucose positron emission tomography |
| ESTRO | European Society for Radiotherapy and Oncology |
| ICI | Immune checkpoint inhibitor |
| CCI | Coverage Compromise Index |
| WBRT | Whole-brain radiation therapy |
| SRS | Stereotactic radiosurgery |
| ASTRO | American Society for Radiation Oncology |
| HER2 | Human epidermal growth factor receptor 2 |
References
- Hellman, S.; Weichselbaum, R.R. Oligometastases. J. Clin. Oncol. 1995, 13, 8–10. [Google Scholar] [CrossRef]
- Dingemans, A.-M.C.; Hendriks, L.E.L.; Berghmans, T.; Levy, A.; Hasan, B.; Faivre-Finn, C.; Giaj-Levra, M.; Giaj-Levra, N.; Girard, N.; Greillier, L.; et al. Definition of synchronous oligometastatic non-small cell lung cancer-A consensus report. J. Thorac. Oncol. 2019, 14, 2109–2119. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.R.; Blumenschein, G.R., Jr.; Lee, J.J.; Hernandez, M.; Ye, R.; Camidge, D.R.; Doebele, R.C.; Skoulidis, F.; Gaspar, L.E.; Gibbons, D.L.; et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: A multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016, 17, 1672–1682. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.R.; Tang, C.; Zhang, J.; Blumenschein, G.R., Jr.; Hernandez, M.; Lee, J.J.; Ye, R.; Palma, D.A.; Louie, A.V.; Camidge, D.R.; et al. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: Long-term results of a multi-institutional, phase II, randomized study. J. Clin. Oncol. 2019, 37, 1558–1565. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, P.; Wardak, Z.; Gerber, D.E.; Tumati, V.; Ahn, C.; Hughes, R.S.; Dowell, J.E.; Cheedella, N.; Nedzi, L.; Westover, K.D.; et al. Consolidative radiotherapy for limited metastatic non-small-cell lung cancer: A phase 2 randomized clinical trial. JAMA Oncol. 2018, 4, e173501. [Google Scholar] [CrossRef] [PubMed]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): A randomised, phase 2, open-label trial. Lancet 2019, 393, 2051–2058. [Google Scholar] [CrossRef]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: Long-term results of the SABR-COMET phase II randomized trial. J. Clin. Oncol. 2020, 38, 2830–2838. [Google Scholar] [CrossRef]
- Wang, X.S.; Bai, Y.F.; Verma, V.; Yu, R.L.; Tian, W.; Ao, R.; Deng, Y.; Zhu, X.Q.; Liu, H.; Pan, H.X.; et al. Randomized trial of first-line tyrosine kinase inhibitor with or without radiotherapy for synchronous oligometastatic EGFR-mutated non-small cell lung cancer. J. Natl. Cancer Ins. 2023, 115, 742–748. [Google Scholar] [CrossRef]
- Olson, R.; Senan, S.; Harrow, S.; Gaede, S.; Louie, A.; Haasbee, C.; Mulroy, L.; Lock, M.; Rodrigues, G.; Yaremko, B.; et al. Quality of life outcomes after stereotactic ablative radiation therapy (SABR) versus standard of care treatments in the oligometastatic setting: A secondary analysis of the SABR-COMET randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 943–947. [Google Scholar] [CrossRef]
- Gerull, W.D.; Puri, V.; Kozower, B.D. The epidemiology and biology of pulmonary metastases. J. Thorac. Dis. 2021, 13, 2585–2589. [Google Scholar] [CrossRef]
- Gits, H.C.; Khosravi Flanigan, M.A.; Kapplinger, J.D.; Reisenauer, J.S.; Eiken, P.W.; Breen, W.G.; Vu, L.H.; Welch, B.T.; Harmsen, W.S.; Day, C.N.; et al. Sublobar resection, stereotactic body radiation therapy, and percutaneous ablation provide comparable outcomes for lung metastasis-directed therapy. Chest 2024, 165, 1247–1259. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dong, X.; Yan, S.; Liu, B.; Li, X.; Li, S.; Lv, C.; Cui, X.; Tao, Y.; Yu, R.; et al. Comparison of the long-term survival outcome of surgery versus stereotactic body radiation therapy as initial local treatment for pulmonary oligometastases from colorectal cancer: A propensity score analysis. Int. J. Radiat. Oncol. Biol. Phys. 2025, 121, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Laeseke, P.; Ng, C.; Ferko, N.; Naghi, A.; Wright, G.W.J.; Wang, D.; Laidlaw, A.; Kalsekar, I.; Amos, T.; Laxmanan, B.; et al. Stereotactic body radiation therapy and thermal ablation for treatment of patients with pulmonary metastases: A systematic literature review and meta-analysis. BMC Pulm. Med. 2025, 25, 188. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kang, K.M.; Choi, H.S.; Ha, I.B.; Jeong, H.; Song, J.H.; Jang, I.S.; Kim, S.H.; Lee, J.W.; Rhee, D.Y.; et al. Comparison of stereotactic body radiotherapy versus metastasectomy outcomes in patients with pulmonary metastases. Thorac. Cancer 2018, 9, 1671–1679. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, R.; Suzuki, O.; Kanou, T.; Ose, N.; Funaki, S.; Shintani, Y.; Minami, M.; Tamari, K.; Otani, K.; Seo, Y.; et al. The short-term outcomes of pulmonary metastasectomy or stereotactic body radiation therapy for pulmonary metastasis from epithelial tumors. J. Cardiothorac. Surg. 2020, 15, 43. [Google Scholar] [CrossRef]
- Song, Y.; Kim, Y.J.; Choi, S.; Yun, J.K.; Ahn, J.H.; Kim, J.E.; Lee, J.S.; Kim, W.; Do, K.H.; Chung, H.W.; et al. Stereotactic ablative radiotherapy for pulmonary metastasis from sarcoma: A retrospective comparison with metastasectomy. Clin. Exp. Metastasis 2024, 42, 2. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.S.; Yun, J.K.; Jung, J.; Park, J.H.; Song, S.Y.; Choi, E.K.; Choi, S.; Kim, H.R.; Kim, Y.H.; Kim, D.K.; et al. Comparison of metastasectomy and stereotactic body radiation therapy for pulmonary oligometastasis from hepatocellular carcinoma: A propensity score-weighted analysis. Int. J. Radiat. Oncol. Biol. Phys. 2025, 121, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.J.; Yang, J.T.; Shaverdian, N.; Patel, J.; Shepherd, A.F.; Eng, J.; Guttmann, D.; Yeh, R.; Gelblum, D.Y.; Namakydoust, A.; et al. Standard-of-care systemic therapy with or without stereotactic body radiotherapy in patients with oligoprogressive breast cancer or non-small-cell lung cancer (Consolidative Use of Radiotherapy to Block [CURB] oligoprogression): An open-label, randomised, controlled, phase 2 study. Lancet 2024, 403, 171–182. [Google Scholar] [PubMed]
- Siva, S.; Sakyanun, P.; Mai, T.; Wong, W.; Lim, A.; Ludbrook, J.; Bettington, C.; Rezo, A.; Pryor, D.; Hardcastle, N.; et al. Long-term outcomes of TROG 13.01 SAFRON II randomized trial of single- versus multifraction stereotactic ablative body radiotherapy for pulmonary oligometastases. J. Clin. Oncol. 2023, 41, 3493–3498. [Google Scholar] [CrossRef]
- Lourenco, R.D.A.; Khoo, T.; Crothers, A.; Haas, M.; Montgomery, R.; Ball, D.; Bressel, M.; Siva, S. Cost-effectiveness of single versus multifraction SABR for pulmonary oligometastases: The SAFRON II trial. Int. J. Radiat. Oncol. Biol. Phys. 2022, 114, 968–976. [Google Scholar] [CrossRef]
- Mayinger, M.; Kotecha, R.; Sahgal, A.; Kim, M.S.; Lo, S.S.; Louie, A.V.; Scorsetti, M.; Slotman, B.; Guckenberger, M. Stereotactic body radiotherapy for lung oligo-metastases: Systematic review and International Stereotactic Radiosurgery Society practice guidelines. Lung Cancer 2023, 182, 107284. [Google Scholar] [CrossRef] [PubMed]
- Kroeze, S.G.C.; Pavic, M.; Stellamans, K.; Lievens, Y.; Becherini, C.; Scorsetti, M.; Alongi, F.; Ricardi, U.; Jereczek-Fossa, B.A.; Westhoff, P.; et al. Metastases-directed stereotactic body radiotherapy in combination with targeted therapy or immunotherapy: Systematic review and consensus recommendations by the EORTC-ESTRO OligoCare consortium. Lancet Oncol. 2023, 24, e121–e132. [Google Scholar] [CrossRef] [PubMed]
- Klement, R.J.; Hoerner-Rieber, J.; Adebahr, S.; Andratschke, N.; Blanck, O.; Boda-Heggemann, J.; Duma, M.; Eble, M.J.; Eich, H.C.; Flentje, M.; et al. Stereotactic body radiotherapy (SBRT) for multiple pulmonary oligometastases: Analysis of number and timing of repeat SBRT as impact factors on treatment safety and efficacy. Radiother. Oncol. 2018, 127, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Spigel, D.R.; Faivre-Finn, C.; Gray, J.E.; Vicente, D.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.F.; Garassino, M.C.; Hui, R.; Quantin, X.; et al. Five-year survival outcomes from the PACIFIC trial: Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J. Clin. Oncol. 2022, 40, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Kurishima, K.; Nakazawa, K.; Kagohashi, K.; Ishikawa, H.; Satoh, H.; Hizawa, N. Specific organ metastases and survival in metastatic non-small-cell lung cancer. Mol. Clin. Oncol. 2014, 3, 217–221. [Google Scholar] [CrossRef]
- Yamazawa, H.; Ishii, Y.; Kitamura, S. A clinical study on liver metastases in patients with primary lung cancer. Haigan 1996, 36, 33–40. [Google Scholar] [CrossRef]
- Nakazawa, K.; Kurishima, K.; Tamura, T.; Kagohashi, K.; Ishikawa, H.; Satoh, H.; Hizawa, N. Specific organ metastases and survival in small cell lung cancer. Oncol. Lett. 2012, 4, 617–620. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomised, controlled, open-label, phase 3 trial. Lancet 2019, 394, 1929–1939. [Google Scholar] [CrossRef]
- Scorsetti, M.; Clerici, E.; Comito, T. Stereotactic body radiation therapy for liver metastases. J. Gastrointest. Oncol. 2014, 5, 190–197. [Google Scholar] [CrossRef]
- Høyer, M.; Swaminath, A.; Bydder, S.; Lock, M.; Romero, A.M.; Kavanagh, B.; Goodman, K.A.; Okunieff, P.; Dawson, L.A. Radiotherapy for liver metastases: A review of evidence. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 1047–1057. [Google Scholar] [CrossRef]
- Withers, H.R.; Taylor, J.M.; Maciejewski, B. Treatment volume and tissue tolerance. Int. J. Radiat. Oncol. Biol. Phys. 1988, 14, 751–759. [Google Scholar] [CrossRef]
- Berkovic, P.; Gulyban, A.; Nguyen, P.V.; Dechambre, D.; Martinive, P.; Jansen, N.; Lakosi, F.; Janvary, L.; Coucke, P.A. Stereotactic robotic body radiotherapy for patients with unresectable hepatic oligorecurrence. Clin. Color. Cancer 2017, 16, 349–357. [Google Scholar] [CrossRef]
- Scorsetti, M.; Comito, T.; Clerici, E.; Franzese, C.; Tozzi, A.; Iftode, C.; Di Brina, L.; Navarria, P.; Mancosu, P.; Reggiori, G.; et al. Phase II trial on SBRT for unresectable liver metastases: Long-term outcome and prognostic factors of survival after 5 years of follow-up. Radiat. Oncol. 2018, 13, 234. [Google Scholar] [CrossRef] [PubMed]
- Rusthoven, K.E.; Kavanagh, B.D.; Cardenes, H.; Stieber, V.W.; Burri, S.H.; Feigenberg, S.J.; Chidel, M.A.; Pugh, T.J.; Franklin, W.; Kane, M.; et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J. Clin. Oncol. 2009, 27, 1572–1578. [Google Scholar] [CrossRef]
- Jackson, W.C.; Tao, Y.; Mendiratta-Lala, M.; Bazzi, L.; Wahl, D.R.; Schipper, M.J.; Feng, M.; Cuneo, K.C.; Lawrence, T.S.; Owen, D. Comparison of stereotactic body radiation therapy and radiofrequency ablation in the treatment of intrahepatic metastases. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.S.; Kim, M.S.; Yoo, H.J.; Jang, W.I.; Paik, E.K.; Han, C.J.; Lee, B.H. Radiofrequency ablation versus stereotactic body radiotherapy for small hepatocellular carcinoma: A Markov model-based analysis. Cancer Med. 2016, 5, 3094–3101. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, T.; Wang, J.; Li, W.; Zhang, A.; He, W.; Zhang, D.; Li, D.; Ding, J.; Duan, X. Biologically effective dose (BED) of stereotactic body radiation therapy (SBRT) was an important factor of therapeutic efficacy in patients with hepatocellular carcinoma (≤5 cm). BMC Cancer 2019, 19, 846. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, S.; Oshiro, Y.; Nakayama, H.; Fukuda, K.; Mizumoto, M.; Abei, M.; Shoda, J.; Matsuzaki, Y.; Thono, E.; Tokita, M.; et al. Proton beam therapy for large hepatocellular carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 460–466. [Google Scholar] [CrossRef]
- Shiba, S.; Wakatsuki, M.; Toyama, S.; Terashima, K.; Uchida, H.; Katoh, H.; Shibuya, K.; Okazaki, S.; Miyasaka, Y.; Ohno, T.; et al. Carbon-ion radiotherapy for oligometastatic liver disease: A national multicentric study by the Japan Carbon-Ion Radiation Oncology Study Group (J-CROS). Cancer Sci. 2023, 114, 3679–3686. [Google Scholar] [CrossRef]
- Lee, M.T.; Kim, J.J.; Dinniwell, R.; Brierley, J.; Lockwood, G.; Wong, R.; Cummings, B.; Ringash, J.; Tse, R.V.; Knox, J.J.; et al. Phase I study of individualized stereotactic body radiotherapy of liver metastases. J. Clin. Oncol. 2009, 27, 1585–1591. [Google Scholar] [CrossRef]
- Folkert, M.R.; Meyer, J.J.; Aguilera, T.A.; Yokoo, T.; Sanford, N.N.; Rule, W.G.; Mansour, J.; Yopp, A.; Polanco, P.; Hannan, R.; et al. Long-term results of a phase 1 dose-escalation trial and subsequent institutional experience of single-fraction stereotactic ablative radiation therapy for liver metastases. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 1387–1395. [Google Scholar] [CrossRef]
- Mahadevan, A.; Blanck, O.; Lanciano, R.; Peddada, A.; Sundararaman, S.; D’Ambrosio, D.; Sharma, S.; Perry, D.; Kolker, J.; Davis, J. Stereotactic body radiotherapy (SBRT) for liver metastasis—Clinical outcomes from the international multi-institutional RSSearch® patient registry. Radiat. Oncol. 2018, 13, 26. [Google Scholar] [CrossRef]
- Yamashita, H.; Onishi, H.; Matsumoto, Y.; Murakami, N.; Matsuo, Y.; Nomiya, T.; Nakagawa, K.; Japanese Radiological Society multi-institutional SBRT study group (JRS-SBRTSG). Local effect of stereotactic body radiotherapy for primary and metastatic liver tumors in 130 Japanese patients. Radiat. Oncol. 2014, 9, 112. [Google Scholar] [CrossRef]
- Kok, E.N.D.; Jansen, E.P.M.; Heeres, B.C.; Kok, N.F.M.; Janssen, T.; van Werkhoven, E.; Sanders, F.R.K.; Ruers, T.J.M.; Nowee, M.E.; Kuhlmann, K.F.D.; et al. High versus low dose stereotactic body radiation therapy for hepatic metastases. Clin. Transl. Radiat. Oncol. 2019, 20, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Fode, M.M.; Høyer, M. Survival and prognostic factors in 321 patients treated with stereotactic body radiotherapy for oligo-metastases. Radiother. Oncol. 2015, 114, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Goodman, K.A.; Wiegner, E.A.; Maturen, K.E.; Zhang, Z.; Mo, Q.; Yang, G.; Gibbs, I.C.; Fisher, G.A.; Koong, A.C. Dose-escalation study of single-fraction stereotactic body radiotherapy for liver malignancies. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.A.; Caudell, J.J.; El-Haddad, G.; Berglund, A.E.; Welsh, E.A.; Yue, B.; Hoffe, S.E.; Naghavi, A.O.; Abuodeh, Y.A.; Frakes, J.M.; et al. Radiosensitivity differences between liver metastases based on primary histology suggest implications for clinical outcomes after stereotactic body radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 1399–1404. [Google Scholar] [CrossRef]
- Mheid, S.; Allen, S.; Ng, S.S.W.; Hall, W.A.; Sanford, N.N.; Aguilera, T.A.; Elamir, A.M.; Bahij, R.; Intven, M.P.W.; Radhakrishna, G.; et al. Local control following stereotactic body radiation therapy for liver oligometastases: Lessons from a quarter century. Curr. Oncol. 2023, 30, 9230–9243. [Google Scholar] [CrossRef]
- Clerici, E.; Comito, T.; Franzese, C.; Di Brina, L.; Tozzi, A.; Iftode, C.; Navarria, P.; Mancosu, P.; Reggiori, G.; Tomatis, S.; et al. Role of stereotactic body radiation therapy in the treatment of liver metastases: Clinical results and prognostic factors. Strahlenther. Onkol. 2020, 196, 325–333. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Meng, M.B.; Liu, C.L.; Wang, H.H.; Jiang, C.; Song, Y.C.; Zhuang, H.Q.; Yang, D.; Wang, J.S.; Wei, W.; et al. Stereotactic body radiation therapy using the CyberKnife(®) system for patients with liver metastases. OncoTargets Ther. 2014, 7, 915–923. [Google Scholar] [CrossRef]
- He, J.; Li, Y.; An, J.; Hu, L.; Zhang, J. Surgical treatment in non-small cell lung cancer with pulmonary oligometastasis. World J. Surg. Oncol. 2017, 15, 36. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.J.; Kim, M.S.; Jang, W.I.; Seo, Y.S.; Cho, C.K.; Yoo, H.J.; Paik, E.K. Stereotactic body radiation therapy for liver oligo-recurrence and oligo-progression from various tumors. Radiat. Oncol. J. 2017, 35, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Abrams, H.L.; Spiro, R.; Godlstein, N. Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer 1950, 3, 74–85. [Google Scholar] [CrossRef]
- Eberhardt, W.E.; Mitchell, A.; Crowley, J.; Kondo, H.; Kim, Y.T.; Turrisi, A., 3rd; Goldstraw, P.; Rami-Porta, R. International Association for Study of Lung Cancer Staging and Prognostic Factors Committee, Advisory Board Members, and Participating Institutions. The IASLC lung cancer staging project: Proposals for the revision of the M descriptors in the forthcoming eighth edition of the TNM classification of lung cancer. J. Thorac. Oncol. 2015, 10, 1515–1522. [Google Scholar] [PubMed]
- Lam, K.-Y.; Lo, C.-Y. Metastatic tumours of the adrenal glands: A 30-year experience in a teaching hospital. Clin. Endocrinol. 2002, 56, 95–101. [Google Scholar] [CrossRef]
- Soffen, E.M.; Solin, L.J.; Rubenstein, J.H.; Hanks, G.E. Palliative radiotherapy for symptomatic adrenal metastases. Cancer 1990, 65, 1318–1320. [Google Scholar] [CrossRef]
- Kong, J.; Odisho, T.; Alhajahjeh, A.; Maqsood, H.A.; Al-Share, B.A.; Shahait, M.; Abubaker, A.; Kim, S.; Shahait, A. Long-term survival following adrenalectomy for secondary adrenal tumors: A systematic review and meta-analysis. Am. J. Surg. 2024, 237, 115809. [Google Scholar] [CrossRef]
- Chen, W.C.; Baal, J.D.; Baal, U.; Pai, J.; Gottschalk, A.; Boreta, L.; Braunstein, S.E.; Raleigh, D.R. Stereotactic body radiation therapy of adrenal metastases: A pooled meta-analysis and systematic review of 39 studies with 1006 patients. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 48–61. [Google Scholar] [CrossRef]
- Metman, M.J.H.; Viëtor, C.L.; Seinen, A.J.; Berends, A.M.A.; Hemmer, P.H.J.; Kerstens, M.N.; Feelders, R.A.; Franssen, G.J.H.; van Ginhoven, T.M.; Kruijff, S. Outcomes after surgical treatment of metastatic disease in the adrenal gland; valuable for the patient? Cancers 2021, 14, 156. [Google Scholar] [CrossRef]
- Holy, R.; Piroth, M.; Pinkawa, M.; Eble, M.J. Stereotactic body radiation therapy (SBRT) for treatment of adrenal gland metastases from non-small cell lung cancer. Strahlenther. Onkol. 2011, 187, 245–251. [Google Scholar] [CrossRef]
- Gamsiz, H.; Beyzadeoglu, M.; Sager, O.; Demiral, S.; Dincoglan, F.; Uysal, B.; Onal, E.; Dirican, B. Evaluation of stereotactic body radiation therapy in the management of adrenal metastases from non-small cell lung cancer. Tumori 2015, 101, 98–103. [Google Scholar] [CrossRef]
- Zhao, X.; Zhu, X.; Fei, J.; Ren, H.; Cao, Y.; Ju, X.; Yuan, Z.; Zhang, H. Short-term outcomes and clinical efficacy of stereotactic body radiation therapy (SBRT) in treatment of adrenal gland metastases from lung cancer. Radiat. Oncol. 2018, 13, 205. [Google Scholar] [CrossRef]
- Arcidiacono, F.; Aristei, C.; Marchionni, A.; Italiani, M.; Fulcheri, C.P.L.; Saldi, S.; Casale, M.; Ingrosso, G.; Anselmo, P.; Maranzano, E. Stereotactic body radiotherapy for adrenal oligometastasis in lung cancer patients. Br. J. Radiol. 2020, 93, 1115. [Google Scholar] [CrossRef] [PubMed]
- Rzazade, R.; Pham, N.T.; Turna, M.; Canoglu, M.D.; Kucukmorkoc, E.; Berberoglu, K.; Caglar, H.B. Stereotactic body radiotherapy in patients with adrenal gland metastases of oligometastatic and oliogoprogressive lung cancer. J. Radiosurg. SBRT 2022, 8, 275–282. [Google Scholar] [PubMed]
- Saito, M.; Ozawa, S.; Komiyama, T.; Kokubo, M.; Shioyama, Y.; Matsuo, Y.; Mizowaki, T.; Kimura, T.; Harada, H.; Igaki, H.; et al. A Japanese national survey on IMRT/SBRT in 2023 by the JASTRO high-precision external beam radiotherapy group. J. Radiat. Res. 2025, 66, 234–252. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Kishi, K.; Du, K.; Komaki, R.; Mizoe, J.; Aikawa, G.; Zheng, W.; Pan, C. Risk factors of local control in adrenal metastases treated by stereotactic body radiation therapy—A systematic review and meta-analysis. Front. Oncol. 2023, 13, 1193574. [Google Scholar] [CrossRef]
- Seidenwurm, D.J.; Elmer, E.B.; Kaplan, L.M.; Williams, E.K.; Morris, D.G.; Hoffman, A.R. Metastases to the adrenal glands and the development of Addison’s disease. Cancer 1984, 54, 552–557. [Google Scholar] [CrossRef]
- Redman, B.G.; Pazdur, R.; Zingas, A.P.; Loredo, R. Prospective evaluation of adrenal insufficiency in patients with adrenal metastasis. Cancer 1987, 60, 103–107. [Google Scholar] [CrossRef]
- Hamidi, O.; Miljanic, M.; Tumyan, G.; Christie, A.; Mirfakhraee, S.; Ali, S.; Dohopolski, M.; Gottumukkala, S.; Brugarolas, J.; Timmerman, R.; et al. Adrenal insufficiency following stereotactic ablative radiotherapy (SAbR) of adrenal gland metastases. Cancers 2024, 16, 3140. [Google Scholar] [CrossRef] [PubMed]
- Grouthier, V.; Lebrun-Vignes, B.; Moey, M.; Johnson, D.B.; Moslehi, J.J.; Salem, J.E.; Bachelot, A. Immune Checkpoint Inhibitor-Associated Primary Adrenal Insufficiency: WHO VigiBase Report Analysis. Oncologist 2020, 25, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Santini, D.; Barni, S.; Intagliata, S.; Falcone, A.; Ferraù, F.; Galetta, D.; Moscetti, L.; La Verde, N.; Ibrahim, T.; Petrelli, F.; et al. Natural history of non-small-cell lung cancer with bone metastases. Sci. Rep. 2015, 5, 18670. [Google Scholar] [CrossRef]
- Sahgal, A.; Myrehaug, S.D.; Siva, S.; Masucci, G.L.; Maralani, P.J.; Brundage, M.; Butler, J.; Chow, E.; Fehlings, M.G.; Foote, M.; et al. Stereotactic body radiotherapy versus conventional external beam radiotherapy in patients with painful spinal metastases: An open-label, multicentre, randomised, controlled, phase 2/3 trial. Lancet Oncol. 2021, 22, 1023–1033. [Google Scholar] [CrossRef]
- Zeng, K.L.; Myrehaug, S.; Soliman, H.; Husain, Z.A.; Tseng, C.L.; Detsky, J.; Ruschin, M.; Atenafu, E.G.; Witiw, C.D.; Larouche, J.; et al. Mature local control and reirradiation rates comparing spine stereotactic body radiation therapy with conventional palliative external beam radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2022, 114, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Zelefsky, M.J.; Yamada, Y.; Greco, C.; Lis, E.; Schöder, H.; Lobaugh, S.; Zhang, Z.; Braunstein, S.; Bilsky, M.H.; Powell, S.N.; et al. Phase 3 multi-center, prospective, randomized trial comparing single-dose 24 Gy radiation therapy to a 3-fraction SBRT regimen in the treatment of oligometastatic cancer. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Soltys, S.G.; Grimm, J.; Milano, M.T.; Xue, J.; Sahgal, A.; Yorke, E.; Yamada, Y.; Ding, G.X.; Li, X.A.; Lovelock, D.M.; et al. Stereotactic body radiation therapy for spinal metastases: Tumor control probability analyses and recommended reporting standards. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.K.; Louie, A.V.; Kotecha, R.; Saxena, A.; Zhang, Y.; Guckenberger, M.; Kim, M.S.; Scorsetti, M.; Slotman, B.J.; Lo, S.S.; et al. Stereotactic body radiotherapy for non-spine bone metastases: A meta-analysis and international stereotactic radiosurgery society (ISRS) clinical practice guidelines. Radiother. Oncol. 2025, 205, 110717. [Google Scholar] [CrossRef] [PubMed]
- Thureau, S.; Marchesi, V.; Vieillard, M.H.; Perrier, L.; Lisbona, A.; Leheurteur, M.; Tredaniel, J.; Culine, S.; Dubray, B.; Bonnet, N.; et al. Efficacy of extracranial stereotactic body radiation therapy (SBRT) added to standard treatment in patients with solid tumors (breast, prostate and non-small cell lung cancer) with up to 3 bone-only metastases: Study protocol for a randomised phase III trial (STEREO-OS). BMC Cancer 2021, 21, 117. [Google Scholar]
- Ong, W.L.; Wong, S.; Soliman, H.; Myrehaug, S.; Tseng, C.L.; Detsky, J.; Husain, Z.; Maralani, P.; Ma, L.; Lo, S.S.; et al. Radiation myelopathy following stereotactic body radiation therapy for spine metastases. J. Neurooncol. 2022, 159, 23–31. [Google Scholar] [CrossRef]
- Jackson, C.B.; Boe, L.A.; Zhang, L.; Apte, A.; Ruppert, L.M.; Haseltine, J.M.; Mueller, B.A.; Schmitt, A.M.; Yang, J.T.; Newman, W.C.; et al. Radiation Myelitis Risk After Hypofractionated Spine Stereotactic Body Radiation Therapy. JAMA Oncol. 2025, 11, 128–134. [Google Scholar] [CrossRef]
- Spieler, B.; Samuels, S.E.; Llorente, R.; Yechieli, R.; Ford, J.C.; Mellon, E.A. Advantages of radiation therapy simulation with 0.35 tesla magnetic resonance imaging for stereotactic ablation of spinal metastases. Pract. Radiat. Oncol. 2020, 10, 339–344. [Google Scholar] [CrossRef]
- Oztek, M.A.; Mayr, N.A.; Mossa-Basha, M.; Nyflot, M.; Sponseller, P.A.; Wu, W.; Hofstetter, C.P.; Saigal, R.; Bowen, S.R.; Hippe, D.S.; et al. The dancing cord: Inherent spinal cord motion and its effect on cord dose in spine stereotactic body radiation therapy. Neurosurgery 2020, 87, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Cox, B.W.; Spratt, D.E.; Lovelock, M.; Bilsky, M.H.; Lis, E.; Ryu, S.; Sheehan, J.; Gerszten, P.C.; Chang, E.; Gibbs, I.; et al. International Spine Radiosurgery Consortium consensus guidelines for target volume definition in spinal stereotactic radiosurgery. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, e597–e605. [Google Scholar] [CrossRef] [PubMed]
- Kowalchuk, R.O.; Mullikin, T.C.; Spears, G.M.; Johnson-Tesch, B.A.; Rose, P.S.; Siontis, B.L.; Kim, D.K.; Costello, B.A.; Morris, J.M.; Gao, R.W.; et al. Assessment of minimum target dose as a predictor of local failure after spine SBRT. Radiother. Oncol. 2024, 195, 110260. [Google Scholar] [CrossRef] [PubMed]
- Guckenberger, M.; Lievens, Y.; Bouma, A.B.; Collette, L.; Dekker, A.; deSouza, N.M.; Dingemans, A.C.; Fournier, B.; Hurkmans, C.; Lecouvet, F.E.; et al. Characterisation and classification of oligometastatic disease: A European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol. 2020, 21, e18–e28. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.; Jiang, W.; Mou, B.; Lund, C.R.; Liu, M.; Bergman, A.M.; Schellenberg, D.; Alexander, A.S.; Carolan, H.; Atrchian, S.; et al. Progression-free survival and local control after SABR for up to 5 oligometastases: An analysis from the population-based phase 2 SABR-5 trial. Int. J. Radiat. Oncol. Biol. Phys. 2022, 114, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Willmann, J.; Vlaskou Badra, E.; Adilovic, S.; Ahmadsei, M.; Christ, S.M.; van Timmeren, J.E.; Kroeze, S.G.C.; Mayinger, M.; Guckenberger, M.; Andratschke, N. Evaluation of the prognostic value of the ESTRO EORTC classification of oligometastatic disease in patients treated with stereotactic body radiotherapy: A retrospective single center study. Radiother. Oncol. 2022, 168, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Olson, R.; Jiang, W.; Liu, M.; Bergman, A.; Schellenberg, D.; Mou, B.; Alexander, A.; Carolan, H.; Hsu, F.; Miller, S.; et al. Treatment with stereotactic ablative radiotherapy for up to 5 oligometastases in patients with cancer primary toxic effect results of the nonrandomized Phase 2 SABR-5 clinical trial. JAMA Oncol. 2022, 8, 1644–1650. [Google Scholar] [CrossRef]
- Baker, S.; Mou, B.; Jiang, W.; Liu, M.; Bergman, A.M.; Schellenberg, D.; Alexander, A.S.; Carolan, H.; Atrchian, S.; Berrang, T.; et al. Predictors of early polymetastatic relapse after SABR for up to 5 oligometastases: A secondary analysis of the phase II SABR-5 trial. Int. J. Radiat. Oncol. Biol. Phys. 2022, 114, 856–861. [Google Scholar] [CrossRef]
- Franceschini, D.; Teriaca, M.A.; Mancosu, P.; Bertolini, A.; Faro, L.L.; Spoto, R.; Dominici, L.; Badalamenti, M.; Bellu, L.; Dei, D.; et al. Prospective phase II trial on ablative stereotactic body radiation therapy (SBRT) for medically inoperable thoracic nodes metastases. Radiother. Oncol. 2024, 197, 110335. [Google Scholar] [CrossRef]
- Shahi, J.; Poon, I.; Ung, Y.C.; Tsao, M.; Bjarnason, G.A.; Malik, N.H.; Zhang, L.; Louie, A.V.; Cheung, P. Stereotactic body radiation therapy for mediastinal and hilar lymph node metastases. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 764–774. [Google Scholar] [CrossRef]
- Cereno, R.E.; Mou, B.; Baker, S.; Chng, N.; Arbour, G.; Bergman, A.; Liu, M.; Schellenberg, D.; Matthews, Q.; Huang, V.; et al. Should organs at risk (OARs) be prioritized over target volume coverage in stereotactic ablative radiotherapy (SABR) for oligometastases? a secondary analysis of the population-based phase II SABR-5 trial. Radiother. Oncol. 2023, 182, 109576. [Google Scholar] [CrossRef] [PubMed]
- Skakodub, A.; Walch, H.; Tringale, K.R.; Eichholz, J.; Imber, B.S.; Vasudevan, H.N.; Li, B.T.; Moss, N.S.; Hei Yu, K.K.; Mueller, B.A.; et al. Genomic analysis and clinical correlations of non-small cell lung cancer brain metastasis. Nat. Commun. 2023, 14, 4980. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.; Baxter, D.H.; Olson, K.B. The management of metastases to the brain by irradiation and corticosteroids. Am. J. Roentgenol. Radium Ther. Nucl. Med. 1971, 111, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Pitz, M.W.; Desai, A.; Grossman, S.A.; Blakeley, J.O. Tissue concentration of systemically administered antineoplastic agents in human brain tumors. J. Neurooncol. 2011, 104, 629–638. [Google Scholar] [CrossRef]
- Iyengar, P.; All, S.; Berry, M.F.; Boike, T.P.; Bradfield, L.; Dingemans, A.-M.C.; Feldman, J.; Gomez, D.R.; Hesketh, P.J.; Jabbour, S.K.; et al. Treatment of oligometastatic non-small cell lung cancer: An ASTRO/ESTRO clinical practice guideline. Pract. Radiat. Oncol. 2023, 13, 393–412. [Google Scholar] [CrossRef]
- Gondi, V.; Bauman, G.; Bradfield, L.; Burri, S.H.; Cabrera, A.R.; Cunningham, D.A.; Eaton, B.R.; Hattangadi-Gluth, J.A.; Kim, M.M.; Kotecha, R.; et al. Radiation therapy for brain metastases: An ASTRO clinical practice guideline. Pract. Radiat. Oncol. 2022, 12, 265–282. [Google Scholar] [CrossRef]
- Brown, P.D.; Ballman, K.V.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Whitton, A.C.; Greenspoon, J.; Parney, I.F.; Laack, N.N.I.; Ashman, J.B.; et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): A multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1049–1060. [Google Scholar] [CrossRef]
- Aoyama, H.; Shirato, H.; Tago, M.; Nakagawa, K.; Toyoda, T.; Hatano, K.; Kenjyo, M.; Oya, N.; Hirota, S.; Shioura, H.; et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs. stereotactic radiosurgery alone for treatment of brain metastases: A randomized controlled trial. JAMA 2006, 295, 2483–2491. [Google Scholar] [CrossRef]
- Muacevic, A.; Wowra, B.; Siefert, A.; Tonn, J.-C.; Steiger, H.-J.; Kreth, F.W. Microsurgery plus whole brain irradiation versus Gamma Knife surgery alone for treatment of single metastases to the brain: A randomized controlled multicentre phase III trial. J. Neurooncol. 2008, 87, 299–307. [Google Scholar] [CrossRef]
- Chang, E.L.; Wefel, J.S.; Hess, K.R.; Allen, P.K.; Lang, F.F.; Kornguth, D.G.; Arbuckle, R.B.; Swint, J.M.; Shiu, A.S.; Maor, M.H.; et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009, 10, 1037–1044. [Google Scholar] [CrossRef]
- Aoyama, H.; Tago, M.; Kato, N.; Toyoda, T.; Kenjyo, M.; Hirota, S.; Shioura, H.; Inomata, T.; Kunieda, E.; Hayakawa, K.; et al. Neurocognitive function of patients with brain metastasis who received either whole brain radiotherapy plus stereotactic radiosurgery or radiosurgery alone. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 1388–1395. [Google Scholar] [CrossRef]
- Aoyama, H.; Tago, M.; Shirato, H. Japanese radiation oncology study group 99-1 (JROSG 99-1) investigators stereotactic radiosurgery with or without whole-brain radiotherapy for brain metastases: Secondary analysis of the JROSG 99-1 randomized clinical trial. JAMA Oncol. 2015, 1, 457–464. [Google Scholar] [CrossRef]
- Lim, S.H.; Lee, J.Y.; Lee, M.-Y.; Kim, H.S.; Lee, J.; Sun, J.-M.; Ahn, J.S.; Um, S.-W.; Kim, H.; Kim, B.S.; et al. A randomized phase III trial of stereotactic radiosurgery (SRS) versus observation for patients with asymptomatic cerebral oligo-metastases in non-small-cell lung cancer. Ann. Oncol. 2015, 26, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.D.; Jaeckle, K.; Ballman, K.V.; Farace, E.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Barker, F.G., II; Deming, R.; Burri, S.H.; et al. Effect of radiosurgery alone vs. radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: A randomized clinical trial. JAMA 2016, 316, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Churilla, T.M.; Ballman, K.V.; Brown, P.D.; Twohy, E.L.; Jaeckle, K.; Farace, E.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Garces, Y.I.; et al. Stereotactic radiosurgery with or without whole-brain radiation therapy for limited brain metastases: A secondary analysis of the North Central Cancer Treatment Group N0574 (alliance) randomized controlled trial. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 1173–1178. [Google Scholar] [CrossRef]
- Bodensohn, R.; Kaempfel, A.-L.; Boulesteix, A.-L.; Orzelek, A.M.; Corradini, S.; Fleischmann, D.F.; Forbrig, R.; Garny, S.; Hadi, I.; Hofmaier, J.; et al. Stereotactic radiosurgery versus whole-brain radiotherapy in patients with 4-10 brain metastases: A nonrandomized controlled trial. Radiother. Oncol. 2023, 186, 109744. [Google Scholar] [CrossRef]
- Scorsetti, M.; Navarria, P.; Cozzi, L.; Clerici, E.; Bellu, L.; Franceschini, D.; Marzo, A.M.; Franzese, C.; Torri, V.; Reggiori, G.; et al. Radiosurgery of limited brain metastases from primary solid tumor: Results of the randomized phase III trial (NCT02355613) comparing treatments executed with a specialized or a C-arm linac-based platform. Radiat. Oncol. 2023, 18, 28. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Serizawa, T.; Shuto, T.; Akabane, A.; Higuchi, Y.; Kawagishi, J.; Yamanaka, K.; Sato, Y.; Jokura, H.; Yomo, S.; et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): A multi-institutional prospective observational study. Lancet Oncol. 2014, 15, 387–395. [Google Scholar] [CrossRef]
- Yamamoto, M.; Serizawa, T.; Higuchi, Y.; Sato, Y.; Kawagishi, J.; Yamanaka, K.; Shuto, T.; Akabane, A.; Jokura, H.; Yomo, S.; et al. A multi-institutional prospective observational study of stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901 study update): Irradiation-related complications and long-term maintenance of mini-mental state examination scores. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 31–40. [Google Scholar] [CrossRef]
- Korytko, T.; Radivoyevitch, T.; Colussi, V.; Wessels, B.W.; Pillai, K.; Maciunas, R.J.; Einstein, D.B. 12 Gy Gamma Knife radiosurgical volume is a predictor for radiation necrosis in non-AVM intracranial tumors. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 419–424. [Google Scholar] [CrossRef]
- Blonigen, B.J.; Steinmetz, R.D.; Levin, L.; Lamba, M.A.; Warnick, R.E.; Breneman, J.C. Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 996–1001. [Google Scholar] [CrossRef]
- Minniti, G.; Clarke, E.; Lanzetta, G.; Osti, M.F.; Trasimeni, G.; Bozzao, A.; Romano, A.; Enrici, R.M. Stereotactic radiosurgery for brain metastases: Analysis of outcome and risk of brain radionecrosis. Radiat. Oncol. 2011, 6, 48. [Google Scholar] [CrossRef]
- Williams, B.J.; Suki, D.; Fox, B.D.; Pelloski, C.E.; Maldaun, M.V.C.; Sawaya, R.E.; Lang, F.F.; Rao, G. Stereotactic radiosurgery for metastatic brain tumors: A comprehensive review of complications: Clinical article. J. Neurosurg. 2009, 111, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Redmond, K.J.; Gui, C.; Benedict, S.; Milano, M.T.; Grimm, J.; Vargo, J.A.; Soltys, S.G.; Yorke, E.; Jackson, A.; El Naqa, I.; et al. Tumor control probability of radiosurgery and fractionated stereotactic radiosurgery for brain metastases. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 53–67. [Google Scholar] [CrossRef]
- Minniti, G.; Scaringi, C.; Paolini, S.; Lanzetta, G.; Romano, A.; Cicone, F.; Osti, M.; Enrici, R.M.; Esposito, V. Single-fraction versus multifraction (3 × 9 Gy) stereotactic radiosurgery for large (>2 cm) brain metastases: A comparative analysis of local control and risk of radiation-induced brain necrosis. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Remick, J.S.; Kowalski, E.; Khairnar, R.; Sun, K.; Morse, E.; Cherng, H.R.R.; Poirier, Y.; Lamichhane, N.; Becker, S.J.; Chen, S.; et al. A multi-center analysis of single-fraction versus hypofractionated stereotactic radiosurgery for the treatment of brain metastasis. Radiat. Oncol. 2020, 15, 128. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.K.; Seto, K.; Nozaki, A.; Torikai, K.; Suzuki, Y.; Saitoh, J.; Noda, S.; Nakano, T. Three-fraction CyberKnife radiotherapy for brain metastases in critical areas: Referring to the risk evaluating radiation necrosis and the surrounding brain volumes circumscribed with a single dose equivalence of 14 Gy (V14). J. Radiat. Res. 2013, 54, 727–735. [Google Scholar] [CrossRef]
- Ryken, T.C.; Kuo, J.S.; Prabhu, R.S.; Sherman, J.H.; Kalkanis, S.N.; Olson, J.J. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guidelines on the Role of Steroids in the Treatment of Adults with Metastatic Brain Tumors. Neurosurgery 2019, 84, E189–E191. [Google Scholar] [CrossRef]
- Vellayappan, B.; Lim-Fat, M.J.; Kotecha, R.; De Salles, A.; Fariselli, L.; Levivier, M.; Ma, L.; Paddick, I.; Pollock, B.E.; Regis, J.; et al. A Systematic Review Informing the Management of Symptomatic Brain Radiation Necrosis after Stereotactic Radiosurgery and International Stereotactic Radiosurgery Society Recommendations. Int. J. Radiat. Oncol. Biol. Phys. 2024, 118, 14–28. [Google Scholar] [CrossRef]
- Koide, Y.; Nagai, N.; Adachi, S.; Ito, M.; Kawamura, M.; Ito, M.; Ito, F.; Shindo, Y.; Aoyama, T.; Shimizu, H.; et al. Impact of concurrent antibody-drug conjugates and radiotherapy on symptomatic radiation necrosis in breast cancer patients with brain metastases: A multicenter retrospective study. J. Neurooncol. 2024, 168, 415–423. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, W.S.; Kwon, D.H.; Cho, Y.H.; Choi, C.M. Effects of an epithelial growth factor receptor-tyrosine kinase inhibitor add-on in stereotactic radiosurgery for brain metastases originating from non-small-cell lung cancer. J. Korean Neurosurg. Soc. 2015, 58, 205–210. [Google Scholar] [CrossRef]
- Tatineni, V.; O’Shea, P.J.; Saxena, S.; Khosla, A.A.; Ozair, A.; Kotecha, R.R.; Jia, X.; Rauf, Y.; Murphy, E.S.; Chao, S.T.; et al. Combination of EGFR-directed tyrosine kinase inhibitors (EGFR-TKI) with radiotherapy in brain metastases from non-small cell lung cancer: A 2010-2019 retrospective cohort study. Cancers 2023, 15, 3015. [Google Scholar] [CrossRef] [PubMed]
- Chiou, G.Y.; Chiang, C.L.; Yang, H.C.; Shen, C.I.; Wu, H.M.; Chen, Y.W.; Chen, C.J.; Luo, Y.H.; Hu, Y.S.; Lin, C.J.; et al. Combined stereotactic radiosurgery and tyrosine kinase inhibitor therapy versus tyrosine kinase inhibitor therapy alone for the treatment of non-small cell lung cancer patients with brain metastases. J. Neurosurg. 2022, 137, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Miller, J.A.; Kotecha, R.; Xiao, R.; Juloori, A.; Ward, M.C.; Ahluwalia, M.S.; Mohammadi, A.M.; Peereboom, D.M.; Murphy, E.S.; et al. The risk of radiation necrosis following stereotactic radiosurgery with concurrent systemic therapies. J. Neurooncol. 2017, 133, 357–368. [Google Scholar] [CrossRef]
- Badrigilan, S.; Meola, A.; Chang, S.D.; Rezaeian, S.; Nemati, H.; Almasi, T.; Rostampour, N. Stereotactic radiosurgery with immune checkpoint inhibitors for brain metastases: A meta-analysis study. Br. J. Neurosurg. 2022, 37, 1533–1543. [Google Scholar] [CrossRef]
- Martin, A.M.; Cagney, D.N.; Catalano, P.J.; Alexander, B.M.; Redig, A.J.; Schoenfeld, J.D.; Aizer, A.A. Immunotherapy and symptomatic radiation necrosis in patients with brain metastases treated with stereotactic radiation. JAMA Oncol. 2018, 4, 1123–1124. [Google Scholar] [CrossRef]
- Skrepnik, T.; Sundararajan, S.; Cui, H.; Stea, B. Improved time to disease progression in the brain in patients with melanoma brain metastases treated with concurrent delivery of radiosurgery and ipilimumab. Oncoimmunology 2017, 6, e1283461. [Google Scholar] [CrossRef] [PubMed]
- McClelland, S., III; Lautenschlaeger, T.; Zang, Y.; Hanna, N.H.; Shiue, K.; Kamer, A.P.; Agrawal, N.; Ellsworth, S.G.; Rhome, R.M.; Watson, G.A. Radiosurgery dose reduction for brain metastases on immunotherapy (RADREMI): A prospective phase I study protocol. Rep. Pract. Oncol. Radiother. 2020, 25, 500–506. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhuang, H. Effect of stereotactic radiotherapy on immune microenvironment of lung cancer. Front. Immunol. 2022, 13, 1025872. [Google Scholar] [CrossRef]
- Chang, J.Y.; Lin, S.H.; Dong, W.; Liao, Z.; Gandhi, S.J.; Gay, C.M.; Zhang, J.; Chun, S.G.; Elamin, Y.Y.; Fossella, F.V.; et al. Stereotactic ablative radiotherapy with or without immunotherapy for early-stage or isolated lung parenchymal recurrent node-negative non-small-cell lung cancer: An open-label, randomised, phase 2 trial. Lancet 2023, 402, 871–881. [Google Scholar] [CrossRef]
- Robinson, C.G.; Xing, L.; Tanaka, H.; Tasaka, S.; Badiyan, S.N.; Nasrallah, H.; Biswas, T.; Shtivelband, M.; Schuette, W.; Shi, A.; et al. Phase 3 study of durvalumab with SBRT for unresected stage I/II, lymph-node negative NSCLC (PACIFIC-4/RTOG3515). J. Clin. Oncol. 2023, 41, TPS8607. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef] [PubMed]
- Bauml, J.M.; Mick, R.; Ciunci, C.; Aggarwal, C.; Davis, C.; Evans, T.; Deshpande, C.; Miller, L.; Patel, P.; Alley, E.; et al. Pembrolizumab after completion of locally ablative therapy for oligometastatic non-small cell lung cancer: A phase 2 trial. JAMA Oncol. 2019, 5, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Y.; Yang, Z.; Hu, M.; Lu, J.; Zhang, Y.; Qian, F.; Zhang, B.; Wang, S.; Wang, K.; et al. Local consolidative therapy for synchronous oligometastatic non-small cell lung cancer treated with first-line pembrolizumab: A retrospective observational study. Thorac. Cancer 2022, 13, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gao, M.; Huang, Z.; Yu, J.; Meng, X. SBRT combined with PD-1/PD-L1 inhibitors in NSCLC treatment: A focus on the mechanisms, advances, and future challenges. J. Hematol. Oncol. 2020, 13, 105. [Google Scholar] [CrossRef]
- Zayed, S.; Louie, A.V.; Breadner, D.A.; Palma, D.A.; Correa, R.J.M. Radiation and immune checkpoint inhibitors in the treatment of oligometastatic non-small-cell lung cancer: A practical review of rationale, recent data, and research questions. Ther. Adv. Med. Oncol. 2023, 15, 17588359231183668. [Google Scholar] [CrossRef]
- Miyawaki, T.; Kenmotsu, H.; Harada, H.; Ohde, Y.; Chiba, Y.; Haratani, K.; Okimoto, T.; Sakamoto, T.; Wakuda, K.; Ito, K.; et al. Phase II study of multidisciplinary therapy combined with pembrolizumab for patients with synchronous oligometastatic non-small cell lung cancer TRAP OLIGO study (WJOG11118L). BMC Cancer 2021, 21, 1121. [Google Scholar] [CrossRef]
- Nakamura, M.; Ishikawa, H.; Ohnishi, K.; Mori, Y.; Baba, K.; Nakazawa, K.; Shiozawa, T.; Sekine, I.; Maruo, K.; Okumura, T.; et al. Effects of lymphopenia on survival in proton therapy with chemotherapy for non-small cell lung cancer. J. Radiat. Res. 2023, 64, 438–447. [Google Scholar] [CrossRef]
- Sukhera, J. Narrative reviews: Flexible, rigorous, and practical. J. Grad. Med. Educ. 2022, 14, 414–417. [Google Scholar] [CrossRef]
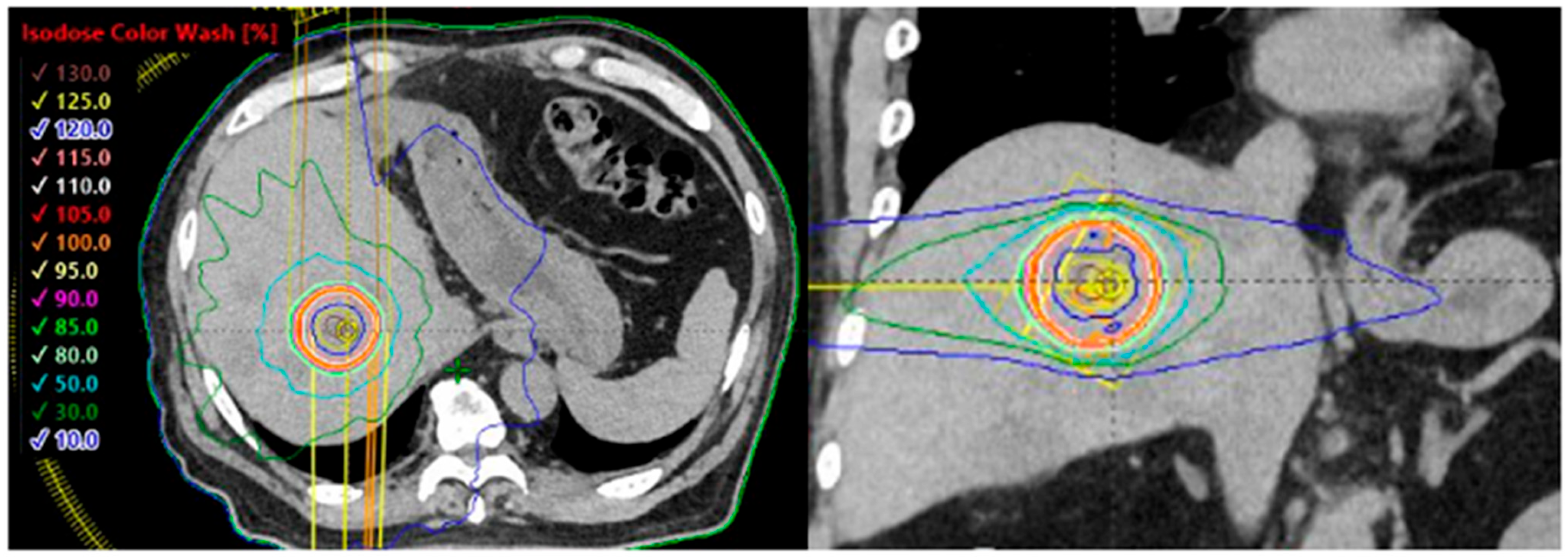

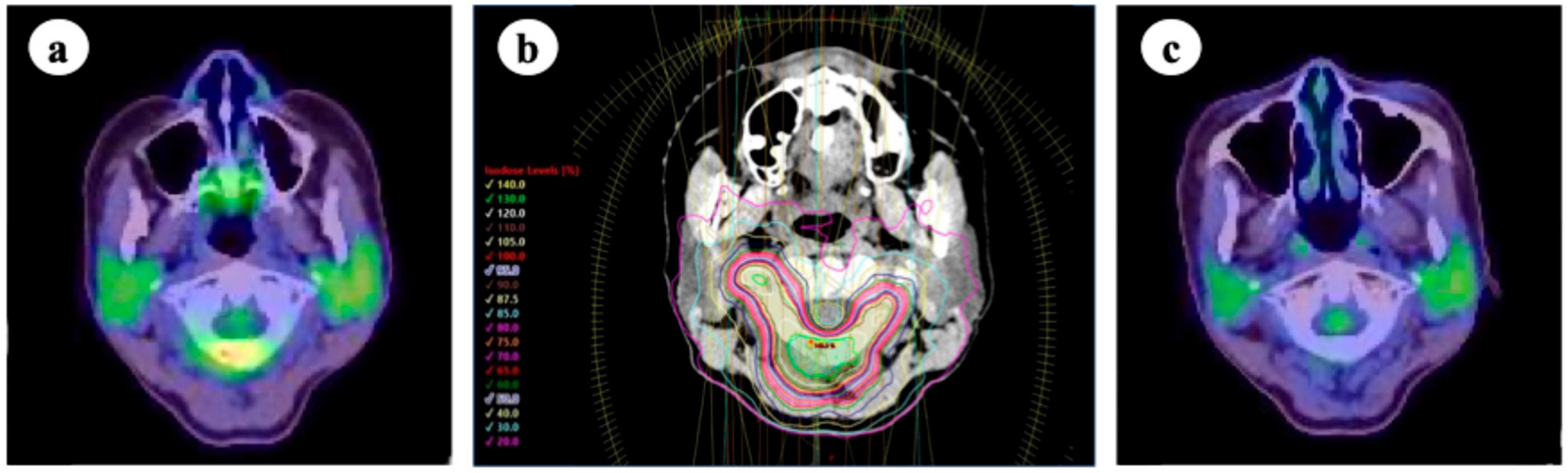
| Author | Year | Patient Number | Lung Cancer (%) | Treatment Modalities | Overall Survival | Local Control | Grade ≥ 3 AEs |
|---|---|---|---|---|---|---|---|
| Lee [14] | 2018 | 21 | 13.3% | SBRT (60 Gy in 3 fr or 48 Gy in 4 fr) | 2 y: 68.2% | 2 y: 75.3% | 4.8% |
| 9.5% | Wedge resection, lobectomy | 2 y: 81.8% | 2 y: 91.5% | 10.0% | |||
| Kanzaki [15] | 2020 | 82 | N/A | SBRT (52 Gy in 4 fr or 60 Gy in 10 fr) | 3 y: 52% | 3 y: 92% | N/A |
| N/A | Wedge resection, segmentectomy, lobectomy | 3 y: 77% | 3 y: 88% | N/A | |||
| Gits [11] | 2024 | 644 | N/A | SBRT | 2 y: 63.3% * | N/A | 2.6% |
| N/A | Sublobar resection | 2 y: 80.3% * | N/A | 2.0% | |||
| N/A | Percutaneous thermal ablation | 2 y: 83.8% * | N/A | 2.4% | |||
| Song [16] | 2024 | 54 | 0% (Sarcoma) | SBRT (48–60 Gy in 3–10 fr) | 3 y: 57.3% * | 3 y: 92.3% | 0% |
| Wedge resection, segmentectomy, lobectomy | 3 y: 84.6% * | 3 y: 92.9% | N/A | ||||
| Wang [12] | 2025 | 335 | 0% (CRC) | SBRT (30–70 Gy in 1–10 fr) | 3 y: 78.9% * | N/A | 0% |
| Wedge resection, segmentectomy, lobectomy | 3 y: 85.9% * | N/A | 0% | ||||
| Shin [17] | 2025 | 209 | 0% (HCC) | SBRT (40–60 Gy in 3–6 fr) | 2 y: 83.0% * | 2 y: 97.8% | 0% |
| Wedge resection, segmentectomy, lobectomy | 2 y: 72.6% * | 2 y: 98.0% | 0% |
| Author | Year | Trial | Patient (Tumor) Number | Lung Cancer (%) | Dose Prescription | Follow-Up | Overall Survival | Local Control | Grade ≥ 3 AEs |
|---|---|---|---|---|---|---|---|---|---|
| Rusthoven [34] | 2009 | Phase 1/2 | 47 (63) | 10 | 36–60 Gy in 3 fr | 16 m | Median: 20.5 m | 1 y/2 y: 95%/92% | <2% with no RILD |
| Lee [40] | 2009 | Phase 1 | 68 (143) | 2 | 27.7–60 Gy in 6 fr | 11 m | Median: 17.6 m | 1 y: 71% | 10% with no RILD |
| Scorsetti [33] | 2018 | Phase 2 | 61 (76) | N/A | 52.5–75 Gy in 3 fr | 61 m | 1 y/3 y: 85.2%/31.1% | 1 y/3 y: 94%/78% | 1.6% |
| Folkert [41] | 2021 | Phase 1 | 33 (39) | N/A | 35–40 Gy in 1 fr | 26 m | 2 y: 82% | 4 y: 96.6% | 0% |
| Mahadevan [42] | 2018 | Retrospective | 427 (568) | 52 | 45 (12–60) Gy in 3 (1–5) fr | 14 m | Median: 22 m | Median: 55 m | N/A |
| Author | Year | Patient Number | Dose Prescription | Overall Survival | Local Control | Grade ≥ 3 AEs |
|---|---|---|---|---|---|---|
| Holy [60] | 2011 | 13 | 15–40 Gy in 3–6 fr | Median: 23 m | Overall: 77% | N/A |
| Gamsiz [61] | 2015 | 15 | 30 Gy in 3 fr | 16 m: 33% | 16 m: 87% | 0% |
| Zhao [62] | 2018 | 32 | 32–50 Gy in 3–8 fr | 1 y: 58% | 1 y: 97% | 0.3% |
| Arcidiacono [63] | 2020 | 37 | 30–50 Gy in 5 fr | 2 y: 68% | 2 y: 54% | 0% |
| Rzazade [64] | 2022 | 44 | 45–50 Gy in 5 fr | 2 y: 57% | 2 y: 91% | 0% |
| Author | Year | Patient Number | Lung Cancer (%) | BMs | Dose Prescription | Overall Survival | Local Control | Intracranial Progression-Free Survival | Radiation Necrosis |
|---|---|---|---|---|---|---|---|---|---|
| Aoyama [98] | 2006 | 67 | 67% | 1–4 (<3 cm) | <2 cm: 22–25 Gy in 1 f >2 cm: 18–20 Gy in 1 fr | 1 y: 28.4% | 91% | 1 y: 23.6% | Grade 4: 1.5% |
| Muacevic [99] | 2008 | 31 | 32.3% | 1 (<3 cm) | 14–27 Gy in 1 fr | Median: 10.3 m | 1 y: 96.8% | 1 y: 74.2% | Grade 4: 3.2% |
| Chang [100] | 2009 | 30 | 43% | 1–3 | 15–20 Gy in 1 fr | Median: 15.2 m 1 y: 63% | 1 y: 67% | 1 y: 27% | Grade 4: 6.7% |
| Aoyama [101] Aoyama [102] | 2007 2015 | 45 | 100%, excluding SCLC | 1–4 (<3 cm) | <2 cm: 22–25 Gy in 1 fr >2 cm: 18–20 Gy in 1 fr | Median: 8.6 m 1y: 28.4% | 91% | Median: 6.2 m 1 y: 23.6% | Grade >3: 2.2% |
| Lim [103] | 2015 | 49 | 100%, excluding SCLC | 1–4 (<3 cm) | NA | Median: 14.6 m 1 y: 57% | 1 y: 84.6% | Median: 9.4 m 1 y: 44.1% | N/A |
| Brown [104] Churilla [105] | 2016 2017 | 111 | 72.1% | 1–3 (<3 cm) | <2 cm: 24 Gy in 1 f >2 cm: 20 Gy in 1 fr | Median: 10.4 m 1 y: 31.7% | 1 y: 72.8% | 1 y: 50.5% | Grade >3: 1.8% |
| Bodensohn [106] | 2023 | 40 | 60%, excluding SCLC | 4–10 (<2.5 cm) | 15–20 Gy in 1 fr | Median: 10.4 m 1 y: 48.2% | 100% | Median: 7.1 m | Grade >3: 0% |
| Scorsetti [107] | 2023 | 251 | 57%, excluding SCLC | 1–4 (<3 cm) | ≤2 cm: 24 Gy in 1 f >2.1–3 cm: 20 Gy in 1 fr | 1 y: 65.7% | 1 y: 98.2% | 1 y: 63.5% | Grade 3: 4.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shirai, K.; Endo, M.; Aoki, S.; Kishi, N.; Fukuda, Y.; Nonaka, T.; Ishikawa, H. Efficacy of Radiotherapy for Oligometastatic Lung Cancer and Irradiation Methods Based on Metastatic Site. Cancers 2025, 17, 2569. https://doi.org/10.3390/cancers17152569
Shirai K, Endo M, Aoki S, Kishi N, Fukuda Y, Nonaka T, Ishikawa H. Efficacy of Radiotherapy for Oligometastatic Lung Cancer and Irradiation Methods Based on Metastatic Site. Cancers. 2025; 17(15):2569. https://doi.org/10.3390/cancers17152569
Chicago/Turabian StyleShirai, Katsuyuki, Masashi Endo, Shuri Aoki, Noriko Kishi, Yukiko Fukuda, Tetsuo Nonaka, and Hitoshi Ishikawa. 2025. "Efficacy of Radiotherapy for Oligometastatic Lung Cancer and Irradiation Methods Based on Metastatic Site" Cancers 17, no. 15: 2569. https://doi.org/10.3390/cancers17152569
APA StyleShirai, K., Endo, M., Aoki, S., Kishi, N., Fukuda, Y., Nonaka, T., & Ishikawa, H. (2025). Efficacy of Radiotherapy for Oligometastatic Lung Cancer and Irradiation Methods Based on Metastatic Site. Cancers, 17(15), 2569. https://doi.org/10.3390/cancers17152569







