Recent Advances in Nanoparticle and Nanocomposite-Based Photodynamic Therapy for Cervical Cancer: A Review
Simple Summary
Abstract
1. Introduction
2. Architecture of Nanoparticles and Nanocomposites in the PDT of Cervical Cancer
3. Photosensitizers Used in Nanoplatforms for PDT of Cervical Cancer
4. Strategies for Delivering Nanosystems for Cervical Cancer
5. Other Strategies to Enhance the Effectiveness of PDT Using Nanosystems
6. Analysis of the Effectiveness of Nanosystems in PDT of Cervical Cancer
7. Safety Assessment of Nanosystems
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, M.; Wentzensen, N. From Human Papillomavirus to Cervical Cancer. Obstet. Gynecol. 2010, 116, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Ghittoni, R.; Accardi, R.; Hasan, U.; Gheit, T.; Sylla, B.; Tommasino, M. The Biological Properties of E6 and E7 Oncoproteins from Human Papillomaviruses. Virus Genes 2010, 40, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Meijer, C.J.L.M.; Steenbergen, R.D.M. Gynaecological Cancer: Novel Molecular Subtypes of Cervical Cancer—Potential Clinical Consequences. Nat. Rev. Clin. Oncol. 2017, 14, 397–398. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Integrated Genomic and Molecular Characterization of Cervical Cancer. Nature 2017, 543, 378–384. [Google Scholar] [CrossRef]
- Fleischmann, M.; Chatzikonstantinou, G.; Fokas, E.; Wichmann, J.; Christiansen, H.; Strebhardt, K.; Rödel, C.; Tselis, N.; Rödel, F. Molecular Markers to Predict Prognosis and Treatment Response in Uterine Cervical Cancer. Cancers 2021, 13, 5748. [Google Scholar] [CrossRef]
- Jiang, W.; Xiang, L.; Pei, X.; He, T.; Shen, X.; Wu, X.; Yang, H. Mutational analysis of KRAS and its clinical implications in cervical cancer patients. J. Gynecol. Oncology 2018, 29, e4. [Google Scholar] [CrossRef]
- Bahar, M.E.; Kim, H.J.; Kim, D.R. Targeting the RAS/RAF/MAPK Pathway for Cancer Therapy: From Mechanism to Clinical Studies. Signal Transduct. Target. Therapy 2023, 8, 455. Available online: https://www.nature.com/articles/s41392-023-01705-z (accessed on 29 July 2025). [CrossRef]
- Wang, B.; Li, X.; Liu, L.; Wang, M. β-Catenin: Oncogenic Role and Therapeutic Target in Cervical Cancer. Biol. Res. 2020, 53, 33. [Google Scholar] [CrossRef]
- Garg, P.; Krishna, M.; Subbalakshmi, A.R.; Ramisetty, S.; Mohanty, A.; Kulkarni, P.; Horne, D.; Salgia, R.; Singhal, S.S. Emerging Biomarkers and Molecular Targets for Precision Medicine in Cervical Cancer. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2024, 1879, 189106. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Rader, J.S.; Zhang, F.; Liapis, H.; Koki, A.T.; Masferrer, J.L.; Subbaramaiah, K.; Dannenberg, A.J. Cyclooxygenase-2 Is Overexpressed in Human Cervical Cancer. Clin. Cancer Res. 2001, 7, 429–434. [Google Scholar] [PubMed]
- Iida, K.; Nakayama, K.; Rahman, M.T.; Rahman, M.; Ishikawa, M.; Katagiri, A.; Yeasmin, S.; Otsuki, Y.; Kobayashi, H.; Nakayama, S.; et al. EGFR Gene Amplification Is Related to Adverse Clinical Outcomes in Cervical Squamous Cell Carcinoma, Making the EGFR Pathway a Novel Therapeutic Target. Br. J. Cancer 2011, 105, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.K.; Kornaga, E.N.; Klimowicz, A.C.; Enwere, E.K.; Dean, M.; Bebb, G.D.; Phan, T.; Ghatage, P.; Magliocco, A.M.; Lees-Miller, S.P.; et al. Expression of DNA Damage Response Proteins in Cervical Cancer Patients Treated with Radical Chemoradiotherapy. Gynecol. Oncol. 2017, 145, 176–184. [Google Scholar] [CrossRef]
- Jia, W.; Wu, Y.; Zhang, Q.; Gao, G.E.; Zhang, C.; Xiang, Y. Expression Profile of Circulating microRNAs as a Promising Fingerprint for Cervical Cancer Diagnosis and Monitoring. Mol. Clin. Oncol. 2015, 3, 851–858. [Google Scholar] [CrossRef]
- Liu, M.; Jia, J.; Wang, X.; Liu, Y.; Wang, C.; Fan, R. Long Non-Coding RNA HOTAIR Promotes Cervical Cancer Progression through Regulating BCL2 via Targeting miR-143-3p. Cancer Biol. Ther. 2018, 19, 391–399. [Google Scholar] [CrossRef]
- Paskeh, M.D.A.; Mirzaei, S.; Gholami, M.H.; Zarrabi, A.; Zabolian, A.; Hashemi, M.; Hushmandi, K.; Ashrafizadeh, M.; Aref, A.R.; Samarghandian, S. Cervical Cancer Progression Is Regulated by SOX Transcription Factors: Revealing Signaling Networks and Therapeutic Strategies. Biomed. Pharmacother. 2021, 144, 112335. [Google Scholar] [CrossRef]
- Truong, D.H.; Tran, P.T.T.; Tran, T.H. Nanoparticles as Carriers of Photosensitizers to Improve Photodynamic Therapy in Cancer. Pharm. Dev. Technol. 2024, 29, 221–235. [Google Scholar] [CrossRef]
- Moan, J.; Peng, Q. An Outline of the Hundred-Year History of PDT. Anticancer Res. 2003, 23, 3591–3600. [Google Scholar]
- Juarranz, A.; Jaén, P.; Sanz-Rodríguez, F.; Cuevas, J.; González, S. Photodynamic Therapy of Cancer. Basic Principles and Applications. Clin. Transl. Oncol. 2008, 10, 148–154. [Google Scholar] [CrossRef]
- Estébanez, S.; Lorente, C.; Kaufman, T.S.; Larghi, E.L.; Thomas, A.H.; Serrano, M.P. Photophysical and Photochemical Properties of 3-Methylpterin as a New and More Stable Pterin-Type Photosensitizer. Photochem. Photobiol. 2018, 94, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.; Wang, W.; Wang, J.; Gong, C.; Zhang, D.; Zhang, X. Probe-Free Direct Identification of Type I and Type II Photosensitized Oxidation Using Field-Induced Droplet Ionization Mass Spectrometry. Angew. Chem. Int. Engl. 2020, 59, 21515–21519. [Google Scholar] [CrossRef]
- Oleinick, N.L.; Morris, R.L.; Belichenko, I. The Role of Apoptosis in Response to Photodynamic Therapy: What, Where, Why, and How. Photochem. Photobiol. Sci. 2002, 1, 1–21. [Google Scholar] [CrossRef]
- Krammer, B. Vascular Effects of Photodynamic Therapy. Anticancer Res. 2001, 21, 4271–4277. [Google Scholar]
- Yi, G.; Hong, S.H.; Son, J.; Yoo, J.; Park, C.; Choi, Y.; Koo, H. Recent Advances in Nanoparticle Carriers for Photodynamic Therapy. Quant. Imaging Med. Surg. 2018, 8, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Zhou, X.; Zhang, J.; Shi, Y.; Zhong, L. Metal Nanoparticles for Photodynamic Therapy: A Potential Treatment for Breast Cancer. Molecules 2021, 26, 6532. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.-J.; Yang, X.-X.; Liu, R.-Q.; Zhao, D.; Guo, C.-X.; Zhu, A.-C.; Wen, M.-N.; Liu, Z.; Qu, G.-F.; Meng, H.-X. Pathological Mechanism of Photodynamic Therapy and Photothermal Therapy Based on Nanoparticles. Int. J. Nanomed. 2020, 15, 6827–6838. [Google Scholar] [CrossRef]
- Kah, G.; Chandran, R.; Abrahamse, H. Curcumin a Natural Phenol and Its Therapeutic Role in Cancer and Photodynamic Therapy: A Review. Pharmaceutics 2023, 15, 639. [Google Scholar] [CrossRef]
- Chen, H.; Tian, J.; He, W.; Guo, Z. H2O2-Activatable and O2-Evolving Nanoparticles for Highly Efficient and Selective Photodynamic Therapy against Hypoxic Tumor Cells. J. Am. Chem. Soc. 2015, 137, 1539–1547. [Google Scholar] [CrossRef]
- Jiang, W.; Liang, M.; Lei, Q.; Li, G.; Wu, S. The Current Status of Photodynamic Therapy in Cancer Treatment. Cancers 2023, 15, 585. [Google Scholar] [CrossRef]
- Auffan, M.; Rose, J.; Bottero, J.-Y.; Lowry, G.V.; Jolivet, J.-P.; Wiesner, M.R. Towards a Definition of Inorganic Nanoparticles from an Environmental, Health and Safety Perspective. Nat. Nanotech. 2009, 4, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Varghese, N.; Francis, T.; Shelly, M.; Nair, A.B. Nanocomposites of Polymer Matrices: Nanoscale Processing Request PDF. In Nanoscale Processing; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Idris, N.M.; Gnanasammandhan, M.K.; Zhang, J.; Ho, P.C.; Mahendran, R.; Zhang, Y. In Vivo Photodynamic Therapy Using Upconversion Nanoparticles as Remote-Controlled Nanotransducers. Nat. Med. 2012, 18, 1580–1585. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, S.; Bu, W.; Chen, Y.; Xiao, Q.; Liu, J.; Xing, H.; Zhou, L.; Peng, W.; Shi, J. A Uniform Sub-50 Nm-Sized Magnetic/Upconversion Fluorescent Bimodal Imaging Agent Capable of Generating Singlet Oxygen by Using a 980 Nm Laser. Chemistry 2012, 18, 7082–7090. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Koo, H.; Sun, I.-C.; Yuk, S.H.; Choi, K.; Kim, K.; Kwon, I.C. Tumor-Targeting Multi-Functional Nanoparticles for Theragnosis: New Paradigm for Cancer Therapy. Adv. Drug. Deliv. Rev. 2012, 64, 1447–1458. [Google Scholar] [CrossRef]
- Wáng, Y.X.J.; Idée, J.-M. A Comprehensive Literatures Update of Clinical Researches of Superparamagnetic Resonance Iron Oxide Nanoparticles for Magnetic Resonance Imaging. Quant Imaging Med. Surg. 2017, 7, 88–122. [Google Scholar] [CrossRef]
- Wang, A.Z.; Langer, R.; Farokhzad, O.C. Nanoparticle Delivery of Cancer Drugs. Annu. Rev. Med. 2012, 63, 185–198. [Google Scholar] [CrossRef]
- Hong, H.; Wang, F.; Zhang, Y.; Graves, S.A.; Eddine, S.B.Z.; Yang, Y.; Theuer, C.P.; Nickles, R.J.; Wang, X.; Cai, W. Red Fluorescent Zinc Oxide Nanoparticle: A Novel Platform for Cancer Targeting. ACS Appl. Mater. Interfaces 2015, 7, 3373–3381. [Google Scholar] [CrossRef]
- Bazak, R.; Houri, M.; El Achy, S.; Kamel, S.; Refaat, T. Cancer Active Targeting by Nanoparticles: A Comprehensive Review of Literature. J. Cancer. Res. Clin. Oncol. 2015, 141, 769–784. [Google Scholar] [CrossRef]
- Byrne, J.D.; Betancourt, T.; Brannon-Peppas, L. Active Targeting Schemes for Nanoparticle Systems in Cancer Therapeutics. Adv. Drug. Deliv. Rev. 2008, 60, 1615–1626. [Google Scholar] [CrossRef]
- Fang, C.; Zhang, M. Nanoparticle-Based Theragnostics: Integrating Diagnostic and Therapeutic Potentials in Nanomedicine. J. Control. Release 2010, 146, 2–5. [Google Scholar] [CrossRef]
- Pan, D.; Lanza, G.M.; Wickline, S.A.; Caruthers, S.D. Nanomedicine: Perspective and Promises with Ligand-Directed Molecular Imaging. Eur. J. Radiol. 2009, 70, 274–285. [Google Scholar] [CrossRef]
- Lee, D.E.; Koo, H.; Sun, I.C.; Ryu, J.H.; Kim, K.; Kwon, I.C. Multifunctional Nanoparticles for Multimodal Imaging and Theragnosis. Chem. Soc. Rev. 2012, 41, 2656–2672. [Google Scholar] [CrossRef]
- Zhou, T.-J.; Xing, L.; Fan, Y.-T.; Cui, P.-F.; Jiang, H.-L. Inhibition of Breast Cancer Proliferation and Metastasis by Strengthening Host Immunity with a Prolonged Oxygen-Generating Phototherapy Hydrogel. J. Control. Release 2019, 309, 82–93. [Google Scholar] [CrossRef]
- van Nostrum, C.F. Polymeric Micelles to Deliver Photosensitizers for Photodynamic Therapy. Adv. Drug. Deliv. Rev. 2004, 56, 9–16. [Google Scholar] [CrossRef]
- Choi, J.; Kim, H.; Choi, Y. Theranostic Nanoparticles for Enzyme-Activatable Fluorescence Imaging and Photodynamic/Chemo Dual Therapy of Triple-Negative Breast Cancer. Quant Imaging Med. Surg. 2015, 5, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, T.; Zhou, H.; Liu, C. Advancements in Nanocarrier Delivery Systems for Photodynamic Therapy in Lung Cancer. Int. J. Nanomed. 2025, 20, 6853–6874. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Sun, C.; Jiang, G.; Xin, Y. Recent Developments of Nanoparticles in the Treatment of Photodynamic Therapy for Cervical Cancer. Anticancer Agents Med. Chem. 2019, 19, 1809–1819. [Google Scholar] [CrossRef]
- Ghosh Chaudhuri, R.; Paria, S. Core/Shell Nanoparticles: Classes, Properties, Synthesis Mechanisms, Characterization, and Applications. Chem. Rev. 2012, 112, 2373–2433. [Google Scholar] [CrossRef]
- Almeida, D.R.S.; Gil, J.F.; Guillot, A.J.; Li, J.; Pinto, R.J.B.; Santos, H.A.; Gonçalves, G. Advances in Microfluidic-Based Core@ Shell Nanoparticles Fabrication for Cancer Applications. Adv. Healthc. Mater. 2024, 13, 2400946. [Google Scholar] [CrossRef]
- Lin, Z.; Zhu, T.; Zhong, X. NIR-Triggered NO Production Combined with Photodynamic Therapy for Tumor Treatment. Photodiagn. Photodyn. Ther. 2024, 49, 104241. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.; Chen, H.; Wu, Y.; Liu, J.; Che, H.; Zhang, Y.; Zhu, X. Motor-Cargo Structured Nanotractors for Augmented NIR Phototherapy via Gas-Boosted Tumor Penetration and Respiration-Impaired Mitochondrial Dysfunction. Adv. Healthc. Mater. 2024, 13, 2402063. [Google Scholar] [CrossRef] [PubMed]
- Nsubuga, A.; Morice, K.; Fayad, N.; Pini, F.; Josserand, V.; Le Guével, X.; Alhabi, A.; Henry, M.; Puchán Sánchez, D.; Plassais, N.; et al. Sub 20 Nm Upconversion Photosensitizers for Near-Infrared Photodynamic Theranostics. Adv. Funct. Mater. 2025, 35, 2410077. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Y.; Du, S.; Ren, J.; Jiang, H.; Zhang, L.; Zhu, J. Mitochondria-Targeting Upconversion Nanoparticles@MOF for Multiple-Enhanced Photodynamic Therapy in Hypoxic Tumor. ACS Appl. Mater. Interfaces 2023, 15, 35884–35894. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S. Perspectives for Upconverting Nanoparticles. ACS Nano 2017, 11, 10644–10653. [Google Scholar] [CrossRef]
- Ash, C.; Dubec, M.; Donne, K.; Bashford, T. Effect of Wavelength and Beam Width on Penetration in Light-Tissue Interaction Using Computational Methods. Lasers. Med. Sci. 2017, 32, 1909–1918. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, W.; Fan, M.; Xu, Z.; Jiang, Y.; Li, Z.; Zhai, P.; Zhang, X.; Chen, T.; Zhang, Y.; et al. AIEgen Photosensitizer-Loaded Silica Nanoparticles for Lysosomes-Targeting Photodynamic Therapy in Tumor. ACS Appl. Nano Mater. 2024, 7, 23504–23512. [Google Scholar] [CrossRef]
- Liu, J.; Chen, J.; Li, T.; Meng, P.; Lin, X.; Zhai, Y.; Zhu, Y.; Li, D.; Yang, D.; Zhang, S. Red Blood Cell Membrane-Camouflaged Red Phosphorus-Modified C3N4 for Enhanced Tumoral Photodynamic Therapy. Adv. Mater. Interfaces 2025, 12, 2500271. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, R.; Wang, X.; Duan, X.; Yan, X.; Liu, C.; Tian, W. Hyaluronic Acid Coated Nano-Particles for H2O2-Elevation Augmented Photo-/Chemodynamic Therapy. Int. J. Biol. Macromol. 2023, 245, 125523. [Google Scholar] [CrossRef]
- Albayati, T.M.; Alardhi, S.M.; Khalbas, A.H.; Humdi, Z.J.; Ali, N.S.; Salih, I.K.; Saady, N.M.C.; Zendehboudi, S.; Abdulrahman, M.A. Comprehensive Review of Mesoporous Silica Nanoparticles: Drug Loading, Release, and Applications as Hemostatic Agents. ChemistrySelect 2024, 9, e202400450. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T.; et al. Mesoporous Silica Nanoparticles in Drug Delivery and Biomedical Applications. Nanomedicine 2015, 11, 313–327. [Google Scholar] [CrossRef]
- Janczyk, A.; Wolnicka-Glubisz, A.; Chmura, A.; Elas, M.; Matuszak, Z.; Stochel, G.; Urbanska, K. NO-Dependent Phototoxicity of Roussin’s Black Salt against Cancer Cells. Nitric. Oxide. 2004, 10, 42–50. [Google Scholar] [CrossRef]
- Chen, J.; Chen, L.; Wu, Y.; Fang, Y.; Zeng, F.; Wu, S.; Zhao, Y. A H2O2-Activatable Nanoprobe for Diagnosing Interstitial Cystitis and Liver Ischemia-Reperfusion Injury via Multispectral Optoacoustic Tomography and NIR-II Fluorescent Imaging. Nat. Commun. 2021, 12, 6870. [Google Scholar] [CrossRef]
- Xiong, J.; Wu, M.; Chen, J.; Liu, Y.; Chen, Y.; Fan, G.; Liu, Y.; Cheng, J.; Wang, Z.; Wang, S.; et al. Cancer-Erythrocyte Hybrid Membrane-Camouflaged Magnetic Nanoparticles with Enhanced Photothermal-Immunotherapy for Ovarian Cancer. ACS Nano 2021, 15, 19756–19770. [Google Scholar] [CrossRef]
- Guo, J.; Agola, J.O.; Serda, R.; Franco, S.; Lei, Q.; Wang, L.; Minster, J.; Croissant, J.G.; Butler, K.S.; Zhu, W.; et al. Biomimetic Rebuilding of Multifunctional Red Blood Cells: Modular Design Using Functional Components. ACS Nano 2020, 14, 7847–7859. [Google Scholar] [CrossRef] [PubMed]
- Luk, B.T.; Fang, R.H.; Hu, C.-M.J.; Copp, J.A.; Thamphiwatana, S.; Dehaini, D.; Gao, W.; Zhang, K.; Li, S.; Zhang, L. Safe and Immunocompatible Nanocarriers Cloaked in RBC Membranes for Drug Delivery to Treat Solid Tumors. Theranostics 2016, 6, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.-Y.; Su, Y.-L.; Cheng, W.; Hu, P.-F.; Chiang, C.-S.; Chen, W.-T.; Hu, S.-H. Graphene Quantum Dots-Mediated Theranostic Penetrative Delivery of Drug and Photolytics in Deep Tumors by Targeted Biomimetic Nanosponges. Nano Lett. 2019, 19, 69–81. [Google Scholar] [CrossRef]
- Knopf-Marques, H.; Pravda, M.; Wolfova, L.; Velebny, V.; Schaaf, P.; Vrana, N.E.; Lavalle, P. Hyaluronic Acid and Its Derivatives in Coating and Delivery Systems: Applications in Tissue Engineering, Regenerative Medicine and Immunomodulation. Adv. Healthc. Mater. 2016, 5, 2841–2855. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Fu, J.; Li, R.; Zhang, F.; Ling, G.; Zhang, P. A Potential Carrier for Anti-Tumor Targeted Delivery-Hyaluronic Acid Nanoparticles. Carbohydr. Polym. 2019, 208, 356–364. [Google Scholar] [CrossRef]
- Hu, C.; Cun, X.; Ruan, S.; Liu, R.; Xiao, W.; Yang, X.; Yang, Y.; Yang, C.; Gao, H. Enzyme-Triggered Size Shrink and Laser-Enhanced NO Release Nanoparticles for Deep Tumor Penetration and Combination Therapy. Biomaterials 2018, 168, 64–75. [Google Scholar] [CrossRef]
- Chen, B.; Cao, J.; Zhang, K.; Zhang, Y.-N.; Lu, J.; Iqbal, M.Z.; Zhang, Q.; Kong, X. Synergistic Photodynamic and Photothermal Therapy of BODIPY-Conjugated Hyaluronic Acid Nanoparticles. J. Biomater. Sci. Polym. 2021, 32, 2028–2045. [Google Scholar] [CrossRef]
- Salathia, S.; Gigliobianco, M.R.; Casadidio, C.; Di Martino, P.; Censi, R. Hyaluronic Acid-Based Nanosystems for CD44 Mediated Anti-Inflammatory and Antinociceptive Activity. Int. J. Mol. Sci. 2023, 24, 7286. [Google Scholar] [CrossRef]
- Cen, Y.; Deng, W.-J.; Yang, Y.; Yu, R.-Q.; Chu, X. Core–Shell–Shell Multifunctional Nanoplatform for Intracellular Tumor-Related mRNAs Imaging and Near-Infrared Light Triggered Photodynamic–Photothermal Synergistic Therapy. Anal. Chem. 2017, 89, 10321–10328. [Google Scholar] [CrossRef]
- Han, R.; Yi, H.; Shi, J.; Liu, Z.; Wang, H.; Hou, Y.; Wang, Y. pH-Responsive Drug Release and NIR-Triggered Singlet Oxygen Generation Based on a Multifunctional Core-Shell-Shell Structure. Phys. Chem. Chem. Phys. PCCP 2016, 18, 25497–25503. [Google Scholar] [CrossRef]
- Ling, B.; Wang, Y.; Dong, H.; Chen, H.; Wang, L. Enzyme-Triggered Aggregation of Upconversion Nanoparticles for Targeted Photodynamic Therapy via NIR Irradiation. Nanoscale Adv. 2025, 7, 3068–3076. [Google Scholar] [CrossRef]
- Ling, B.; Yang, L.; Wang, C.; Dong, L.; Yang, Y.; Wang, L.; Zhang, J.; Yuan, Y. Cascade-Responsive Upconversion Nanoplatform for Efficient Cell Nucleus Targeting and Boosted Photodynamic Tumor Therapy. ACS Mater. Lett. 2024, 6, 5256–5265. [Google Scholar] [CrossRef]
- Xing, H.; Zhang, S.; Bu, W.; Zheng, X.; Wang, L.; Xiao, Q.; Ni, D.; Zhang, J.; Zhou, L.; Peng, W.; et al. Ultrasmall NaGdF4 Nanodots for Efficient MR Angiography and Atherosclerotic Plaque Imaging. Adv. Mater. 2014, 26, 3867–3872. [Google Scholar] [CrossRef]
- Ma, R.-F.; Zhang, Q.; Wang, Y.; Xu, Z.-R. Visualizing Mitochondrial ATP Fluctuations in Single Cells during Photodynamic Therapy by In-Situ SERS Three-Dimensional Imaging. Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 2024, 323, 124910. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Chen, S. Janus Nanoparticles: Preparation, Characterization, and Applications. Chem. Asian J. 2014, 9, 418–430. [Google Scholar] [CrossRef]
- Zhang, X.; Fu, Q.; Duan, H.; Song, J.; Yang, H. Janus Nanoparticles: From Fabrication to (bio)Applications. ACS Nano 2021, 15, 6147–6191. [Google Scholar] [CrossRef]
- Tran, L.-T.; Lesieur, S.; Faivre, V. Janus Nanoparticles: Materials, Preparation and Recent Advances in Drug Delivery. Expert Opin. Drug. Deliv. 2014, 11, 1061–1074. [Google Scholar] [CrossRef] [PubMed]
- Krafft, C.; Popp, J. The Many Facets of Raman Spectroscopy for Biomedical Analysis. Anal. Bioanal. Chem. 2015, 407, 699–717. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, S.; Kim, J.H.; Bulte, J.W.M.; Barman, I. Surface-Enhanced Raman Scattering: An Emerging Tool for Sensing Cellular Function. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1802. [Google Scholar] [CrossRef]
- Zhang, Y.; Hong, H.; Myklejord, D.V.; Cai, W. Molecular Imaging with SERS-Active Nanoparticles. Small 2011, 7, 3261–3269. [Google Scholar] [CrossRef]
- Chandra, A.; Kumar, V.; Garnaik, U.C.; Dada, R.; Qamar, I.; Goel, V.K.; Agarwal, S. Unveiling the Molecular Secrets: A Comprehensive Review of Raman Spectroscopy in Biological Research. ACS Omega 2024, 9, 50049–50063. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Wang, Y.; Jiang, Y.; Luo, S.; Li, M.; Cao, Y.; Ma, Y.; Tang, B. A Novel Multi-Carboxyl Functionalized MOF Platform for Effective Photodynamic Therapy with Hypoxia Modulation Based on Prominent Self-Oxygen Generation. Inorg. Chem. Front. 2024, 11, 1186–1197. [Google Scholar] [CrossRef]
- Khalil, I.E.; Fonseca, J.; Reithofer, M.R.; Eder, T.; Chin, J.M. Tackling Orientation of Metal-Organic Frameworks (MOFs): The Quest to Enhance MOF Performance. Coord. Chem. Rev. 2023, 481, 215043. [Google Scholar] [CrossRef]
- Zheng, Q.; Liu, X.; Zheng, Y.; Yeung, K.W.K.; Cui, Z.; Liang, Y.; Li, Z.; Zhu, S.; Wang, X.; Wu, S. The Recent Progress on Metal–Organic Frameworks for Phototherapy. Chem. Soc. Rev. 2021, 50, 5086–5125. [Google Scholar] [CrossRef]
- Feng, D.; Gu, Z.-Y.; Li, J.-R.; Jiang, H.-L.; Wei, Z.; Zhou, H.-C. Zirconium-Metalloporphyrin PCN-222: Mesoporous Metal–Organic Frameworks with Ultrahigh Stability as Biomimetic Catalysts. Angew. Chem. Int. Ed. 2012, 51, 10307–10310. [Google Scholar] [CrossRef]
- Saikolappan, S.; Kumar, B.; Shishodia, G.; Koul, S.; Koul, H.K. Reactive Oxygen Species and Cancer: A Complex Interaction. Cancer Lett. 2019, 452, 132–143. [Google Scholar] [CrossRef]
- Yang, N.; Xiao, W.; Song, X.; Wang, W.; Dong, X. Recent Advances in Tumor Microenvironment Hydrogen Peroxide-Responsive Materials for Cancer Photodynamic Therapy. Nano-Micro Lett. 2020, 12, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Tian, S.; Qiu, W.; Mo, L.; Lin, W. Hierarchical MOF@ AuNP/Hairpin Nanotheranostic for Enhanced Photodynamic Therapy via O2 Self-Supply and Cancer-Related MicroRNA Imaging In Vivo. Anal. Chem. 2023, 95, 16279–16288. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Y.; Ren, X.; Jia, R.; Si, L.; Bao, J.; Shi, Y.; Sun, J.; Zhong, Y.; Duan, P.-C.; et al. Microemulsion-Assisted Self-Assembly of Indium Porphyrin Photosensitizers with Enhanced Photodynamic Therapy. ACS Nano 2024, 18, 3161–3172. [Google Scholar] [CrossRef]
- Magna, G.; Monti, D.; Di Natale, C.; Paolesse, R.; Stefanelli, M. The Assembly of Porphyrin Systems in Well-Defined Nanostructures: An Update. Molecules 2019, 24, 4307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, P.; Dong, H.; Zhen, Y.; Liu, M.; Hu, W. Porphyrin Supramolecular 1D Structures via Surfactant-assisted Self-assembly. Adv. Mater. 2015, 27, 5379–5387. [Google Scholar] [CrossRef]
- Feng, X.; Liu, C.; Wang, X.; Jiang, Y.; Yang, G.; Wang, R.; Zheng, K.; Zhang, W.; Wang, T.; Jiang, J. Functional Supramolecular Gels Based on the Hierarchical Assembly of Porphyrins and Phthalocyanines. Front. Chem. 2019, 7, 336. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wang, Y.; Xu, Y.; Yan, Y.; Huang, J. Out-of-Plane Coordinated Porphyrin Nanotubes with Enhanced Singlet Oxygen Generation Efficiency. Sci. Rep. 2016, 6, 31339. [Google Scholar] [CrossRef]
- Subedi, D.R.; Reid, R.; D’Souza, P.F.; Nesterov, V.N.; D’Souza, F. Singlet Oxygen Generation in Peripherally Modified Platinum and Palladium Porphyrins: Effect of Triplet Excited State Lifetimes and Meso-Substituents on 1O2 Quantum Yields. Chempluschem 2022, 87, e202200010. [Google Scholar] [CrossRef] [PubMed]
- Ohno, O.; Kaizu, Y.; Kobayashi, H. J-aggregate Formation of a Water-soluble Porphyrin in Acidic Aqueous Media. J. Chem. Phys. 1993, 99, 4128–4139. [Google Scholar] [CrossRef]
- Ohno, O.; Kaizu, Y.; Kobayashi, H. J- and H-Aggregates of Porphyrin-Surfactant Complexes: Time-Resolved Fluorescence and Other Spectroscopic Studies. J. Phys. Chem. B 1993, 99, 4128–4139. [Google Scholar] [CrossRef]
- Yu, Q.; Wu, C.; Wang, F. J- and H-Aggregation of 5,10,15,20-Tetrakis-(4-Sulfonatophenyl)Porphyrin within Stimuli Responsive Complex Micelles via Electrostatic Interaction. Asian J. Chem. 2014, 26, 4269–4272. [Google Scholar] [CrossRef]
- Fan-Lin Meng, F.-L.; Qian, H.-L.; Yan, X.-P. Conjugation-Regulating Synthesis of High Photosensitizing Activity Porphyrin-Based Covalent Organic Frameworks for Photodynamic Inactivation of Bacteria. Talanta 2021, 223, 122536. [Google Scholar]
- Xu, S.; Yuan, Y.; Cai, X.; Zhang, C.-J.; Hu, F.; Liang, J.; Zhang, G.; Zhang, D.; Liu, B. Tuning the Singlet-Triplet Energy Gap: A Unique Approach to Efficient Photosensitizers with Aggregation-Induced Emission (AIE) Characteristics. Chem. Sci. 2015, 6, 5824–5830. [Google Scholar] [CrossRef] [PubMed]
- Jacques, S.L. Optical Properties of Biological Tissues: A Review. Phys. Med. Biol. 2013, 58, R37. [Google Scholar] [CrossRef]
- Elfeky, S.A.; Mahmoud, S.E.; Youssef, A.F. Applications of CTAB Modified Magnetic Nanoparticles for Removal of Chromium (VI) from Contaminated Water. J. Adv. Res. 2017, 8, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, Y.; Pan, Z.; Xu, C.; Zhang, X.; Li, M.; Wang, W.; Jia, F.; Wu, Y. OXPHOS-Targeted Nanoparticles for Boosting Photodynamic Therapy against Hypoxia Tumor. Int. J. Pharm. 2024, 654, 123943. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, W.; Liu, K.; Wang, S.; Li, C.; Wu, F.; Wang, S.; Tang, Y. Double-Layer Hollow Mesoporous Silica Nanoparticles for Ultrasound-Guided Photodynamic Treatment. Biomed. Mater. 2024, 19, 045006. [Google Scholar] [CrossRef]
- Croissant, J.G.; Fatieiev, Y.; Almalik, A.; Khashab, N.M. Mesoporous Silica and Organosilica Nanoparticles: Physical Chemistry, Biosafety, Delivery Strategies, and Biomedical Applications. Adv. Healthc. Mater. 2018, 7, 1700831. [Google Scholar] [CrossRef]
- Teng, Z.; Li, W.; Tang, Y.; Elzatahry, A.; Lu, G.; Zhao, D. Mesoporous Organosilica Hollow Nanoparticles: Synthesis and Applications. Adv. Mater. 2019, 31, 1707612. [Google Scholar] [CrossRef]
- Teng, Z.; Su, X.; Zheng, Y.; Zhang, J.; Liu, Y.; Wang, S.; Wu, J.; Chen, G.; Wang, J.; Zhao, D.; et al. A Facile Multi-Interface Transformation Approach to Monodisperse Multiple-Shelled Periodic Mesoporous Organosilica Hollow Spheres. J. Am. Chem. Soc. 2015, 137, 7935–7944. [Google Scholar] [CrossRef]
- Liu, Y.; Miyoshi, H.; Nakamura, M. Encapsulated Ultrasound Microbubbles: Therapeutic Application in Drug/Gene Delivery. J. Control. Release 2006, 114, 89–99. [Google Scholar] [CrossRef]
- Gao, X.; Guo, D.; Mao, X.; Shan, X.; He, X.; Yu, C. Perfluoropentane-Filled Chitosan Poly-Acrylic Acid Nanobubbles with High Stability for Long-Term Ultrasound Imaging in Vivo. Nanoscale 2021, 13, 5333–5343. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, K.; Yang, X.; Liu, M.; Cui, Y.; Li, Y.; Li, C.; Liu, S.; Lu, Y.; Zhang, Z.; et al. Embedding Atomically Dispersed Manganese/Gadolinium Dual Sites in Oxygen Vacancy-Enriched Biodegradable Bimetallic Silicate Nanoplatform for Potentiating Catalytic Therapy. Adv. Sci. 2024, 11, 2307424. [Google Scholar] [CrossRef]
- Zhuang, G.; Chen, Y.; Zhuang, Z.; Yu, Y.; Yu, J. Oxygen Vacancies in Metal Oxides: Recent Progress towards Advanced Catalyst Design. Sci. China Mater. 2020, 63, 2089–2118. [Google Scholar] [CrossRef]
- Pacchioni, G. Oxygen Vacancy: The Invisible Agent on Oxide Surfaces. Chemphyschem 2003, 4, 1041–1047. [Google Scholar] [CrossRef]
- Bi, X.; Du, G.; Kalam, A.; Sun, D.; Yu, Y.; Su, Q.; Xu, B.; Al-Sehemi, A.G. Tuning Oxygen Vacancy Content in TiO2 Nanoparticles to Enhance the Photocatalytic Performance. Chem. Eng. Sci. 2021, 234, 116440. [Google Scholar] [CrossRef]
- Meyerstein, D. Re-Examining Fenton and Fenton-like Reactions. Nat. Rev. Chem. 2021, 5, 595–597. [Google Scholar] [CrossRef]
- Liu, S.; Hu, Y.; Hu, C.; Xiong, Y.; Duan, M. Quantum Yields of Singlet Oxygen of Tetrakis (4-Carboxyphenyl) Porphyrin in Different Solvents. J. Porphyr. Phthalocyanines 2019, 23, 1084–1091. [Google Scholar] [CrossRef]
- Park, J.; Jiang, Q.; Feng, D.; Zhou, H.-C. Controlled Generation of Singlet Oxygen in Living Cells with Tunable Ratios of the Photochromic Switch in Metal–Organic Frameworks. Angew. Chem. Int. Ed. 2016, 55, 7188–7193. [Google Scholar] [CrossRef]
- Singh, S.; Aggarwal, A.; Bhupathiraju, N.V.S.D.K.; Arianna, G.; Tiwari, K.; Drain, C.M. Glycosylated Porphyrins, Phthalocyanines, and Other Porphyrinoids for Diagnostics and Therapeutics. Chem. Rev. 2015, 115, 10261–10306. [Google Scholar] [CrossRef] [PubMed]
- Eçik, E.T.; Bulut, O.; Kazan, H.H.; Şenkuytu, E.; Çoşut, B. Design of Novel Photosensitizers and Controlled Singlet Oxygen Generation for Photodynamic Therapy. N. J. Chem. 2021, 45, 16298–16305. [Google Scholar] [CrossRef]
- Li, X.; Zheng, B.-D.; Peng, X.-H.; Li, S.-Z.; Ying, J.-W.; Zhao, Y.; Huang, J.-D.; Yoon, J. Phthalocyanines as Medicinal Photosensitizers: Developments in the Last Five Years. Coord. Chem. Rev. 2019, 379, 147–160. [Google Scholar] [CrossRef]
- Leng, W.; Myers Kelley, A. Resonance Hyper-Raman Spectra of Zinc Phthalocyanine. J. Phys. Chem. A 2008, 112, 5925–5929. [Google Scholar] [CrossRef] [PubMed]
- Hak, A.; Ali, M.S.; Sankaranarayanan, S.A.; Shinde, V.R.; Rengan, A.K. Chlorin E6: A Promising Photosensitizer in Photo-Based Cancer Nanomedicine. ACS Appl. Bio Mater. 2023, 6, 349–364. [Google Scholar] [CrossRef]
- Alves, C.G.; Lima-Sousa, R.; de Melo-Diogo, D.; Louro, R.O.; Correia, I.J. IR780 Based Nanomaterials for Cancer Imaging and Photothermal, Photodynamic and Combinatorial Therapies. Int. J. Pharm. 2018, 542, 164–175. [Google Scholar] [CrossRef]
- Hishikawa, H.; Kaibori, M.; Tsuda, T.; Matsui, K.; Okumura, T.; Ozeki, E.; Yoshii, K. Near-Infrared Fluorescence Imaging and Photodynamic Therapy with Indocyanine Green Lactosomes Has Antineoplastic Effects for Gallbladder Cancer. Oncotarget 2019, 10, 5622–5631. [Google Scholar] [CrossRef]
- Shirata, C.; Kaneko, J.; Inagaki, Y.; Kokudo, T.; Sato, M.; Kiritani, S.; Akamatsu, N.; Arita, J.; Sakamoto, Y.; Hasegawa, K.; et al. Near-Infrared Photothermal/Photodynamic Therapy with Indocyanine Green Induces Apoptosis of Hepatocellular Carcinoma Cells through Oxidative Stress. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Millard, M.; Bernhard, Y.; Canilho, N.; Grandemange, S.; Parant, S.; Mourer, M.; Lassalle, H.-P.; Pasc, A. Enhanced Stability and Photothermal Efficiency of Indocyanine Green J-Aggregates by Nanoformulation with Calix(4)arene for Photothermal Therapy of Cancers. Colloids Surf. B Biointerfaces 2023, 230, 113516. [Google Scholar] [CrossRef]
- Castán, J.M.A.; Amruth, C.; Josse, P.; Galan, L.A.; Marqués, P.S.; Allain, M.; Maury, O.; Bahers, T.L.; Blanchard, P.; Monnereau, C.; et al. Thiochromenocarbazole Imide: A New Organic Dye with First Utility in Large Area Flexible Electroluminescent Devices. Mater. Chem. Front. 2022, 6, 1912–1919. [Google Scholar] [CrossRef]
- Andrés Castán, J.M.; Abidi, S.; Ghanem, T.; Touihri, S.; Blanchard, P.; Welch, G.C.; Zagranyarski, Y.; Boixel, J.; Walker, B.; Josse, P.; et al. N-Annulation of the BTI Rylene Imide Organic Building Block: Impact on the Optoelectronic Properties of π-Extended Molecular Structures. Colorants 2023, 2, 22–30. [Google Scholar] [CrossRef]
- Turksoy, A.; Yildiz, D.; Akkaya, E.U. Photosensitization and Controlled Photosensitization with BODIPY Dyes. Coord. Chem. Rev. 2019, 379, 47–64. [Google Scholar] [CrossRef]
- Kamkaew, A.; Lim, S.H.; Lee, H.B.; Kiew, L.V.; Chung, L.Y.; Burgess, K. BODIPY Dyes in Photodynamic Therapy. Chem. Soc. Rev. 2012, 42, 77–88. [Google Scholar] [CrossRef]
- Chan, M.-H.; Liu, R.-S.; Hsiao, M. Graphitic Carbon Nitride-Based Nanocomposites and Their Biological Applications: A Review. Nanoscale 2019, 11, 14993–15003. [Google Scholar] [CrossRef]
- Ji, T.; Zhao, Y.; Ding, Y.; Nie, G. Using Functional Nanomaterials to Target and Regulate the Tumor Microenvironment: Diagnostic and Therapeutic Applications. Adv. Mater. 2013, 25, 3508–3525. [Google Scholar] [CrossRef] [PubMed]
- Du, J.Z.; Mao, C.Q.; Yuan, Y.Y.; Yang, X.Z.; Wang, J. Tumor Extracellular Acidity-Activated Nanoparticles as Drug Delivery Systems for Enhanced Cancer Therapy. Biotechnol. Adv. 2014, 32, 789–803. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Nakamura, H.; Fang, J. The EPR Effect for Macromolecular Drug Delivery to Solid Tumors: Improvement of Tumor Uptake, Lowering of Systemic Toxicity, and Distinct Tumor Imaging in Vivo. Adv. Drug Deliv. Rev. 2013, 65, 71–79. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A New Concept for Macromolecular Therapeutics in Cancer Chemotherapy: Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Rao, C.; Liao, D.; Pan, Y.; Zhong, Y.; Zhang, W.; Ouyang, Q.; Liu, J. Novel Formulations of Metal-Organic Frameworks for Controlled Drug Delivery. Expert. Opin. Drug. Deliv. 2022, 19, 1183–1202. [Google Scholar] [CrossRef]
- Yousefi, Q.; Nezamzadeh-Ejhieh, A. A Comprehensive Study on RSM Optimization of the Influencing Variables on Loading/Releasing of Ciprofloxacin onto/from Magnetic Iron Nanoparticles. Solid State Sci. 2024, 154, 107584. [Google Scholar] [CrossRef]
- Tong, F.; Wang, Y.; Gao, H. Progress and Challenges in the Translation of Cancer Nanomedicines. Curr. Opin. Biotechnol. 2024, 85, 103045. [Google Scholar] [CrossRef]
- Jeon, S.; Jun, E.; Chang, H.; Yhee, J.Y.; Koh, E.Y.; Kim, Y.; Kim, S.C. Prediction the Clinical EPR Effect of Nanoparticles in Patient-Derived Xenograft Models. J. Control. Release 2022, 351, 37–49. [Google Scholar] [CrossRef]
- Fang, J.; Islam, W.; Maeda, H. Exploiting the Dynamics of the EPR Effect and Strategies to Improve the Therapeutic Effects of Nanomedicines by Using EPR Effect Enhancers. Adv. Drug. Deliv. Rev. 2020, 157, 142–160. [Google Scholar] [CrossRef]
- Maeda, H. Toward a Full Understanding of the EPR Effect in Primary and Metastatic Tumors as Well as Issues Related to Its Heterogeneity. Adv. Drug Deliv. Rev. 2015, 91, 3–6. [Google Scholar] [CrossRef]
- Lammers, T.; Kiessling, F.; Hennink, W.E.; Storm, G. Drug Targeting to Tumors: Principles, Pitfalls and (Pre-) Clinical Progress. J. Control. Release 2012, 161, 175–187. [Google Scholar] [CrossRef]
- Nguyen, L.N.M.; Ngo, W.; Lin, Z.P.; Sindhwani, S.; MacMillan, P.; Mladjenovic, S.M.; Chan, W.C.W. The Mechanisms of Nanoparticle Delivery to Solid Tumours. Nat. Rev. Bioeng. 2024, 2, 201–213. [Google Scholar] [CrossRef]
- Kadkhoda, J.; Tarighatnia, A.; Nader, N.D.; Aghanejad, A. Targeting Mitochondria in Cancer Therapy: Insight into Photodynamic and Photothermal Therapies. Life Sci. 2022, 307, 120898. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, M.D.; Xu, L.; Li, C.; Leong, M.M.L.; Xu, D.D. Targeting Mitochondria for Cancer Photodynamic Therapy. Photodiagnosis Photodyn. Ther. 2022, 38, 102830. [Google Scholar] [CrossRef]
- Dickinson, B.C.; Chang, C.J. A Targetable Fluorescent Probe for Imaging Hydrogen Peroxide in the Mitochondria of Living Cells. J. Am. Chem. Soc. 2008, 130, 9638–9639. [Google Scholar] [CrossRef]
- Hu, Q.; Gao, M.; Feng, G.; Liu, B. Mitochondria-Targeted Cancer Therapy Using a Light-Up Probe with Aggregation-Induced-Emission Characteristics. Angew. Chem. Int. Ed. 2014, 53, 14225–14229. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. Selective Targeting of Bioactive Compounds to Mitochondria. Trends. Biotechnol. 1997, 15, 326–330. [Google Scholar] [CrossRef]
- Smith, R.A.J.; Porteous, C.M.; Gane, A.M.; Murphy, M.P. Delivery of Bioactive Molecules to Mitochondria In Vivo. Proc. Natl. Acad. Sci. USA 2003, 100, 5407–5412. [Google Scholar] [CrossRef]
- Zielonka, J.; Sikora, A.; Hardy, M.; Ouari, O.; Vasquez-Vivar, J.; Cheng, G.; Lopez, M.; Kalyanaraman, B. Mitochondria-Targeted Triphenylphosphonium-Based Compounds: Syntheses, Mechanisms of Action, and Therapeutic and Diagnostic Applications. Chem. Rev. 2017, 117, 10043–10120. [Google Scholar] [CrossRef]
- Nixon, G.L.; Moss, D.M.; Shone, A.E.; Lalloo, D.G.; Fisher, N.; O’Neill, P.M.; Ward, S.A.; Biagini, G.A. Antimalarial Pharmacology and Therapeutics of Atovaquone. J. Antimicrob. Chemother. 2013, 68, 977–985. [Google Scholar] [CrossRef]
- Ashton, T.M.; Fokas, E.; Kunz-Schughart, L.A.; Folkes, L.K.; Anbalagan, S.; Huether, M.; Kelly, C.J.; Pirovano, G.; Buffa, F.M.; Hammond, E.M.; et al. The Anti-Malarial Atovaquone Increases Radiosensitivity by Alleviating Tumour Hypoxia. Nat. Commun. 2016, 7, 12308. [Google Scholar] [CrossRef]
- Gong, J.; Cheng, D.; Liu, C.; Wu, S.; Sun, N.; Zhao, L.; Li, J.; Xing, Y.; Zhao, J. Hybrid Cell Membrane-Coated Nanoparticles for Synergizing Sonodynamic Therapy and Immunotherapy against Triple-Negative Breast Cancer. Adv. Healthc. Mater. 2025, 14, 2404184. [Google Scholar] [CrossRef]
- Lu, L.; Liu, G.; Lin, C.; Li, K.; He, T.; Zhang, J.; Luo, Z.; Cai, K. Mitochondrial Metabolism Targeted Nanoplatform for Efficient Triple-Negative Breast Cancer Combination Therapy. Adv. Heal. Mater. 2021, 10, e2100978. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Shao, Y.; Kim, J.H.; Pu, Z.; Zhao, X.; Huang, H.; Xiong, T.; Kang, Y.; Li, G.; Shao, K.; et al. Unimolecular Photodynamic O2-Economizer To Overcome Hypoxia Resistance in Phototherapeutics. J. Am. Chem. Soc. 2020, 142, 5380–5388. [Google Scholar] [CrossRef] [PubMed]
- Larue, L.; Myrzakhmetov, B.; Ben-Mihoub, A.; Moussaron, A.; Thomas, N.; Arnoux, P.; Baros, F.; Vanderesse, R.; Acherar, S.; Frochot, C. Fighting Hypoxia to Improve PDT. Pharmaceuticals 2019, 12, 163. [Google Scholar] [CrossRef] [PubMed]
- Che, J.; Okeke, C.I.; Hu, Z.-B.; Xu, J. DSPE-PEG: A Distinctive Component in Drug Delivery System. Curr. Pharm. Des. 2015, 21, 1598–1605. [Google Scholar] [CrossRef]
- Sodroski, J.; Patarca, R.; Rosen, C.; Wong-Staal, F.; Haseltine, W. Location of the Trans-Activating Region on the Genome of Human T-Cell Lymphotropic Virus Type III. Science 1985, 229, 74–77. [Google Scholar] [CrossRef]
- Wadia, J.S.; Dowdy, S.F. Transmembrane Delivery of Protein and Peptide Drugs by TAT-Mediated Transduction in the Treatment of Cancer. Adv. Drug. Deliv. Rev. 2005, 57, 579–596. [Google Scholar] [CrossRef]
- Bartlett, D.W.; Su, H.; Hildebrandt, I.J.; Weber, W.A.; Davis, M.E. Impact of Tumor-Specific Targeting on the Biodistribution and Efficacy of siRNA Nanoparticles Measured by Multimodality in Vivo Imaging. Proc. Natl. Acad. Sci. USA 2007, 104, 15549–15554. [Google Scholar] [CrossRef]
- Kamaly, N.; Xiao, Z.; Valencia, P.M.; Radovic-Moreno, A.F.; Farokhzad, O.C. Targeted Polymeric Therapeutic Nanoparticles: Design, Development and Clinical Translation. Chem. Soc. Rev. 2012, 41, 2971–3010. [Google Scholar] [CrossRef]
- Marques, A.C.; Costa, P.J.; Velho, S.; Amaral, M.H. Functionalizing Nanoparticles with Cancer-Targeting Antibodies: A Comparison of Strategies. J. Control. Release 2020, 320, 180–200. [Google Scholar] [CrossRef]
- Mahmoudian, F.; Ahmari, A.; Shabani, S.; Sadeghi, B.; Fahimirad, S.; Fattahi, F. Aptamers as an Approach to Targeted Cancer Therapy. Cancer Cell Int. 2024, 24, 108. [Google Scholar] [CrossRef]
- Saga, T.; Neumann, R.D.; Heya, T.; Sato, J.; Kinuya, S.; Le, N.; Paik, C.H.; Weinstein, J.N. Targeting Cancer Micrometastases with Monoclonal Antibodies: A Binding-Site Barrier. Proc. Natl. Acad. Sci. USA 1995, 92, 8999–9003. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Juweid, M.; Neumann, R.; Paik, C.; Perez-Bacete, M.J.; Sato, J.; Van Osdol, W.; Weinstein, J.N. Micropharmacology of Monoclonal Antibodies in Solid Tumors: Direct Experimental Evidence for a Binding Site Barrier. Cancer Res. 1992, 52, 5144–5153. [Google Scholar] [PubMed]
- Mattheolabakis, G.; Milane, L.; Singh, A.; Amiji, M.M. Hyaluronic Acid Targeting of CD44 for Cancer Therapy: From Receptor Biology to Nanomedicine. J. Drug. Target. 2015, 23, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Zhou, Y.; Jiang, J.; Yuan, L.; Xue, M. CD44 Affects the Expression Level of FOS-like Antigen 1 in Cervical Cancer Tissues. Mol. Med. Rep. 2014, 9, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, H.K.; Raju, K.; Sheela, S.R. Association of P16, Ki-67, and CD44 Expression in High-Grade Squamous Intraepithelial Neoplasia and Squamous Cell Carcinoma of the Cervix. J. Cancer Res. Ther. 2023, 19, S260. [Google Scholar] [CrossRef]
- Du, J.; Lane, L.A.; Nie, S. Stimuli-Responsive Nanoparticles for Targeting the Tumor Microenvironment. J. Control. Release 2015, 219, 205–214. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Y.; Tan, J.; Zhang, Y.; Zhuang, J.; Ge, M.; Shi, B.; Li, J.; Xu, G.; Xu, S.; Fan, C.; et al. Visualizing Glioma Margins by Real-Time Tracking of γ-Glutamyltranspeptidase Activity. Biomaterials 2018, 173, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Feng, D.; Liu, T.-F.; Su, J.; Yuan, S.; Chen, Y.-P.; Bosch, M.; Zou, X.; Zhou, H.-C. A Series of Highly Stable Mesoporous Metalloporphyrin Fe-MOFs. J. Am. Chem. Soc. 2014, 136, 13983–13986. [Google Scholar] [CrossRef]
- Yu, F.; Zhu, Y.; Liu, Y.; Qiu, G.; Shang, X.; Meng, T.; Yuan, H.; Hu, F. Poly-γ-Glutamic Acid Derived Nanopolyplexes for up-Regulation of Gamma-Glutamyl Transpeptidase to Augment Tumor Active Targeting and Enhance Synergistic Antitumor Therapy by Regulating Intracellular Redox Homeostasis. Biomater Sci. 2020, 8, 5955–5968. [Google Scholar] [CrossRef]
- Wan, D.; Wu, Y.; Liu, Y.; Liu, Y.; Pan, J. Advances in 2,3-Dimethylmaleic Anhydride (DMMA)-Modified Nanocarriers in Drug Delivery Systems. Pharmaceutics 2024, 16, 809. [Google Scholar] [CrossRef]
- Liang, Y.; Wu, J.; Yan, Y.; Wang, Y.; Zhao, H.; Wang, X.; Chang, S.; Li, S. Charge-Reversal Nano-Drug Delivery Systems in the Tumor Microenvironment: Mechanisms, Challenges, and Therapeutic Applications. Int. J. Mol. Sci. 2024, 25, 9779. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Choi, S.Y.C.; Niu, X.; Kang, N.; Xue, H.; Killam, J.; Wang, Y. Lactic Acid and an Acidic Tumor Microenvironment Suppress Anticancer Immunity. Int. J. Mol. Sci. 2020, 21, 8363. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wu, Q.; Ren, X.; Niu, M.; Ren, J.; Meng, X. Tunable Zeolitic Imidazolate Framework-8 Nanoparticles for Biomedical Applications. Small Methods 2024, 8, 2301270. [Google Scholar] [CrossRef]
- Hu, Y.; Miao, Y.; Zhang, J.; Chen, Y.; Qiu, L.; Lin, J.; Ye, D. Alkaline Phosphatase Enabled Fluorogenic Reaction and in Situ Coassembly of Near-Infrared and Radioactive Nanoparticles for in Vivo Imaging. Nano Lett. 2021, 21, 10377–10385. [Google Scholar] [CrossRef]
- Dong, L.; Miao, Q.; Hai, Z.; Yuan, Y.; Liang, G. Enzymatic Hydrogelation-Induced Fluorescence Turn-Off for Sensing Alkaline Phosphatase in Vitro and in Living Cells. Anal. Chem. 2015, 87, 6475–6478. [Google Scholar] [CrossRef]
- Gillies, R.J.; Schomack, P.A.; Secomb, T.W.; Raghunand, N. Causes and Effects of Heterogeneous Perfusion in Tumors, Neoplasia 1. Neoplasia 1999, 1, 197–207. [Google Scholar] [CrossRef]
- Matsumura, Y. Cancer Stromal Targeting (CAST) Therapy. Adv. Drug Deliv. Rev. 2012, 64, 710–719. [Google Scholar] [CrossRef]
- Choi, N.-E.; Lee, J.-Y.; Park, E.-C.; Lee, J.-H.; Lee, J. Recent Advances in Organelle-Targeted Fluorescent Probes. Molecules 2021, 26, 217. [Google Scholar] [CrossRef]
- Ding, Q.; Zhang, Z.; Li, M.; Zhu, J.-H.; Fu, W.; He, M.; Bai, Y.; Zhang, Z.; Li, S.; Wang, L.; et al. Subcellular Targeting Strategies: Chemical Structure-Based Design Concepts for Bioimaging and Theranostics. Cell Biomater. 2025, 1, 100001. [Google Scholar] [CrossRef]
- Chauhan, N.; Patro, B.S. Emerging Roles of Lysosome Homeostasis (Repair, Lysophagy and Biogenesis) in Cancer Progression and Therapy. Cancer Lett. 2024, 584, 216599. [Google Scholar] [CrossRef] [PubMed]
- Iulianna, T.; Kuldeep, N.; Eric, F. The Achilles’ Heel of Cancer: Targeting Tumors via Lysosome-Induced Immunogenic Cell Death. Cell Death. Dis. 2022, 13, 509. [Google Scholar] [CrossRef] [PubMed]
- Aebisher, D.; Woźnicki, P.; Bartusik-Aebisher, D. Photodynamic Therapy and Adaptive Immunity Induced by Reactive Oxygen Species: Recent Reports. Cancers 2024, 16, 967. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, D.; Liu, Y.; Jiang, B.; Jiang, W.; Yan, X.; Fan, K. Platinum-Carbon-Integrated Nanozymes for Enhanced Tumor Photodynamic and Photothermal Therapy. Nanoscale 2020, 12, 13548–13557. [Google Scholar] [CrossRef]
- Er, E.; Çelikkan, H.; Erk, N. A Novel Electrochemical Nano-Platform Based on Graphene/Platinum Nanoparticles/Nafion Composites for the Electrochemical Sensing of Metoprolol. Sens. Actuators B Chem. 2017, 238, 779–787. [Google Scholar] [CrossRef]
- Wang, R.; Chen, H.; Yan, W.; Zheng, M.; Zhang, T.; Zhang, Y. Ferrocene-Containing Hybrids as Potential Anticancer Agents: Current Developments, Mechanisms of Action and Structure-Activity Relationships. Eur. J. Med. Chem. 2020, 190, 112109. [Google Scholar] [CrossRef]
- Han, R.; Li, X.; Gao, X.; Lv, G. Cinnamaldehyde: Pharmacokinetics, Anticancer Properties and Therapeutic Potential (Review). Mol. Med. Rep. 2024, 30, 1–14. [Google Scholar] [CrossRef]
- Peng, J.; Song, X.; Yu, W.; Pan, Y.; Zhang, Y.; Jian, H.; He, B. The Role and Mechanism of Cinnamaldehyde in Cancer. J. Food Drug. Anal. 2024, 32, 140–154. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, X.; Cheng, Y.; Lu, X.; Meng, J.; Chen, Z.; Li, M.; Lin, C.; Wang, Y.; Yang, J. CuWO4 Nanodots for NIR-Induced Photodynamic and Chemodynamic Synergistic Therapy. ACS Appl. Mater. Interfaces 2021, 13, 22150–22158. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liu, M.; Cheng, J.; Yin, J.; Huang, C.; Cui, H.; Zhang, X.; Zhao, G. Acidity-Triggered Tumor-Targeted Nanosystem for Synergistic Therapy via a Cascade of ROS Generation and NO Release. ACS Appl. Mater. Interfaces 2020, 12, 28975–28984. [Google Scholar] [CrossRef]
- Zhang, Z.; Peng, H. Deeper and Wider Siamese Networks for Real-Time Visual Tracking. In Proceedings of the 2019 IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), Long Beach, CA, USA, 16–20 June 2019; pp. 4586–4595. [Google Scholar]
- Malhotra, J.D.; Kaufman, R.J. Endoplasmic Reticulum Stress and Oxidative Stress: A Vicious Cycle or a Double-Edged Sword? Antioxid. Redox. Signal. 2007, 9, 2277–2293. [Google Scholar] [CrossRef]
- Ying, L.; Hofseth, L.J. An Emerging Role for Endothelial Nitric Oxide Synthase in Chronic Inflammation and Cancer. Cancer Res. 2007, 67, 1407–1410. [Google Scholar] [CrossRef]
- Oronsky, B.; Fanger, G.R.; Oronsky, N.; Knox, S.; Scicinski, J. The Implications of Hyponitroxia in Cancer. Transl. Oncol. 2014, 7, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Fukumura, D.; Kashiwagi, S.; Jain, R.K. The Role of Nitric Oxide in Tumour Progression. Nat. Rev. Cancer 2006, 6, 521–534. [Google Scholar] [CrossRef]
- Basudhar, D.; Somasundaram, V.; De Oliveira, G.A.; Kesarwala, A.; Heinecke, J.L.; Cheng, R.Y.; Glynn, S.A.; Ambs, S.; Wink, D.A.; Ridnour, L.A. Nitric Oxide Synthase-2-Derived Nitric Oxide Drives Multiple Pathways of Breast Cancer Progression. Antioxid. Redox. Signal. 2017, 26, 1044–1058. [Google Scholar] [CrossRef]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of Oxidative Stress as an Anticancer Strategy. Nat. Rev. Drug. Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, W.; Wang, R.; Hopkins, S.P.; Spagnoli, J.C.; Racin, M.; Bai, L.; Li, L.; Jiang, W.; Yang, X.; et al. Nanoparticles Encapsulating Nitrosylated Maytansine To Enhance Radiation Therapy. ACS Nano 2020, 14, 1468–1481. [Google Scholar] [CrossRef]
- Wang, L.; Chang, Y.; Feng, Y.; Li, X.; Cheng, Y.; Jian, H.; Ma, X.; Zheng, R.; Wu, X.; Xu, K.; et al. Nitric Oxide Stimulated Programmable Drug Release of Nanosystem for Multidrug Resistance Cancer Therapy. Nano Lett. 2019, 19, 6800–6811. [Google Scholar] [CrossRef] [PubMed]
- Friebe, A.; Koesling, D. Regulation of Nitric Oxide-Sensitive Guanylyl Cyclase. Circ. Res. 2003, 93, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Lv, T.; Ma, Y.; Xu, J.; Zhang, Y.; Hou, Y.; Huang, Z.; Ding, Y. Nanoscale Coordination Polymers for Synergistic NO and Chemodynamic Therapy of Liver Cancer. Nano Lett. 2019, 19, 2731–2738. [Google Scholar] [CrossRef] [PubMed]

| Inclusion Criteria |
|---|
| Articles describing photodynamic therapy |
| Articles describing cancer therapy |
| Articles describing nanoparticles and nanocomposites |
| Articles published from 2023 to July 2025 |
| Exclusion criteria |
| Articles describing photodynamic therapy combined with other forms of therapy (chemotherapy, radiation therapy, gene therapy, photothermal therapy, etc.) |
| Articles describing cancers other than cervical cancer |
| Articles other than original research papers |
| Articles in which the results of therapy were described only in vitro |
| Articles in a language other than English |
| Type | Structure Type | Name | Photosensitizer | Ref. |
|---|---|---|---|---|
| Nanoparticles | Core–shell | TPP-UCNPs@MOF-Pt | TCPP (porphyrin derivative) | [54] |
| Fc-CA-PCN-HA | TCPP (porphyrin derivative) | [59] | ||
| MT@SiO2-MP NPs | MEO-TTMN (AIE-type) | [57] | ||
| UCNP-M-TCI | M-TCI (maleimide–TCI) | [53] | ||
| UM-RZ | Zinc phthalocyanine | [52] | ||
| Core–shell–shell | UCNP@SiO2-Bodipy@FFYp | Bodipy-I | [75] | |
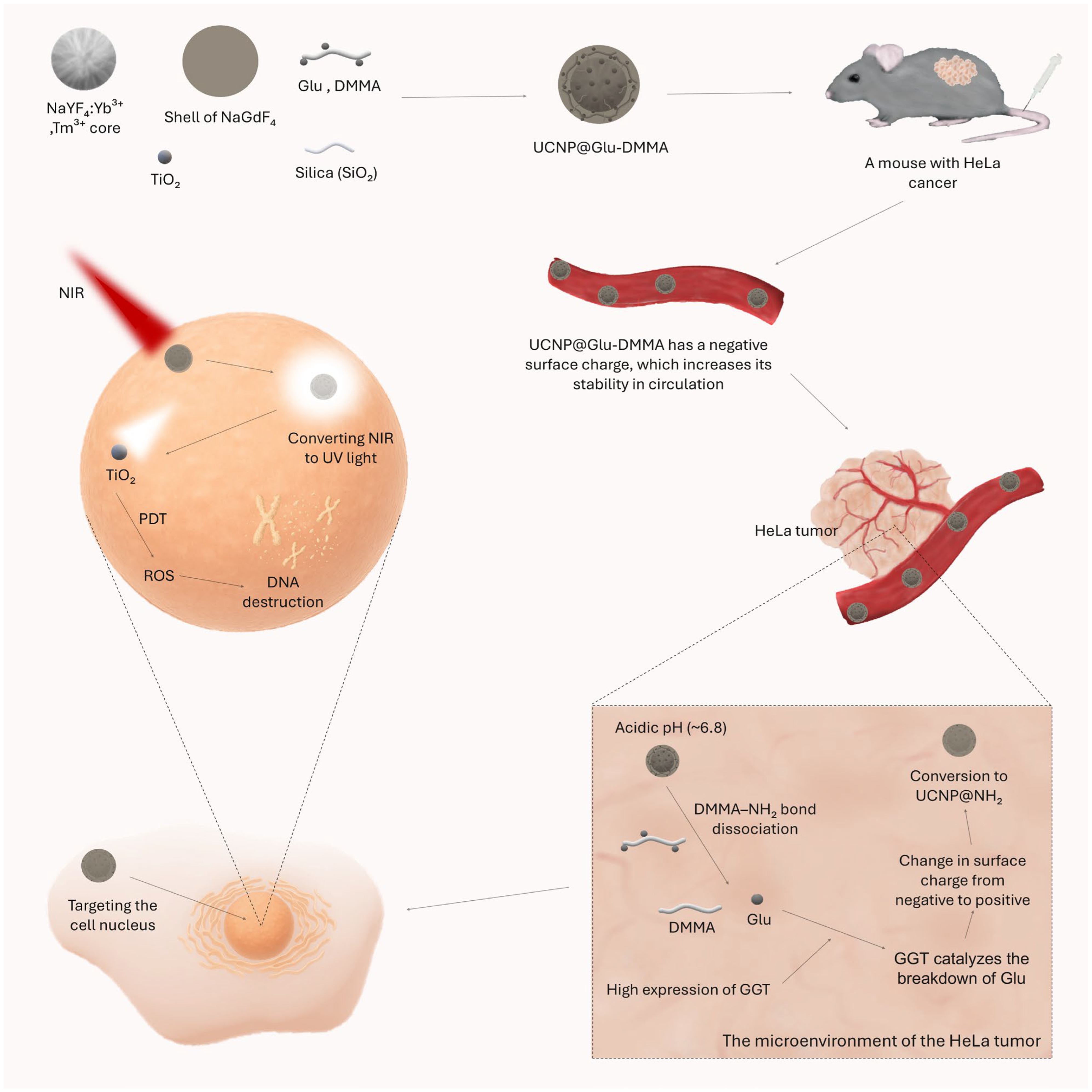
| Core–shell–shell–shell | UCNP@Glu-DMMA | TiO2 | [76] | |
| Janus | JMDA | No classic—the whole structure acts as a photosensitizer | [78] | |
| MOF | PMOF@AuNP/hairpin | TCPP (porphyrin derivative) | [92] | |
| Supramolecular | TAT-InTPP | InTPP (mesotetraphenylporphyrinium indium(III) hydrochloride) | [93] | |
| Polymer | TNPs/IA | IR-780 | [106] | |
| Mesoporous silica | DSi@Z/P | Zinc phthalocyanine | [107] | |
| Nanocomposites | Core–shell | C3N4-RP@RBCm | C3N4-RP (graphitic carbon nitride + red phosphorus) | [58] |
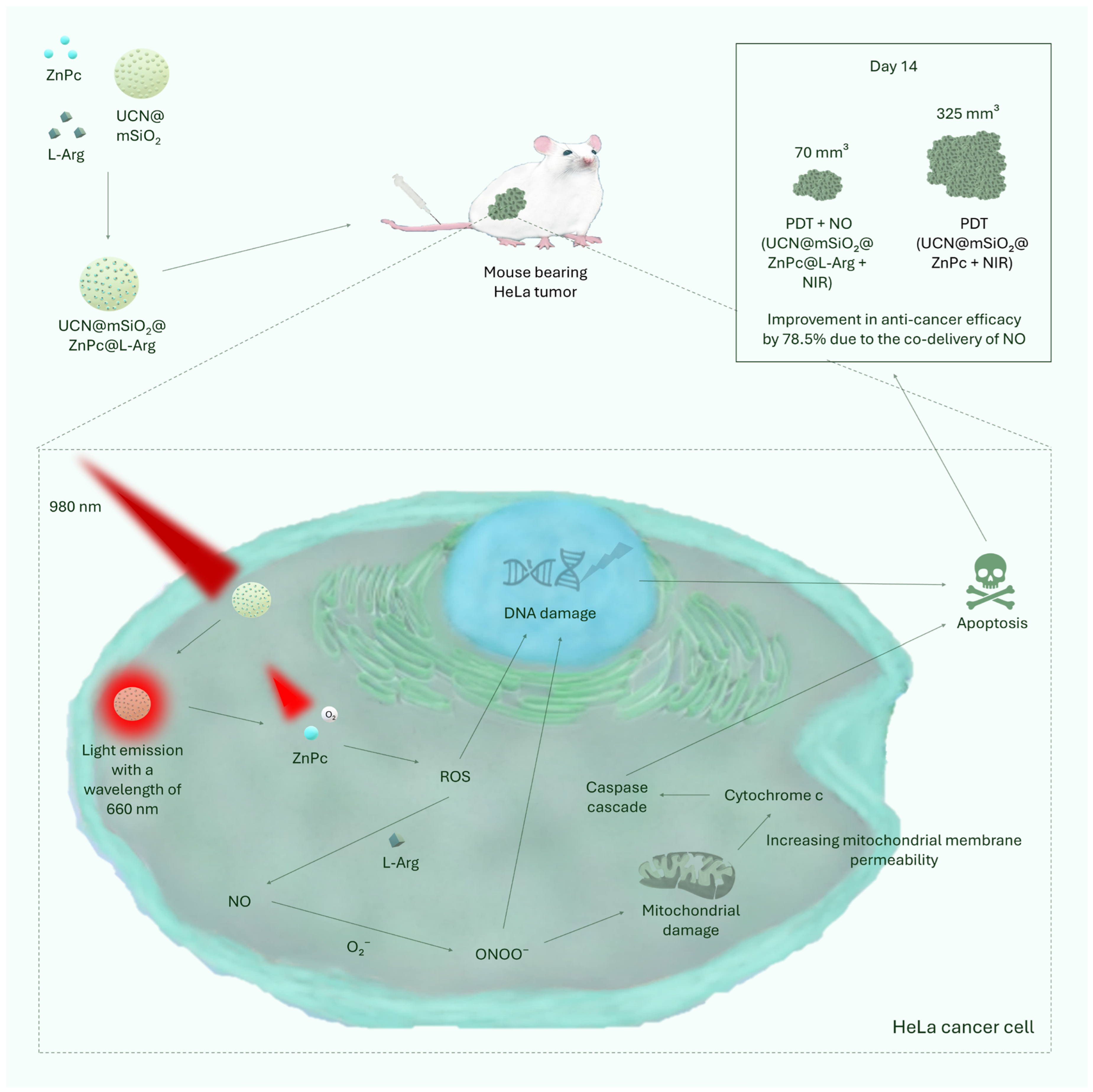
| UCN@mSiO2@ZnPc@L-Arg | Zinc phthalocyanine | [51] | ||
| MOF | IMF | Indocyanine green | [86] | |
| Mesoporous silica | PMnSAGMSNs-V@Ce6 | Chlorin e6 | [113] |
| Author | Name | Approximate Initial Tumor Volume | Therapeutic Regimen | Effectiveness | Ref. |
|---|---|---|---|---|---|
| Gao et al. | IMF | 75–100 mm3 | One-off | 2 of the 3 tumors have completely disappeared; the third has shrunk significantly after 10 days | [86] |
| Gao et al. | TNPs/IA | 100 mm3 | One-off | ~10 mm3 after 14 days | [106] |
| Liu et al. | C3N4-RP@RBCm | 80 mm3 | One-off | ~35–45 mm3 after 14 days | [58] |
| Yang et al. | TAT-InTPP | 100 mm3 | 3 injections—on days 0, 2, 4 | ~65 mm3 after 11 days | [93] |
| Ling et al. | UCNP@SiO2-Bodipy@FFYp | 60 mm3 | 4 injections—on days 0, 1, 3, 5 | ~39 mm3 after 15 days | [75] |
| Chen et al. | TPP-UCNPs@MOF-Pt | 100 mm3 | One-off | ~70 mm3 after 14 days | [54] |
| Lin et al. | UCN@mSiO2@ZnPc@L-Arg | 80 mm3 | 6 injections—on days 0, 2, 4, 6, 8, 12 | ~70 mm3 after 14 days | [51] |
| Bai et al. | Fc-CA-PCN-HA | 100 mm3 | 5 injections—on days 0, 4, 8, 12, 16 | ~100–120 mm3 after 20 days | [59] |
| Ling et al. | UCNP@Glu-DMMA | 100 mm3 | One-off | ~130 mm3 after 16 days | [76] |
| Wang et al. | UM-RZ | 100 mm3 | One-off | ~150 mm3 after 14 days | [52] |
| Ma et al. | JMDA | 100 mm3 | 4 injections—no information on which days | ~150 mm3 after 14 days | [78] |
| Zhao et al. | MT@SiO2-MP NPs | 50 mm3 | One-off | ~75 mm3 after 15 days | [57] |
| Yang et al. | PMOF@AuNP/hairpin | 100 mm3 | One-off | ~150–200 mm3 after 15 days | [92] |
| Ye et al. | PMnSAGMSNs-V@Ce6 | 70 mm3 | One-off | ~105–140 mm3 after 14 days | [113] |
| Chen et al. | DSi@Z/P | 700–800 mm3 | One-off | ~1840 mm3 after 14 days | [107] |
| Nsubuga et al. | UCNP-M-TCI | 100 mm3 | One-off | No statistically significant inhibition of tumor growth | [53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartusik-Aebisher, D.; Saad, M.A.; Przygórzewska, A.; Aebisher, D. Recent Advances in Nanoparticle and Nanocomposite-Based Photodynamic Therapy for Cervical Cancer: A Review. Cancers 2025, 17, 2572. https://doi.org/10.3390/cancers17152572
Bartusik-Aebisher D, Saad MA, Przygórzewska A, Aebisher D. Recent Advances in Nanoparticle and Nanocomposite-Based Photodynamic Therapy for Cervical Cancer: A Review. Cancers. 2025; 17(15):2572. https://doi.org/10.3390/cancers17152572
Chicago/Turabian StyleBartusik-Aebisher, Dorota, Mohammad A. Saad, Agnieszka Przygórzewska, and David Aebisher. 2025. "Recent Advances in Nanoparticle and Nanocomposite-Based Photodynamic Therapy for Cervical Cancer: A Review" Cancers 17, no. 15: 2572. https://doi.org/10.3390/cancers17152572
APA StyleBartusik-Aebisher, D., Saad, M. A., Przygórzewska, A., & Aebisher, D. (2025). Recent Advances in Nanoparticle and Nanocomposite-Based Photodynamic Therapy for Cervical Cancer: A Review. Cancers, 17(15), 2572. https://doi.org/10.3390/cancers17152572








