Effectiveness of the Human Papillomavirus Vaccine in Extended Age Groups: A Real-World Analysis Based on the Korean HPV Cohort Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Ethical Statement
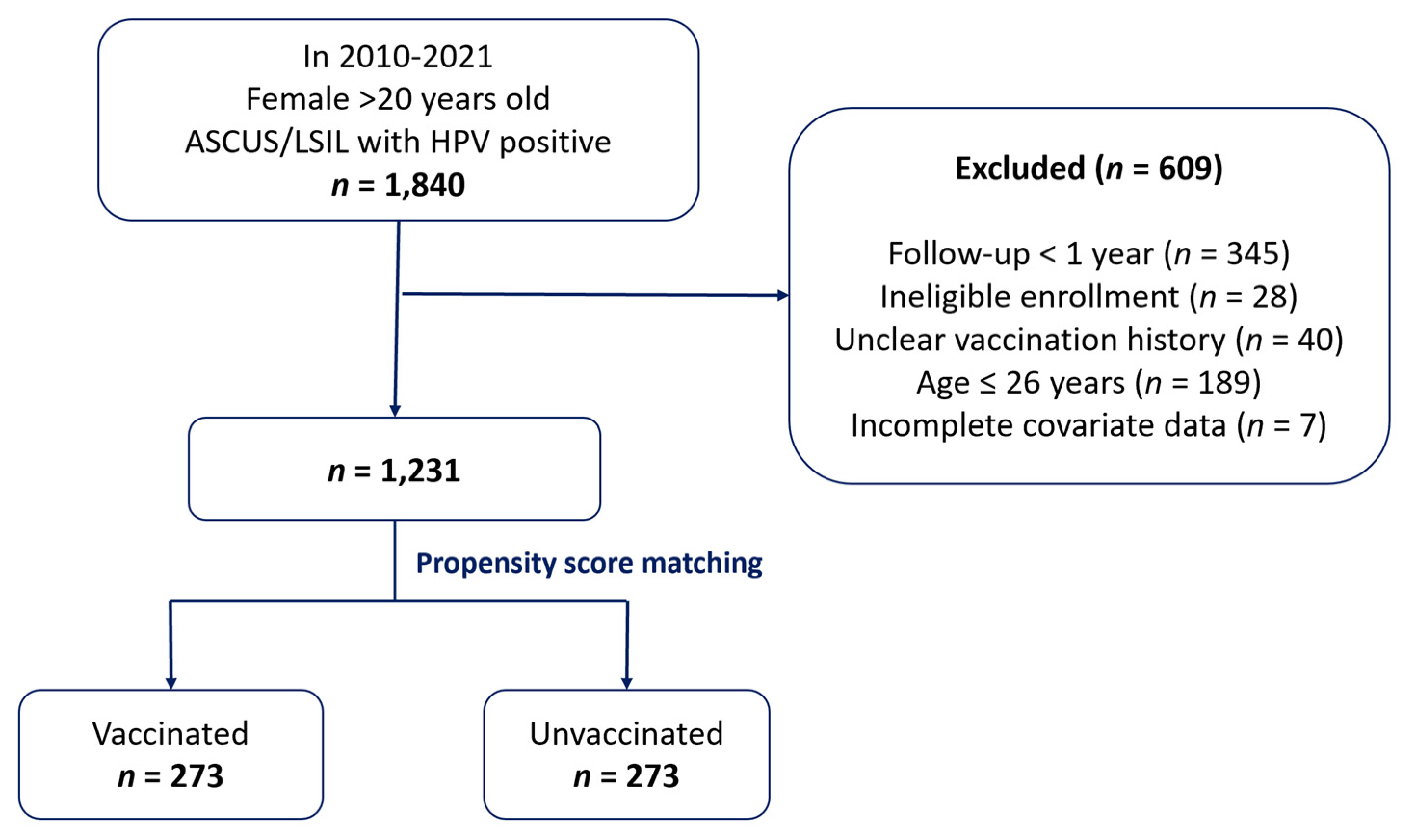
2.3. Cohorts
2.4. Study Population
2.5. Study Outcomes and Covariates
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. HPV Persistence Outcome
3.3. Subgroup Analysis
3.4. Cox Proportional Hazards Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASCUS | Atypical squamous cells of undetermined significance |
| BMI | Body mass index |
| CIN2+ | Cervical intraepithelial neoplasia grade 2 or worse |
| CI | Confidence interval |
| HR | Hazard ratio |
| HPV | Human Papillomavirus |
| HSIL | High-grade squamous intraepithelial lesions |
| IRB | Institutional review board |
| IRR | Incidence rate ratio |
| LSILs | Low-grade squamous intraepithelial lesions |
| OR | Odds ratio |
| PSM | Propensity score matching |
| RCT | Randomized controlled trial |
| RR | Relative risk |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Park, E.H.; Jung, K.W.; Park, N.J.; Kang, M.J.; Yun, E.H.; Kim, H.J.; Kim, J.E.; Kong, H.J.; Im, J.S.; Seo, H.G.; et al. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2021. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2024, 56, 357–371. [Google Scholar] [CrossRef]
- Lei, J.; Ploner, A.; Elfström, K.M.; Wang, J.; Roth, A.; Fang, F.; Sundström, K.; Dillner, J.; Sparén, P. HPV Vaccination and the risk of invasive cervical Cancer. N. Engl. J. Med. 2020, 383, 1340–1348. [Google Scholar] [CrossRef]
- Lehtinen, M.; Lagheden, C.; Luostarinen, T.; Eriksson, T.; Apter, D.; Bly, A.; Gray, P.; Harjula, K.; Heikkilä, K.; Hokkanen, M.; et al. Human papillomavirus vaccine efficacy against invasive, HPV-positive cancers: Population-based follow-up of a cluster-randomised trial. BMJ Open 2021, 11, e050669. [Google Scholar] [CrossRef]
- Arbyn, M.; Xu, L.; Simoens, C.; Martin-Hirsch, P.P. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst. Rev. 2018, 5, Cd009069. [Google Scholar] [CrossRef]
- Ellingson, M.K.; Sheikha, H.; Nyhan, K.; Oliveira, C.R.; Niccolai, L.M. Human papillomavirus vaccine effectiveness by age at vaccination: A systematic review. Hum. Vaccines Immunother. 2023, 19, 2239085. [Google Scholar] [CrossRef]
- Petrosky, E.; Bocchini, J.A.; Hariri, S., Jr.; Chesson, H.; Curtis, C.R.; Saraiya, M.; Unger, E.R.; Markowitz, L.E. Centers for Disease Control and Prevention (CDC). Use of 9-valent human papillomavirus (HPV) vaccine: Updated HPV vaccination recommendations of the advisory committee on immunization practices. Morb. Mortal. Wkly. Rep. 2015, 64, 300–304. [Google Scholar]
- Park, N.; Ban, S.; Kwon, S.; Kwon, G.Y.; Park, Y.; Lee, H.; Lee, J.; Ki, M.; An, H.; Luu, N.M.; et al. Impact assessment of the national HPV immunization program in the Republic of Korea. Public Health Wkly. Rep. 2022, 15, 1850–1865. [Google Scholar]
- Cho, H.W.; Min, K.J.; Kwon, S.H.; Kim, K.; Kim, S.; Seong, S.J.; Song, Y.J.; Lee, K.H.; Lee, S.W.; Lee, J.W.; et al. Updated clinical guideline for human papillomavirus vaccine: The Korean Society of Gynecologic Oncology guidelines. J. Gynecol. Oncol. 2021, 32, e94. [Google Scholar] [CrossRef] [PubMed]
- Kamolratanakul, S.; Pitisuttithum, P. Human Papillomavirus Vaccine Efficacy and Effectiveness against Cancer. Vaccines 2021, 9, 1413. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.C.; Lee, S.Y.; Koo, Y.J.; Kim, T.J.; Hur, S.Y.; Hong, S.R.; Kim, S.S.; Kee, M.K.; Rhee, J.E.; Lee, J.S.; et al. Establishment of a Korea HPV cohort study. J. Gynecol. Oncol. 2013, 24, 59–65. [Google Scholar] [CrossRef]
- Benedetto, U.; Head, S.J.; Angelini, G.D.; Blackstone, E.H. Statistical primer: Propensity score matching and its alternatives. Eur. J. Cardiothorac. Surg. 2018, 53, 1112–1117. [Google Scholar] [CrossRef]
- Ebrahim, E.A.; Cengiz, M.A.; Terzi, E. The best fit Bayesian hierarchical generalized linear model selection using information complexity criteria in the MCMC approach. J. Appl. Math 2024, 2024, 1459524. [Google Scholar] [CrossRef]
- Okunade, K.S. Human papillomavirus and cervical cancer. J. Obstet. Gynaecol. 2020, 40, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Kuebler, U.; Fischer, S.; Mernone, L.; Breymann, C.; Abbruzzese, E.; Ehlert, U. Is stress related to the presence and persistence of oncogenic human papillomavirus infection in young women? BMC Cancer. 2021, 21, 419. [Google Scholar] [CrossRef]
- Basu, P.; Malvi, S.G.; Joshi, S.; Bhatla, N.; Muwonge, R.; Lucas, E.; Verma, Y.; Esmy, P.O.; Poli, U.R.R.; Shah, A.; et al. Vaccine efficacy against persistent human papillomavirus (HPV) 16/18 infection at 10 years after one, two, and three doses of quadrivalent HPV vaccine in girls in India: A multicentre, prospective, cohort study. Lancet Oncol. 2021, 22, 1518–1529. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.J.; Jeong, O.; Seong, J.; Lee, J.; Lee, S.Y.; Hur, S.; Ryou, S. HPV vaccination status and effectiveness in Korean women with HPV16/18 infection (2010-2021): A retrospective study. J. Gynecol. Oncol. 2024, 35, e56. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, N.; Manalastas, R., Jr.; Pitisuttithum, P.; Tresukosol, D.; Monsonego, J.; Ault, K.; Clavel, C.; Luna, J.; Myers, E.; Hood, S.; et al. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine in women aged 24-45 years: A randomised, double-blind trial. Lancet 2009, 373, 1949–1957. [Google Scholar] [CrossRef]
- Pinto, L.A.; Dillner, J.; Beddows, S.; Unger, E.R. Immunogenicity of HPV prophylactic vaccines: Serology assays and their use in HPV vaccine evaluation and development. Vaccine 2018, 36, 4792–4799. [Google Scholar] [CrossRef]
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N. Engl. J. Med. 2007, 356, 1915–1927. [Google Scholar] [CrossRef]
- Paavonen, J.; Naud, P.; Salmerón, J.; Wheeler, C.M.; Chow, S.N.; Apter, D.; Kitchener, H.; Castellsague, X.; Teixeira, J.C.; Skinner, S.R.; et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): Final analysis of a double-blind, randomised study in young women. Lancet 2009, 374, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, D.J.; Lee, H.J. Assessment of malignant potential for HPV types 16, 52, and 58 in the uterine cervix within a Korean cohort. Sci. Rep. 2024, 14, 14619. [Google Scholar] [CrossRef]
- Seong, J.; Ryou, S.; Lee, J.; Yoo, M.; Hur, S.; Choi, B.S. Enhanced disease progression due to persistent HPV-16/58 infections in Korean women: A systematic review and the Korea HPV cohort study. Virol. J. 2021, 18, 188. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.R.; Joura, E.A.; Yen, G.P.; Kothari, S.; Luxembourg, A.; Saah, A.; Walia, A.; Perez, G.; Khoury, H.; Badgley, D.; et al. Systematic literature review of cross-protective effect of HPV vaccines based on data from randomized clinical trials and real-world evidence. Vaccine 2021, 39, 2224–2236. [Google Scholar] [CrossRef]
- Shing, J.Z.; Porras, C.; Pinheiro, M.; Herrero, R.; Hildesheim, A.; Liu, D.; Gail, M.H.; Romero, B.; Schiller, J.T.; Zúñiga, M.; et al. Differential long-term bivalent HPV vaccine cross-protection by variants in the Costa Rica HPV vaccine trial. NPJ Vaccines 2024, 9, 101. [Google Scholar] [CrossRef]
- Wright, T.C., Jr.; Behrens, C.M.; Ranger-Moore, J.; Rehm, S.; Sharma, A.; Stoler, M.H.; Ridder, R. Triaging HPV-positive women with p16/Ki-67 dual-stained cytology: Results from a sub-study nested into the ATHENA trial. Gynecol. Oncol. 2017, 144, 51–56. [Google Scholar] [CrossRef]
- Calder, P.C.; Carr, A.C.; Gombart, A.F.; Eggersdorfer, M. Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. Nutrients 2020, 12, 1181. [Google Scholar] [CrossRef]
- She, Y.; Mangat, R.; Tsai, S.; Proctor, S.D.; Richard, C. The interplay of obesity, dyslipidemia and immune dysfunction: A brief overview on pathophysiology, animal models, and nutritional modulation. Front. Nutr. 2022, 9, 840209. [Google Scholar] [CrossRef]
- Kjaer, S.K.; Dehlendorff, C.; Belmonte, F.; Baandrup, L. Real-world effectiveness of Human Papillomavirus vaccination against cervical cancer. J. Natl. Cancer Inst. 2021, 113, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Joura, E.A.; Giuliano, A.R.; Iversen, O.E.; Bouchard, C.; Mao, C.; Mehlsen, J.; Moreira, E.D., Jr.; Ngan, Y.; Petersen, L.K.; Lazcano-Ponce, E.; et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N. Engl. J. Med. 2015, 372, 711–723. [Google Scholar] [CrossRef]
- Kim, J.J.; Simms, K.T.; Killen, J.; Smith, M.A.; Burger, E.A.; Sy, S.; Regan, C.; Canfell, K. Human papillomavirus vaccination for adults aged 30 to 45 years in the United States: A cost-effectiveness analysis. PLoS Med. 2021, 18, e1003534. [Google Scholar] [CrossRef] [PubMed]
- Chesson, H.W.; Meites, E.; Ekwueme, D.U.; Saraiya, M.; Markowitz, L.E. Cost-effectiveness of HPV vaccination for adults through age 45 years in the United States: Estimates from a simplified transmission model. Vaccine. 2020, 38, 8032–8039. [Google Scholar] [CrossRef] [PubMed]


| Variable | Cohort | p-Value a | |
|---|---|---|---|
| Unvaccinated | Vaccinated | ||
| (n = 891) [Mean ± SD; n (%)] | (n = 340) [Mean ± SD; n (%)] | ||
| Age at enrollment (y) | 44.19 ± 8.62 | 35.13 ± 6.50 | <0.001 |
| Age at vaccination (y) | not applicable | 34.24 ± 6.45 | not applicable |
| Height (cm) | 159.51 ± 5.22 | 161.72 ± 5.16 | <0.001 |
| Weight (kg) | 56.59 ± 7.37 | 54.75 ± 7.65 | <0.001 |
| <0.001 | |||
| ≤Normal weight | 704 (79.01) | 259 (76.18) | |
| Underweight | 57 (6.40) | 52 (15.29) | |
| Overweight | 130 (14.59) | 29 (8.53) | |
| Education level | <0.001 | ||
| ≤Middle school | 124 (13.92) | 6 (1.76) | |
| High school | 321 (36.03) | 54 (15.88) | |
| College | 139 (15.60) | 88 (25.88) | |
| University | 243 (27.27) | 150 (44.12) | |
| Graduate school | 64 (7.18) | 42 (12.35) | |
| Smoking | <0.001 | ||
| Yes (five or more packs of cigarettes in entire lifetime) | 100 (11.22) | 65 (19.12) | |
| No | 791 (88.78) | 275 (80.88) | |
| Drinking | <0.001 | ||
| Yes | 583 (65.43) | 281 (82.65) | |
| Past (1 year of sobriety since the last survey) | 71 (7.97) | 20 (5.88) | |
| None (never consumed alcohol in entire life) | 237 (26.60) | 39 (11.47) | |
| Regular exercise | <0.001 | ||
| Yes (exercise regularly enough to sweat) | 424 (47.59) | 119 (35.00) | |
| No | 467 (52.41) | 221 (65.00) | |
| Pregnancy history | <0.001 | ||
| Yes | 773 (86.76) | 202 (59.41) | |
| No | 118 (13.24) | 138 (40.59) | |
| Oral contraception use | <0.001 | ||
| Yes | 122 (13.69) | 75 (22.06) | |
| No | 769 (86.31) | 265 (77.94) | |
| Family history of malignancy | 0.696 | ||
| Yes | 265 (29.74) | 265 (29.74) | |
| No | 626 (70.26) | 235 (69.12) | |
| Variable | OR | 95% CI | p-Value |
|---|---|---|---|
| Age | 1.67 | 1.28, 2.20 | <0.001 |
| Vaccination status | 0.297 | ||
| Unvaccinated | − | − | |
| Vaccinated | 0.84 | 0.60, 1.17 | |
| HPV risk | 0.033 | ||
| Non-HPV 16/18 | − | − | |
| HPV 16/18 | 0.66 | 0.45, 0.97 | |
| BMI | |||
| Normal weight | − | − | |
| Underweight | 0.47 | 0.29, 0.76 | 0.006 |
| Overweight | 0.77 | 0.47, 1.27 | |
| Age × Vaccination status | |||
| Age × Vaccinated | 0.61 | 0.42, 0.88 | 0.009 |
| BMI × HPV risk | 0.015 | ||
| Underweight × HPV 16/18 | 3.83 | 1.53, 9.85 | |
| Overweight × HPV 16/18 | 0.96 | 0.25, 3.53 |
| Variable | OR | 95% CI | p-Value |
|---|---|---|---|
| Age | 4.58 | 2.54, 8.50 | 0 |
| Vaccination status | 0.04 | ||
| Unvaccinated | − | − | |
| Vaccinated | 0.46 | 0.22, 0.96 | |
| HPV risk | 0.117 | ||
| Non-HPV 16/18 | − | − | |
| HPV 16/18 | 0.68 | 0.42, 1.10 | |
| BMI | 0.078 | ||
| Normal weight | − | − | |
| Underweight | 0.57 | 0.34, 0.94 | |
| Overweight | 1.08 | 0.60, 1.94 | |
| Age × vaccination status | 0.003 | ||
| Age × Vaccinated | 0.33 | 0.15, 0.68 |
| Variable | OR | 95% CI | p-Value |
|---|---|---|---|
| Vaccination age | 0.72 | 0.44, 1.17 | 0.186 |
| HPV risk | 0.891 | ||
| Non-HPV 16/18 | − | − | |
| HPV 16/18 | 1.05 | 0.50, 2.29 | |
| BMI | 0.169 | ||
| Normal weight | − | − | |
| Underweight | 0.62 | 0.33, 1.13 | |
| Overweight | 0.6 | 0.27, 1.31 | |
| Vaccination age × HPV risk | <0.001 | ||
| Vaccination age × HPV 16/18 | 15.55 | 3.68, 83.48 |
| Variable | HR | 95% CI | p-Value |
|---|---|---|---|
| HPV risk | 0.094 | ||
| non-HPV 16/18 | − | − | |
| HPV 16/18 | 2.39 | 0.86, 6.63 | |
| BMI | 0.54 | 0.20, 1.47 | 0.227 |
| Vaccination status | 0.011 | ||
| unvaccinated | − | − | |
| vaccinated | 0.38 | 0.18, 0.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, H.; Lee, S.; Choi, S.; Hur, S.Y. Effectiveness of the Human Papillomavirus Vaccine in Extended Age Groups: A Real-World Analysis Based on the Korean HPV Cohort Study. Cancers 2025, 17, 2561. https://doi.org/10.3390/cancers17152561
Song H, Lee S, Choi S, Hur SY. Effectiveness of the Human Papillomavirus Vaccine in Extended Age Groups: A Real-World Analysis Based on the Korean HPV Cohort Study. Cancers. 2025; 17(15):2561. https://doi.org/10.3390/cancers17152561
Chicago/Turabian StyleSong, Heekyoung, Sanha Lee, Suein Choi, and Soo Young Hur. 2025. "Effectiveness of the Human Papillomavirus Vaccine in Extended Age Groups: A Real-World Analysis Based on the Korean HPV Cohort Study" Cancers 17, no. 15: 2561. https://doi.org/10.3390/cancers17152561
APA StyleSong, H., Lee, S., Choi, S., & Hur, S. Y. (2025). Effectiveness of the Human Papillomavirus Vaccine in Extended Age Groups: A Real-World Analysis Based on the Korean HPV Cohort Study. Cancers, 17(15), 2561. https://doi.org/10.3390/cancers17152561






