Optimising Exercise for Managing Chemotherapy-Induced Peripheral Neuropathy in People Diagnosed with Cancer
Simple Summary
Abstract
1. Introduction
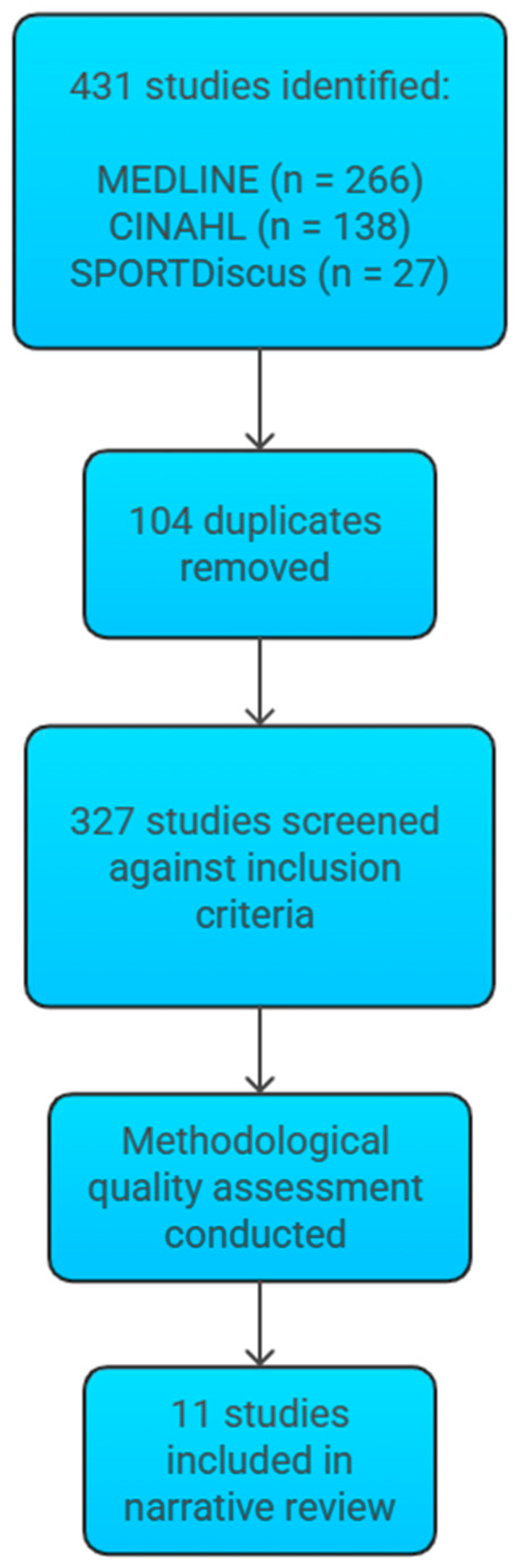
2. Methods
3. Results
4. Discussion
4.1. Multimodal Exercise in the Management of CIPN
4.2. Aerobic Exercise in the Management of CIPN
4.3. Resistance Exercise in the Management of CIPN
4.4. Balance and Sensorimotor Training in the Management of CIPN
4.5. Yoga in the Management of CIPN
4.6. Key Findings
4.7. Strengths and Limitations
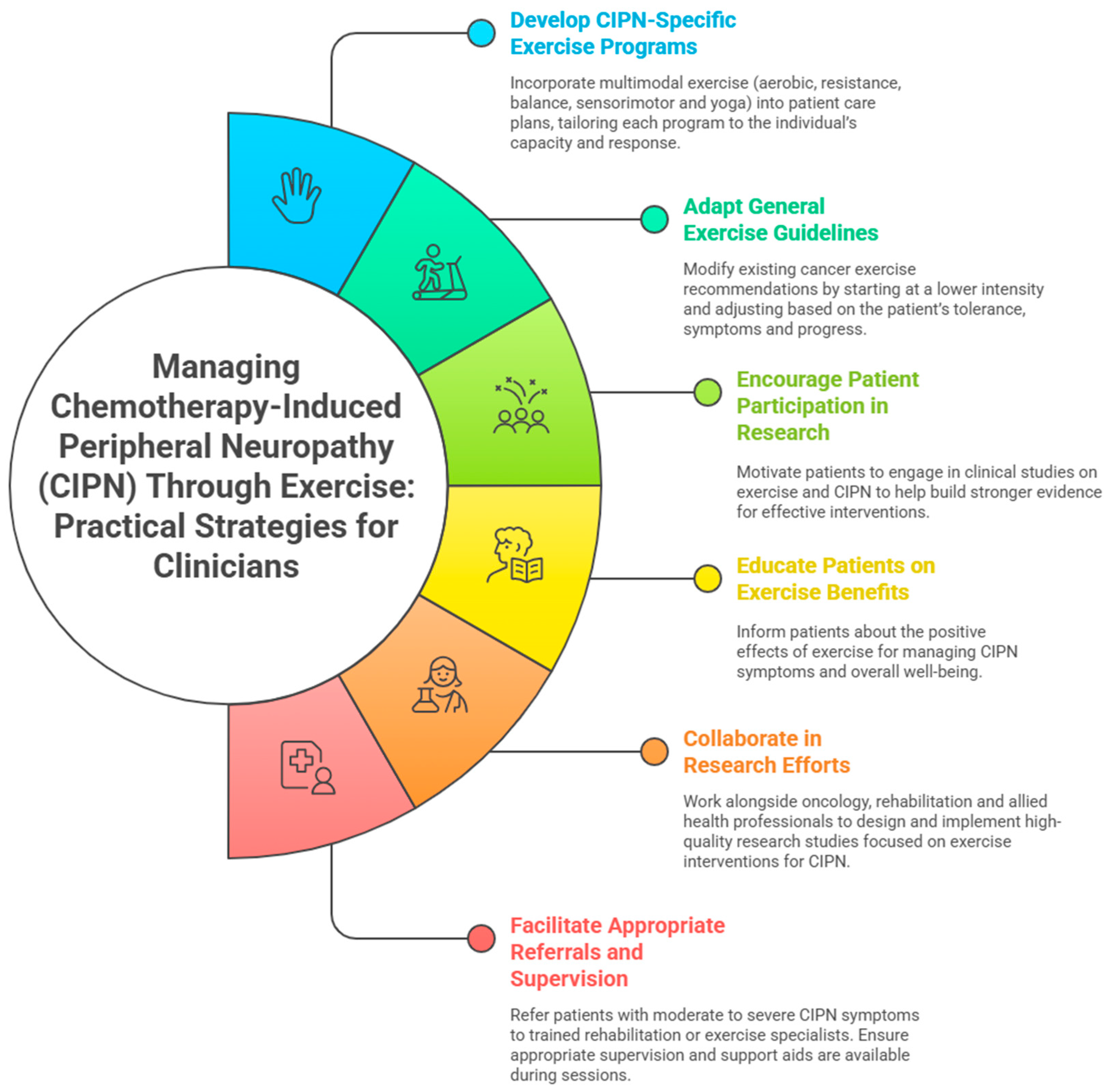
4.8. Practical Applications
4.9. Future Research Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hou, S.; Huh, B.; Kim, H.K.; Kim, K.H.; Abdi, S. Treatment of Chemotherapy-Induced Peripheral Neuropathy: Systematic Review and Recommendations. Pain Physician 2018, 21, 571–592. [Google Scholar] [CrossRef]
- Zajączkowska, R.; Kocot-Kępska, M.; Leppert, W.; Wrzosek, A.; Mika, J.; Wordliczek, J. Mechanisms of Chemotherapy-Induced Peripheral Neuropathy. Int. J. Mol. Sci. 2019, 20, 1451. [Google Scholar] [CrossRef] [PubMed]
- Seretny, M.; Currie, G.L.; Sena, E.S.; Ramnarine, S.; Grant, R.; MacLeod, M.R.; Colvin, L.A.; Fallon, M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain 2014, 155, 2461–2470. [Google Scholar] [CrossRef]
- D’Souza, R.S.; Saini, C.; Hussain, N.; Javed, S.; Prokop, L.; Her, Y.F. Global estimates of prevalence of chronic painful neuropathy among patients with chemotherapy-induced peripheral neuropathy: Systematic review and meta-analysis of data from 28 countries, 2000–2024. Reg. Anesth. Pain Med. 2025, 2024, 106229. [Google Scholar]
- de Arenas-Arroyo, S.N.; Cavero-Redondo, I.; Torres-Costoso, A.; Reina-Gutiérrez, S.; Lorenzo-García, P.; Martínez-Vizcaíno, V. Effects of exercise interventions to reduce chemotherapy-induced peripheral neuropathy severity: A meta-analysis. Scand. J. Med. Sci. Sports 2023, 33, 1040–1053. [Google Scholar] [CrossRef] [PubMed]
- Park, S.B.; Goldstein, D.; Krishnan, A.V.; Lin, C.S.; Friedlander, M.L.; Cassidy, J.; Koltzenburg, M.; Kiernan, M.C. Chemotherapy-induced peripheral neurotoxicity: A critical analysis. CA Cancer J. Clin. 2013, 63, 419–437. [Google Scholar] [CrossRef]
- Mols, F.; Beijers, T.; Vreugdenhil, G.; van de Poll-Franse, L. Chemotherapy-induced peripheral neuropathy and its association with quality of life: A systematic review. Support. Care Cancer 2014, 22, 2261–2269. [Google Scholar] [CrossRef]
- Hong, J.S.; Tian, J.; Wu, L.H. The Influence of Chemotherapy-Induced Neurotoxicity on Psychological Distress and Sleep Sisturbance in Cancer Patients. Curr. Oncol. 2014, 21, 174–180. [Google Scholar] [CrossRef]
- Kim, E.Y.; Hong, S.J. Real-Life Experiences of Chemotherapy-Induced Peripheral Neuropathy in Patients with Cancer: A Qualitative Meta-Synthesis Study. Semin. Oncol. Nurs. 2023, 39, 151499. [Google Scholar] [CrossRef]
- Kolb, N.A.; Smith, A.G.; Singleton, J.R.; Beck, S.L.; Stoddard, G.J.; Brown, S.; Mooney, K. The Association of Chemotherapy-Induced Peripheral Neuropathy Symptoms and the Risk of Falling. JAMA Neurol. 2016, 73, 860–866. [Google Scholar] [CrossRef]
- Burgess, J.; Ferdousi, M.; Gosal, D.; Boon, C.; Matsumoto, K.; Marshall, A.; Mak, T.; Marshall, A.; Frank, B.; Malik, R.A.; et al. Chemotherapy-Induced Peripheral Neuropathy: Epidemiology, Pathomechanisms and Treatment. Oncol. Ther. 2021, 9, 385–450. [Google Scholar] [CrossRef]
- Tamburin, S.; Park, S.B.; Schenone, A.; Mantovani, E.; Hamedani, M.; Alberti, P.; Yildiz-Kabak, V.; Kleckner, I.R.; Kolb, N.; Mazzucchelli, M.; et al. Rehabilitation, exercise, and related non-pharmacological interventions for chemotherapy-induced peripheral neurotoxicity: Systematic review and evidence-based recommendations. Crit. Rev. Oncol. Hematol. 2022, 171, 103575. [Google Scholar] [CrossRef]
- Cormie, P.; Atkinson, M.; Bucci, L.; Cust, A.; Eakin, E.; Hayes, S.; McCarthy, A.L.; Murnane, A.; Patchell, S.; Adams, D. Clinical Oncology Society of Australia position statement on exercise in cancer care. Med. J. Aust. 2018, 209, 184–187. [Google Scholar] [CrossRef]
- Segal, R.; Zwaal, C.; Green, E.; Tomasone, J.R.; Loblaw, A.; Petrella, T. Exercise for people with cancer: A systematic review. Curr. Oncol. 2017, 24, e290–e315. [Google Scholar] [CrossRef]
- Hayes, S.C.; Newton, R.U.; Spence, R.R.; Galvão, D.A. The Exercise and Sports Science Australia position statement: Exercise medicine in cancer management. J. Sci. Med. Sport 2019, 22, 1175–1199. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.H.; Park, S.B.; Streckmann, F.; Wiskemann, J.; Mohile, N.; Kleckner, A.S.; Colloca, L.; Dorsey, S.G.; Kleckner, I.R. Mechanisms, Mediators, and Moderators of the Effects of Exercise on Chemotherapy-Induced Peripheral Neuropathy. Cancers 2022, 14, 1224. [Google Scholar] [CrossRef]
- Guo, S.; Han, W.; Wang, P.; Wang, X.; Fang, X. Effects of exercise on chemotherapy-induced peripheral neuropathy in cancer patients: A systematic review and meta-analysis. J. Cancer Surviv. 2023, 17, 318–331. [Google Scholar] [CrossRef] [PubMed]
- Amarelo, A.; da Mota, M.C.C.; Amarelo, B.L.P.; Ferreira, M.C.; Fernandes, C.S. Effects of Physical Exercise on Chemotherapy-Induced Peripheral Neuropathy: A Systematic Review and Meta-Analysis. Pain Manag. Nurs. 2025, 26, 212–221. [Google Scholar] [CrossRef]
- Barker, T.H.; Stone, J.C.; Sears, K.; Klugar, M.; Tufanaru, C.; Leonardi-Bee, J.; Aromataris, E.; Munn, Z. The revised JBI critical appraisal tool for the assessment of risk of bias for randomized controlled trials. JBI Evid. Synth. 2023, 21, 494–506. [Google Scholar] [CrossRef]
- Barker, T.H.; Habibi, N.; Aromataris, E.; Stone, J.C.; Leonardi-Bee, J.; Sears, K.; Hasanoff, S.; Klugar, M.; Tufanaru, C.; Moola, S.; et al. The revised JBI critical appraisal tool for the assessment of risk of bias for quasi-experimental studies. JBI Evid. Synth. 2024, 22, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, C.; Munn, Z.; Porritt, K. Qualitative research synthesis: Methodological guidance for systematic reviewers utilizing meta-aggregation. JBI Evid. Implement. 2015, 13, 179–187. [Google Scholar] [CrossRef]
- Bland, K.A.; Kirkham, A.A.; Bovard, J.; Shenkier, T.; Zucker, D.; McKenzie, D.C.; Davis, M.K.; Gelmon, K.A.; Campbell, K.L. Effect of Exercise on Taxane Chemotherapy-Induced Peripheral Neuropathy in Women with Breast Cancer: A Randomized Controlled Trial. Clin. Breast Cancer 2019, 19, 411–422. [Google Scholar] [CrossRef]
- Ikio, Y.; Sagari, A.; Nakashima, A.; Matsuda, D.; Sawai, T.; Higashi, T. Efficacy of combined hand exercise intervention in patients with chemotherapy-induced peripheral neuropathy: A pilot randomized controlled trial. Support. Care Cancer 2022, 30, 4981–4992. [Google Scholar] [CrossRef]
- Kneis, S.; Wehrle, A.; Müller, J.; Maurer, C.; Ihorst, G.; Gollhofer, A.; Bertz, H. It’s never too late-balance and endurance training improves functional performance, quality of life, and alleviates neuropathic symptoms in cancer survivors suffering from chemotherapy-induced peripheral neuropathy: Results of a randomized controlled trial. BMC Cancer 2019, 19, 414. [Google Scholar] [CrossRef] [PubMed]
- Vollmers, P.L.; Mundhenke, C.; Maass, N.; Bauerschlag, D.; Kratzenstein, S.; Röcken, C.; Schmidt, T. Evaluation of the effects of sensorimotor exercise on physical and psychological parameters in breast cancer patients undergoing neurotoxic chemotherapy. J. Cancer Res. Clin. Oncol. 2018, 144, 1785–1792. [Google Scholar] [CrossRef]
- Zimmer, P.; Trebing, S.; Timmers-Trebing, U.; Schenk, A.; Paust, R.; Bloch, W.; Rudolph, R.; Streckmann, F.; Baumann, F.T. Eight-week, multimodal exercise counteracts a progress of chemotherapy-induced peripheral neuropathy and improves balance and strength in metastasized colorectal cancer patients: A randomized controlled trial. Support. Care Cancer 2018, 26, 615–624. [Google Scholar] [CrossRef]
- Bao, T.; Zhi, I.; Baser, R.; Hooper, M.; Chen, C.; Piulson, L.; Li, Q.S.; Galantino, M.L.; Blinder, V.; Robson, M.; et al. Yoga for Chemotherapy-Induced Peripheral Neuropathy and Fall Risk: A Randomized Controlled Trial. JNCI Cancer Spectr. 2020, 4, pkaa048. [Google Scholar] [CrossRef] [PubMed]
- Galantino, M.L.; Tiger, R.; Brooks, J.; Jang, S.; Wilson, K. Impact of Somatic Yoga and Meditation on Fall Risk, Function, and Quality of Life for Chemotherapy-Induced Peripheral Neuropathy Syndrome in Cancer Survivors. Integr. Cancer Ther. 2019, 18, 1534735419850627. [Google Scholar] [CrossRef] [PubMed]
- Cao, A.; Cartmel, B.; Li, F.-Y.; Gottlieb, L.T.; Harrigan, M.; Ligibel, J.A.; Gogoi, R.; Schwartz, P.E.; Esserman, D.A.; Irwin, M.L.; et al. Effect of Exercise on Chemotherapy-Induced Peripheral Neuropathy Among Patients Treated for Ovarian Cancer: A Secondary Analysis of a Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e2326463. [Google Scholar] [CrossRef]
- Chen, S.C.; Huang, H.P.; Huang, W.S.; Lin, Y.C.; Chu, T.P.; Beaton, R.D.; Jane, S.W. Non-randomized preliminary study of an education and elastic-band resistance exercise program on severity of neuropathy, physical function, muscle strength and endurance & quality of life in colorectal cancer patients experiencing oxaliplatin-induced peripheral neuropathy. Eur. J. Oncol. Nurs. 2020, 49, 101834. [Google Scholar] [CrossRef]
- Schwenk, M.; Grewal, G.S.; Holloway, D.; Muchna, A.; Garland, L.; Najafi, B. Interactive Sensor-Based Balance Training in Older Cancer Patients with Chemotherapy-Induced Peripheral Neuropathy: A Randomized Controlled Trial. Gerontology 2016, 62, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Streckmann, F.; Lehmann, H.C.; Balke, M.; Schenk, A.; Oberste, M.; Heller, A.; Schürhörster, A.; Elter, T.; Bloch, W.; Baumann, F.T. Sensorimotor training and whole-body vibration training have the potential to reduce motor and sensory symptoms of chemotherapy-induced peripheral neuropathy-a randomized controlled pilot trial. Support. Care Cancer 2019, 27, 2471–2478. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Hariton, E.; Locascio, J.J. Randomised controlled trials-the gold standard for effectiveness research: Study design: Randomised controlled trials. BJOG 2018, 125, 1716. [Google Scholar] [CrossRef] [PubMed]
| Exercise Modality | Study | Exercise Prescription | Benefits |
|---|---|---|---|
| Multimodal | Bland et al., 2019 [22] | Type: Aerobic—cycle ergometer/walking Resistance—full body exercises using machines, free weights, and resistance bands Balance—progressively more difficult exercises on progressively unstable surfaces Frequency: five days per week Intensity: Aerobic—50–75% heart rate reserve/12 to 14 on Borg Scale Resistance—50–65% of 1 repetition maximum Time: Aerobic—40 min | ↓ Symptom severity ↑ Quality of life ↑ Chemotherapy completion rate |
| Ikio et al., 2022 [23] | Type: Resistance—grip and pinching movements Manual dexterity—origami and paper tearing Sensory function—material identification and tactile perception practice Frequency: at least 3 times per week Intensity: 40–60% of maximum muscle strength Time: 30 min | ↑ Strength ↑ Upper extremity function | |
| Kneis et al., 2019 [24] | Type: Aerobic—stationary bike Balance—progressively reducing support surface and visual input, adding motor/cognitive tasks, and instability induction Frequency: twice per week Intensity: moderate intensity/below the individual anaerobic threshold Time: 60 min | ↑ Balance ↓ Symptom severity ↑ Cardiorespiratory fitness | |
| Vollmers et al., 2018 [25] | Type: Sensorimotor—static and dynamic single-leg stance and smovey exercises on posturomed devices Resistance—full body exercises Aerobic—warm-up Frequency: twice per week Intensity: RPE of 13–15 on the Borg Scale | ↑ Strength ↑ Balance | |
| Zimmer et al., 2018 [26] | Type: Balance—balance pads, balancing on lines Coordination—cherry pit pillows, Brasils Aerobic—cross trainer/bicycle ergometer/walking Resistance—circuit-based full body exercises Stretching—cool-down Frequency: twice per week Intensity: Aerobic—RPE of 12–13 on Borg scale/60–70% of maximum heart rate Resistance—Borg CR10 scale level 6/60–80% of 1 repetition maximum Time: 60 min | ↑ Balance ↑ Strength ↓ Symptom severity ↑ Quality of life | |
| Yoga | Bao et al., 2020 [27] | Type: breathwork and modifiable postures Frequency: daily Time: 60 min | ↓ Pain ↑ Quality of life ↑ Stability/balance ↑ Physical performance |
| Galantino et al., 2019 [28] | Type: somatic seated and supine movements Frequency: twice per week Time: 180 min per week | ↑ Flexibility ↑ Balance ↑ Physical function ↓ Pain ↑ Vibration sense ↓ Symptom severity | |
| Aerobic | Cao et al., 2023 [29] | Type: brisk walking Intensity: moderate Time: 150 min per week | ↓ Symptom severity |
| Resistance | Chen et al., 2020 [30] | Type: lower-limb elastic band exercises Frequency: 3 per week Intensity: low intensity Time: 40 min | ↑ Strength ↑ Aerobic endurance ↓ Symptom severity ↑ Quality of life |
| Balance | Schwenk et al., 2016 [31] | Type: interactive, sensor-based, cognitively challenging, dynamic weight-shifting tasks Frequency: twice per week Time: 45 mins | ↑ Balance |
| Sensorimotor | Streckmann et al., 2019 [32] | Type: progressively more difficult balance exercises on progressively unstable surfaces Frequency: twice per week Time: each exercise performed 3 times for 20 s with a rest of 40 s between each set and 1 min between each exercise | ↓ Symptom severity |
| Study | Criteria 1 | Criteria 2 | Criteria 3 | Criteria 4 | Criteria 5 | Criteria 6 | Criteria 7 | Criteria 8 | Criteria 9 | Criteria 10 | Criteria 11 | Criteria 12 | Criteria 13 | Summary |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bao et al., 2020 [27] | Yes | Yes | Yes | No | No | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | 10/13—potential bias due to lack of blinding of participants, assessors, and individual delivering treatment |
| Bland et al., 2019 [22] | Yes | Yes | Yes | No | No | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | 10/13—potential bias due to lack of blinding of participants, assessors, and individual delivering treatment |
| Cao et al., 2023 [29] | Yes | Unclear | Yes | No | No | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | 9/13—potential bias due to lack of blinding of participants and individual delivering treatment, and insufficient information on assessor blinding and allocation concealment |
| Ikio et al., 2022 [23] | Yes | Yes | Yes | No | N/A | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 11/13—minimal bias due to lack of participant blinding |
| Kneis et al., 2019 [24] | Yes | Yes | Yes | No | No | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | 10/13—potential bias due to lack of blinding of participants and individual delivering treatment, and insufficient information on assessor blinding |
| Schwenk et al., 2016 [31] | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 11/13—minimal bias due to lack of blinding of the participant and individual delivering treatment |
| Streckmann et al., 2019 [32] | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 11/13—minimal bias due to lack of blinding of the participant and individual delivering treatment |
| Vollmers et al., 2018 [25] | Yes | Unclear | Yes | No | No | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | 9/13—potential bias due to lack of blinding of participants and individual delivering treatment, and insufficient information on assessor blinding and allocation concealment |
| Zimmer et al., 2018 [26] | Yes | Yes | Yes | No | No | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | 10/13—potential bias due to lack of blinding of participants, assessors, and individual delivering treatment |
| Study | Criteria 1 | Criteria 2 | Criteria 3 | Criteria 4 | Criteria 5 | Criteria 6 | Criteria 7 | Criteria 8 | Criteria 9 | Summary |
|---|---|---|---|---|---|---|---|---|---|---|
| Chen et al., 2020 [30] | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8/9—minimal bias due to the lack of a control group |
| Galantino et al., 2019 [28] | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8/9—minimal bias due to the lack of a control group |
| Study | Criteria 1 | Criteria 2 | Criteria 3 | Criteria 4 | Criteria 5 | Criteria 6 | Criteria 7 | Criteria 8 | Criteria 9 | Criteria 10 | Summary |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Galantino et al., 2019 [28] | Unclear | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | 7/10—potential bias due to insufficient information on philosophical perspectives and potential influence of the researchers’ beliefs and values |
| Key Findings | Details |
|---|---|
| Positive impact of exercise on CIPN | Exercise, including aerobic, resistance, balance, sensorimotor, and yoga, showed improvements. Multimodal exercise is recommended. Benefits include improvements in symptom severity, quality of life, chemotherapy completion rate, strength, function, balance, and aerobic fitness. |
| Lack of standardised guidelines | Absence of established CIPN-specific exercise guidelines. Studies are varied in design, population, and exercise prescriptions, making it challenging to identify a definitive, evidence-based approach. |
| Research gaps | Optimal exercise prescriptions for CIPN management and the physiological mechanisms underlying exercise-induced changes in CIPN. |
| Need for robust studies | The field is relatively under-researched, and there is a need for more RCTs with larger sample sizes. |
| Practical Applications | Details |
|---|---|
| Developing CIPN-specific guidelines | Incorporate multimodal exercise regimens (aerobic, resistance, balance, sensorimotor, and yoga exercises) for patients with CIPN. Tailor exercise based on individual capacity and response. |
| General exercise guidelines for CIPN patients | Adapt general cancer exercise recommendations cautiously. Start at a lower intensity and progress based on tolerance and patient response. |
| Encouraging patient participation in future research | Encourage patients to participate in research studies to increase sample sizes and obtain more diverse data for better evidence and precise exercise guidelines. |
| Integrating balance and sensorimotor training | Emphasise balance and sensorimotor exercises in rehabilitation programs to enhance stability, reduce fall risk, and improve neuromuscular function. |
| Collaborative research efforts | Healthcare institutions, oncology clinics, and rehabilitation centres could collaborate for high-quality randomised controlled trials on CIPN and exercise. |
| Facilitate referrals and supervision | Clinicians should refer patients with CIPN, especially those with severe symptoms or high fall risk, to rehabilitation or exercise specialists for tailored programs. Supervision and use of support aids are recommended to reduce fall risk and enhance safety. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sidhu, D.; Cochrane Wilkie, J.; Buchan, J.; Toohey, K. Optimising Exercise for Managing Chemotherapy-Induced Peripheral Neuropathy in People Diagnosed with Cancer. Cancers 2025, 17, 2533. https://doi.org/10.3390/cancers17152533
Sidhu D, Cochrane Wilkie J, Buchan J, Toohey K. Optimising Exercise for Managing Chemotherapy-Induced Peripheral Neuropathy in People Diagnosed with Cancer. Cancers. 2025; 17(15):2533. https://doi.org/10.3390/cancers17152533
Chicago/Turabian StyleSidhu, Dhiaan, Jodie Cochrane Wilkie, Jena Buchan, and Kellie Toohey. 2025. "Optimising Exercise for Managing Chemotherapy-Induced Peripheral Neuropathy in People Diagnosed with Cancer" Cancers 17, no. 15: 2533. https://doi.org/10.3390/cancers17152533
APA StyleSidhu, D., Cochrane Wilkie, J., Buchan, J., & Toohey, K. (2025). Optimising Exercise for Managing Chemotherapy-Induced Peripheral Neuropathy in People Diagnosed with Cancer. Cancers, 17(15), 2533. https://doi.org/10.3390/cancers17152533








