Supercharged Natural Killer (sNK) Cells Inhibit Melanoma Tumor Progression and Restore Endogenous NK Cell Function in Humanized BLT Mice
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines, Reagents, and Antibodies
2.2. Purification of Human NK Cells and Monocytes
2.3. Probiotic Bacteria AJ2 Sonication
2.4. Generation of Osteoclasts and Expansion of Human NK Cells
2.5. Analysis of Melanoma Growth in Humanized BLT Mice
2.6. Cell Dissociation and Cell Culture of Tissues from Hu-BLT Mice
2.7. Purification of NK Cells and CD3+ T Cells from Hu-BLT Mice
2.8. Enzyme-Linked Immunosorbent Assays (ELISAs) and Multiplex Cytokine Assay
2.9. Surface Staining Assays
2.10. 51Cr Release Cytotoxicity Assay
Total cpm—spontaneous cpm
2.11. Statistical Analysis
3. Results
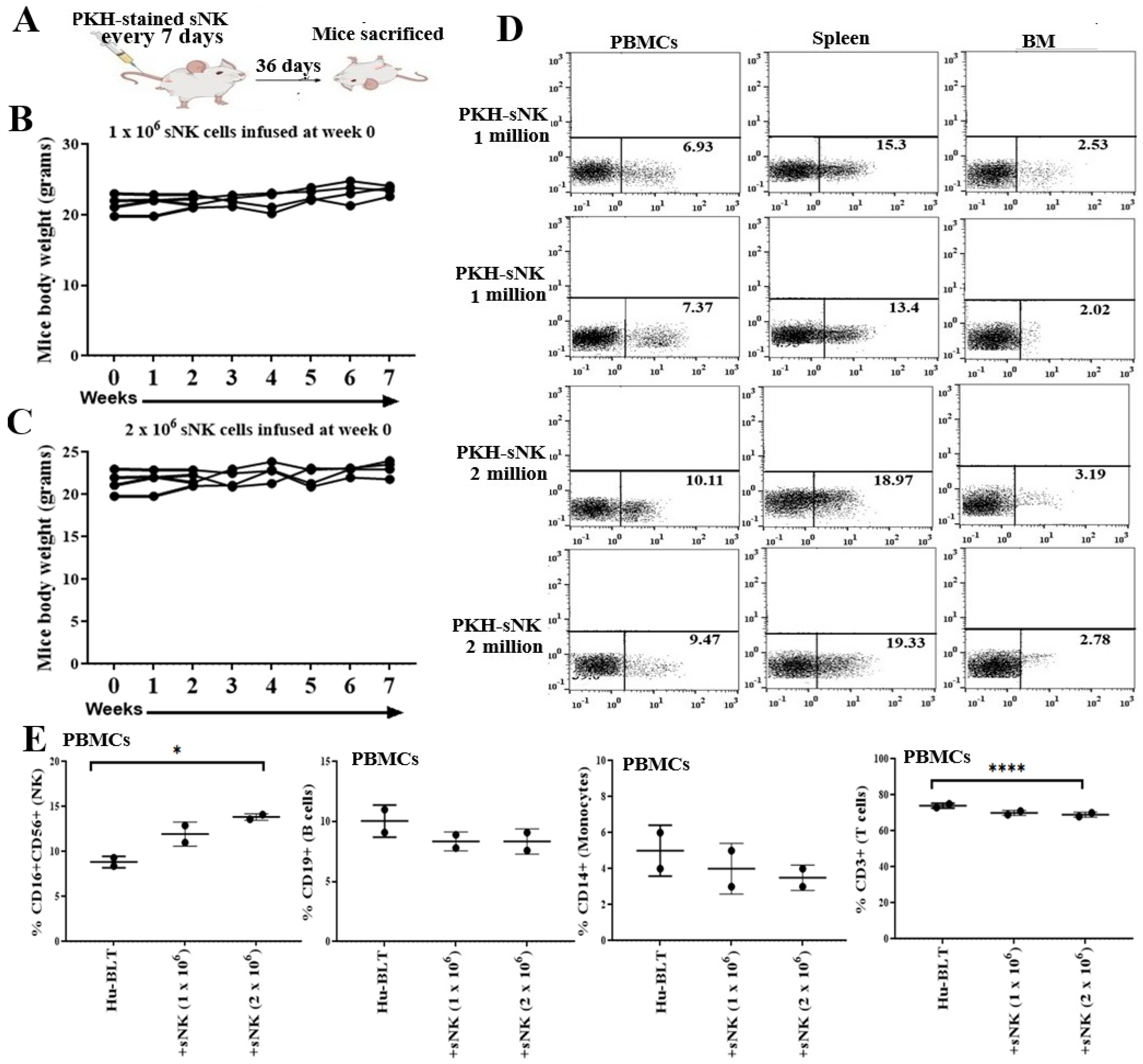
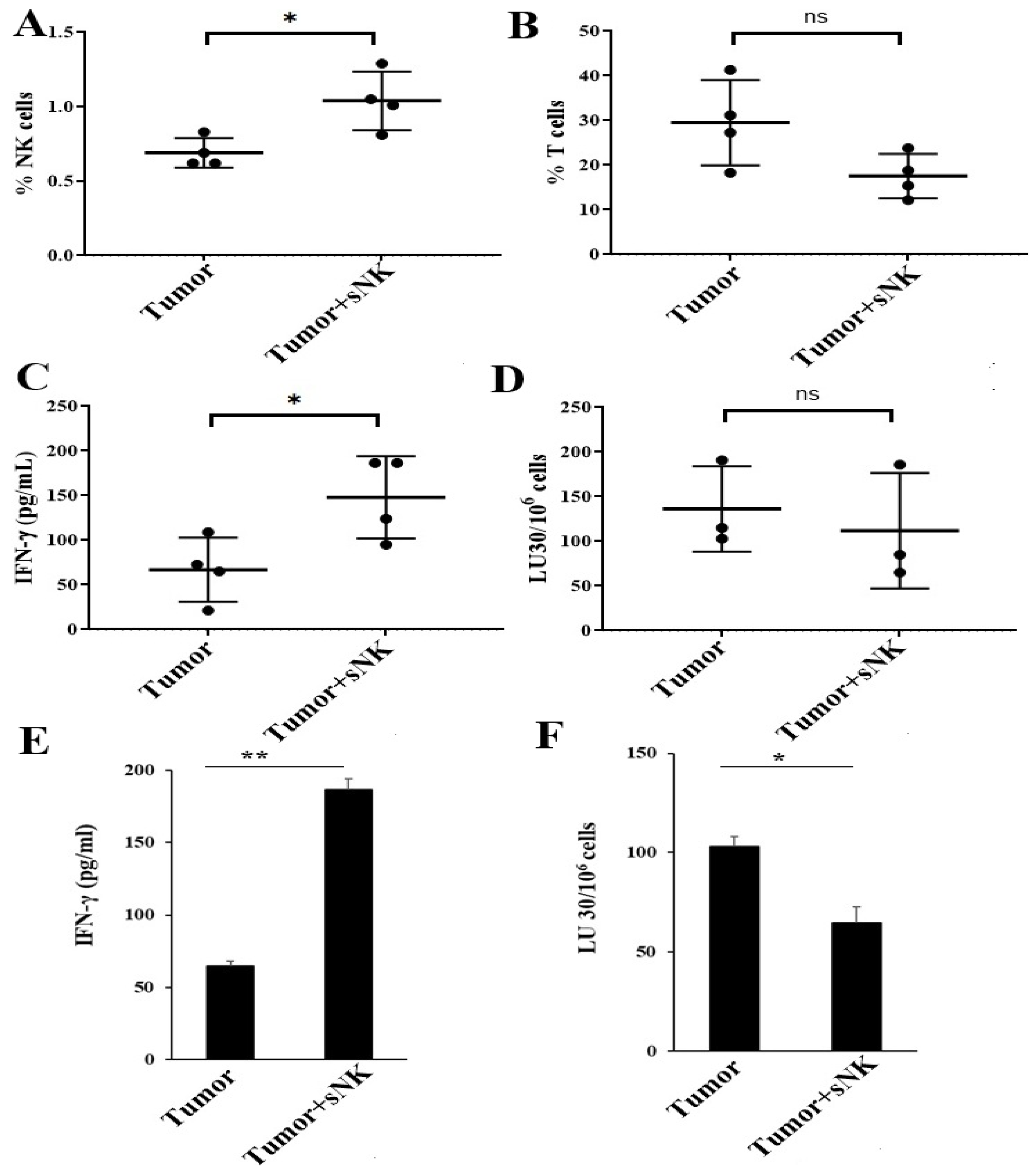
3.1. Safety and Biodistribution Profile of Supercharged NK Cells Infused into Healthy Hu-BLT Mice
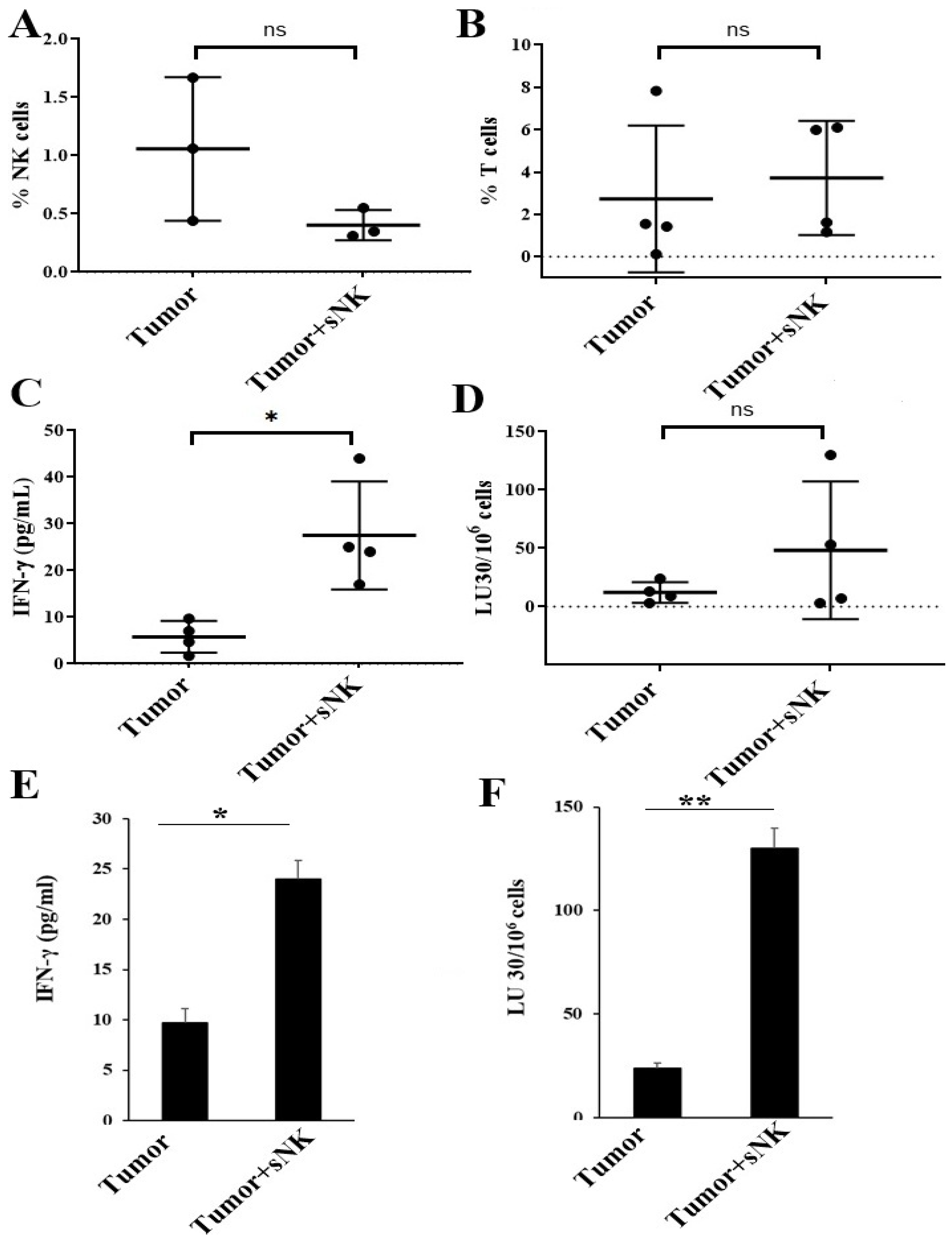
3.2. Supercharged NK Cells Induced Effects on the Immune Cell Function of Disease-Free Hu-BLT Mice
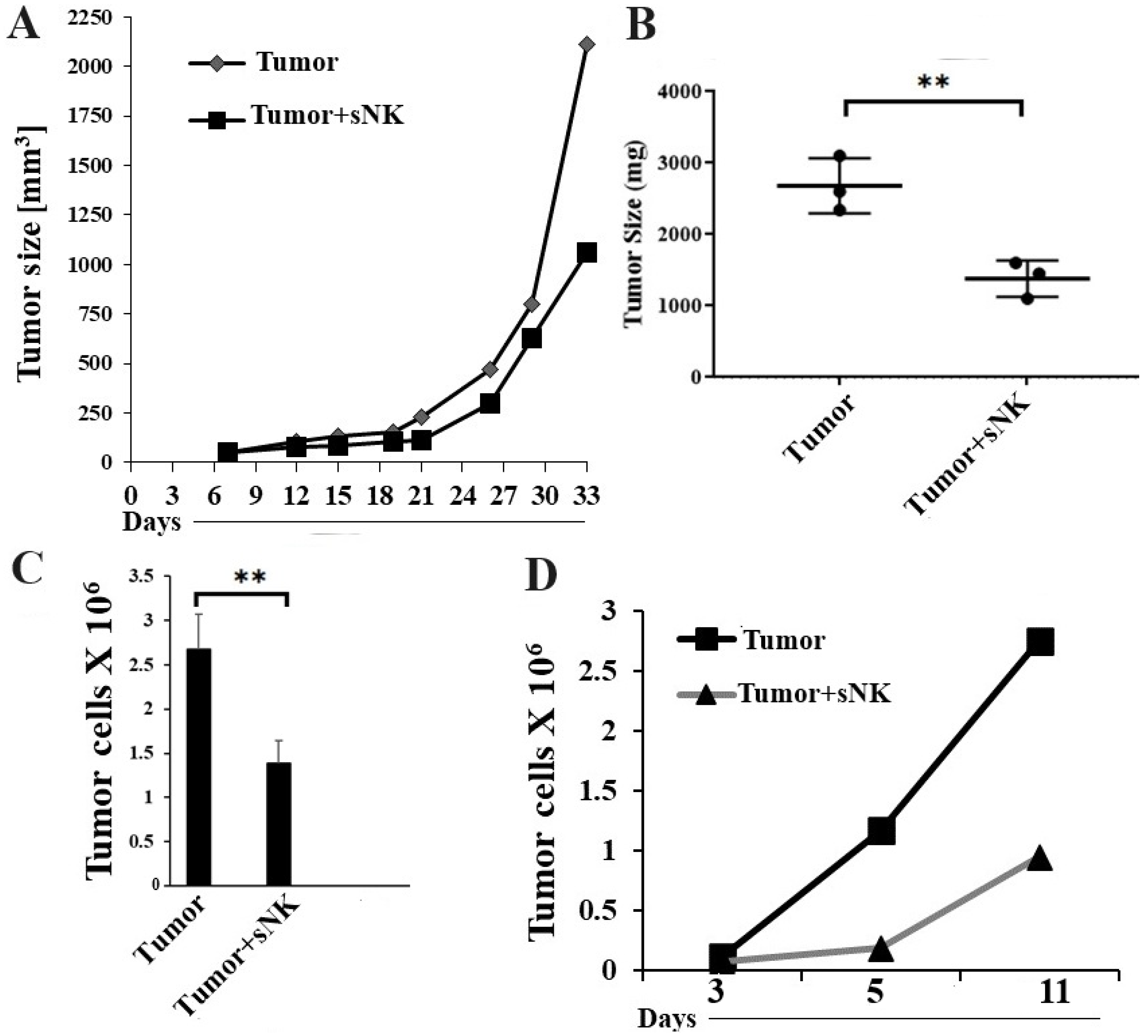
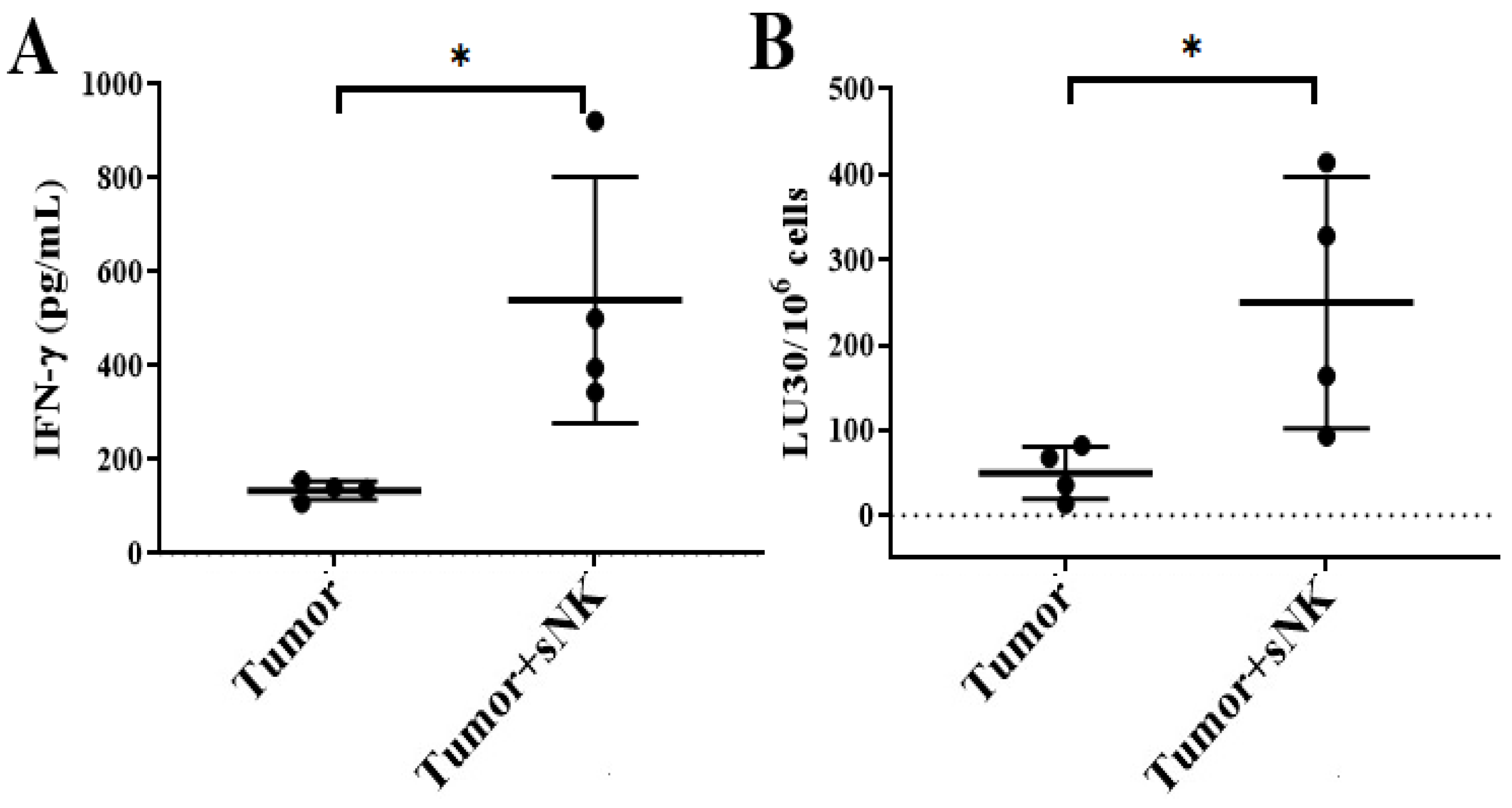
3.3. Infusion of Supercharged NK Cells Inhibited Melanoma Tumor Growth in Hu-BLT Mice
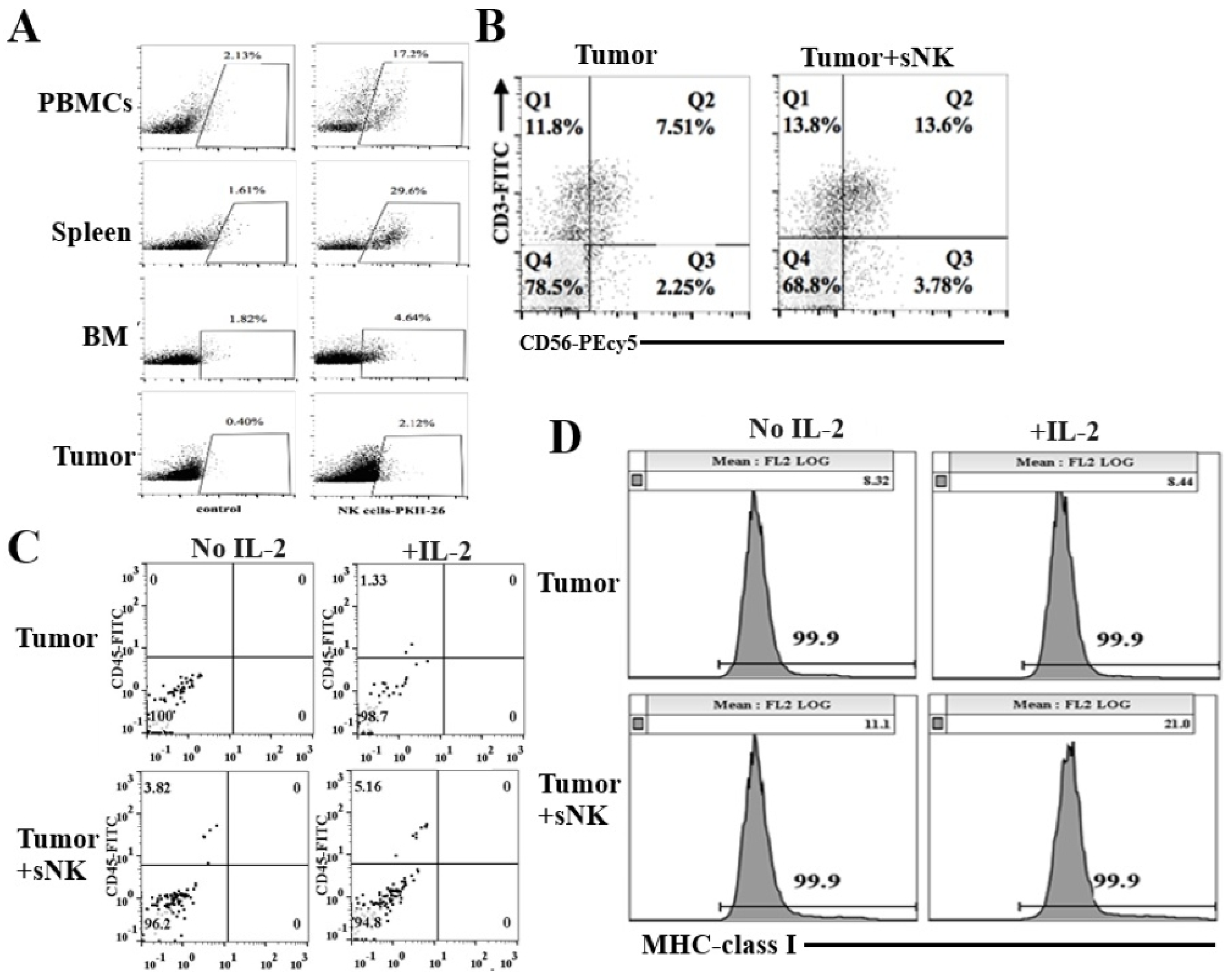
3.4. sNK Cells Circulated Through PBMCs, the Spleen, and Bone Marrow; Were Found Within the Tumors of Hu-BLT Mice; and Were Responsible for the Induction of In Vivo Differentiation of Melanoma Tumors
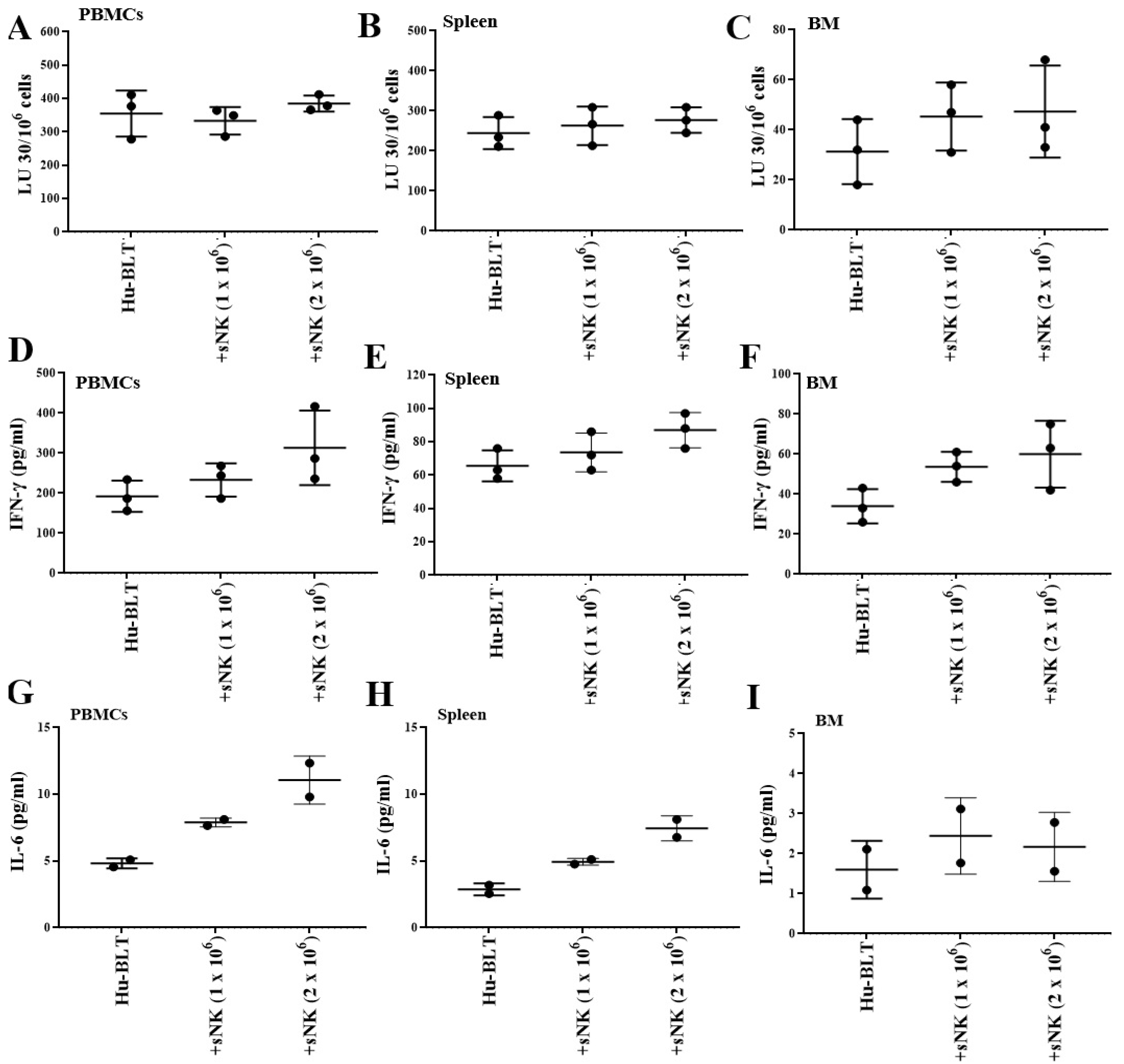
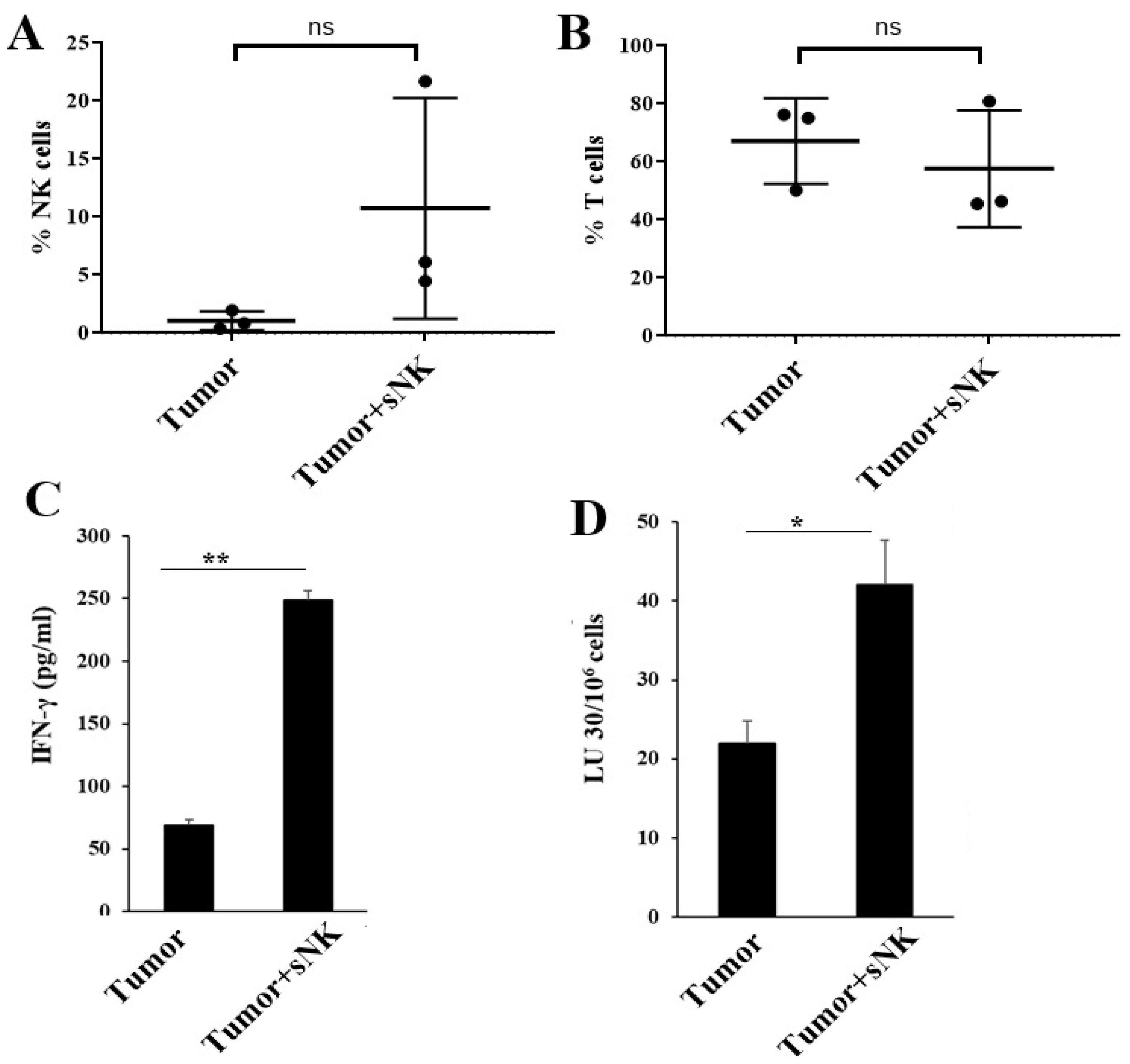
3.5. sNK Cell Infusion Increased Percentages of NK Cells and Restored IFN-γ Secretion Within the Tissue Compartments of Melanoma Tumor-Bearing Mice
3.6. sNK Cell Infusion Restored IFN-γ Secretion in NK and T Cells of Melanoma Tumor-Bearing Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NK cells | Natural killer cells |
| sNK cells | Supercharged NK cells |
| CSCs | Cancer stem-like cells |
| Hu-BLT | Humanized bone marrow/liver/thymus |
| IFN-γ | Interferon-gamma |
| IL-2 | Interleukin 2 |
| OCs | Osteoclasts |
| PBMCs | Peripheral blood-derived mononuclear cells |
| OSCSCs | Oral squamous cancer stem-like cells |
| ELISAs | Enzyme-Linked Immunosorbent Assays |
| Cr | Chromium |
References
- Kibbi, N.; Kluger, H.; Choi, J.N. Melanoma: Clinical Presentations. Cancer Treat. Res. 2016, 167, 107–129. [Google Scholar] [CrossRef]
- Dzwierzynski, W.W. Managing malignant melanoma. Plast. Reconstr. Surg. 2013, 132, 446e–460e. [Google Scholar] [CrossRef] [PubMed]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Barsouk, A. Epidemiology of Melanoma. Med. Sci. 2021, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Scarpati, G.D.; Fusciello, C.; Sabbatino, F.; Ferrone, S.; Caponigro, F.; Perri, F.; Carlomagno, C.; Pepe, S. Multidisciplinary approach to patient with malignant melanoma. Anticancer Agents Med. Chem. 2013, 13, 887–900. [Google Scholar] [CrossRef][Green Version]
- Mackiewicz, J.; Mackiewicz, A. Programmed cell death 1 checkpoint inhibitors in the treatment of patients with advanced melanoma. Contemp. Oncol. 2017, 21, 1–5. [Google Scholar] [CrossRef]
- Azijli, K.; Stelloo, E.; Peters, G.J.; AJ, V.D.E. New developments in the treatment of metastatic melanoma: Immune checkpoint inhibitors and targeted therapies. Anticancer Res. 2014, 34, 1493–1505. [Google Scholar]
- Rosenberg, S.A. IL-2: The first effective immunotherapy for human cancer. J. Immunol. 2014, 192, 5451–5458. [Google Scholar] [CrossRef]
- Eddy, K.; Chen, S. Overcoming Immune Evasion in Melanoma. Int. J. Mol. Sci. 2020, 21, 8984. [Google Scholar] [CrossRef]
- Kwiatkowska-Borowczyk, E.P.; Gąbka-Buszek, A.; Jankowski, J.; Mackiewicz, A. Immunotargeting of cancer stem cells. Contemp. Oncol. 2015, 19, A52–A59. [Google Scholar] [CrossRef]
- Brinckerhoff, C.E. Cancer Stem Cells (CSCs) in melanoma: There’s smoke, but is there fire? J. Cell Physiol. 2017, 232, 2674–2678. [Google Scholar] [CrossRef]
- Li, H.; He, M.; Zhao, P.; Liu, P.; Chen, W.; Xu, X. Chelerythrine Chloride Inhibits Stemness of Melanoma Cancer Stem-Like Cells (CSCs) Potentially via Inducing Reactive Oxygen Species and Causing Mitochondria Dysfunction. Comput. Math. Methods Med. 2022, 2022, 4000733. [Google Scholar] [CrossRef] [PubMed]
- Sample, A.; He, Y.Y. Mechanisms and prevention of UV-induced melanoma. Photodermatol. Photoimmunol. Photomed. 2018, 34, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Bernerd, F.; Passeron, T.; Castiel, I.; Marionnet, C. The Damaging Effects of Long UVA (UVA1) Rays: A Major Challenge to Preserve Skin Health and Integrity. Int. J. Mol. Sci. 2022, 23, 8243. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.M.; Rajasekaran, K.; Thakar, M.S.; Malarkannan, S. Clinical relevance of natural killer cells following hematopoietic stem cell transplantation. J. Cancer 2013, 4, 25–35. [Google Scholar] [CrossRef]
- Fildes, J.E.; Yonan, N.; Leonard, C.T. Natural killer cells and lung transplantation, roles in rejection, infection, and tolerance. Transpl. Immunol. 2008, 19, 1–11. [Google Scholar] [CrossRef]
- Farag, S.S.; Caligiuri, M.A. Human natural killer cell development and biology. Blood Rev. 2006, 20, 123–137. [Google Scholar] [CrossRef]
- Lopez-Soto, A.; Gonzalez, S.; Smyth, M.J.; Galluzzi, L. Control of Metastasis by NK Cells. Cancer Cell 2017, 32, 135–154. [Google Scholar] [CrossRef]
- Solana, R.; Casado, J.G.; Delgado, E.; DelaRosa, O.; Marín, J.; Durán, E.; Pawelec, G.; Tarazona, R. Lymphocyte activation in response to melanoma: Interaction of NK-associated receptors and their ligands. Cancer Immunol. Immunother. 2007, 56, 101–109. [Google Scholar] [CrossRef]
- Bui, V.T.; Tseng, H.-C.; Kozlowska, A.; Maung, P.O.; Kaur, K.; Topchyan, P.; Jewett, A. Augmented IFN-γ and TNF-α Induced by Probiotic Bacteria in NK Cells Mediate Differentiation of Stem-Like Tumors Leading to Inhibition of Tumor Growth and Reduction in Inflammatory Cytokine Release; Regulation by IL-10. Front. Immunol. 2015, 6, 576. [Google Scholar] [CrossRef]
- Ebert, L.M.; Meuter, S.; Moser, B. Homing and function of human skin gammadelta T cells and NK cells: Relevance for tumor surveillance. J. Immunol. 2006, 176, 4331–4336. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, L.F.; Lu, Y.; Luoma, A.; Ito, Y.; Pan, D.; Pyrdol, J.W.; Yoon, C.H.; Yuan, G.C.; Wucherpfennig, K.W. Discovery of specialized NK cell populations infiltrating human melanoma metastases. JCI Insight 2019, 4, e133103. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.; Hong, D.L.; Atzberger, A.; Kollnberger, S.; Filer, A.D.; Buckley, C.D.; McMichael, A.; Enver, T.; Bowness, P. CD56bright human NK cells differentiate into CD56dim cells: Role of contact with peripheral fibroblasts. J. Immunol. 2007, 179, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Burke, S.; Lakshmikanth, T.; Colucci, F.; Carbone, E. New views on natural killer cell-based immunotherapy for melanoma treatment. Trends Immunol. 2010, 31, 339–345. [Google Scholar] [CrossRef]
- Jurisic, V.; Srdic, T.; Konjevic, G.; Markovic, O.; Colovic, M. Clinical stage-depending decrease of NK cell activity in multiple myeloma patients. Med. Oncol. 2007, 24, 312–317. [Google Scholar] [CrossRef]
- Larsen, S.K.; Gao, Y.; Basse, P.H. NK cells in the tumor microenvironment. Crit. Rev. Oncog. 2014, 19, 91–105. [Google Scholar] [CrossRef]
- Imai, K.; Matsuyama, S.; Miyake, S.; Suga, K.; Nakachi, K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: An 11-year follow-up study of a general population. Lancet 2000, 356, 1795–1799. [Google Scholar] [CrossRef]
- Bruno, A.; Ferlazzo, G.; Albini, A.; Noonan, D.M. A think tank of TINK/TANKs: Tumor-infiltrating/tumor-associated natural killer cells in tumor progression and angiogenesis. J. Natl. Cancer Inst. 2014, 106, dju200. [Google Scholar] [CrossRef]
- Gross, E.; Sunwoo, J.B.; Bui, J.D. Cancer immunosurveillance and immunoediting by natural killer cells. Cancer J. 2013, 19, 483–489. [Google Scholar] [CrossRef]
- Mirjacic Martinovic, K.M.; Babovic, N.; Dzodic, R.R.; Jurisic, V.B.; Tanic, N.T.; Konjevic, G.M. Decreased expression of NKG2D, NKp46, DNAM-1 receptors, and intracellular perforin and STAT-1 effector molecules in NK cells and their dim and bright subsets in metastatic melanoma patients. Melanoma Res. 2014, 24, 295–304. [Google Scholar] [CrossRef]
- Gubbels, J.A.; Felder, M.; Horibata, S.; Belisle, J.A.; Kapur, A.; Holden, H.; Petrie, S.; Migneault, M.; Rancourt, C.; Connor, J.P.; et al. MUC16 provides immune protection by inhibiting synapse formation between NK and ovarian tumor cells. Mol. Cancer 2010, 9, 11. [Google Scholar] [CrossRef]
- Balsamo, M.; Scordamaglia, F.; Pietra, G.; Manzini, C.; Cantoni, C.; Boitano, M.; Queirolo, P.; Vermi, W.; Facchetti, F.; Moretta, A.; et al. Melanoma-associated fibroblasts modulate NK cell phenotype and antitumor cytotoxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 20847–20852. [Google Scholar] [CrossRef] [PubMed]
- Castriconi, R.; Cantoni, C.; Della Chiesa, M.; Vitale, M.; Marcenaro, E.; Conte, R.; Biassoni, R.; Bottino, C.; Moretta, L.; Moretta, A. Transforming growth factor beta 1 inhibits expression of NKp30 and NKG2D receptors: Consequences for the NK-mediated killing of dendritic cells. Proc. Natl. Acad. Sci. USA 2003, 100, 4120–4125. [Google Scholar] [CrossRef] [PubMed]
- Pietra, G.; Manzini, C.; Rivara, S.; Vitale, M.; Cantoni, C.; Petretto, A.; Balsamo, M.; Conte, R.; Benelli, R.; Minghelli, S.; et al. Melanoma cells inhibit natural killer cell function by modulating the expression of activating receptors and cytolytic activity. Cancer Res. 2012, 72, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Krockenberger, M.; Dombrowski, Y.; Weidler, C.; Ossadnik, M.; Honig, A.; Hausler, S.; Voigt, H.; Becker, J.C.; Leng, L.; Steinle, A.; et al. Macrophage migration inhibitory factor contributes to the immune escape of ovarian cancer by down-regulating NKG2D. J. Immunol. 2008, 180, 7338–7348. [Google Scholar] [CrossRef]
- Vitale, M.; Cantoni, C.; Pietra, G.; Mingari, M.C.; Moretta, L. Effect of tumor cells and tumor microenvironment on NK-cell function. Eur. J. Immunol. 2014, 44, 1582–1592. [Google Scholar] [CrossRef]
- Gallois, A.; Silva, I.; Osman, I.; Bhardwaj, N. Reversal of natural killer cell exhaustion by TIM-3 blockade. Oncoimmunology 2014, 3, e946365. [Google Scholar] [CrossRef]
- Hersey, P.; Edwards, A.; Honeyman, M.; McCarthy, W.H. Low natural-killer-cell activity in familial melanoma patients and their relatives. Br. J. Cancer 1979, 40, 113–122. [Google Scholar] [CrossRef]
- Igarashi, T.; Wynberg, J.; Srinivasan, R.; Becknell, B.; McCoy, J.P., Jr.; Takahashi, Y.; Suffredini, D.A.; Linehan, W.M.; Caligiuri, M.A.; Childs, R.W. Enhanced cytotoxicity of allogeneic NK cells with killer immunoglobulin-like receptor ligand incompatibility against melanoma and renal cell carcinoma cells. Blood 2004, 104, 170–177. [Google Scholar] [CrossRef]
- White, D.; Jones, D.B.; Cooke, T.; Kirkham, N. Natural killer (NK) activity in peripheral blood lymphocytes of patients with benign and malignant breast disease. Br. J. Cancer 1982, 46, 611–616. [Google Scholar] [CrossRef]
- Santos, M.F.; Mannam, V.K.; Craft, B.S.; Puneky, L.V.; Sheehan, N.T.; Lewis, R.E.; Cruse, J.M. Comparative analysis of innate immune system function in metastatic breast, colorectal, and prostate cancer patients with circulating tumor cells. Exp. Mol. Pathol. 2014, 96, 367–374. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Tsujimoto, H.; Ono, S.; Shinomiya, N.; Miyazaki, H.; Hiraki, S.; Takahata, R.; Yoshida, K.; Saitoh, D.; Yamori, T.; et al. Abdominal Infection Suppresses the Number and Activity of Intrahepatic Natural Killer Cells and Promotes Tumor Growth in a Murine Liver Metastasis Model. Ann. Surg. Oncol. 2016, 23 (Suppl. S2), S257–S265. [Google Scholar] [CrossRef]
- Baskic, D.; Vujanovic, L.; Arsenijevic, N.; Whiteside, T.L.; Myers, E.N.; Vujanovic, N.L. Suppression of natural killer-cell and dendritic-cell apoptotic tumoricidal activity in patients with head and neck cancer. Head Neck 2013, 35, 388–398. [Google Scholar] [CrossRef]
- Mickel, R.A.; Kessler, D.J.; Taylor, J.M.; Lichtenstein, A. Natural killer cell cytotoxicity in the peripheral blood, cervical lymph nodes, and tumor of head and neck cancer patients. Cancer Res. 1988, 48, 5017–5022. [Google Scholar]
- Ghoneum, M.; Gill, G.; Perry, L. Natural killer cell activity in patients with carcinoma of the larynx and hypopharynx. Laryngoscope 1986, 96, 1300. [Google Scholar]
- Tartter, P.I.; Steinberg, B.; Barron, D.M.; Martinelli, G. The prognostic significance of natural killer cytotoxicity in patients with colorectal cancer. Arch. Surg. 1987, 122, 1264–1268. [Google Scholar] [CrossRef]
- Izawa, S.; Kono, K.; Mimura, K.; Kawaguchi, Y.; Watanabe, M.; Maruyama, T.; Fujii, H. H2O2 production within tumor microenvironment inversely correlated with infiltration of CD56dim NK cells in gastric and esophageal cancer: Possible mechanisms of NK cell dysfunction. Cancer Immunol. Immunother. 2011, 60, 1801–1810. [Google Scholar] [CrossRef]
- Nolibe, D.; Poupon, M.F. Enhancement of pulmonary metastases induced by decreased lung natural killer cell activity. J. Natl. Cancer Inst. 1986, 77, 99–103. [Google Scholar] [PubMed]
- Kaur, K.; Ko, M.W.; Ohanian, N.; Cook, J.; Jewett, A. Osteoclast-expanded super-charged NK-cells preferentially select and expand CD8+ T cells. Sci. Rep. 2020, 10, 20363. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, L.; Capanni, M.; Urbani, E.; Perruccio, K.; Shlomchik, W.D.; Tosti, A.; Posati, S.; Rogaia, D.; Frassoni, F.; Aversa, F.; et al. Effectiveness of Donor Natural Killer Cell Alloreactivity in Mismatched Hematopoietic Transplants. Science 2002, 295, 2097–2100. [Google Scholar] [CrossRef] [PubMed]
- Venstrom, J.M.; Pittari, G.; Gooley, T.A.; Chewning, J.H.; Spellman, S.; Haagenson, M.; Gallagher, M.M.; Malkki, M.; Petersdorf, E.; Dupont, B.; et al. HLA-C-dependent prevention of leukemia relapse by donor activating KIR2DS1. N. Engl. J. Med. 2012, 367, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Iliopoulou, E.G.; Kountourakis, P.; Karamouzis, M.V.; Doufexis, D.; Ardavanis, A.; Baxevanis, C.N.; Rigatos, G.; Papamichail, M.; Perez, S.A. A phase I trial of adoptive transfer of allogeneic natural killer cells in patients with advanced non-small cell lung cancer. Cancer Immunol. Immunother. 2010, 59, 1781–1789. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.S.; Soignier, Y.; Panoskaltsis-Mortari, A.; McNearney, S.A.; Yun, G.H.; Fautsch, S.K.; McKenna, D.; Le, C.; Defor, T.E.; Burns, L.J.; et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005, 105, 3051–3057. [Google Scholar] [CrossRef] [PubMed]
- Re, F.; Staudacher, C.; Zamai, L.; Vecchio, V.; Bregni, M. Killer cell Ig-like receptors ligand-mismatched, alloreactive natural killer cells lyse primary solid tumors. Cancer 2006, 107, 640–648. [Google Scholar] [CrossRef]
- Geller, M.A.; Cooley, S.; Judson, P.L.; Ghebre, R.; Carson, L.F.; Argenta, P.A.; Jonson, A.L.; Panoskaltsis-Mortari, A.; Curtsinger, J.; McKenna, D.; et al. A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy 2011, 13, 98–107. [Google Scholar] [CrossRef]
- Casado, J.G.; Pawelec, G.; Morgado, S.; Sanchez-Correa, B.; Delgado, E.; Gayoso, I.; Duran, E.; Solana, R.; Tarazona, R. Expression of adhesion molecules and ligands for activating and costimulatory receptors involved in cell-mediated cytotoxicity in a large panel of human melanoma cell lines. Cancer Immunol. Immunother. 2009, 58, 1517–1526. [Google Scholar] [CrossRef]
- Vetter, C.S.; Groh, V.; thor Straten, P.; Spies, T.; Bröcker, E.B.; Becker, J.C. Expression of stress-induced MHC class I related chain molecules on human melanoma. J. Invest. Dermatol. 2002, 118, 600–605. [Google Scholar] [CrossRef]
- Cagnano, E.; Hershkovitz, O.; Zilka, A.; Bar-Ilan, A.; Golder, A.; Sion-Vardy, N.; Bogdanov-Berezovsky, A.; Mandelboim, O.; Benharroch, D.; Porgador, A. Expression of ligands to NKp46 in benign and malignant melanocytes. J. Investig. Dermatol. 2008, 128, 972–979. [Google Scholar] [CrossRef]
- Byrd, A.; Hoffmann, S.C.; Jarahian, M.; Momburg, F.; Watzl, C. Expression analysis of the ligands for the Natural Killer cell receptors NKp30 and NKp44. PLoS ONE 2007, 2, e1339. [Google Scholar] [CrossRef]
- Li, H.; Hong, S.; Qian, J.; Zheng, Y.; Yang, J.; Yi, Q. Cross talk between the bone and immune systems: Osteoclasts function as antigen-presenting cells and activate CD4+ and CD8+ T cells. Blood 2010, 116, 210–217. [Google Scholar] [CrossRef]
- Kaur, K.; Cook, J.; Park, S.H.; Topchyan, P.; Kozlowska, A.; Ohanian, N.; Fang, C.; Nishimura, I.; Jewett, A. Novel Strategy to Expand Super-Charged NK Cells with Significant Potential to Lyse and Differentiate Cancer Stem Cells: Differences in NK Expansion and Function between Healthy and Cancer Patients. Front. Immunol. 2017, 8, 297. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Rowland, I.; Yaqoob, P. Comparative effects of six probiotic strains on immune function in vitro. Br. J. Nutr. 2012, 108, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.W.; Mei, A.; Senjor, E.; Nanut, M.P.; Gao, L.W.; Wong, P.; Chen, P.C.; Cohn, W.; Whitelegge, J.P.; Kos, J.; et al. Osteoclast-expanded supercharged NK cells perform superior antitumour effector functions. BMJ Oncol. 2025, 4, e000676. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Chen, P.C.; Ko, M.W.; Mei, A.; Senjor, E.; Malarkannan, S.; Kos, J.; Jewett, A. Sequential therapy with supercharged NK cells with either chemotherapy drug cisplatin or anti-PD-1 antibody decreases the tumor size and significantly enhances the NK function in Hu-BLT mice. Front. Immunol. 2023, 14, 1132807. [Google Scholar] [CrossRef]
- Romee, R.; Rosario, M.; Berrien-Elliott, M.M.; Wagner, J.A.; Jewell, B.A.; Schappe, T.; Leong, J.W.; Abdel-Latif, S.; Schneider, S.E.; Willey, S.; et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci. Transl. Med. 2016, 8, 357ra123. [Google Scholar] [CrossRef]
- Terrén, I.; Orrantia, A.; Astarloa-Pando, G.; Amarilla-Irusta, A.; Zenarruzabeitia, O.; Borrego, F. Cytokine-Induced Memory-Like NK Cells: From the Basics to Clinical Applications. Front. Immunol. 2022, 13, 884648. [Google Scholar] [CrossRef]
- Kaur, K.; Safaie, T.; Ko, M.-W.; Wang, Y.; Jewett, A. ADCC against MICA/B Is Mediated against Differentiated Oral and Pancreatic and Not Stem-Like/Poorly Differentiated Tumors by the NK Cells; Loss in Cancer Patients due to Down-Modulation of CD16 Receptor. Cancers 2021, 13, 239. [Google Scholar] [CrossRef]
- Huerta-Yepez, S.; Chen, P.C.; Kaur, K.; Jain, Y.; Singh, T.; Esedebe, F.; Liao, Y.J.; DiBernardo, G.; Moatamed, N.A.; Mei, A.; et al. Supercharged NK cells, unlike primary activated NK cells, effectively target ovarian cancer cells irrespective of MHC-class I expression. BMJ Oncol. 2025, 4, e000618. [Google Scholar] [CrossRef]
- Hiraga, T.; Ito, S.; Nakamura, H. Cancer stem-like cell marker CD44 promotes bone metastases by enhancing tumorigenicity, cell motility, and hyaluronan production. Cancer Res. 2013, 73, 4112–4122. [Google Scholar] [CrossRef]
- Shimizu, S.; Hong, P.; Arumugam, B.; Pokomo, L.; Boyer, J.; Koizumi, N.; Kittipongdaja, P.; Chen, A.; Bristol, G.; Galic, Z.; et al. A highly efficient short hairpin RNA potently down-regulates CCR5 expression in systemic lymphoid organs in the hu-BLT mouse model. Blood 2010, 115, 1534–1544. [Google Scholar] [CrossRef]
- Vatakis, D.N.; Koya, R.C.; Nixon, C.C.; Wei, L.; Kim, S.G.; Avancena, P.; Bristol, G.; Baltimore, D.; Kohn, D.B.; Ribas, A.; et al. Antitumor activity from antigen-specific CD8 T cells generated in vivo from genetically engineered human hematopoietic stem cells. Proc. Natl. Acad. Sci. USA 2011, 108, E1408–E1416. [Google Scholar] [CrossRef] [PubMed]
- Vatakis, D.N.; Bristol, G.C.; Kim, S.G.; Levin, B.; Liu, W.; Radu, C.G.; Kitchen, S.G.; Zack, J.A. Using the BLT humanized mouse as a stem cell based gene therapy tumor model. J. Vis. Exp. 2012, e4181. [Google Scholar] [CrossRef]
- Jewett, A.; Bonavida, B. Target-induced inactivation and cell death by apoptosis in a subset of human NK cells. J. Immunol. 1996, 156, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Tarazona, R.; Duran, E.; Solana, R. Natural Killer Cell Recognition of Melanoma: New Clues for a More Effective Immunotherapy. Front. Immunol. 2015, 6, 649. [Google Scholar] [CrossRef] [PubMed]
- Schlecker, E.; Fiegler, N.; Arnold, A.; Altevogt, P.; Rose-John, S.; Moldenhauer, G.; Sucker, A.; Paschen, A.; von Strandmann, E.P.; Textor, S.; et al. Metalloprotease-mediated tumor cell shedding of B7-H6, the ligand of the natural killer cell-activating receptor NKp30. Cancer Res. 2014, 74, 3429–3440. [Google Scholar] [CrossRef]
- Dhar, P.; Wu, J.D. NKG2D and its ligands in cancer. Curr. Opin. Immunol. 2018, 51, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Carré, T.; Thiery, J.; Janji, B.; Terry, S.; Gros, G.; Meurice, G.; Kieda, C.; Olive, D.; Chouaib, S. Selection of tumor-resistant variants following sustained natural killer cell-mediated immune stress. Oncol. Rep. 2021, 45, 582–594. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Kato, S.; Nesline, M.K.; Conroy, J.M.; DePietro, P.; Pabla, S.; Kurzrock, R. Indoleamine 2,3-dioxygenase (IDO) inhibitors and cancer immunotherapy. Cancer Treat. Rev. 2022, 110, 102461. [Google Scholar] [CrossRef]
- Kaur, K.; Kozlowska, A.K.; Topchyan, P.; Ko, M.W.; Ohanian, N.; Chiang, J.; Cook, J.; Maung, P.O.; Park, S.H.; Cacalano, N.; et al. Probiotic-Treated Super-Charged NK Cells Efficiently Clear Poorly Differentiated Pancreatic Tumors in Hu-BLT Mice. Cancers 2019, 12, 63. [Google Scholar] [CrossRef]
- Kaur, K.; Topchyan, P.; Kozlowska, A.K.; Ohanian, N.; Chiang, J.; Maung, P.O.; Park, S.H.; Ko, M.W.; Fang, C.; Nishimura, I.; et al. Super-charged NK cells inhibit growth and progression of stem-like/poorly differentiated oral tumors in vivo in humanized BLT mice; effect on tumor differentiation and response to chemotherapeutic drugs. Oncoimmunology 2018, 7, e1426518. [Google Scholar] [CrossRef]
- Decombis, S.; Smolander, J.; Bouhlal, J.; Klievink, J.; Lähteenmäki, H.; Jokinen, E.; Lin, L.; Laitinen, H.; Matjusinski, K.; Holappa, E.; et al. Bone Marrow Stromal Cells Hamper Leukemia Cell Induced NK Cell Activation and Cytotoxicity. Blood 2024, 144, 4814. [Google Scholar] [CrossRef]
- Aref, S.; Khaled, N.; Al Gilany, A.H.; Ayed, M.; Abouzeid, T.; Attia, D. Impact of Bone Marrow Natural Killer Cells (NK); Soluble TNF-α and IL-32 Levels in Myelodysplastic Syndrome Patients. Asian Pac. J. Cancer Prev. 2020, 21, 2949–2953. [Google Scholar] [CrossRef]
- Kaur, K.; Celis, A.P.; Jewett, A. Natural Killer Cell-Secreted IFN-γ and TNF-α Mediated Differentiation in Lung Stem-like Tumors, Leading to the Susceptibility of the Tumors to Chemotherapeutic Drugs. Cells 2025, 14, 90. [Google Scholar] [CrossRef]
- Breznik, B.; Ko, M.-W.; Tse, C.; Chen, P.-C.; Senjor, E.; Majc, B.; Habič, A.; Angelillis, N.; Novak, M.; Župunski, V.; et al. Infiltrating natural killer cells bind, lyse and increase chemotherapy efficacy in glioblastoma stem-like tumorospheres. Commun. Biol. 2022, 5, 436. [Google Scholar] [CrossRef]
- Jewett, A. First in Human Clinical Study Demonstrating the Safety and Efficacy of NK101 (supercharged NK cells) in the Treatment of Patients with Cancer. South East Eur. J. Immunol. 2025, 8, 040. [Google Scholar] [CrossRef]
| IFN-γ | ITAC | GMCSF | FRACTALKINE | IL-10 | MIP-3a | IL-12 | IL-13 | IL-17A | IL-1b | IL-21 | IL-4 | IL-23 | MIP-1a | IL-8 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hu-BLT | 7 | 34 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 3 |
| +sNK (1 million) | 12 | 17 | 8 | 28 | 1 | 17 | 2 | 4 | 18 | 3 | 11 | 4 | 26 | 5 | 36 |
| +sNK (2 million) | 18 | 13 | 11 | 37 | 3 | 23 | 7 | 3 | 21 | 7 | 9 | 9 | 88 | 7 | 58 |
| Serum | IFN-γ | TNF-α | IL-10 | IL-12 | IL-17A | IL-1b | IL-2 |
|---|---|---|---|---|---|---|---|
| Tumor | 5.5 ± 3 | 7 ± 1.4 | 33.5 ± 16 | 3 ± 1.4 | 6 ± 0.1 | 2 ± 1.1 | 5 ± 2.1 |
| Tumor + sNK | 60 | 26 | 17 | 24 | 48 | 16 | 16 |
| Serum | GMCSF | MIP-1b | ITAC | Fractalkine | MIP-3A | ||
| Tumor | 291.5 ± 168 | 6.5 ± 0.7 | 16 ± 7 | 109 ± 12 | 4 ± 1.1 | ||
| Tumor + sNK | 328 | 33 | 32 | 320 | 27 |
| PBMCs | IFN-γ | TNF-α | IL-6 | IFN-a | VEGF | GMCSF | IP-10 | Eotaxin | Fractalkine |
|---|---|---|---|---|---|---|---|---|---|
| Tumor | 0.9 ± 0.7 | 2.5 ± 1.6 | 5 ± 3.3 | 10 ± 5.8 | 57 ± 10 | 0.5 ± 0.5 | 3.2 ± 0.1 | 3.8 ± 1 | 14 ± 5 |
| Tumor + sNK | 2 ± 0.7 | 4.1 ± 3 | 7.7 ± 2.3 | 24 ± 2 | 33.6 ± 12 | 3.1 ± 1.9 | 6 ± 2.5 | 4.3 ± 0.6 | 22 ± 7 |
| Spleen | IFN-γ | TNF-α | IL-6 | IL-10 | GMCSF | MCP-1 | MIP-1a | MIP-1b | MDC | IP-10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Tumor | 3 ± 2 | 3.7 ± 1.2 | 1.9 ± 1 | 2.5 ± 0.6 | 6.15 ± 2.8 | 5.3 ± 3.5 | 202 ± 165 | 63 ± 17 | 211 ± 91 | 14.7 ± 4.5 |
| Tumor + sNK | 6.5 ± 2.5 | 6.9 ± 1.8 | 4.9 ± 1.1 | 24 ± 18 | 12.2 ± 0.1 | 14.1 ± 2.3 | 805 ± 296 | 118 ± 48 | 405 ± 147 | 34 ± 21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, K.; Topchyan, P.; Jewett, A. Supercharged Natural Killer (sNK) Cells Inhibit Melanoma Tumor Progression and Restore Endogenous NK Cell Function in Humanized BLT Mice. Cancers 2025, 17, 2430. https://doi.org/10.3390/cancers17152430
Kaur K, Topchyan P, Jewett A. Supercharged Natural Killer (sNK) Cells Inhibit Melanoma Tumor Progression and Restore Endogenous NK Cell Function in Humanized BLT Mice. Cancers. 2025; 17(15):2430. https://doi.org/10.3390/cancers17152430
Chicago/Turabian StyleKaur, Kawaljit, Paytsar Topchyan, and Anahid Jewett. 2025. "Supercharged Natural Killer (sNK) Cells Inhibit Melanoma Tumor Progression and Restore Endogenous NK Cell Function in Humanized BLT Mice" Cancers 17, no. 15: 2430. https://doi.org/10.3390/cancers17152430
APA StyleKaur, K., Topchyan, P., & Jewett, A. (2025). Supercharged Natural Killer (sNK) Cells Inhibit Melanoma Tumor Progression and Restore Endogenous NK Cell Function in Humanized BLT Mice. Cancers, 17(15), 2430. https://doi.org/10.3390/cancers17152430








