Multiparametric Analysis of PET and Quantitative MRI for Identifying Intratumoral Habitats and Characterizing Trastuzumab-Induced Alterations
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model and Treatment Response Assessment
2.2. Magnetic Resonance Imaging
2.3. PET/CT Imaging
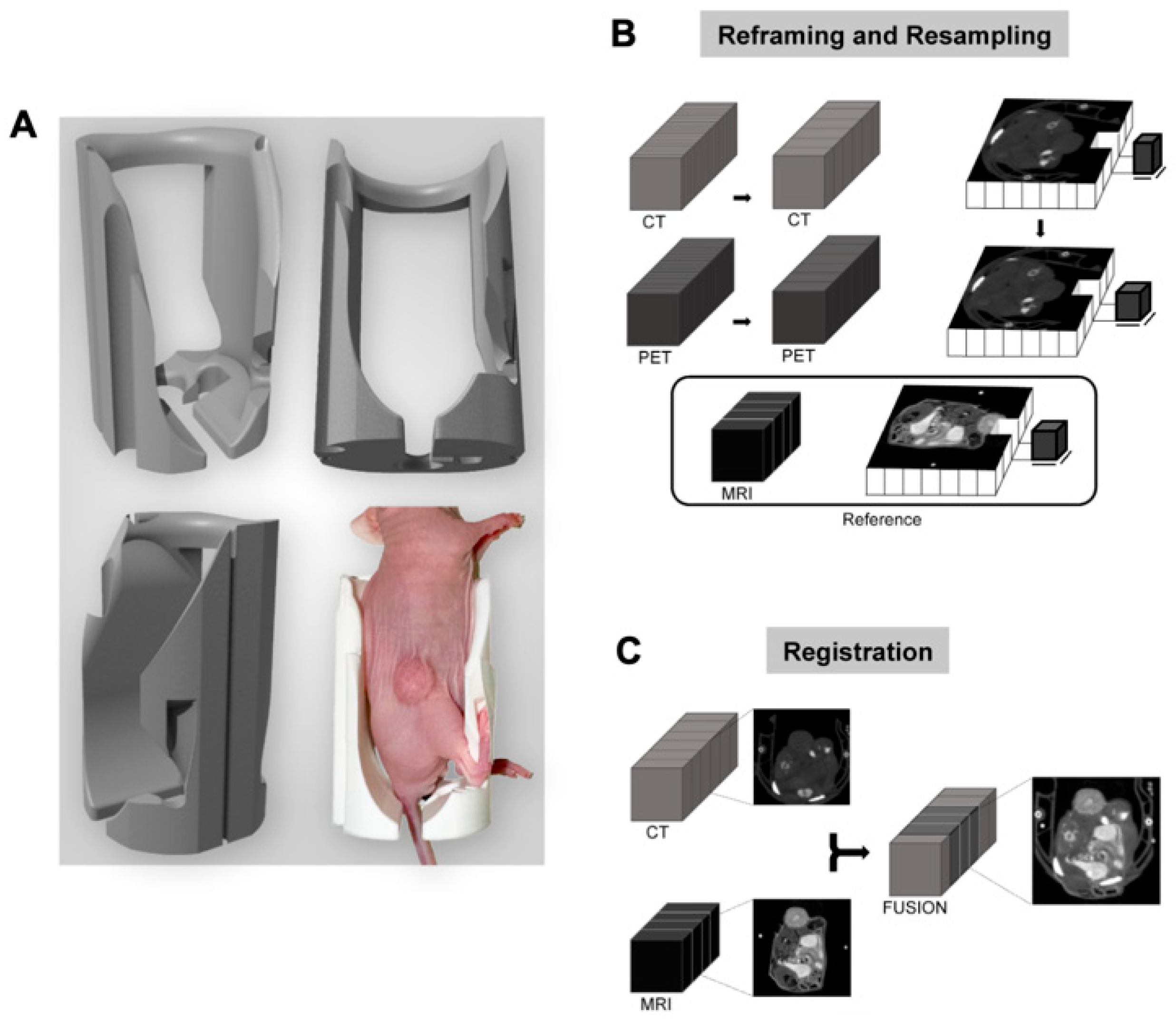
2.4. Developing a Multimodal Registration Pipeline
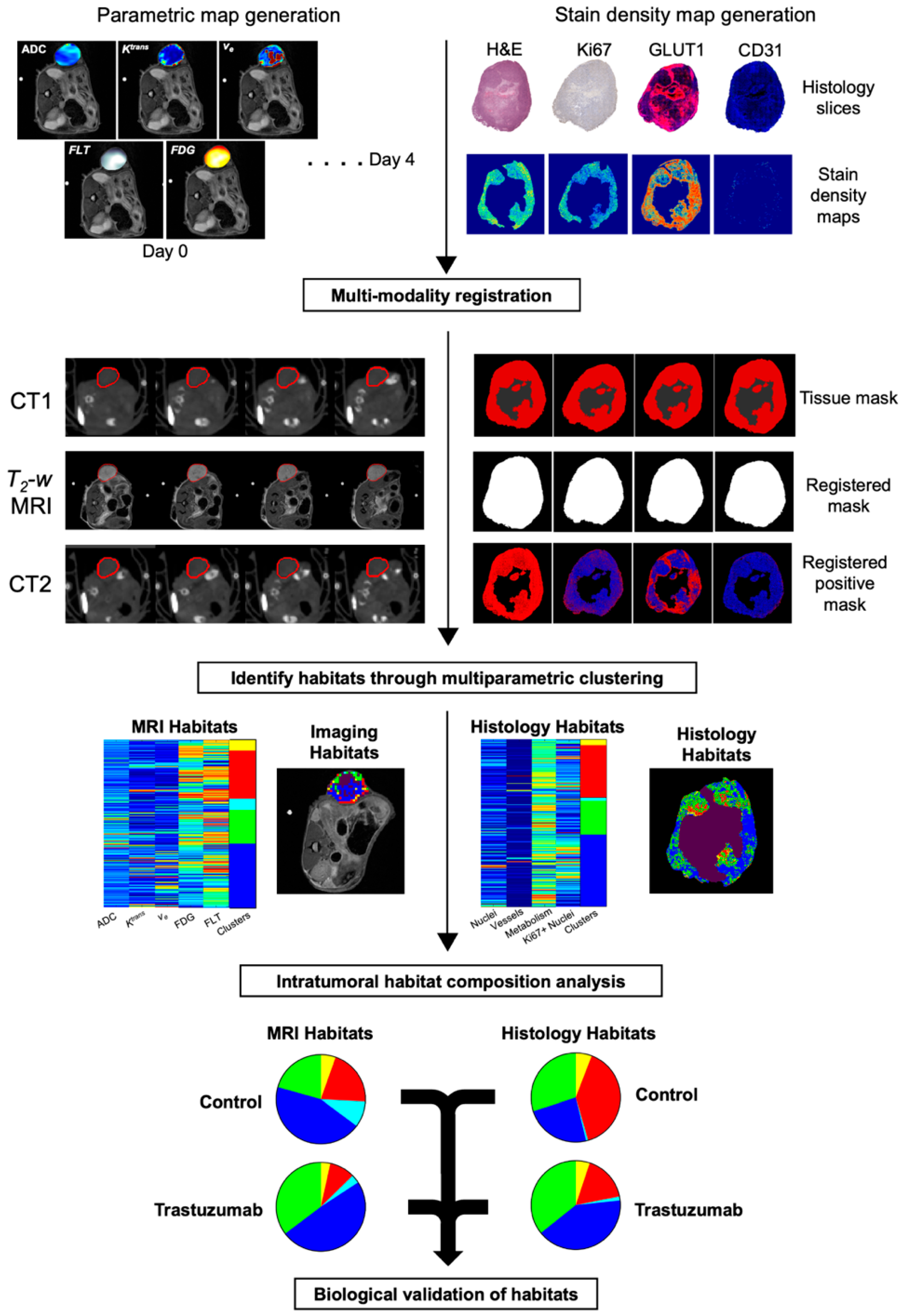
2.5. Discovery of Intratumoral Imaging Habitats
2.6. Immunohistochemical Staining and Imaging Processing
2.7. Correlating PET/MR Imaging Habitats with Histological Habitats
3. Statistical Analysis
4. Results
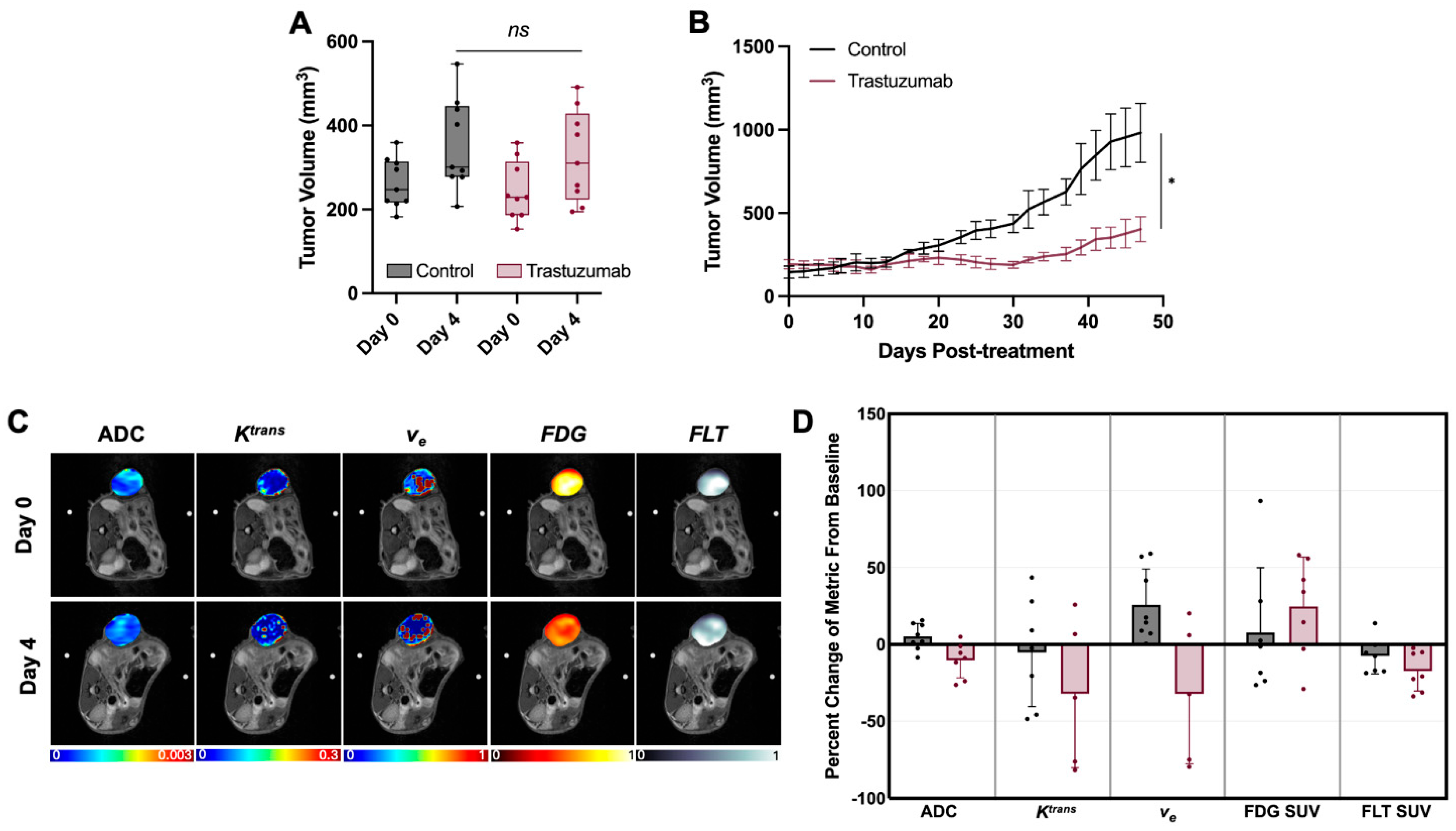
4.1. Early Treatment Induced Alterations Are Masked When Measured as Single Imaging Metric Changes
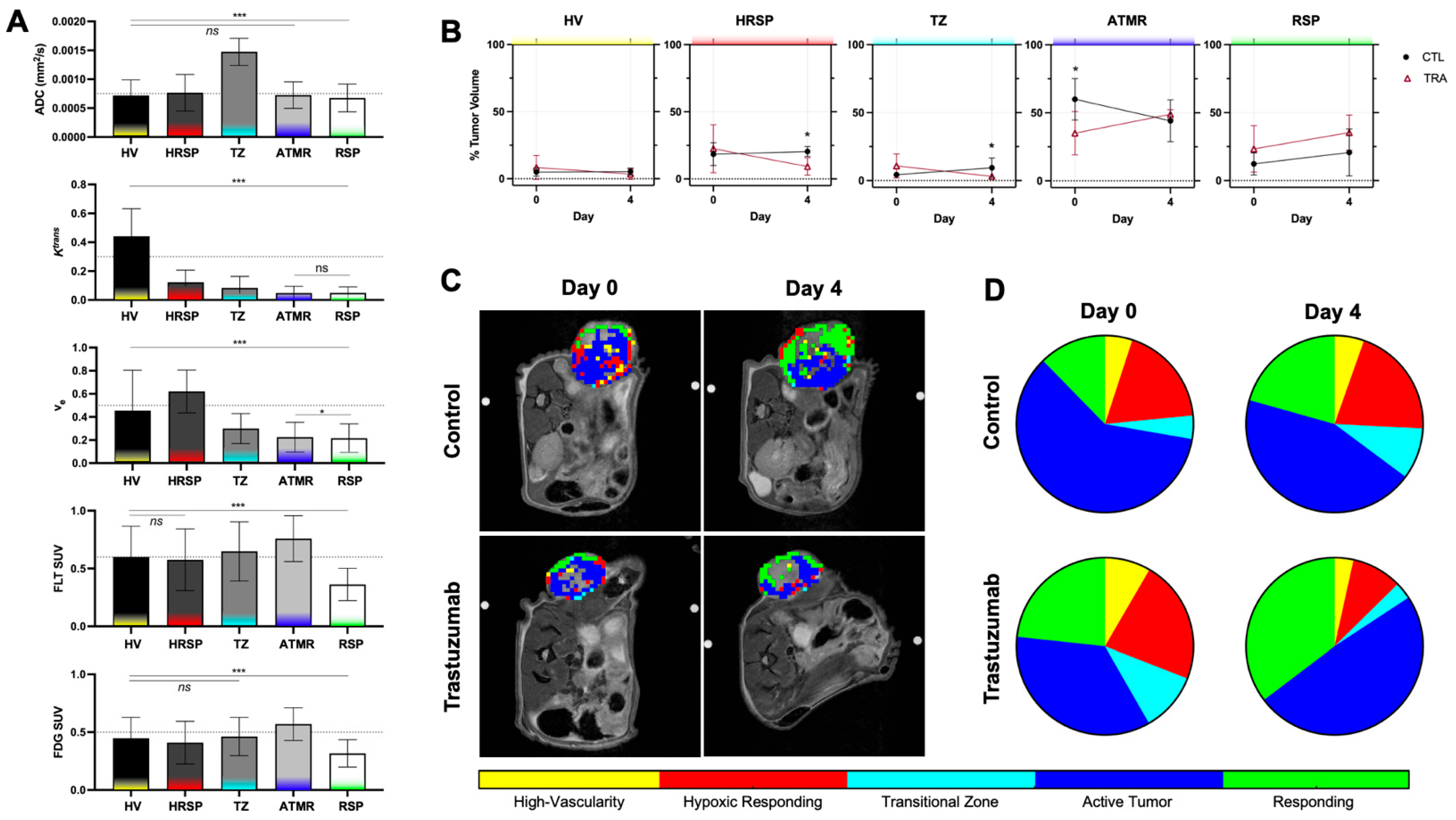
4.2. Discovery of PET and MRI Tumor Habitats
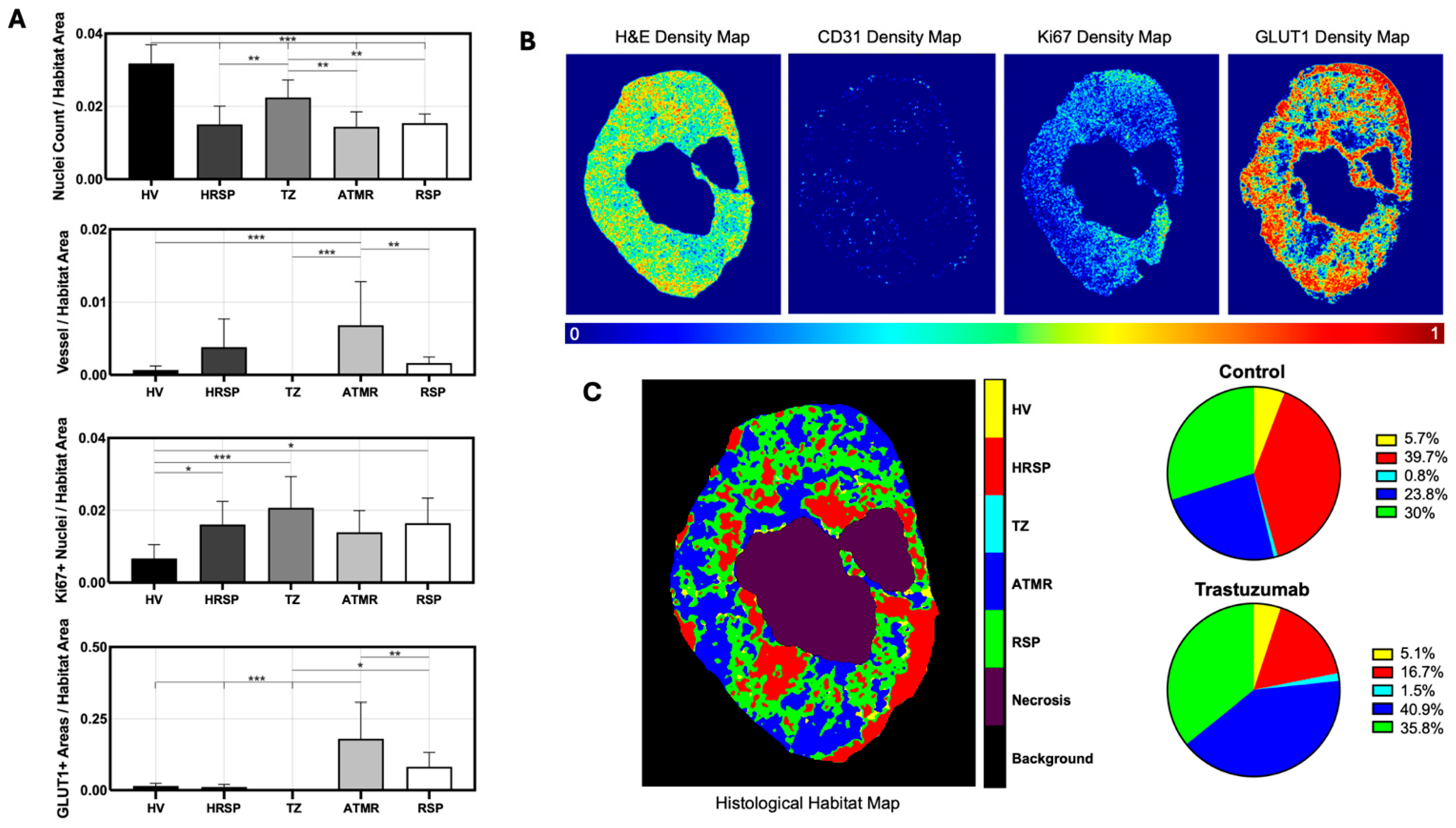
4.3. Discovery of Histological Tumor Habitats
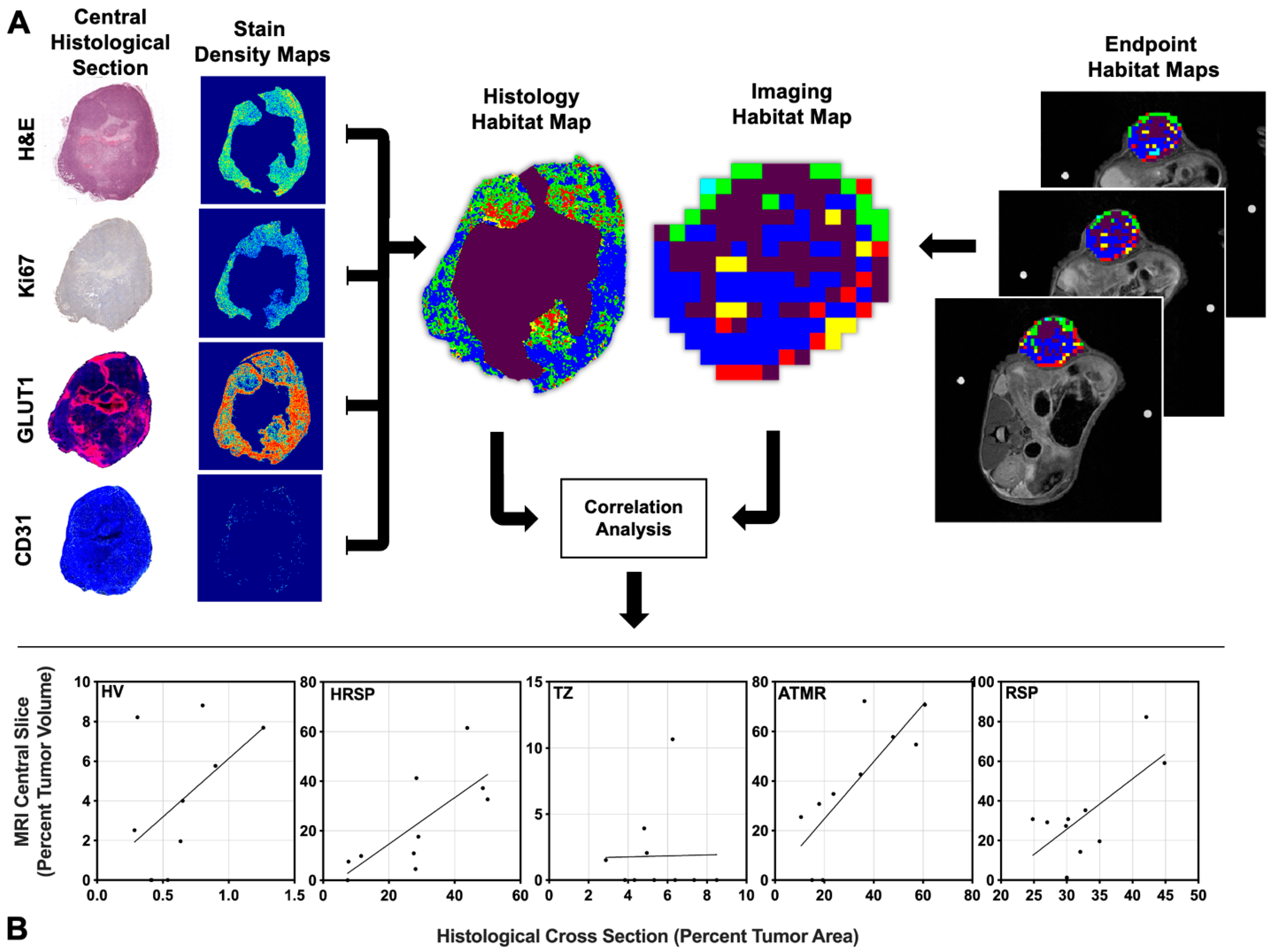
4.4. Positive Linear Correlations Between Imaging-Derived and Histology-Derived Habitats
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramón, Y.; Cajal, S.; Sesé, M.; Capdevila, C.; Aasen, T.; De Mattos-Arruda, L.; Diaz-Cano, S.J.; Hernández-Losa, J.; Castellví, J. Clinical implications of intratumor heterogeneity: Challenges and opportunities. J. Mol. Med. 2020, 98, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Gianni, L. HER2-positive breast cancer. Lancet 2017, 389, 2415–2429. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Hammond, M.E.H.; Schwartz, J.N.; Hagerty, K.L.; Allred, D.C.; Cote, R.J.; Dowsett, M.; Fitzgibbons, P.L.; Hanna, W.M.; Langer, A.; et al. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer. J. Clin. Oncol. 2007, 25, 118–145. [Google Scholar] [CrossRef] [PubMed]
- Gajria, D.; Chandarlapaty, S. HER2-amplified breast cancer: Mechanisms of trastuzumab resistance and novel targeted therapies. Expert Rev. Anticancer Ther. 2011, 11, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Narayan, M.; Wilken, J.A.; Harris, L.N.; Baron, A.T.; Kimbler, K.D.; Maihle, N.J. Trastuzumab-Induced HER Reprogramming in “Resistant” Breast Carcinoma Cells. Cancer Res. 2009, 69, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Shah, C.; Miller, T.W.; Wyatt, S.K.; McKinley, E.T.; Olivares, M.G.; Sanchez, V.; Nolting, D.D.; Buck, J.R.; Zhao, P.; Ansari, M.S.; et al. Imaging biomarkers predict response to anti-HER2 (ErbB2) therapy in preclinical models of breast cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 4712–4721. [Google Scholar] [CrossRef] [PubMed]
- Mansur, A.; Song, P.N.; Lu, Y.; Burns, A.C.; Sligh, L.; Yang, E.S.; Sorace, A.G. Combination Therapy with Trastuzumab and Niraparib: Quantifying Early Proliferative Alterations in HER2+ Breast Cancer Models. Biomedicines 2023, 11, 2090. [Google Scholar] [CrossRef] [PubMed]
- Whisenant, J.G.; McIntyre, J.O.; Peterson, T.E.; Kang, H.; Sánchez, V.; Manning, H.C.; Arteaga, C.L.; Yankeelov, T.E. Utility of 18FLT-PET to Assess Treatment Response in Trastuzumab-resistant and Sensitive HER2-overexpressingHuman Breast Cancer Xenografts. Mol. Imaging Biol. MIB Off. Publ. Acad. Mol. Imaging 2015, 17, 119–128. [Google Scholar] [CrossRef][Green Version]
- McLarty, K.; Fasih, A.; Scollard, D.A.; Done, S.J.; Vines, D.C.; Green, D.E.; Costantini, D.L.; Reilly, R.M. 18F-FDG Small-Animal PET/CT Differentiates Trastuzumab-Responsive from Unresponsive Human Breast Cancer Xenografts in Athymic Mice. J. Nucl. Med. 2009, 50, 1848–1856. [Google Scholar] [CrossRef] [PubMed]
- Kazerouni, A.S.; Hormuth, D.A.; Davis, T.; Bloom, M.J.; Mounho, S.; Rahman, G.; Virostko, J.; Yankeelov, T.E.; Sorace, A.G. Quantifying Tumor Heterogeneity via MRI Habitats to Characterize Microenvironmental Alterations in HER2+ Breast Cancer. Cancers 2022, 14, 1837. [Google Scholar] [CrossRef] [PubMed]
- Sorace, A.G.; Syed, A.K.; Barnes, S.L.; Quarles, C.C.; Sanchez, V.; Kang, H.; Yankeelov, T.E. Quantitative [18F]FMISO PET Imaging Shows Reduction of Hypoxia Following Trastuzumab in a Murine Model of HER2+ Breast Cancer. Mol. Imaging Biol. 2017, 19, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Galbán, C.J.; Ma, B.; Malyarenko, D.; Pickles, M.D.; Heist, K.; Henry, N.L.; Schott, A.F.; Neal, C.H.; Hylton, N.M.; Rehemtulla, A.; et al. Multi-Site Clinical Evaluation of DW-MRI as a Treatment Response Metric for Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy. PLoS ONE 2015, 10, e0122151. [Google Scholar] [CrossRef] [PubMed]
- Jardim-Perassi, B.V.; Huang, S.; Dominguez-Viqueira, W.; Poleszczuk, J.; Budzevich, M.M.; Abdalah, M.A.; Pillai, S.R.; Ruiz, E.; Bui, M.M.; Zuccari, D.A.P.C.; et al. Multiparametric MRI and Coregistered Histology Identify Tumor Habitats in Breast Cancer Mouse Models. Cancer Res. 2019, 79, 3952–3964. [Google Scholar] [CrossRef] [PubMed]
- Whisenant, J.G.; Sorace, A.G.; McIntyre, J.O.; Kang, H.; Sánchez, V.; Loveless, M.E.; Yankeelov, T.E. Evaluating treatment response using DW-MRI and DCE-MRI in trastuzumab responsive and resistant HER2-overexpressing human breast cancer xenografts. Transl. Oncol. 2014, 7, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Abramson, R.G.; Arlinghaus, L.R.; Kang, H.; Chakravarthy, A.B.; Abramson, V.G.; Farley, J.; Mayer, I.A.; Kelley, M.C.; Meszoely, I.M.; et al. Multiparametric Magnetic Resonance Imaging for Predicting Pathological Response After the First Cycle of Neoadjuvant Chemotherapy in Breast Cancer. Investig. Radiol. 2015, 50, 195. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kang, H.; Arlinghaus, L.R.; Abramson, R.G.; Chakravarthy, A.B.; Abramson, V.G.; Farley, J.; Sanders, M.; Yankeelov, T.E. Analyzing Spatial Heterogeneity in DCE- and DW-MRI Parametric Maps to Optimize Prediction of Pathologic Response to Neoadjuvant Chemotherapy in Breast Cancer. Transl. Oncol. 2014, 7, 14–22. [Google Scholar] [CrossRef] [PubMed]
- An, Y.-S.; Kang, D.K.; Jung, Y.S.; Han, S.; Kim, T.H. Tumor metabolism and perfusion ratio assessed by 18F-FDG PET/CT and DCE-MRI in breast cancer patients: Correlation with tumor subtype and histologic prognostic factors. Eur. J. Radiol. 2015, 84, 1365–1370. [Google Scholar] [CrossRef] [PubMed]
- Tofts, P.S.; Brix, G.; Buckley, D.L.; Evelhoch, J.L.; Henderson, E.; Knopp, M.V.; Larsson, H.B.W.; Lee, T.-Y.; Mayr, N.A.; Parker, G.J.M.; et al. Estimating kinetic parameters from dynamic contrast-enhanced t1-weighted MRI of a diffusable tracer: Standardized quantities and symbols. J. Magn. Reson. Imaging 1999, 10, 223–232. [Google Scholar] [CrossRef]
- Loveless, M.E.; Halliday, J.; Liess, C.; Xu, L.; Dortch, R.D.; Whisenant, J.; Waterton, J.C.; Gore, J.C.; Yankeelov, T.E. A quantitative comparison of the influence of individual versus population-derived vascular input functions on dynamic contrast enhanced-MRI in small animals. Magn. Reson. Med. 2012, 67, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Loveless, M.E.; Lawson, D.; Collins, M.; Prasad Nadella, M.V.; Reimer, C.; Huszar, D.; Halliday, J.; Waterton, J.C.; Gore, J.C.; Yankeelov, T.E. Comparisons of the Efficacy of a Jak1/2 Inhibitor (AZD1480) with a VEGF Signaling Inhibitor (Cediranib) and Sham Treatments in Mouse Tumors Using DCE-MRI, DW-MRI, and Histology. Neoplasia 2012, 14, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Pineda, F.; Hormuth, D.A.; Karczmar, G.S.; Yankeelov, T.E. Quantitative analysis of vascular properties derived from ultrafast DCE-MRI to discriminate malignant and benign breast tumors. Magn. Reson. Med. 2019, 81, 2147–2160. [Google Scholar] [CrossRef] [PubMed]
- Syed, A.K.; Whisenant, J.G.; Barnes, S.L.; Sorace, A.G.; Yankeelov, T.E. Multiparametric Analysis of Longitudinal Quantitative MRI data to Identify Distinct Tumor Habitats in Preclinical Models of Breast Cancer. Cancers 2020, 12, 1682. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cao, G.; Sun, X.; Lee, J.; Rubin, D.L.; Napel, S.; Kurian, A.W.; Daniel, B.L.; Li, R. Intratumoral Spatial Heterogeneity at Perfusion MR Imaging Predicts Recurrence-free Survival in Locally Advanced Breast Cancer Treated with Neoadjuvant Chemotherapy. Radiology 2018, 288, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Song, P.N.; Mansur, A.; Lu, Y.; Della Manna, D.; Burns, A.; Samuel, S.; Heinzman, K.; Lapi, S.E.; Yang, E.S.; Sorace, A.G. Modulation of the Tumor Microenvironment with Trastuzumab Enables Radiosensitization in HER2+ Breast Cancer. Cancers 2022, 14, 1015. [Google Scholar] [CrossRef] [PubMed]
- Fliedner, F.P.; Engel, T.B.; El-Ali, H.H.; Hansen, A.E.; Kjaer, A. Diffusion weighted magnetic resonance imaging (DW-MRI) as a non-invasive, tissue cellularity marker to monitor cancer treatment response. BMC Cancer 2020, 20, 134. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, P.; Schwenck, J.; Frauenfeld, L.; Divine, M.R.; Agrawal, V.; Kohlhofer, U.; Gatidis, S.; Kontermann, R.; Königsrainer, A.; Quintanilla-Martinez, L.; et al. Quantification of intratumoural heterogeneity in mice and patients via machine-learning models trained on PET–MRI data. Nat. Biomed. Eng. 2023, 7, 1014–1027. [Google Scholar] [CrossRef] [PubMed]
- Erlandsson, K.; Dickson, J.; Arridge, S.; Atkinson, D.; Ourselin, S.; Hutton, B.F. MR Imaging–Guided Partial Volume Correction of PET Data in PET/MR Imaging. PET Clin. 2016, 11, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Ellingson, B.M.; Yao, J.; Raymond, C.; Nathanson, D.A.; Chakhoyan, A.; Simpson, J.; Garner, J.S.; Olivero, A.G.; Mueller, L.U.; Rodon, J.; et al. Multiparametric MR-PET Imaging Predicts Pharmacokinetics and Clinical Response to GDC-0084 in Patients with Recurrent High-Grade Glioma. Clin. Cancer Res. 2020, 26, 3135–3144. [Google Scholar] [CrossRef] [PubMed]
- Hicks, R.M.; Simko, J.P.; Westphalen, A.C.; Nguyen, H.G.; Greene, K.L.; Zhang, L.; Carroll, P.R.; Hope, T.A. Diagnostic Accuracy of 68Ga-PSMA-11 PET/MRI Compared with Multiparametric MRI in the Detection of Prostate Cancer. Radiology 2018, 289, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Romeo, V.; Clauser, P.; Rasul, S.; Kapetas, P.; Gibbs, P.; Baltzer, P.A.T.; Hacker, M.; Woitek, R.; Helbich, T.H.; Pinker, K. AI-enhanced simultaneous multiparametric 18F-FDG PET/MRI for accurate breast cancer diagnosis. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 596–608. [Google Scholar] [CrossRef] [PubMed]
- Sorace, A.G.; Wu, C.; Barnes, S.L.; Jarrett, A.M.; Avery, S.; Patt, D.; Goodgame, B.; Luci, J.J.; Kang, H.; Abramson, R.G.; et al. Repeatability, reproducibility, and accuracy of quantitative mri of the breast in the community radiology setting. J. Magn. Reson. Imaging 2018, 48, 695–707. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansur, A.; Gallegos, C.; Burns, A.; Watts, L.; Lee, S.; Song, P.; Lu, Y.; Sorace, A. Multiparametric Analysis of PET and Quantitative MRI for Identifying Intratumoral Habitats and Characterizing Trastuzumab-Induced Alterations. Cancers 2025, 17, 2422. https://doi.org/10.3390/cancers17152422
Mansur A, Gallegos C, Burns A, Watts L, Lee S, Song P, Lu Y, Sorace A. Multiparametric Analysis of PET and Quantitative MRI for Identifying Intratumoral Habitats and Characterizing Trastuzumab-Induced Alterations. Cancers. 2025; 17(15):2422. https://doi.org/10.3390/cancers17152422
Chicago/Turabian StyleMansur, Ameer, Carlos Gallegos, Andrew Burns, Lily Watts, Seth Lee, Patrick Song, Yun Lu, and Anna Sorace. 2025. "Multiparametric Analysis of PET and Quantitative MRI for Identifying Intratumoral Habitats and Characterizing Trastuzumab-Induced Alterations" Cancers 17, no. 15: 2422. https://doi.org/10.3390/cancers17152422
APA StyleMansur, A., Gallegos, C., Burns, A., Watts, L., Lee, S., Song, P., Lu, Y., & Sorace, A. (2025). Multiparametric Analysis of PET and Quantitative MRI for Identifying Intratumoral Habitats and Characterizing Trastuzumab-Induced Alterations. Cancers, 17(15), 2422. https://doi.org/10.3390/cancers17152422






