Predictive Biomarkers for Immunotherapy in Endometrial Carcinoma
Simple Summary
Abstract
1. Introduction
2. Molecular Subtypes of EC
2.1. POLE-Ultramutated (POLE-mut)
2.2. Mismatch Repair Deficiency (dMMR)/Microsatellite Instability-High (MSI-H)
2.3. Copy-Number Low (NSMP)
2.4. CNH/p53-Mutated
3. Tumor Mutational Burden
4. Immune Micro-Environment in EC
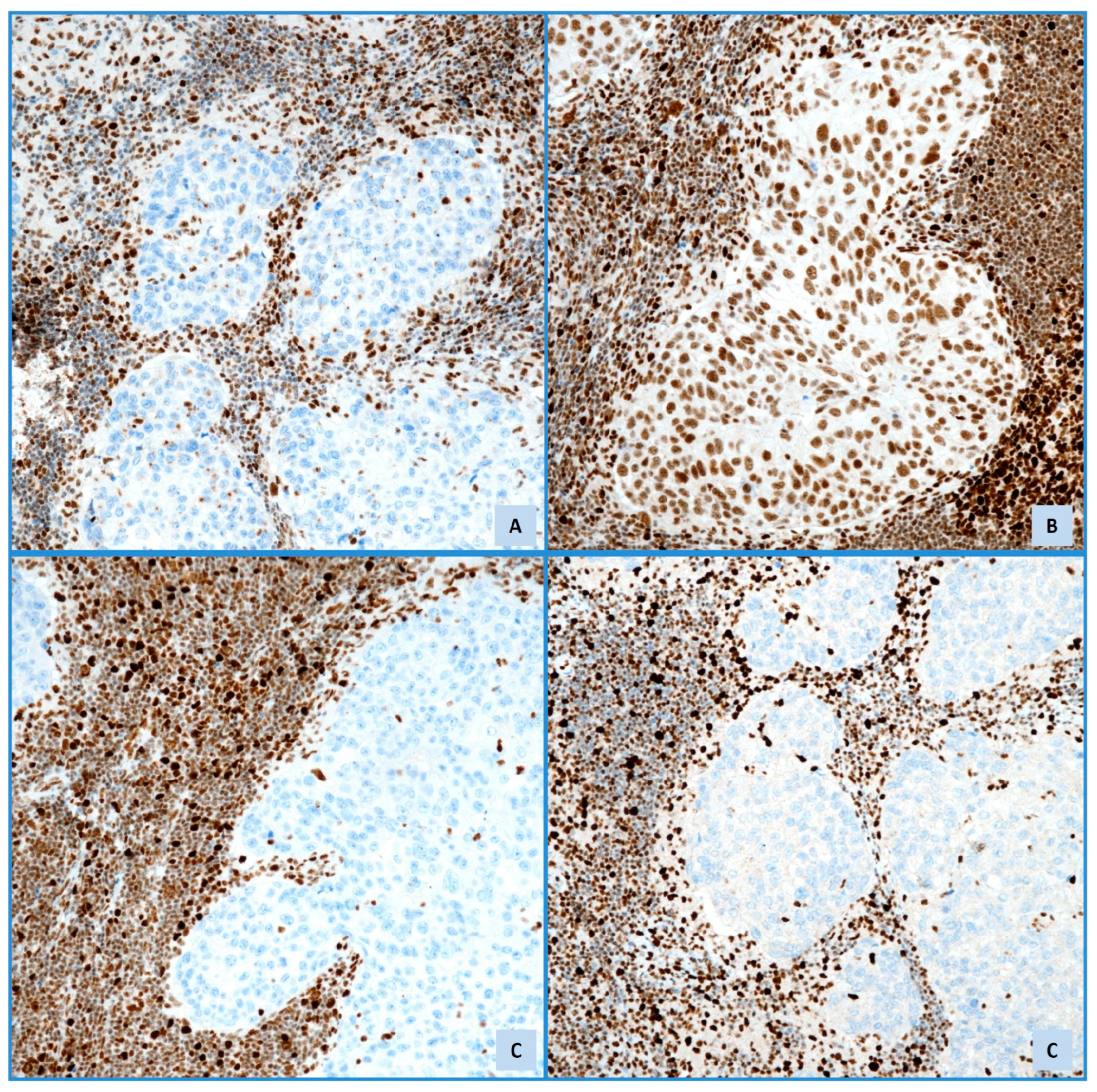
5. PD-L1 Expression
PD-L1 Expression in EC
6. Immunotherapy for EC
6.1. Clinical Immunotherapeutic Trials in EC
| Agent(s)/Trial | Target/Mechanism | Patient Population/Setting | Key Outcomes/Findings | Reference(s) |
|---|---|---|---|---|
| Pembrolizumab (KEYNOTE-028, -158) | Anti-PD-1 | PD-L1 + AEC (028); dMMR/MSI-H solid tumors incl. EC (158). | Showed durable activity and safety in PD-L1 + EC (ORR 13% in 028); Effective in dMMR/MSI-H EC; Led to site-agnostic approval for MSI-H/dMMR tumors. | [24,66,67] |
| Dostarlimab (GARNET) | Anti-PD-1 | Recurrent or advanced dMMR/MSI-H EC. | Clinically meaningful and durable activity with acceptable safety; Approved for dMMR/MSI-H EC. | [36] |
| Durvalumab + Carboplatin/Paclitaxel, followed by maintenance Durvalumab with or without Olaparib DUO-E trial (GOG-3041/ENGOT-EN10 | Anti-PD-1 + | Advanced or recurrent EC (dMMR and pMMR cohorts). | PFS benefit in dMMR, 0.42 [95% CI, 0.22 to 0.80]; 0.41 [95% CI, 0.21 to 0.75]) and pMMR subgroups, 0.77 [95% CI, 0.60 to 0.97]; 0.57; [95% CI, 0.44 to 0.73]); and in PD-L1–positive subgroups, 0.63 [95% CI, 0.48 to 0.83]; 0.42 [95% CI, 0.31 to 0.57]. | [69] |
| Avelumab (Konstantinopoulos et al.). | Anti-PD-L1 | Recurrent/persistent dMMR and pMMR EC. | Promising activity in dMMR EC regardless of PD-L1 status. Low activity in pMMR/non-POLE-mut EC. | [71] |
| Durvalumab (Antill et al.) | Anti-PD-L1 | AEC: dMMR (0–3 prior lines), pMMR (1–3 prior lines). | Promising activity and acceptable safety in dMMR AEC (ORR 47%). Limited activity in pMMR AEC. | [72] |
| RAINBO Program | Various (based on subtype) | Adjuvant setting post-surgery for specific molecular subtypes (p53abn, MMRd, NSMP, POLE-mut). | Ongoing program investigating molecularly-directed adjuvant therapies to improve outcomes and reduce toxicity. | [74] |
| Cabozantinib + Nivolumab (Lheureux et al.) | Multi-kinase inhibitor + Anti-PD-1 | Recurrent EC (immunotherapy-naïve and prior ICI). | Combination significantly improved outcomes (PFS) in heavily pretreated EC vs. historical controls. | [77] |
| Pembrolizumab + Chemotherapy (Eskander et al./KEYNOTE-868/NRG-GY018) | Anti-PD-1 + Carboplatin/Paclitaxel | Advanced or recurrent EC (dMMR and pMMR cohorts); first-line. | Significantly longer PFS vs. chemo alone, especially in dMMR cohort (70% reduction in progression/death risk). Benefit also seen in pMMR cohort (median PFS 13.1 vs. 8.7 mo). | [78] |
| Dostarlimab + Chemotherapy (Mirza et al./RUBY/ENGOT-EN6/GOG3031/NSGO) | Anti-PD-1 + Carboplatin/Paclitaxel | Primary advanced stage III/IV or first recurrent EC (dMMR/MSI-H and overall populations) | Significantly improved PFS vs. chemo alone in both dMMR/MSI-H population (est. 24-mo PFS 61.4% vs. 15.7%) and overall population (est. 24-mo PFS 36.1% vs. 18.1%). Substantial benefit in dMMR/MSI-H. | [79] |
6.2. Role of PD-1/PD-L1 Expression in EC
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Nagel, J.; Paschoalini, R.B.; Barreto, P.S.D.; Credidio, C.H.; Paulino, E.; Del Pilar Estevez-Diz, M. Predictive Biomarkers in Endometrial Carcinomas: A Review of Their Relevance in Daily Anatomic Pathology. Surg. Exp. Pathol. 2024, 7, 21. [Google Scholar] [CrossRef]
- Albertí-Valls, M.; Olave, S.; Olomí, A.; Macià, A.; Eritja, N. Advances in Immunotherapy for Endometrial Cancer: Insights into MMR Status and Tumor Microenvironment. Cancers 2024, 16, 3918. [Google Scholar] [CrossRef] [PubMed]
- Musacchio, L.; Boccia, S.M.; Caruso, G.; Santangelo, G.; Fischetti, M.; Tomao, F.; Perniola, G.; Palaia, I.; Muzii, L.; Pignata, S.; et al. Immune Checkpoint Inhibitors: A Promising Choice for Endometrial Cancer Patients? J. Clin. Med. 2020, 9, 1721. [Google Scholar] [CrossRef] [PubMed]
- Di Dio, C.; Bogani, G.; Di Donato, V.; Cuccu, I.; Muzii, L.; Musacchio, L.; Scambia, G.; Lorusso, D. The Role of Immunotherapy in Advanced and Recurrent MMR Deficient and Proficient Endometrial Carcinoma. Gynecol. Oncol. 2023, 169, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Mirza, M.R.; Lorusso, D.; Shen, Q.; Allonby, O.; Slim, M.; Borkowska, K.; Betts, M.; Coleman, R.L. First-Line Treatments for Advanced or Recurrent Endometrial Cancer: Systematic Literature Review of Clinical Evidence. Crit. Rev. Oncol. Hematol. 2025, 206, 104555. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Angelico, G.; Travaglino, A.; Inzani, F.; Arciuolo, D.; Valente, M.; D’Alessandris, N.; Scaglione, G.; Fiorentino, V.; Raffone, A.; et al. New Pathological and Clinical Insights in Endometrial Cancer in View of the Updated ESGO/ESTRO/ESP Guidelines. Cancers 2021, 13, 2623. [Google Scholar] [CrossRef] [PubMed]
- Pizzimenti, C.; Fiorentino, V.; Ruggeri, C.; Franchina, M.; Ercoli, A.; Tuccari, G.; Ieni, A. Autophagy Involvement in Non-Neoplastic and Neoplastic Endometrial Pathology: The State of the Art with a Focus on Carcinoma. Int. J. Mol. Sci. 2024, 25, 12118. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Angelico, G.; Inzani, F.; Spadola, S.; Arciuolo, D.; Valente, M.; Fiorentino, V.; Mulè, A.; Scambia, G.; Zannoni, G.F. The Many Faces of Endometriosis-Related Neoplasms in the Same Patient: A Brief Report. Gynecol. Obstet. Investig. 2020, 85, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Alexa, M.; Hasenburg, A.; Battista, M.J. The TCGA Molecular Classification of Endometrial Cancer and Its Possible Impact on Adjuvant Treatment Decisions. Cancers 2021, 13, 1478. [Google Scholar] [CrossRef] [PubMed]
- Bokhman, J.V. Two Pathogenetic Types of Endometrial Carcinoma. Gynecol. Oncol. 1983, 15, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Inoue, F.; Sone, K.; Toyohara, Y.; Takahashi, Y.; Kukita, A.; Hara, A.; Taguchi, A.; Tanikawa, M.; Tsuruga, T.; Osuga, Y. Targeting Epigenetic Regulators for Endometrial Cancer Therapy: Its Molecular Biology and Potential Clinical Applications. Int. J. Mol. Sci. 2021, 22, 2305. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.A.; Cancer Genome Atlas Research Network. Integrated Genomic Characterization of Endometrial Carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Vermij, L.; Horeweg, N.; Leon-Castillo, A.; Rutten, T.A.; Mileshkin, L.R.; Mackay, H.J.; Leary, A.; Powell, M.E.; Singh, N.; Crosbie, E.J.; et al. HER2 Status in High-Risk Endometrial Cancers (PORTEC-3): Relationship with Histotype, Molecular Classification, and Clinical Outcomes. Cancers 2020, 13, 44. [Google Scholar] [CrossRef] [PubMed]
- Kommoss, S.; McConechy, M.K.; Kommoss, F.; Leung, S.; Bunz, A.; Magrill, J.; Britton, H.; Kommoss, F.; Grevenkamp, F.; Karnezis, A.; et al. Final Validation of the ProMisE Molecular Classifier for Endometrial Carcinoma in a Large Population-Based Case Series. Ann. Oncol. 2018, 29, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Berek, J.S.; Matias-Guiu, X.; Creutzberg, C.; Fotopoulou, C.; Gaffney, D.; Kehoe, S.; Lindemann, K.; Mutch, D.; Concin, N. FIGO Staging of Endometrial Cancer: 2023. Int. J. Gynecol. Obstet. 2023, 162, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Tuninetti, V.; Pace, L.; Ghisoni, E.; Quarà, V.; Arezzo, F.; Palicelli, A.; Mandato, V.D.; Geuna, E.; Cormio, G.; Biglia, N.; et al. Retrospective Analysis of the Correlation of MSI-h/DMMR Status and Response to Therapy for Endometrial Cancer: RAME Study, a Multicenter Experience. Cancers 2023, 15, 3639. [Google Scholar] [CrossRef] [PubMed]
- Tashireva, L.A.; Larionova, I.V.; Ermak, N.A.; Maltseva, A.A.; Livanos, E.I.; Kalinchuk, A.Y.; Stakheyeva, M.N.; Kolomiets, L.A. Predicting Immunotherapy Efficacy in Endometrial Cancer: Focus on the Tumor Microenvironment. Front. Immunol. 2025, 15, 1523518. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Song, Y.; Deng, W.; Blake, N.; Luo, X.; Meng, J. Potential Predictive Biomarkers in Antitumor Immunotherapy: Navigating the Future of Antitumor Treatment and Immune Checkpoint Inhibitor Efficacy. Front. Oncol. 2024, 14, 1483454. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Hao, Y.; Qi, Y.; Wei, H.; Zhang, J.; Li, H. Molecular Mechanism of Tumor-Infiltrating Immune Cells Regulating Endometrial Carcinoma. Genes Dis. 2025, 12, 101442. [Google Scholar] [CrossRef] [PubMed]
- Martín-López, J.V.; Fishel, R. The Mechanism of Mismatch Repair and the Functional Analysis of Mismatch Repair Defects in Lynch Syndrome. Fam. Cancer 2013, 12, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Travaglino, A.; Raffone, A.; Mascolo, M.; Guida, M.; Insabato, L.; Zannoni, G.F.; Zullo, F. TCGA Molecular Subgroups in Endometrial Undifferentiated/Dedifferentiated Carcinoma. Pathol. Oncol. Res. 2020, 26, 1411–1416. [Google Scholar] [CrossRef] [PubMed]
- Addante, F.; D’Amati, A.; Santoro, A.; Angelico, G.; Inzani, F.; Arciuolo, D.; Travaglino, A.; Raffone, A.; D’Alessandris, N.; Scaglione, G.; et al. Mismatch Repair Deficiency as a Predictive and Prognostic Biomarker in Endometrial Cancer: A Review on Immunohistochemistry Staining Patterns and Clinical Implications. Int. J. Mol. Sci. 2024, 25, 1056. [Google Scholar] [CrossRef] [PubMed]
- Talhouk, A.; Derocher, H.; Schmidt, P.; Leung, S.; Milne, K.; Gilks, C.B.; Anglesio, M.S.; Nelson, B.H.; McAlpine, J.N. Molecular Subtype Not Immune Response Drives Outcomes in Endometrial Carcinoma. Clin. Cancer Res. 2019, 25, 2537–2548. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, J.M.; Panda, A.; Zhong, H.; Hirshfield, K.; Damare, S.; Lane, K.; Sokol, L.; Stein, M.N.; Rodriguez-Rodriquez, L.; Kaufman, H.L.; et al. Immune Activation and Response to Pembrolizumab in POLE-Mutant Endometrial Cancer. J. Clin. Investig. 2016, 126, 2334–2340. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.-P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair–Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Léon-Castillo, A. Update in the Molecular Classification of Endometrial Carcinoma. Int. J. Gynecol. Cancer 2023, 33, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Talhouk, A.; McConechy, M.K.; Leung, S.; Li-Chang, H.H.; Kwon, J.S.; Melnyk, N.; Yang, W.; Senz, J.; Boyd, N.; Karnezis, A.N.; et al. A Clinically Applicable Molecular-Based Classification for Endometrial Cancers. Br. J. Cancer 2015, 113, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Vermij, L.; Jobsen, J.J.; León-Castillo, A.; Brinkhuis, M.; Roothaan, S.; Powell, M.E.; de Boer, S.M.; Khaw, P.; Mileshkin, L.R.; Fyles, A.; et al. Prognostic Refinement of NSMP High-Risk Endometrial Cancers Using Oestrogen Receptor Immunohistochemistry. Br. J. Cancer 2023, 128, 1360–1368. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, A.; Bosse, T.; McAlpine, J.N. The Emerging Role of Molecular Pathology in Directing the Systemic Treatment of Endometrial Cancer. Ther. Adv. Med. Oncol. 2021, 13, 17588359211035959. [Google Scholar] [CrossRef] [PubMed]
- Mendiola, M.; Heredia-Soto, V.; Ruz-Caracuel, I.; Baillo, A.; Ramon-Patino, J.L.; Escudero, F.J.; Miguel, M.; Pelaez-Garcia, A.; Hernandez, A.; Feliu, J.; et al. Comparison of Methods for Testing Mismatch Repair Status in Endometrial Cancer. Int. J. Mol. Sci. 2023, 24, 14468. [Google Scholar] [CrossRef] [PubMed]
- León-Castillo, A.; de Boer, S.M.; Powell, M.E.; Mileshkin, L.R.; Mackay, H.J.; Leary, A.; Nijman, H.W.; Singh, N.; Pollock, P.M.; Bessette, P.; et al. Molecular Classification of the PORTEC-3 Trial for High-Risk Endometrial Cancer: Impact on Prognosis and Benefit From Adjuvant Therapy. J. Clin. Oncol. 2020, 38, 3388–3397. [Google Scholar] [CrossRef] [PubMed]
- Coll-de la Rubia, E.; Martinez-Garcia, E.; Dittmar, G.; Gil-Moreno, A.; Cabrera, S.; Colas, E. Prognostic Biomarkers in Endometrial Cancer: A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 1900. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.S.; Hacker, K.E.; Secord, A.A.; DeLair, D.F.; McCourt, C.; Urban, R. Molecular Testing for Endometrial Cancer: An SGO Clinical Practice Statement. Gynecol. Oncol. 2023, 168, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Sha, D.; Jin, Z.; Budczies, J.; Kluck, K.; Stenzinger, A.; Sinicrope, F.A. Tumor Mutational Burden as a Predictive Biomarker in Solid Tumors. Cancer Discov. 2020, 10, 1808–1825. [Google Scholar] [CrossRef] [PubMed]
- Strickler, J.H.; Hanks, B.A.; Khasraw, M. Tumor Mutational Burden as a Predictor of Immunotherapy Response: Is More Always Better? Clin. Cancer Res. 2021, 27, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Oaknin, A.; Gilbert, L.; Tinker, A.V.; Brown, J.; Mathews, C.; Press, J.; Sabatier, R.; O’Malley, D.M.; Samouelian, V.; Boni, V.; et al. Safety and Antitumor Activity of Dostarlimab in Patients with Advanced or Recurrent DNA Mismatch Repair Deficient/Microsatellite Instability-High (DMMR/MSI-H) or Proficient/Stable (MMRp/MSS) Endometrial Cancer: Interim Results from GARNET—A Phase I, Singl. J. Immunother. Cancer 2022, 10, e003777. [Google Scholar] [CrossRef] [PubMed]
- Mo, S.-F.; Cai, Z.-Z.; Kuai, W.-H.; Li, X.; Chen, Y.-T. Universal Cutoff for Tumor Mutational Burden in Predicting the Efficacy of Anti-PD-(L)1 Therapy for Advanced Cancers. Front. Cell Dev. Biol. 2023, 11, 1209243. [Google Scholar] [CrossRef] [PubMed]
- Dousset, L.; Poizeau, F.; Robert, C.; Mansard, S.; Mortier, L.; Caumont, C.; Routier, É.; Dupuy, A.; Rouanet, J.; Battistella, M.; et al. Positive Association Between Location of Melanoma, Ultraviolet Signature, Tumor Mutational Burden, and Response to Anti–PD-1 Therapy. JCO Precis. Oncol. 2021, 5, 1821–1829. [Google Scholar] [CrossRef] [PubMed]
- McGrail, D.J.; Pilié, P.G.; Rashid, N.U.; Voorwerk, L.; Slagter, M.; Kok, M.; Jonasch, E.; Khasraw, M.; Heimberger, A.B.; Lim, B.; et al. High Tumor Mutation Burden Fails to Predict Immune Checkpoint Blockade Response across All Cancer Types. Ann. Oncol. 2021, 32, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Fumet, J.-D.; Truntzer, C.; Yarchoan, M.; Ghiringhelli, F. Tumour Mutational Burden as a Biomarker for Immunotherapy: Current Data and Emerging Concepts. Eur. J. Cancer 2020, 131, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Vanderstraeten, A.; Tuyaerts, S.; Amant, F. The Immune System in the Normal Endometrium and Implications for Endometrial Cancer Development. J. Reprod. Immunol. 2015, 109, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Laba, S.; Mallett, G.; Amarnath, S. The Depths of PD-1 Function within the Tumor Microenvironment beyond CD8+ T Cells. Semin. Cancer Biol. 2022, 86, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Jubel, J.M.; Barbati, Z.R.; Burger, C.; Wirtz, D.C.; Schildberg, F.A. The Role of PD-1 in Acute and Chronic Infection. Front. Immunol. 2020, 11, 487. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 Pathway: Current Researches in Cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar] [PubMed]
- Salmaninejad, A.; Khoramshahi, V.; Azani, A.; Soltaninejad, E.; Aslani, S.; Zamani, M.R.; Zal, M.; Nesaei, A.; Hosseini, S.M. PD-1 and Cancer: Molecular Mechanisms and Polymorphisms. Immunogenetics 2018, 70, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Germanà, E.; Pepe, L.; Pizzimenti, C.; Ballato, M.; Pierconti, F.; Tuccari, G.; Ieni, A.; Giuffrè, G.; Fadda, G.; Fiorentino, V.; et al. Programmed Cell Death Ligand 1 (PD-L1) Immunohistochemical Expression in Advanced Urothelial Bladder Carcinoma: An Updated Review with Clinical and Pathological Implications. Int. J. Mol. Sci. 2024, 25, 6750. [Google Scholar] [CrossRef] [PubMed]
- Mamat @ Yusof, M.N.; Chew, K.T.; Kampan, N.C.; Shafiee, M.N. Expression of PD-1 and PD-L1 in Endometrial Cancer: Molecular and Clinical Significance. Int. J. Mol. Sci. 2023, 24, 15233. [Google Scholar] [CrossRef] [PubMed]
- Ohaegbulam, K.C.; Assal, A.; Lazar-Molnar, E.; Yao, Y.; Zang, X. Human Cancer Immunotherapy with Antibodies to the PD-1 and PD-L1 Pathway. Trends Mol. Med. 2015, 21, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Liu, Y.; Li, Q.; Li, X.-D.; Zhao, W.-Q.; Zhang, H.; Zhang, X.; Jiang, J.-T.; Wu, C.-P. PD-1/PD-L1 Pathway in Non-Small-Cell Lung Cancer and Its Relation with EGFR Mutation. J. Transl. Med. 2015, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, R.; Martin, A.; Bommarito, D.; Wang, K.; Hansen, S.H.; Freeman, G.J.; Ritz, J. Interferon-γ-Induced Activation of JAK1 and JAK2 Suppresses Tumor Cell Susceptibility to NK Cells through Upregulation of PD-L1 Expression. Oncoimmunology 2015, 4, e1008824. [Google Scholar] [CrossRef]
- Fiorentino, V.; Pizzimenti, C.; Franchina, M.; Pepe, L.; Russotto, F.; Tralongo, P.; Micali, M.G.; Militi, G.B.; Lentini, M. Programmed Cell Death Ligand 1 Immunohistochemical Expression and Cutaneous Melanoma: A Controversial Relationship. Int. J. Mol. Sci. 2024, 25, 676. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Diaz, A.; Shin, D.S.; Moreno, B.H.; Saco, J.; Escuin-Ordinas, H.; Rodriguez, G.A.; Zaretsky, J.M.; Sun, L.; Hugo, W.; Wang, X.; et al. Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression. Cell Rep. 2019, 29, 3766. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, V.; Tralongo, P.; Larocca, L.M.; Pizzimenti, C.; Martini, M.; Pierconti, F. First-Line ICIs in Renal Cell Carcinoma. Hum. Vaccines Immunother. 2023, 19, 2225386. [Google Scholar] [CrossRef] [PubMed]
- Ott, P.A.; Bang, Y.-J.; Berton-Rigaud, D.; Elez, E.; Pishvaian, M.J.; Rugo, H.S.; Puzanov, I.; Mehnert, J.M.; Aung, K.L.; Lopez, J.; et al. Safety and Antitumor Activity of Pembrolizumab in Advanced Programmed Death Ligand 1–Positive Endometrial Cancer: Results From the KEYNOTE-028 Study. J. Clin. Oncol. 2017, 35, 2535–2541. [Google Scholar] [CrossRef] [PubMed]
- Crumley, S.; Kurnit, K.; Hudgens, C.; Fellman, B.; Tetzlaff, M.T.; Broaddus, R. Identification of a Subset of Microsatellite-Stable Endometrial Carcinoma with High PD-L1 and CD8+ Lymphocytes. Mod. Pathol. 2019, 32, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Vanderwalde, A.; Spetzler, D.; Xiao, N.; Gatalica, Z.; Marshall, J. Microsatellite Instability Status Determined by Next-generation Sequencing and Compared with PD-L1 and Tumor Mutational Burden in 11,348 Patients. Cancer Med. 2018, 7, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Joehlin-Price, A.S.; Rhoades, J.; Ayoola-Adeola, M.; Miller, K.; Parwani, A.V.; Backes, F.J.; Felix, A.S.; Suarez, A.A. Programmed Death Ligand 1 Expression Among 700 Consecutive Endometrial Cancers. Int. J. Gynecol. Cancer 2018, 28, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Zong, L.; Sun, Z.; Mo, S.; Lu, Z.; Yu, S.; Xiang, Y.; Chen, J. PD-L1 Expression in Tumor Cells Is Associated with a Favorable Prognosis in Patients with High-Risk Endometrial Cancer. Gynecol. Oncol. 2021, 162, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Minaguchi, T.; Xu, C.; Qi, N.; Itagaki, H.; Shikama, A.; Tasaka, N.; Akiyama, A.; Sakurai, M.; Ochi, H.; et al. PD-L1 and CD4 Are Independent Prognostic Factors for Overall Survival in Endometrial Carcinomas. BMC Cancer 2020, 20, 127. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Nakayama, K.; Ishikawa, M.; Nakamura, K.; Ishibashi, T.; Sanuki, K.; Ono, R.; Sasamori, H.; Minamoto, T.; Iida, K.; et al. Microsatellite Instability Is a Biomarker for Immune Checkpoint Inhibitors in Endometrial Cancer. Oncotarget 2018, 9, 5652–5664. [Google Scholar] [CrossRef] [PubMed]
- Chew, M.; Wong, Y.P.; Karim, N.; Mustangin, M.; Alfian, N.; Tan, G.C. Programmed Death Ligand 1: A Poor Prognostic Marker in Endometrial Carcinoma. Diagnostics 2020, 10, 394. [Google Scholar] [CrossRef] [PubMed]
- Kucukgoz Gulec, U.; Kilic Bagir, E.; Paydas, S.; Guzel, A.B.; Gumurdulu, D.; Vardar, M.A. Programmed Death-1 (PD-1) and Programmed Death-Ligand 1 (PD-L1) Expressions in Type 2 Endometrial Cancer. Arch. Gynecol. Obstet. 2019, 300, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.S.; Filiaci, V.L.; Mannel, R.S.; Cohn, D.E.; Matsumoto, T.; Tewari, K.S.; DiSilvestro, P.; Pearl, M.L.; Argenta, P.A.; Powell, M.A.; et al. Carboplatin and Paclitaxel for Advanced Endometrial Cancer: Final Overall Survival and Adverse Event Analysis of a Phase III Trial (NRG Oncology/GOG0209). J. Clin. Oncol. 2020, 38, 3841–3850. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, S.F.; Bao, W. Molecular Subtypes of Endometrial Cancer: Implications for Adjuvant Treatment Strategies. Int. J. Gynecol. Obstet. 2024, 164, 436–459. [Google Scholar] [CrossRef] [PubMed]
- Nomura, H.; Aoki, D.; Takahashi, F.; Katsumata, N.; Watanabe, Y.; Konishi, I.; Jobo, T.; Hatae, M.; Hiura, M.; Yaegashi, N. Randomized Phase II Study Comparing Docetaxel plus Cisplatin, Docetaxel plus Carboplatin, and Paclitaxel plus Carboplatin in Patients with Advanced or Recurrent Endometrial Carcinoma: A Japanese Gynecologic Oncology Group Study (JGOG2041). Ann. Oncol. 2011, 22, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- Makker, V.; Green, A.K.; Wenham, R.M.; Mutch, D.; Davidson, B.; Miller, D.S. New Therapies for Advanced, Recurrent, and Metastatic Endometrial Cancers. Gynecol. Oncol. Res. Pract. 2017, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Rousset-Rouviere, S.; Rochigneux, P.; Chrétien, A.-S.; Fattori, S.; Gorvel, L.; Provansal, M.; Lambaudie, E.; Olive, D.; Sabatier, R. Endometrial Carcinoma: Immune Microenvironment and Emerging Treatments in Immuno-Oncology. Biomedicines 2021, 9, 632. [Google Scholar] [CrossRef] [PubMed]
- Westin, S.N.; Moore, K.; Chon, H.S.; Lee, J.-Y.; Thomes Pepin, J.; Sundborg, M.; Shai, A.; de la Garza, J.; Nishio, S.; Gold, M.A.; et al. Durvalumab Plus Carboplatin/Paclitaxel Followed by Maintenance Durvalumab With or Without Olaparib as First-Line Treatment for Advanced Endometrial Cancer: The Phase III DUO-E Trial. J. Clin. Oncol. 2024, 42, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Tamura, K.; Katsumata, N.; Matsumoto, K.; Takahashi, S.; Mukai, H.; Nomura, H.; Minami, H. Efficacy and Safety of Nivolumab (Nivo) in Patients (Pts) with Advanced or Recurrent Uterine Cervical or Corpus Cancers. J. Clin. Oncol. 2018, 36, 5594. [Google Scholar] [CrossRef]
- Konstantinopoulos, P.A.; Luo, W.; Liu, J.F.; Gulhan, D.C.; Krasner, C.; Ishizuka, J.J.; Gockley, A.A.; Buss, M.; Growdon, W.B.; Crowe, H.; et al. Phase II Study of Avelumab in Patients With Mismatch Repair Deficient and Mismatch Repair Proficient Recurrent/Persistent Endometrial Cancer. J. Clin. Oncol. 2019, 37, 2786–2794. [Google Scholar] [CrossRef] [PubMed]
- Antill, Y.; Kok, P.-S.; Robledo, K.; Yip, S.; Cummins, M.; Smith, D.; Spurdle, A.; Barnes, E.; Lee, Y.C.; Friedlander, M.; et al. Clinical Activity of Durvalumab for Patients with Advanced Mismatch Repair-Deficient and Repair-Proficient Endometrial Cancer. A Nonrandomized Phase 2 Clinical Trial. J. Immunother. Cancer 2021, 9, e002255. [Google Scholar] [CrossRef] [PubMed]
- Oaknin, A.; Tinker, A.V.; Gilbert, L.; Samouëlian, V.; Mathews, C.; Brown, J.; Barretina-Ginesta, M.-P.; Moreno, V.; Gravina, A.; Abdeddaim, C.; et al. Clinical Activity and Safety of the Anti–Programmed Death 1 Monoclonal Antibody Dostarlimab for Patients With Recurrent or Advanced Mismatch Repair–Deficient Endometrial Cancer. JAMA Oncol. 2020, 6, 1766. [Google Scholar] [CrossRef] [PubMed]
- RAINBO Research Consortium. Refining Adjuvant Treatment in Endometrial Cancer Based on Molecular Features: The RAINBO Clinical Trial Program. Int. J. Gynecol. Cancer 2023, 33, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Makker, V.; Colombo, N.; Herráez, A.C.; Monk, B.J.; Mackay, H.; Santin, A.D.; Miller, D.S.; Moore, R.G.; Baron-Hay, S.; Ray-Coquard, I.; et al. Lenvatinib Plus Pembrolizumab in Previously Treated Advanced Endometrial Cancer: Updated Efficacy and Safety From the Randomized Phase III Study 309/KEYNOTE-775. J. Clin. Oncol. 2023, 41, 2904–2910. [Google Scholar] [CrossRef] [PubMed]
- Makker, V.; Taylor, M.H.; Aghajanian, C.; Oaknin, A.; Mier, J.; Cohn, A.L.; Romeo, M.; Bratos, R.; Brose, M.S.; DiSimone, C.; et al. Lenvatinib Plus Pembrolizumab in Patients With Advanced Endometrial Cancer. J. Clin. Oncol. 2020, 38, 2981–2992. [Google Scholar] [CrossRef] [PubMed]
- Lheureux, S.; Matei, D.; Konstantinopoulos, P.A.; Block, M.S.; Jewell, A.; Gaillard, S.; McHale, M.S.; McCourt, C.K.; Temkin, S.; Girda, E.; et al. A Randomized Phase II Study of Cabozantinib and Nivolumab versus Nivolumab in Recurrent Endometrial Cancer. J. Clin. Oncol. 2020, 38, 6010. [Google Scholar] [CrossRef]
- Eskander, R.N.; Sill, M.W.; Beffa, L.; Moore, R.G.; Hope, J.M.; Musa, F.B.; Mannel, R.; Shahin, M.S.; Cantuaria, G.H.; Girda, E.; et al. Pembrolizumab plus Chemotherapy in Advanced Endometrial Cancer. N. Engl. J. Med. 2023, 388, 2159–2170. [Google Scholar] [CrossRef] [PubMed]
- Mirza, M.R.; Chase, D.M.; Slomovitz, B.M.; dePont Christensen, R.; Novák, Z.; Black, D.; Gilbert, L.; Sharma, S.; Valabrega, G.; Landrum, L.M.; et al. Dostarlimab for Primary Advanced or Recurrent Endometrial Cancer. N. Engl. J. Med. 2023, 388, 2145–2158. [Google Scholar] [CrossRef] [PubMed]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-γ in tumor progression and regression: A review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, C.; Lei, X.; Huang, T.; Zhou, R.; Lu, Y.J. Immune Cytolytic Activity for Comprehensive Insights of the Immune Landscape in Endometrial Carcinoma. J. Oncol. 2022, 2022, 9060243. [Google Scholar] [CrossRef]


| Feature | POLE-Ultramutated (POLE-mut) | Mismatch Repair Deficient (dMMR)/Microsatellite Instability-High (MSI-H) | Copy-Number Low (NSMP/No Specific Molecular Profile) | Copy-Number High (CNH/p53-Mutated) |
|---|---|---|---|---|
| Approx. Frequency | 5–8% overall; ~12% in high-grade [21]. | ~30% overall [22]. | ~50% overall [4]. | ~15% overall [4]. |
| Key Molecular Basis | Pathogenic mutations in the POLE exonuclease domain. | Defective DNA mismatch repair (somatic or Germline–Lynch Syndrome) | Low frequency of copy number alterations; often PTEN/PIK3CA mutations. | High frequency of copy number alterations; often TP53, PPP2R1A, FBXW7 mutations [12]. |
| Mutational Burden | Ultra-high (Ultramutation) [23]. | High (Hypermutation); MSI-H [22,24]. | Low [4]. | Low [12]. |
| Immunogenicity | High due to numerous neoantigens [23]. | High due to numerous neoantigens; high TILs [22,25]. | Lower immunogenic potential [4]. | Less immunogenic [12]. |
| Predicted ICI Response | Favorable response expected [23,26]. | Generally considered the best candidates for ICI therapy [22]. | Less likely to respond; ER-negative may respond better (needs validation) [4,27,28]. | Less likely to respond to monotherapy; combination strategies explored [12]. |
| Common Associations | Often high-grade tumors, undifferentiated, carcinosarcomas [21]. | Endometrioid histology, high TILs, LS association (2–5%) [22]. | Typically ER-positive, endometrioid histology, relatively stable genome [4,28]. | Often serous or serous-like histology, aggressive clinical course [12,29]. |
| Prognosis | Generally favorable [26]. | Variable; dMMR itself can be prognostic [30]. | Intermediate [4]. | Poorest, associated with 50–70% of mortality [29]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pizzimenti, C.; Fiorentino, V.; Pepe, L.; Franchina, M.; Ruggeri, C.; Ercoli, A.; Ciappina, G.; Berretta, M.; Tuccari, G.; Ieni, A. Predictive Biomarkers for Immunotherapy in Endometrial Carcinoma. Cancers 2025, 17, 2420. https://doi.org/10.3390/cancers17152420
Pizzimenti C, Fiorentino V, Pepe L, Franchina M, Ruggeri C, Ercoli A, Ciappina G, Berretta M, Tuccari G, Ieni A. Predictive Biomarkers for Immunotherapy in Endometrial Carcinoma. Cancers. 2025; 17(15):2420. https://doi.org/10.3390/cancers17152420
Chicago/Turabian StylePizzimenti, Cristina, Vincenzo Fiorentino, Ludovica Pepe, Mariausilia Franchina, Chiara Ruggeri, Alfredo Ercoli, Giuliana Ciappina, Massimiliano Berretta, Giovanni Tuccari, and Antonio Ieni. 2025. "Predictive Biomarkers for Immunotherapy in Endometrial Carcinoma" Cancers 17, no. 15: 2420. https://doi.org/10.3390/cancers17152420
APA StylePizzimenti, C., Fiorentino, V., Pepe, L., Franchina, M., Ruggeri, C., Ercoli, A., Ciappina, G., Berretta, M., Tuccari, G., & Ieni, A. (2025). Predictive Biomarkers for Immunotherapy in Endometrial Carcinoma. Cancers, 17(15), 2420. https://doi.org/10.3390/cancers17152420










