The Impact of Neoadjuvant Chemotherapy on Survival Outcomes in Gastric Signet-Ring Cell Carcinoma: An International Multicenter Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Study Outcomes
2.2. Diagnosis and Treatment
2.3. Follow-Up and Statistical Analysis
3. Results
3.1. Study Population
3.2. Treatment and Histopathological Outcomes
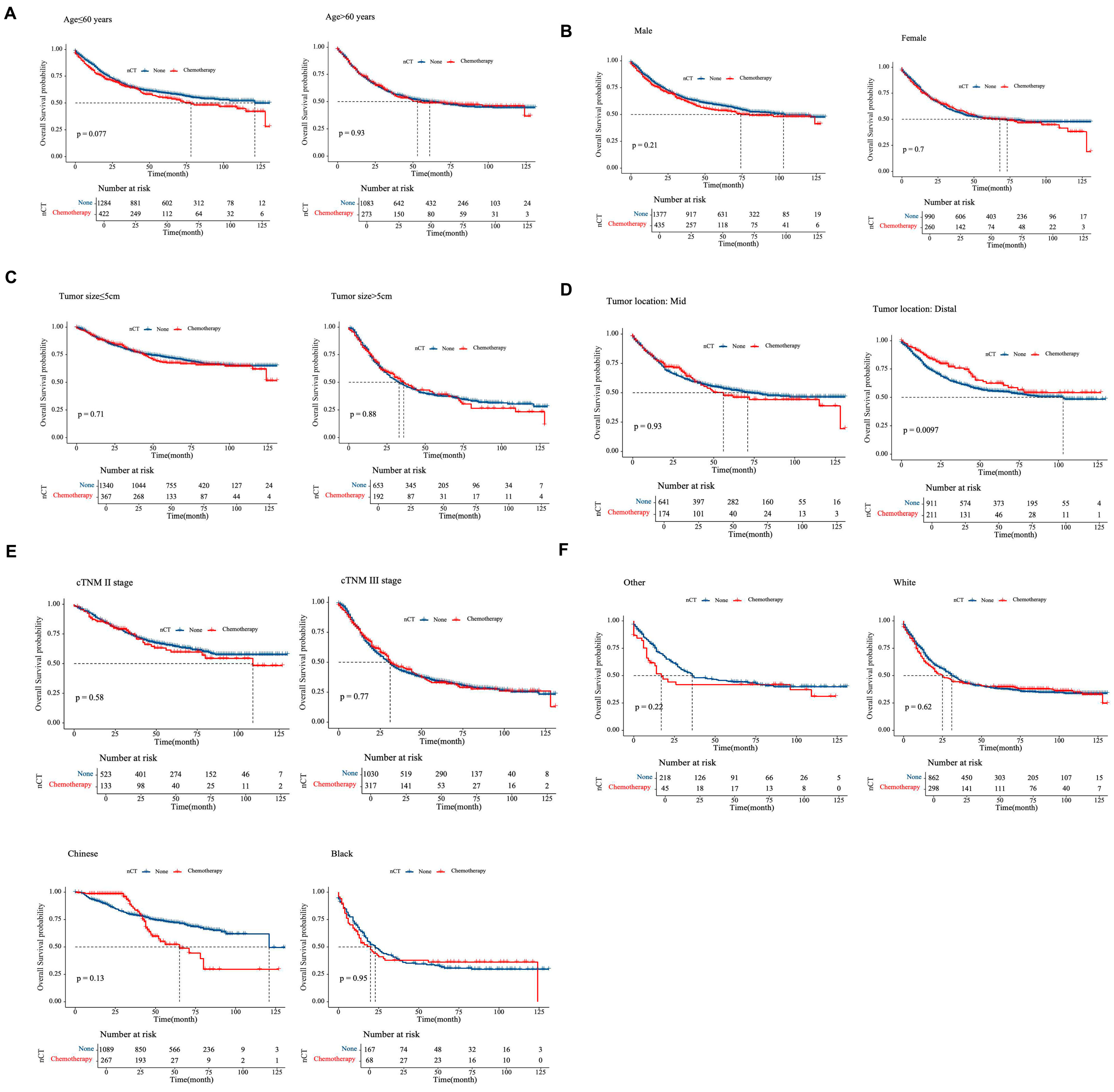
3.3. Outcome and Prognostic Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Efared, B.; Kadi, M.; Tahiri, L.; Lahmidani, N.; Hassani, K.I.M.; Bouhaddouti, H.E.; Benbrahim, Z.; Adil, I.S.; Chbani, L. Gastric Signet Ring Cell Carcinoma: A Comparative Analysis of Clinicopathologic Features. Cancer Control J. Moffitt Cancer Cent. 2020, 27, 1073274820976596. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, Z.; Ma, F.; Xue, L.; Tian, Y. Gastric Signet Ring Cell Carcinoma: Current Management and Future Challenges. Cancer Manag. Res. 2020, 12, 7973–7981. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Zu, H.; Wang, H.; Li, C.; Xue, Y. Clinicopathologic characteristics and prognostic value of various histological types in advanced gastric cancer. Int. J. Clin. Exp. Pathol. 2014, 7, 5692–5700. [Google Scholar] [PubMed]
- Kao, Y.-C.; Fang, W.-L.; Wang, R.-F.; Li, A.F.-Y.; Yang, M.-H.; Wu, C.-W.; Shyr, Y.-M.; Huang, K.-H. Clinicopathological differences in signet ring cell adenocarcinoma between early and advanced gastric cancer. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2019, 22, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Yang, Z.; Feng, Q.; Yu, M.; Zhang, Y.; Mao, C.; Shen, L.; Tang, J. The characteristics and prognostic value of signet ring cell histology in gastric cancer: A retrospective cohort study of 2199 consecutive patients. Medicine 2016, 95, e4052. [Google Scholar] [CrossRef] [PubMed]
- Pernot, S.; Voron, T.; Perkins, G.; Lagorce-Pages, C.; Berger, A.; Taieb, J. Signet-ring cell carcinoma of the stomach: Impact on prognosis and specific therapeutic challenge. World J. Gastroenterol. WJG 2015, 21, 11428–11438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ma, Y.; Liu, B. Prognostic Performance of Three Lymph-Node Staging Systems on Gastric Signet-Ring-Cell Carcinoma. Cancers 2023, 15, 3170. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.-E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.-G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 2019, 393, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van De Velde, C.J.H.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Heger, U.; Blank, S.; Wiecha, C.; Langer, R.; Weichert, W.; Lordick, F.; Bruckner, T.; Dobritz, M.; Burian, M.; Springfeld, C.; et al. Is preoperative chemotherapy followed by surgery the appropriate treatment for signet ring cell containing adenocarcinomas of the esophagogastric junction and stomach? Ann. Surg. Oncol. 2014, 21, 1739–1748. [Google Scholar] [CrossRef] [PubMed]
- Messager, M.; Lefevre, J.H.; Pichot-Delahaye, V.; Souadka, A.; Piessen, G.; Mariette, C.; Arnaud, J.P.; Balon, J.M.; Bonnetain, F.; Borie, F.; et al. The Impact of Perioperative Chemotherapy on Survival in Patients With Gastric Signet Ring Cell Adenocarcinoma: A Multicenter Comparative Study. Ann. Surg. 2011, 254, 684. [Google Scholar] [CrossRef] [PubMed]
- Takiuchi, H.; Hirata, I.; Kawabe, S.; Egashira, Y.; Katsu, K. Immunohistochemical expression of vascular endothelial growth factor can predict response to 5-fluorouracil and cisplatin in patients with gastric adenocarcinoma. Oncol. Rep. 2000, 7, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Piessen, G.; Messager, M.; Leteurtre, E.; Jean-Pierre, T.; Mariette, C. Signet ring cell histology is an independent predictor of poor prognosis in gastric adenocarcinoma regardless of tumoral clinical presentation. Ann. Surg. 2009, 250, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, F.-H.; Xue, L.-Y.; Tian, Y.-T. Neoadjuvant chemotherapy vs upfront surgery for gastric signet ring cell carcinoma: A retrospective, propensity score-matched study. World J. Gastroenterol. 2020, 26, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Gertsen, E.C.; Van Der Veen, A.; Brenkman, H.J.F.; Brosens, L.A.A.; Van Der Post, R.S.; Verhoeven, R.H.A.; Luijten, J.C.H.B.M.; Vissers, P.A.J.; Vegt, E.; Van Hillegersberg, R.; et al. Multimodal Therapy Versus Primary Surgery for Gastric and Gastroesophageal Junction Diffuse Type Carcinoma, with a Focus on Signet Ring Cell Carcinoma: A Nationwide Study. Ann. Surg. Oncol. 2024, 31, 1760–1772. [Google Scholar] [CrossRef] [PubMed]
- Moehler, M.; Baltin, C.T.H.; Ebert, M.; Fischbach, W.; Gockel, I.; Grenacher, L.; Hölscher, A.H.; Lordick, F.; Malfertheiner, P.; Messmann, H.; et al. International comparison of the German evidence-based S3-guidelines on the diagnosis and multimodal treatment of early and locally advanced gastric cancer, including adenocarcinoma of the lower esophagus. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2015, 18, 550–563. [Google Scholar] [CrossRef] [PubMed]
- D’Ugo, D.; Agnes, A.; Grieco, M.; Biondi, A.; Persiani, R. Global updates in the treatment of gastric cancer: A systematic review. Part 2: Perioperative management, multimodal therapies, new technologies, standardization of the surgical treatment and educational aspects. Updates Surg. 2020, 72, 355–378. [Google Scholar] [CrossRef] [PubMed]
- Piessen, G.; Messager, M.; Le Malicot, K.; Robb, W.B.; Di Fiore, F.; Guilbert, M.; Moreau, M.; Christophe, V.; Adenis, A.; Mariette, C. Phase II/III multicentre randomised controlled trial evaluating a strategy of primary surgery and adjuvant chemotherapy versus peri-operative chemotherapy for resectable gastric signet ring cell adenocarcinomas–PRODIGE 19–FFCD1103–ADCI002. BMC Cancer 2013, 13, 281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.S.; Deng, W.Y.; Huang, S.L.; Yang, B.F.; Zhu, F.H.; Jiang, B.; Wang, S.N.; Wang, Y.K. Clinicopathological characteristics of signet-ring cell carcinoma derived from gastric fovelar epithelium. J. Dig. Dis. 2022, 23, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Alberts, S.R.; Cervantes, A.; Van De Velde, C.J.H. Gastric cancer: Epidemiology, pathology and treatment. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2003, 14, ii31–ii36. [Google Scholar] [CrossRef] [PubMed]
- Ychou, M.; Boige, V.; Pignon, J.P.; Conroy, T.; Bouché, O.; Lebreton, G.; Ducourtieux, M.; Bedenne, L.; Fabre, J.M.; Saint-Aubert, B.; et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: An FNCLCC and FFCD multicenter phase III trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Van Hagen, P.; Hulshof, M.C.; Van Lanschot, J.J.; Steyerberg, E.W.; Van Berge Henegouwen, M.I.; Wijnhoven, B.P.; Richel, D.J.; Nieuwenhuijzen, G.A.; Hospers, G.A.; Bonenkamp, J.J.; et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 2012, 366, 2074–2084. [Google Scholar] [CrossRef] [PubMed]
- Schiefer, S.; Crnovrsanin, N.; Kalkum, E.; Vey, J.A.; Nienhüser, H.; Rompen, I.F.; Haag, G.M.; Müller-Stich, B.; Billmann, F.; Schmidt, T.; et al. Is neoadjuvant chemotherapy followed by surgery the appropriate treatment for esophagogastric signet ring cell carcinomas? A systematic review and meta-analysis. Front. Surg. 2024, 11, 1382039. [Google Scholar] [CrossRef] [PubMed]
- Heger, U.; Sisic, L.; Nienhüser, H.; Blank, S.; Hinz, U.; Haag, G.M.; Ott, K.; Ulrich, A.; Büchler, M.W.; Schmidt, T. Neoadjuvant Therapy Improves Outcomes in Locally Advanced Signet-Ring-Cell Containing Esophagogastric Adenocarcinomas. Ann. Surg. Oncol. 2018, 25, 2418–2427. [Google Scholar] [CrossRef] [PubMed]
- Dong, E.; Duan, L.; Wu, B.U. Racial and Ethnic Minorities at Increased Risk for Gastric Cancer in a Regional US Population Study. Clin. Gastroenterol. Hepatol. 2017, 15, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, S.; Zhao, L.; Wang, H.; Wang, L.; Shi, R.; Liang, S.; Li, B.; Chen, B. Screening and identification of vascular endothelial cell targeting peptide in gastric cancer through novel integrated in vitro and in vivo strategy. BMC Cancer 2024, 24, 1595. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Cristescu, R.; Lee, J.; Nebozhyn, M.; Kim, K.-M.; Ting, J.C.; Wong, S.S.; Liu, J.; Yue, Y.G.; Wang, J.; Yu, K.; et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat. Med. 2015, 21, 449–456. [Google Scholar] [CrossRef] [PubMed]

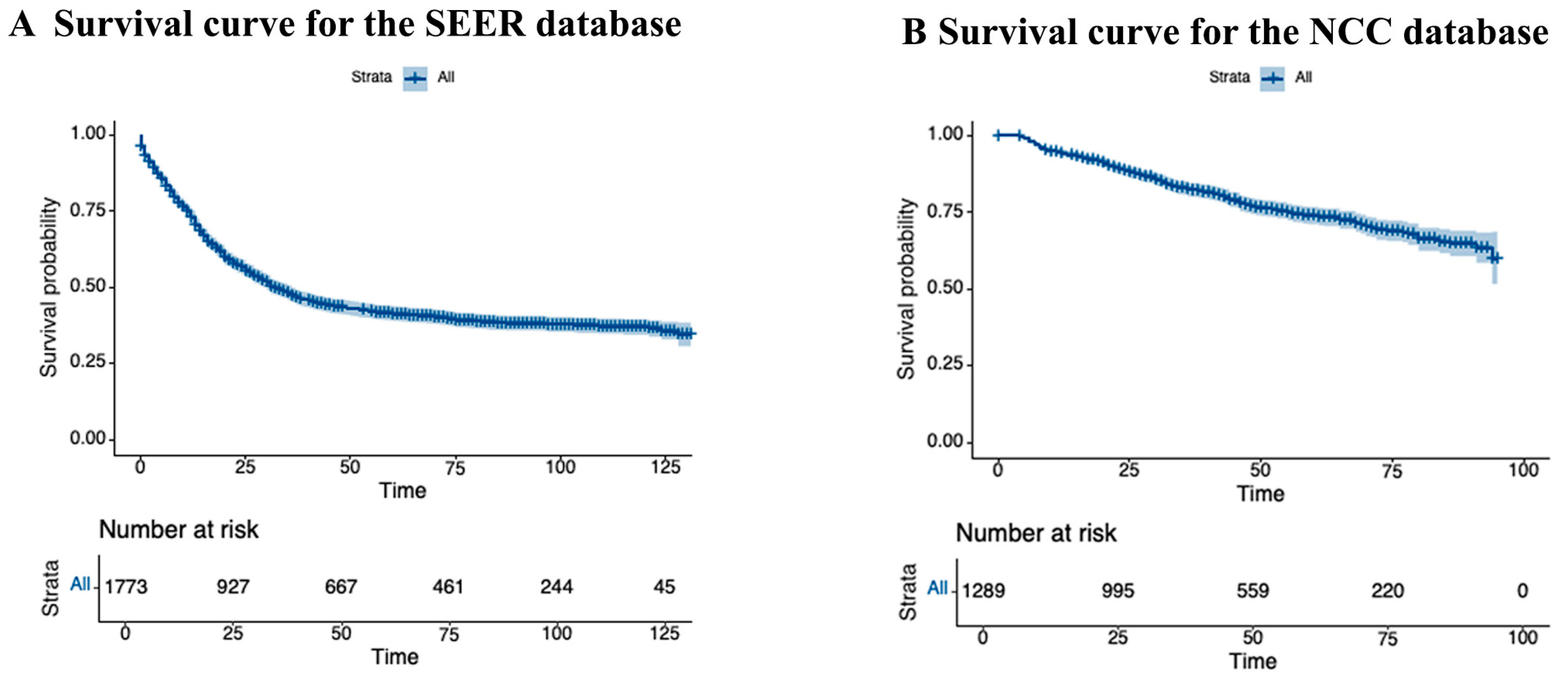
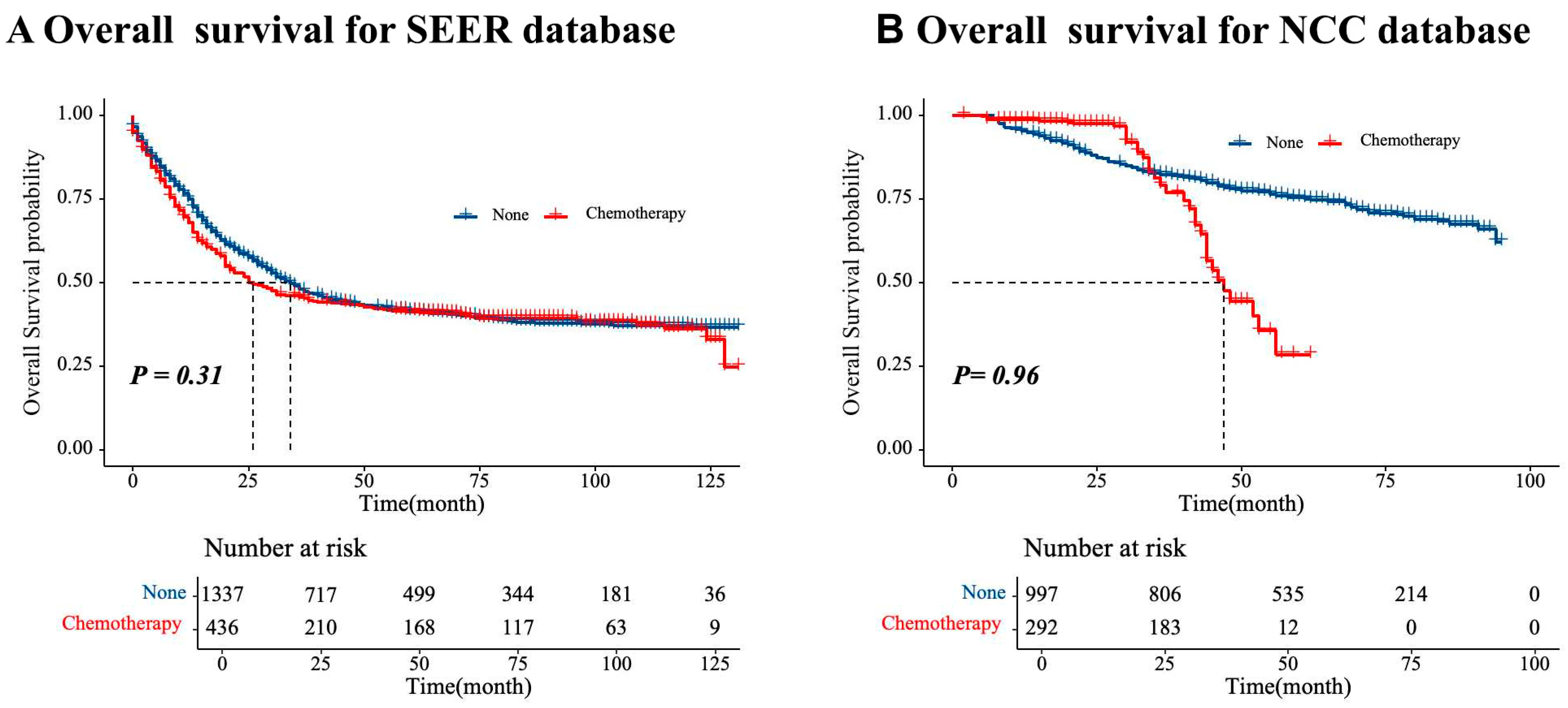
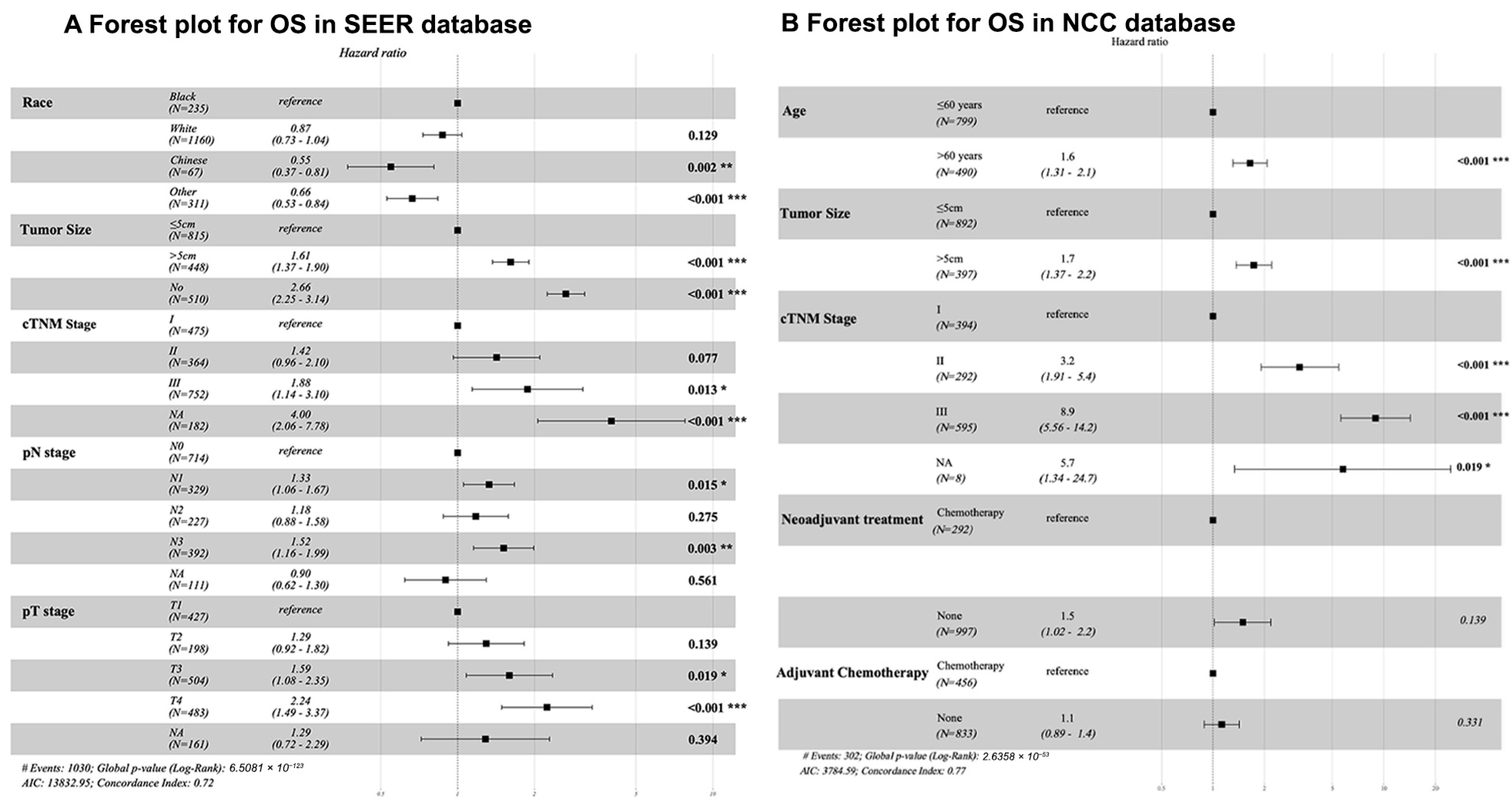
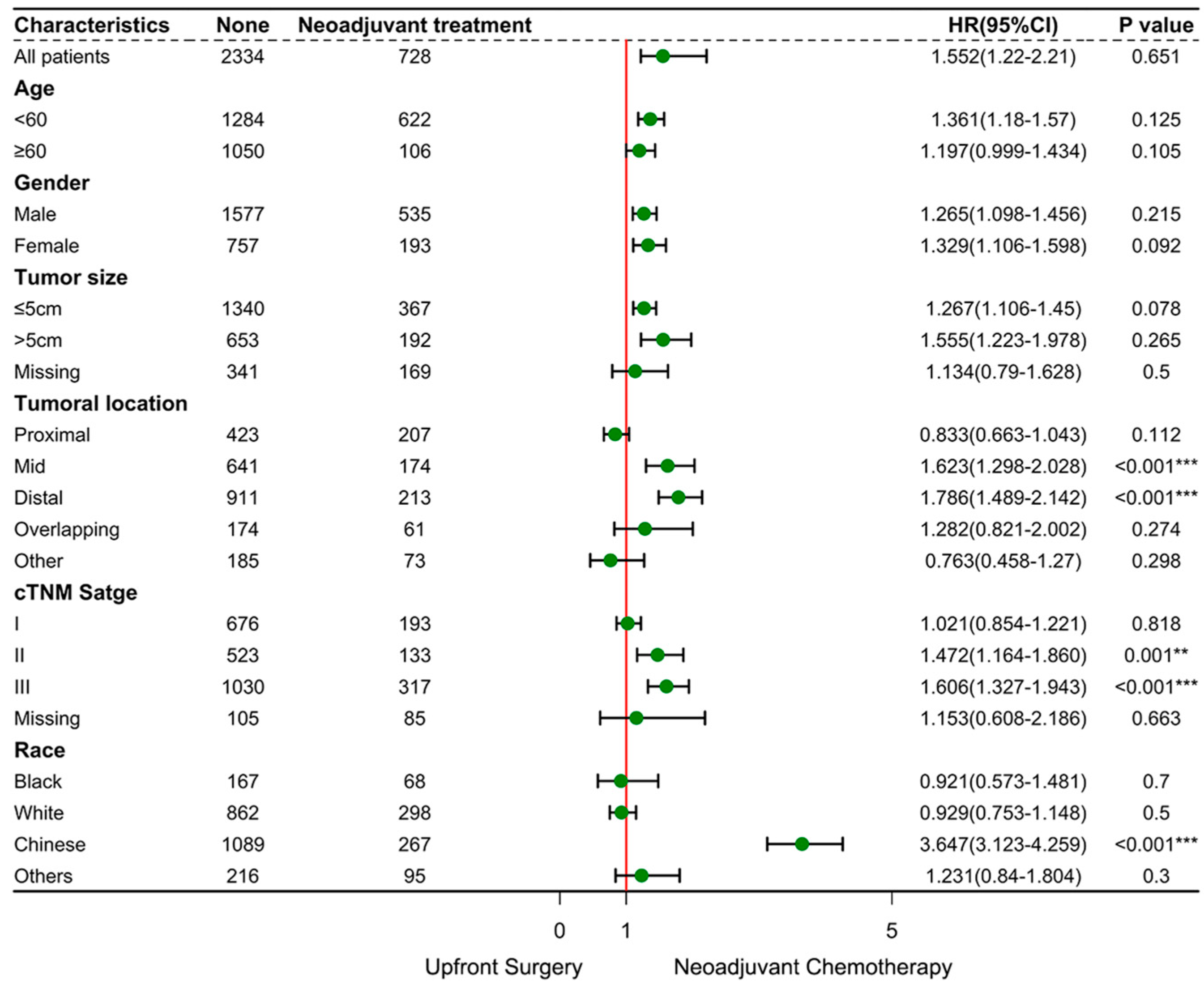
| Variables | SEER Database (N = 1773, %) | NCC Database (N = 1289, %) | p-Value | Total (N = 3062, %) |
|---|---|---|---|---|
| Gender | 0.233 | |||
| Male | 1238 (69.8%) | 874 (67.8%) | 2112 (69.0) | |
| Female | 535 (30.2%) | 415 (32.2%) | 950 (31.0) | |
| Age, years | 0.800 | |||
| ≤60 | 1107 (62.4%) | 799 (62.0%) | 1906 (62.2) | |
| >60 | 666 (37.6%) | 490 (38.0%) | 1156 (37.8) | |
| Race | <0.001 | |||
| Black | 235 (13.3%) | 0 | 235 (7.7) | |
| White | 1160 (65.4%) | 0 | 1160 (37.9) | |
| Chinese | 67 (3.8%) | 1289 (100%) | 1356 (44.3) | |
| Other | 311 (17.5%) | 0 | 311 (10.2) | |
| Tumoral locations | <0.001 | |||
| Proximal | 316 (17.8%) | 314 (24.4%) | 630 (20.6) | |
| Mid | 506 (28.5%) | 309 (24.0%) | 815 (26.6) | |
| Distal | 528 (29.8%) | 596 (46.2%) | 1124 (36.7) | |
| Overlapping | 165 (9.3%) | 70 (5.4%) | 235 (7.7) | |
| Unknown | 258 (14.6%) | 0 | 258 (8.4) | |
| Tumor Size, cm | <0.001 | |||
| ≤5 | 815 (46.0%) | 892 (69.2%) | 1707 (55.7) | |
| >5 | 448 (25.3%) | 397 (30.8%) | 845 (27.6) | |
| NA | 510 (28.8%) | 0 | 510 (16.6) | |
| Year of surgery | 0.227 | |||
| 2011–2014 | 812 (45.8%) | 562 (43.6%) | 1374 (44.8) | |
| 2015–2018 | 961 (54.2%) | 727 (56.4%) | 1688 (55.2) | |
| Pretherapeutic cTNM stage | 0.003 | |||
| I | 475 (26.8%) | 394 (30.6%) | 869 (28.3) | |
| II | 364 (20.5%) | 292 (22.7%) | 656 (21.4) | |
| III | 752 (42.4%) | 595 (46.2%) | 1347 (44.0) | |
| NA | 182 (10.3%) | 8 (0.6%) | 190 (6.2) |
| Variables | SEER Database (n = 1773, %) | NCC Database (n = 1289, %) | p-Value | Total (n = 3062, %) |
|---|---|---|---|---|
| Neoadjuvant treatment | 0.159 | |||
| Chemotherapy | 436 (24.6%) | 292 (22.6%) | 728 (23.8) | |
| None | 1337 (75.4%) | 997 (77.3%) | 2334 (76.2) | |
| Adjuvant treatment | <0.001 | |||
| Chemotherapy | 296 (16.7%) | 456 (35.4%) | 752 (24.6) | |
| Chemoradiotherapy | 388 (21.9%) | 0 | 388 (12.7) | |
| Radiotherapy alone | 54 (3.0%) | 0 | 54 (1.7) | |
| None | 1035 (58.4%) | 833 (64.6%) | 1868 (61.0) | |
| Surgical procedure a | - | |||
| Extended resection to neighboring organs | - | 3 (0.2%) | ||
| Total gastrectomy | - | 772 (59.9) | ||
| Subtotal gastrectomy | - | 429 (33.3) | ||
| Unknown type of procedure | - | 88 (6.8) |
| Variables | SEER Database (n = 1773, %) | NCC Database (n = 1289, %) | p-Value | Total (n = 3062, %) |
|---|---|---|---|---|
| Tumor stage | ||||
| pT | 0.021 | |||
| Tis | 0 | 3 (0.2) | 3 (0.1) | |
| T1 | 427 (24.1) | 384 (29.8) | 811 (26.5) | |
| T2 | 198 (11.2) | 157 (12.2) | 355 (11.6) | |
| T3 | 504 (28.4) | 295 (22.9) | 799 (26.1) | |
| T4 | 483 (27.2) | 449 (34.8) | 932 (30.4) | |
| Unknown | 161 (9.1) | 1 (0.1) | 162 (5.3) | |
| pN | <0.001 | |||
| N0 | 714 (40.3) | 537 (41.7) | 1251 (40.9) | |
| N1 | 329 (18.6) | 179 (13.9) | 508 (16.6) | |
| N2 | 227 (12.8) | 192 (14.9) | 419 (13.7) | |
| N3 | 392 (22.1) | 374 (29.0) | 766 (25.0) | |
| Unknown | 111 (6.3) | 7 (0.5) | 118 (3.8) | |
| Tumor differentiation | <0.001 | |||
| Well | 3 (0.2) | 9 (0.7) | 12 (0.4) | |
| Moderate | 35 (2.0) | 259 (20.1) | 294 (9.6) | |
| Poorly | 1489 (84) | 947 (73.5) | 2436 (79.6) | |
| Undifferentiated | 41 (2.3) | 1 (0.1) | 42 (1.3) | |
| Unknown | 205 (11.6) | 73 (5.7) | 278 (9.1) | |
| HER2 a | - | |||
| Loss of expression | - | 593 (46.0) | ||
| Low expression | - | 497 (38.6) | ||
| Overexpression | - | 189 (14.7) | ||
| Not detected | - | 10 (0.7) | ||
| Number of dissected lymph nodes [mean ± SD] | 27.4 ± 40.1 | 32.0 ± 14.9 | <0.001 | - |
| Number of invaded lymph nodes [mean ± SD] | 19.3 ± 21.5 | 6.0 ± 9.7 | <0.001 | - |
| Variables | SEER Database | NCC Database | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariable Analysis | Multivariable Analysis | Univariable Analysis | Multivariable Analysis | |||||
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Sex | ||||||||
| Male | REF | REF | ||||||
| Female | 1.07 (0.95–1.21) | 0.253 | 0.84 (0.66–1.08) | 0.185 | ||||
| Age (year) | ||||||||
| <60 | REF | REF | ||||||
| >60 | 0.94 (0.83–1.07) | 0.348 | 2.02 (1.61–2.54) | <0.001 | 1.65 (1.31–2.07) | <0.001 | ||
| Race | ||||||||
| Black | REF | REF | ||||||
| White | 0.86 (0.72–1.02) | 0.082 | 0.87 (0.73–1.04) | 0.129 | - | - | - | - |
| Chinese | 0.53 (0.36–0.77) | 0.001 | 0.55 (0.37–0.81) | 0.002 | - | - | - | - |
| Others | 0.56 (0.5–0.70) | <0.001 | 0.66 (0.53–0.84) | <0.001 | - | - | - | - |
| Tumor size (cm) | - | - | - | - | ||||
| <5 | REF | REF | REF | |||||
| >5 | 2.69 (2.31–3.13) | <0.001 | 1.61 (1.37–1.90) | <0.001 | 3.09 (2.46–3.87) | <0.001 | 1.73 (1.37–2.20) | <0.001 |
| Unknown | 3.18 (2.74–3.7) | <0.001 | 2.66 (2.25–3.14) | <0.001 | - | - | - | - |
| Tumor location | ||||||||
| Proximal | REF | REF | ||||||
| Mid | 1.07 (0.89–1.29) | 0.462 | 0.81(0.72–0.93) | 0.425 | ||||
| Distal | 1.1 (0.91–1.32) | 0.318 | 0.15 (0.05–0.35) | 0.150 | ||||
| Overlapping | 1.06 (0.82–1.35) | 0.670 | 0.22 (0.20–5.53) | 0.980 | ||||
| Unknown | 1.02 (0.82–1.27) | 0.872 | 1.25 (0.15–3.69) | 0.115 | ||||
| Histological grade | ||||||||
| Well | REF | REFREF | ||||||
| Moderate | 1.69 (0.23–12.71) | 0.610 | 4.22 (1.23–9.88) | 0.991 | ||||
| Poorly | 2.07 (0.29–14.7) | 0.468 | 6.39 (2.56–17.21) | 0.991 | ||||
| Undifferentiated | 1.83 (0.25–13.53) | 0.555 | 4.81 (4.10–8.20) | 0.989 | ||||
| Unknown | 2.04 (0.29–14.62) | 0.477 | 2.79 (0.55–7.88) | 0.991 | ||||
| pT stage | ||||||||
| T1 | REF | REF | REF | |||||
| T2 | 1.47 (1.11–1.95) | 0.007 | 1.29 (0.92–1.82) | 0.139 | 0.2 (0.03–1.51) | 0.119 | ||
| T3 | 2.81 (2.28–3.47) | <0.001 | 1.59 (1.08–2.35) | 0.019 | 0.47 (0.06–3.50) | 0.459 | ||
| T4 | 4.82 (3.93–5.92) | <0.001 | 2.24 (1.49–3.37) | <0.001 | 1.5 (0.21–10.75) | 0.688 | ||
| Unknown | 7.19 (5.58–9.27) | <0.001 | 1.29 (0.72–2.29) | 0.394 | 2.32 (0.32–16.58) | 0.402 | ||
| pN stage | ||||||||
| N0 | REF | REF | REF | |||||
| N1 | 1.94 (1.62–2.32) | <0.001 | 1.33 (1.06–1.67) | 0.015 | 1.11 (0.08–15.21) | 0.552 | ||
| N2 | 2.06 (1.69–2.51) | <0.001 | 1.18 (0.88–1.58) | 0.275 | 3.36 (0.55–7.55) | 0.152 | ||
| N3 | 3.21 (2.73–3.79) | <0.001 | 1.52 (1.16–1.99) | 0.003 | 0.05 (0.01–2.55) | 0.335 | ||
| Unknown | 4.43 (3.47–5.67) | <0.001 | 0.90 (0.62–1.30) | 0.561 | 5.56 (1.12–7.88) | 0.782 | ||
| cTNM stage | ||||||||
| I | REF | REF | REF | |||||
| II | 2.15 (1.71–2.70) | <0.001 | 1.42 (0.96–2.10) | 0.077 | 3.31 (1.99–5.53) | <0.001 | 3.20 (1.91–5.40) | <0.001 |
| III | 4.69 (3.86–5.70) | <0.001 | 1.88 (1.14–3.20) | 0.013 | 11.06 (7.07–17.31) | <0.001 | 8.90 (5.56–14.20) | <0.001 |
| Unknown | 7.67 (6.01–9.80) | <0.001 | 4.00(2.06–7.78) | <0.001 | 6.09 (1.43–26.02) | 0.015 | 5.70 (1.34–24.70) | 0.019 |
| Year of surgery | ||||||||
| 2011–2014 | REF | REF | ||||||
| 2015–2018 | 0.97 (0.86–1.10) | 0.683 | 0.82 (0.64–1.04) | 0.107 | ||||
| Neoadjuvant treatment | ||||||||
| Chemotherapy | REF | REF | REF | |||||
| None | 0.96 (0.81–1.14) | 0.653 | 1.40 (0.96–2.05) | 0.08 | 1.49 (1.02–2.18) | 0.139 | ||
| Adjuvant chemotherapy | ||||||||
| Presence | REF | REF | REF | |||||
| Absence | 0.94 (0.72–1.23) | 0.638 | 1.40 (0.96–2.05) | 0.08 | 1.10 (0.89–1.40) | 0.331 | ||
| Unknown | 0.91 (0.78–1.07) | 0.257 | - | - | - | - | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Wang, P.; Tian, Y. The Impact of Neoadjuvant Chemotherapy on Survival Outcomes in Gastric Signet-Ring Cell Carcinoma: An International Multicenter Study. Cancers 2025, 17, 2419. https://doi.org/10.3390/cancers17152419
Jiang Y, Wang P, Tian Y. The Impact of Neoadjuvant Chemotherapy on Survival Outcomes in Gastric Signet-Ring Cell Carcinoma: An International Multicenter Study. Cancers. 2025; 17(15):2419. https://doi.org/10.3390/cancers17152419
Chicago/Turabian StyleJiang, Yujuan, Peng Wang, and Yantao Tian. 2025. "The Impact of Neoadjuvant Chemotherapy on Survival Outcomes in Gastric Signet-Ring Cell Carcinoma: An International Multicenter Study" Cancers 17, no. 15: 2419. https://doi.org/10.3390/cancers17152419
APA StyleJiang, Y., Wang, P., & Tian, Y. (2025). The Impact of Neoadjuvant Chemotherapy on Survival Outcomes in Gastric Signet-Ring Cell Carcinoma: An International Multicenter Study. Cancers, 17(15), 2419. https://doi.org/10.3390/cancers17152419







