Apalutamide and Stereotactic Body Radiotherapy in Metastatic Hormone-Sensitive Prostate Cancer: Multicenter Real-World Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Lesion Distribution and Characteristics of Metastatic Disease
3.3. Fractionation Scheme
3.4. PSA Levels
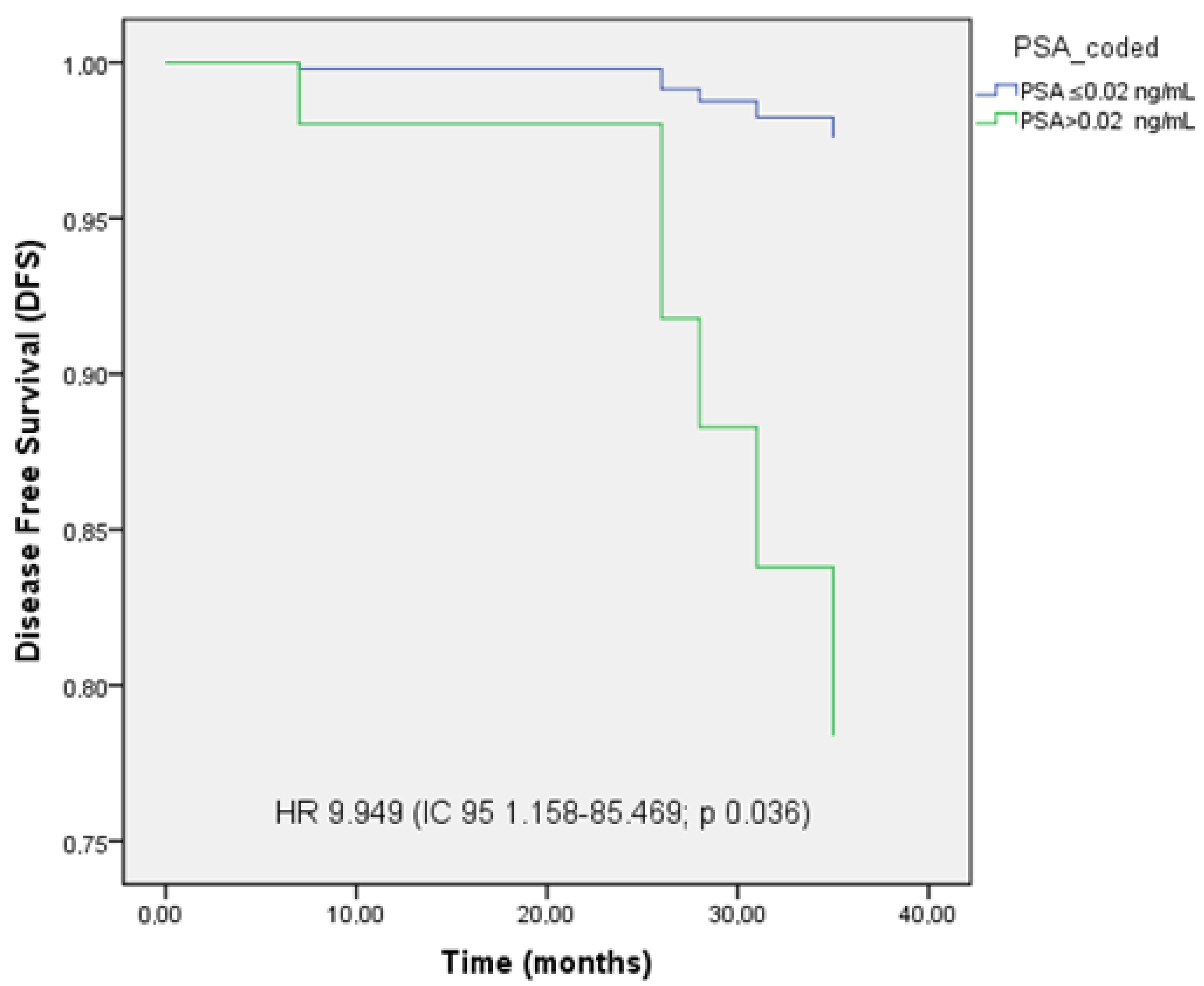
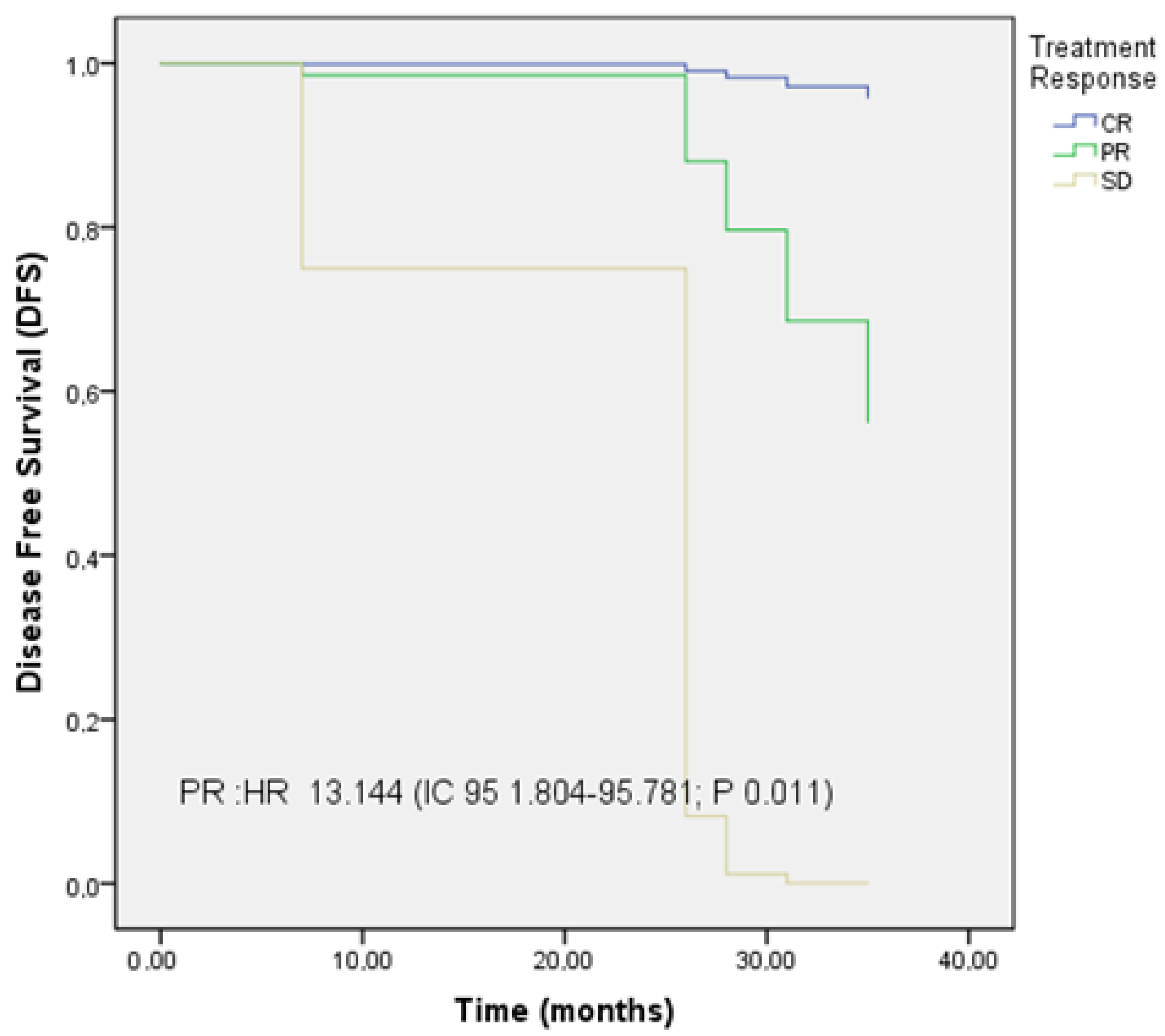
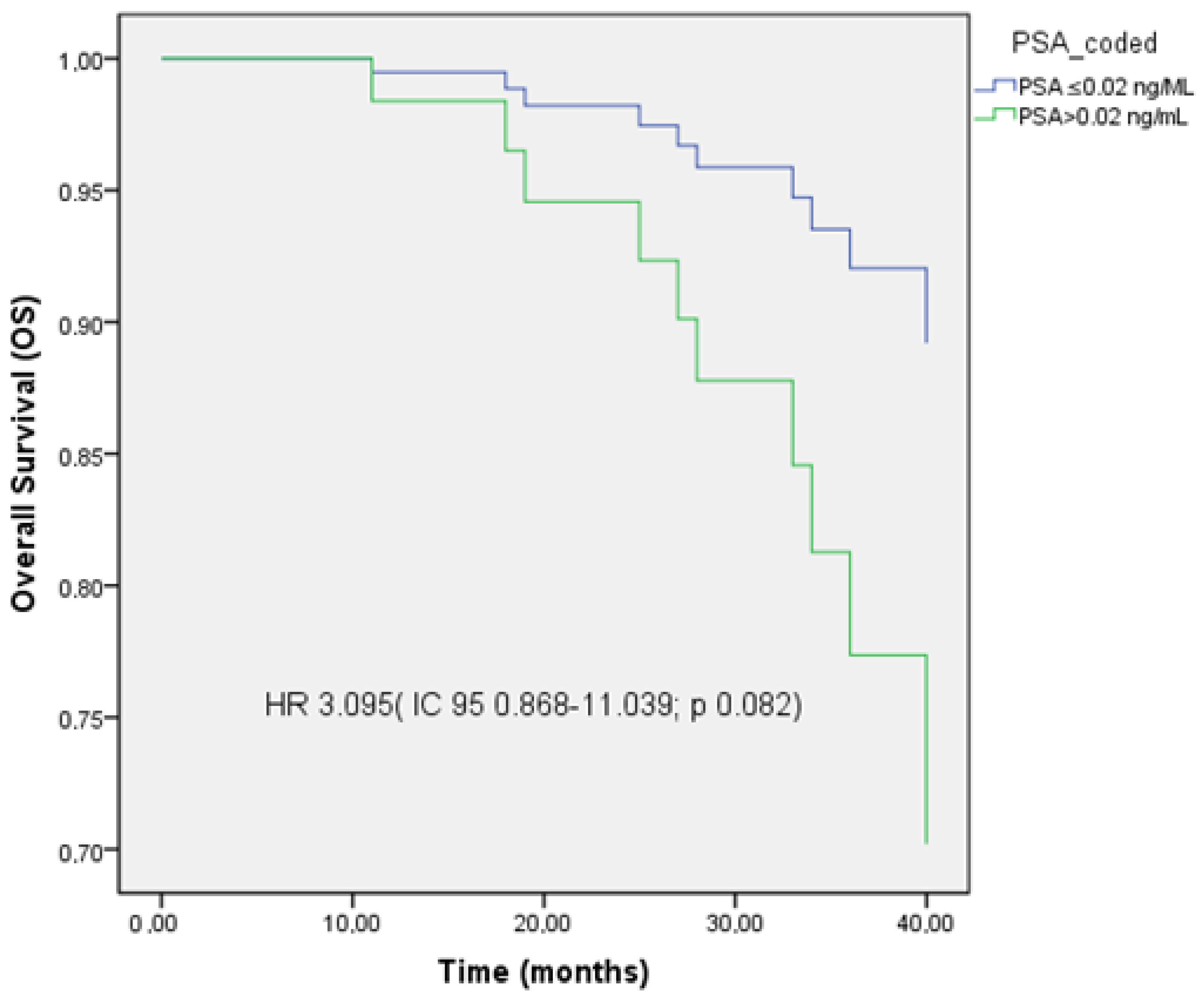
3.5. PFS, LC, and OS
3.6. Acute and Chronic Toxicity After MDT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADT | Androgen Deprivation Therapy |
| ARSI | Androgen Receptor Signaling Inhibitors |
| BED | Biologically Effective Dose |
| BS | Bone Scan |
| CR | Complete Response |
| CT | Computed Tomography |
| CTCAE | Common Terminology Criteria for Adverse Events |
| Fig | Figure |
| G | Grade |
| LC | Local Control |
| mHSPC | Metastatic Hormone-Sensitive Prostate Cancer |
| MDT | Metastasis-Directed Therapy |
| OS | Overall Survival |
| PET/CT | Positron Emission Tomography/Computed Tomography |
| PC | Prostate Cancer |
| PR | Partial Response |
| PSA | Prostate Specific Antigen |
| PFS | Progression-Free Survival |
| rPFS | Radiographic Progression–Free Survival |
| SBRT | Stereotactic Body Radiation Therapy |
| SD | Stable Disease |
| SST | Subsequent Systemic Therapy |
| UL1 PSA | Ultralow 1 Prostate Specific Antigen |
| UL2 PSA | Ultralow 2 Prostate Specific Antigen |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Abate, D.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdel-Rahman, O.; Abdelalim, A.; Abdoli, A.; Abdollahpour, I.; et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-Adjusted life-years for 29 cancer groups, 1990 to 2017: A systematic analysis for the global burden of disease study. JAMA Oncol. 2019, 5, 1749–1768. [Google Scholar] [PubMed]
- Butler, S.S.; Muralidhar, V.; Zhao, S.G.; Sanford, N.N.; Franco, I.; Fullerton, Z.H.; Chavez, J.; D’Amico, A.V.; Feng, F.Y.; Rebbeck, T.R.; et al. Prostate cancer incidence across stage, NCCN risk groups, and age before and after USPSTF Grade D recommendations against prostate-specific antigen screening in 2012. Cancer 2020, 126, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Fedewa, S.A.; Ma, J.; Siegel, R.; Lin, C.C.; Brawley, O.; Ward, E.M. Prostate Cancer Incidence and PSA Testing Patterns in Relation to USPSTF Screening Recommendations. JAMA 2015, 314, 2054–2061. [Google Scholar] [CrossRef]
- Jemal, A.; Culp, M.B.; Ma, J.; Islami, F.; A Fedewa, S. Prostate Cancer Incidence 5 Years After US Preventive Services Task Force Recommendations Against Screening. JNCI J. Natl. Cancer Inst. 2021, 113, 64–71. [Google Scholar] [CrossRef]
- Desai, M.M.; Cacciamani, G.E.; Gill, K.; Zhang, J.; Liu, L.; Abreu, A.; Gill, I.S. Trends in Incidence of Metastatic Prostate Cancer in the US. JAMA Netw. Open 2022, 5, e222246. [Google Scholar] [CrossRef]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Eastham, J.A.; Auffenberg, G.B.; Barocas, D.A.; Chou, R.; Crispino, T.; Davis, J.W.; Eggener, S.; Horwitz, E.M.; Kane, C.J.; Kirkby, E.; et al. Clinically Localized Prostate Cancer: AUA/ASTRO Guideline, Part I: Introduction, Risk Assessment, Staging, and Risk-Based Management. J. Urol. 2022, 208, 10–18. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology. Available online: https://www.nccn.org/ (accessed on 5 September 2024).
- Cornford, P.; Bellmunt, J.; Bolla, M.; Briers, E.; De Santis, M.; Gross, T.; Henry, A.M.; Joniau, S.; Lam, T.B.; Mason, M.D.; et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer. Eur. Urol. 2017, 71, 630–642. Available online: https://pubmed.ncbi.nlm.nih.gov/27591931/ (accessed on 28 October 2024). [CrossRef]
- Dabkara, D.; Mondal, D.; Ghosh, J.; Biswas, B.; Ganguly, S. How I treat Metastatic Hormone-Sensitive Prostate Cancer? Indian J. Med. Paediatr. Oncol. 2021, 42, 100–107. [Google Scholar] [CrossRef]
- Chi, K.N.; Chowdhury, S.; Bjartell, A.; Chung, B.H.; Gomes, A.J.P.d.S.; Given, R.; Juárez, A.; Merseburger, A.S.; Özgüroğlu, M.; Uemura, H.; et al. Apalutamide in Patients with Metastatic Castration-Sensitive Prostate Cancer: Final Survival Analysis of the Randomized, Double-Blind, Phase III TITAN Study. J. Clin. Oncol. 2021, 39, 2294–2303. [Google Scholar] [CrossRef] [PubMed]
- Chi, K.N.; Agarwal, N.; Bjartell, A.; Chung, B.H.; Gomes, A.J.P.D.S.; Given, R.; Soto, A.J.; Merseburger, A.S.; Özgüroglu, M.; Uemura, H.; et al. Apalutamide for Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2019, 381, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Özgüroğlu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): Final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019, 20, 686–700. [Google Scholar] [CrossRef]
- Fizazi, K.; Foulon, S.; Carles, J.; Roubaud, G.; McDermott, R.; Fléchon, A.; Tombal, B.; Supiot, S.; Berthold, D.; Ronchin, P.; et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): A multicentre, open-label, randomised, phase 3 study with a 2 × 2 factorial design. Lancet 2022, 399, 1695–1707. [Google Scholar] [CrossRef]
- Caro Teller, J.M.; Cortijo Cascajares, S.; Escribano Valenciano, I.; Serrano Garrote, O.; Ferrari Piquero, J.M. Uso, efectividad y seguridad de abiraterona en cáncer de próstata. Farm. Hosp. 2014, 38, 118–122. [Google Scholar]
- Davis, I.D.; Martin, A.J.; Stockler, M.R.; Begbie, S.; Chi, K.N.; Chowdhury, S.; Coskinas, X.; Frydenberg, M.; Hague, W.E.; Horvath, L.G.; et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N. Engl. J. Med. 2019, 381, 121–131. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Szmulewitz, R.Z.; Petrylak, D.P.; Holzbeierlein, J.; Villers, A.; Azad, A.; Alcaraz, A.; Alekseev, B.; Iguchi, T.; Shore, N.D.; et al. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy with Enzalutamide or Placebo in Men with Metastatic Hormone-Sensitive Prostate Cancer. J. Clin. Oncol. 2019, 37, 2974–2986. [Google Scholar] [CrossRef]
- Smith, M.R.; Hussain, M.; Saad, F.; Fizazi, K.; Sternberg, C.N.; Crawford, E.D.; Kopyltsov, E.; Park, C.H.; Alekseev, B.; Montesa-Pino, Á.; et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2022, 386, 1132–1142. [Google Scholar] [CrossRef]
- Glicksman, R.M.; Metser, U.; Vines, D.; Valliant, J.; Liu, Z.; Chung, P.W.; Bristow, R.G.; Finelli, A.; Hamilton, R.; Fleshner, N.E.; et al. Curative-intent Metastasis-directed Therapies for Molecularly defined Oligorecurrent Prostate Cancer: A Prospective Phase II Trial Testing the Oligometastasis Hypothesis. Eur. Urol. 2021, 80, 374–382. [Google Scholar] [CrossRef]
- Supiot, S.; Vaugier, L.; Pasquier, D.; Buthaud, X.; Magné, N.; Peiffert, D.; Sargos, P.; Crehange, G.; Pommier, P.; Loos, G.; et al. OLIGOPELVIS GETUG P07, a Multicenter Phase II Trial of Combined High-dose Salvage Radiotherapy and Hormone Therapy in Oligorecurrent Pelvic Node Relapses in Prostate Cancer. Eur. Urol. 2021, 80, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.; Shi, W.Y.; Deek, M.; Radwan, N.; Lim, S.J.; Antonarakis, E.S.; Rowe, S.P.; Ross, A.E.; Gorin, M.A.; Deville, C.; et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J. Clin. Oncol. 2020, 38, 2830–2838. [Google Scholar] [CrossRef]
- ESMO 2023: Effect of Rapid Ultra-Low Prostate-Specific Antigen Decline (UL PSA) in TITAN Patients with Metastatic Castration-Sensitive Prostate Cancer (mCSPC) Who Received Apalutamide Plus Androgen Deprivation Therapy [Internet]. Available online: https://www.urotoday.com/conference-highlights/esmo-2023/esmo-2023-prostate-cancer/147490-esmo-2023-effect-of-rapid-ultra-low-prostate-specific-antigen-decline-ul-psa-in-titan-patients-with-metastatic-castration-sensitive-prostate-cancer-mcspc-who-received-apalutamide-plus-androgen-deprivation-therapy.html (accessed on 28 October 2024).
- López-Abad, A.; Backhaus, M.R.; Gómez, G.S.; Avellaneda, E.C.; Alarcón, C.M.; Cubillana, P.L.; Giménez, P.Y.; Rodríguez, P.d.P.; Fita, M.J.J.; Durán, M.C.; et al. Real-world prostate-specific antigen reduction and survival outcomes of metastatic hormone-sensitive prostate cancer patients treated with apalutamide: An observational, retrospective, and multicentre study. Prostate Int. 2023, 12, 20–26. [Google Scholar] [CrossRef]
- Encarnación Navarro, J.A.; Morillo Macías, V.; Borrás Calbo, M.; De la Fuente Muñoz, I.; Lozano Martínez, A.; García Martínez, V.; Fernández Fornos, L.; Guijarro Roche, M.; Amr Rey, O.; García Gómez, R. Multicenter Real-World Study: 432 Patients with Apalutamide in Metastatic Hormone-Sensitive Prostate Cancer. Curr. Oncol. 2025, 32, 119. [Google Scholar] [CrossRef]
- Tang, C.; Sherry, A.D.; Haymaker, C.; Bathala, T.; Liu, S.; Fellman, B.; Cohen, L.; Aparicio, A.; Zurita, A.J.; Reuben, A.; et al. Addition of Metastasis-Directed Therapy to Intermittent Hormone Therapy for Oligometastatic Prostate Cancer: The EXTEND Phase 2 Randomized Clinical Trial. JAMA Oncol. 2023, 9, 825–834. [Google Scholar] [CrossRef]
- Sathianathen, N.J.; Koschel, S.; Thangasamy, I.A.; Teh, J.; Alghazo, O.; Butcher, G.; Howard, H.; Kapoor, J.; Lawrentschuk, N.; Siva, S.; et al. Indirect Comparisons of Efficacy between Combination Approaches in Metastatic Hormone-sensitive Prostate Cancer: A Systematic Review and Network Meta-analysis. Eur. Urol. 2020, 77, 365–372. [Google Scholar] [CrossRef]
- Marchioni, M.; Di Nicola, M.; Primiceri, G.; Novara, G.; Castellan, P.; Paul, A.K.; Veccia, A.; Autorino, R.; Cindolo, L.; Schips, L. New Antiandrogen Compounds Compared to Docetaxel for Metastatic Hormone Sensitive Prostate Cancer: Results from a Network Meta-Analysis. J. Urol. 2020, 203, 751–758. [Google Scholar] [CrossRef]
- Wang, L.; Paller, C.J.; Hong, H.; De Felice, A.; Alexander, G.C.; Brawley, O. Comparison of Systemic Treatments for Metastatic Castration-Sensitive Prostate Cancer: A Systematic Review and Network Meta-analysis. JAMA Oncol. 2021, 7, 412–420. [Google Scholar] [CrossRef]
- Francolini, G.; Porreca, A.; Facchini, G.; Santini, D.; Bruni, A.; Simoni, N.; Trovò, M.; Osti, M.F.; Fornarini, G.; Sisani, M.; et al. PERSIAN trial (NCT05717660): An ongoing randomized trial testing androgen deprivation therapy, apalutamide and stereotactic body radiotherapy. An alternative “triplet” for oligometastatic hormone sensitive prostate cancer patients. Med. Oncol. 2023, 41, 39. [Google Scholar] [CrossRef]
- Triggiani, L.; Mazzola, R.; Magrini, S.M.; Ingrosso, G.; Borghetti, P.; Trippa, F.; Lancia, A.; Detti, B.; Francolini, G.; Matrone, F.; et al. Metastasis-directed stereotactic radiotherapy for oligoprogressive castration-resistant prostate cancer: A multicenter study. World J. Urol. 2019, 37, 2631–2637. [Google Scholar] [CrossRef] [PubMed]
- Marvaso, G.; Corrao, G.; Zaffaroni, M.; Vincini, M.G.; Lorubbio, C.; Gandini, S.; Fodor, C.; Netti, S.; Zerini, D.; Luzzago, S.; et al. ADT with SBRT versus SBRT alone for hormone-sensitive oligorecurrent prostate cancer (RADIOSA): A randomised, open-label, phase 2 clinical trial. Lancet Oncol. 2025, 26, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Sherry, A.D.; Hwang, H.; Francolini, G.; Livi, L.; Tran, P.T.; Corn, P.G.; Aparicio, A.; Simontacchi, G.; Kiess, A.P.; et al. World-wide oligometastatic prostate cancer (omPC) meta-analysis leveraging individual patient data (IPD) from randomized trials (WOLVERINE): An analysis from the X-MET collaboration. J. Clin. Oncol. 2025, 43 (Suppl. S5), 15. [Google Scholar] [CrossRef]
- Conde-Moreno, A.J.; López-Campos, F.; Hervás, A.; Morillo, V.; Méndez, A.; Puertas, M.D.M.; Valero-Albarrán, J.; Gómez Iturriaga, A.; Rico, M.; Vázquez, M.L.; et al. A Phase II Trial of Stereotactic Body Radiation Therapy and Androgen Deprivation for Oligometastases in Prostate Cancer (SBRT-SG 05). Pract. Radiat. Oncol. 2024, 14, e344–e352. [Google Scholar] [CrossRef]
| n: 134 | |
|---|---|
| Median age at the start of treatment | 73 years (56–87) |
| Type of patient (proportion, n) | |
| Synchronous debut | 24.6% (33) |
| Metachronous biochemical recurrence | 75.4% (101) |
| Diagnostic PSA (median) | 8.38 ng/mL (0.25–158) |
| Metastasis location (proportion, n) | |
| Extrapelvic nodal (M1a) | 26.8% (36) |
| Bone (M1b) | 68.7% (92) |
| Visceral (M1c) | 4.5% (6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Encarnación, J.A.; Morillo Macías, V.; De la Fuente Muñoz, I.; Soria, V.D.; Fernández Fornos, L.; Antequera, M.A.; Rey, O.A.; García Martínez, V.; Alonso-Romero, J.L.; García Gómez, R. Apalutamide and Stereotactic Body Radiotherapy in Metastatic Hormone-Sensitive Prostate Cancer: Multicenter Real-World Study. Cancers 2025, 17, 2216. https://doi.org/10.3390/cancers17132216
Encarnación JA, Morillo Macías V, De la Fuente Muñoz I, Soria VD, Fernández Fornos L, Antequera MA, Rey OA, García Martínez V, Alonso-Romero JL, García Gómez R. Apalutamide and Stereotactic Body Radiotherapy in Metastatic Hormone-Sensitive Prostate Cancer: Multicenter Real-World Study. Cancers. 2025; 17(13):2216. https://doi.org/10.3390/cancers17132216
Chicago/Turabian StyleEncarnación, Juan A., Virginia Morillo Macías, Isabel De la Fuente Muñoz, Violeta Derrac Soria, Luis Fernández Fornos, María Albert Antequera, Osamah Amr Rey, Vicente García Martínez, José L. Alonso-Romero, and Raquel García Gómez. 2025. "Apalutamide and Stereotactic Body Radiotherapy in Metastatic Hormone-Sensitive Prostate Cancer: Multicenter Real-World Study" Cancers 17, no. 13: 2216. https://doi.org/10.3390/cancers17132216
APA StyleEncarnación, J. A., Morillo Macías, V., De la Fuente Muñoz, I., Soria, V. D., Fernández Fornos, L., Antequera, M. A., Rey, O. A., García Martínez, V., Alonso-Romero, J. L., & García Gómez, R. (2025). Apalutamide and Stereotactic Body Radiotherapy in Metastatic Hormone-Sensitive Prostate Cancer: Multicenter Real-World Study. Cancers, 17(13), 2216. https://doi.org/10.3390/cancers17132216






