Clinical Influence of Bile Duct and Duodenum Preservation on Zinc Absorption and Remnant Pancreatic Volume in Duodenum-Preserving Pancreatic Head Resection for Low-Grade Malignant Pancreatic Tumors
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
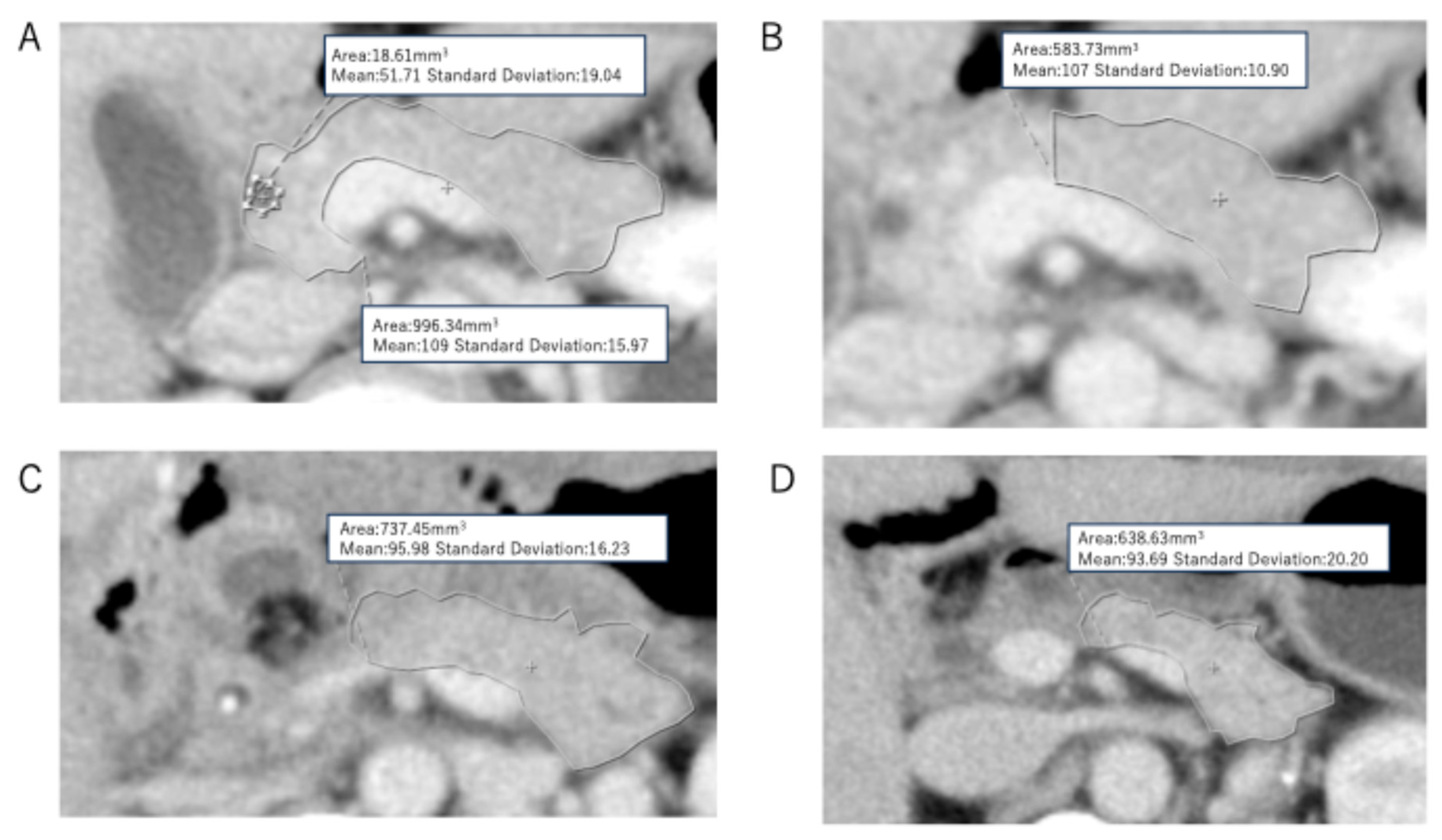
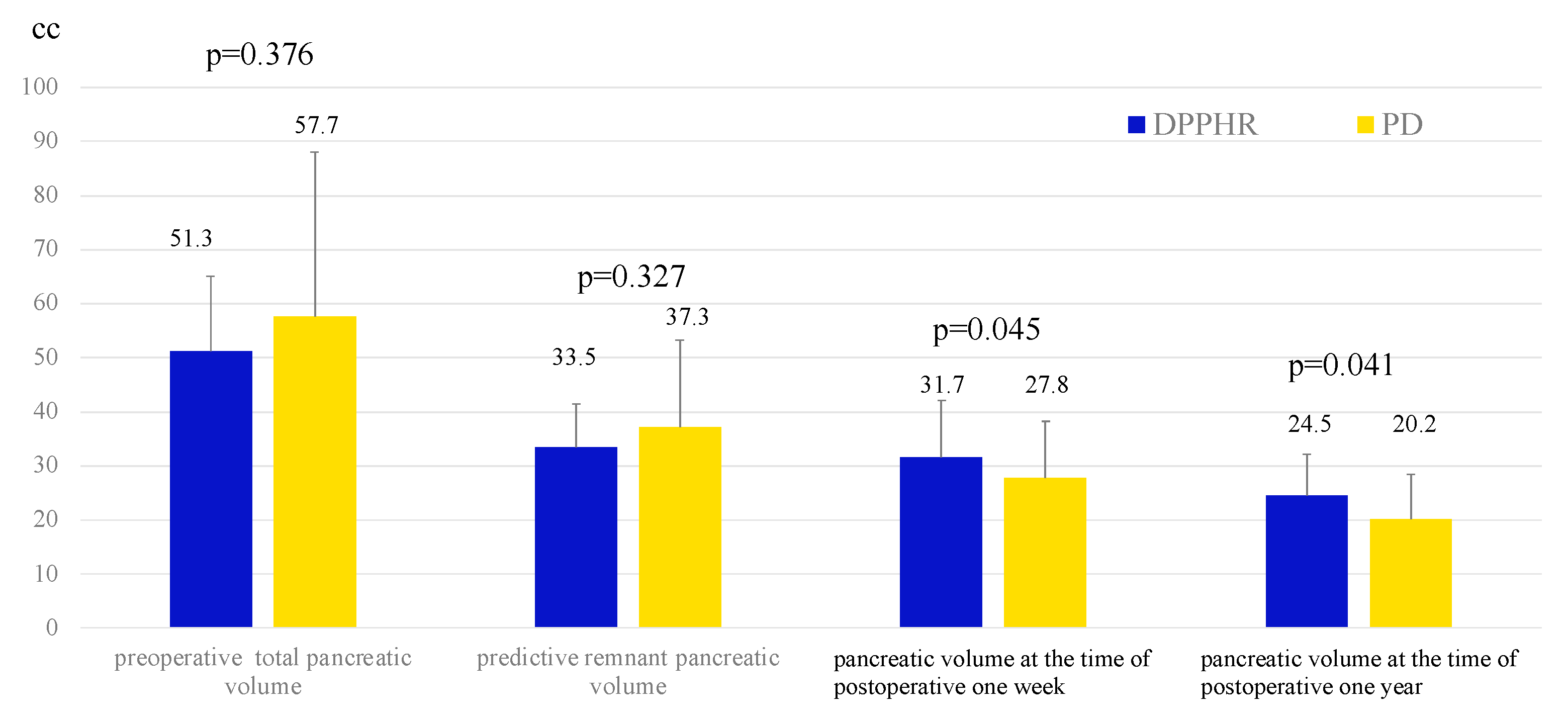
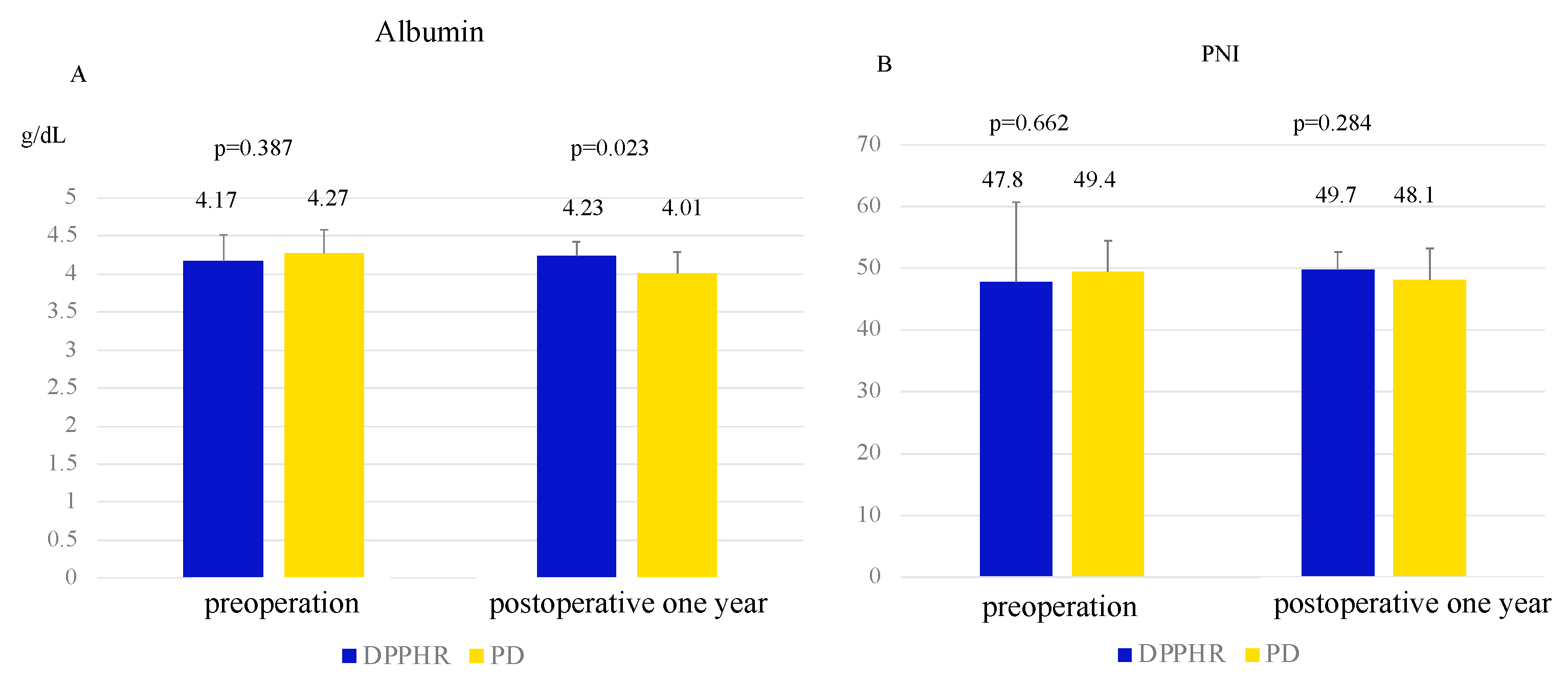
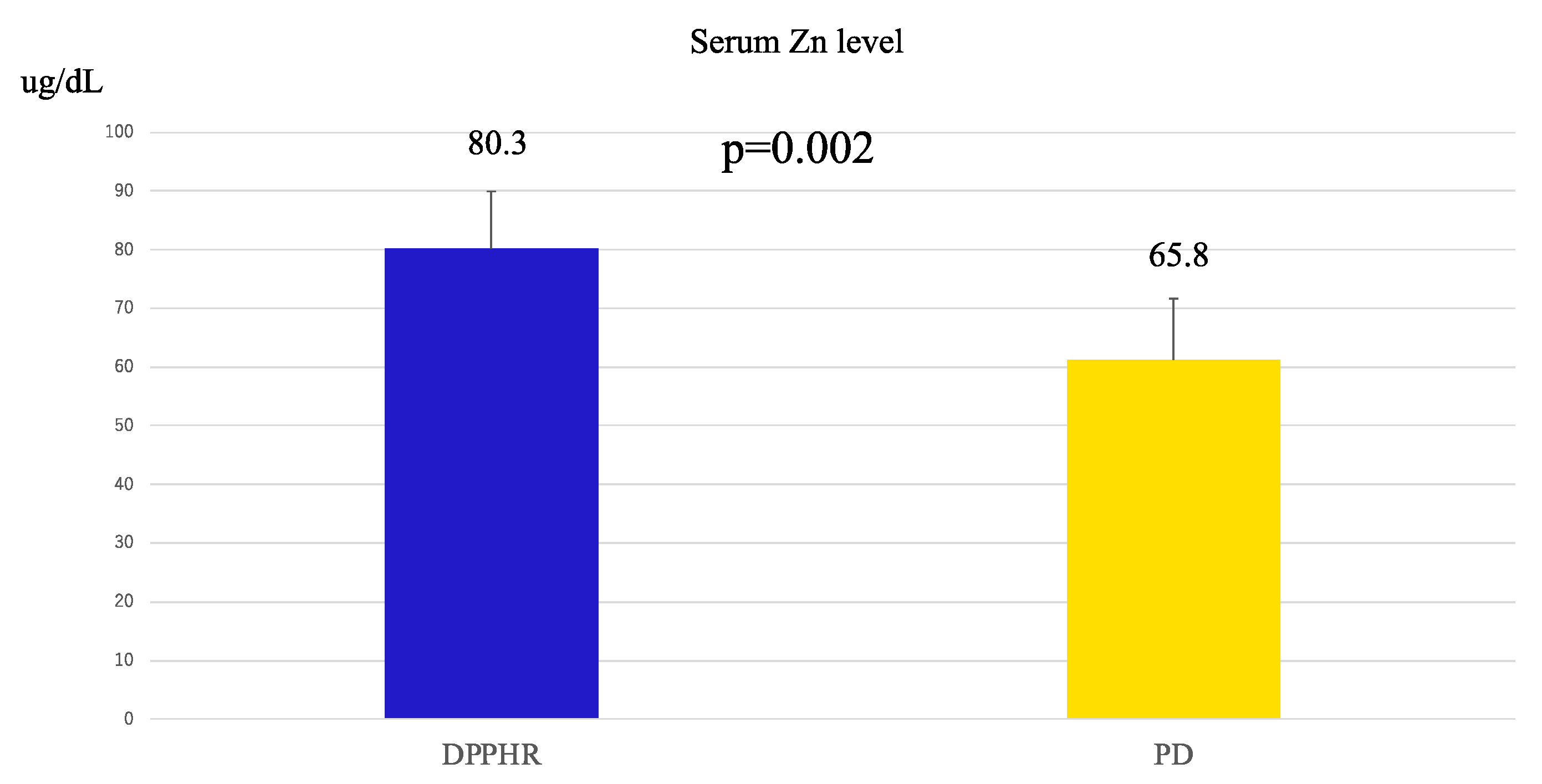
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Beger, H.; Witte, C.; Krautzberger, W.; Bittner, R. Experiences with duodenum-sparing pancreas head resection in chronic pancreatitis. Der. Chir. Z. Fur. Alle Geb. Der. Oper. Medizen 1980, 51, 303–307. [Google Scholar]
- Takada, T.; Yasuda, H.; Uchiyama, K.; Hasegawa, H. Duodenum-preserving pancreatoduodenostomy—A new technique for complete excision of the head of the pancreas with preservation of biliary and alimentary integrity. Hepato-Gastroenterology. 1993, 40, 356–359. [Google Scholar] [PubMed]
- Umemoto, K.; Tsuchikawa, T.; Nakamura, T.; Okamura, K.; Noji, T.; Asano, T.; Nakanishi, Y.; Tanaka, K.; Hirano, S. Postoperative nutritional benefits of proximal parenchymal pancreatectomy for low-grade malignant lesions in the pancreatic head. HPB 2019, 21, 1491–1496. [Google Scholar] [CrossRef]
- Yu, H.-H.; Yang, T.-M.; Shan, Y.-S.; Lin, P.-W. Zinc deficiency in patients undergoing pancreatoduodenectomy for periampullary tumors is associated with pancreatic exocrine insufficiency. World J. Surg. 2011, 35, 2110–2117. [Google Scholar] [CrossRef]
- Takishita, C.; Nagakawa, Y.; Osakabe, H.; Nakagawa, N.; Mitsuka, Y.; Mazaki, J.; Iwasaki, K.; Ishizaki, T.; Kozono, S. Significance of zinc replacement therapy after pancreaticoduodenectomy. Anticancer Res. 2022, 42, 5833–5837. [Google Scholar] [CrossRef]
- Armstrong, T.; Strommer, L.; Ruiz-Jasbon, F.; Shek, F.; Harris, S.; Permert, J.; Johnson, C. Pancreaticoduodenectomy for peri-ampullary neoplasia leads to specific micronutrient deficiencies. Pancreatology 2007, 7, 37–44. [Google Scholar] [CrossRef]
- Hambidge, M. Human zinc deficiency. J. Nutr. 2000, 130, 1344S–1349S. [Google Scholar] [CrossRef]
- Krebs, N.F. Overview of zinc absorption and excretion in the human gastrointestinal tract. J. Nutr. 2000, 130, 1374S–1377S. [Google Scholar] [CrossRef]
- Kato, K.; Isaji, S.; Kawarada, Y.; Hibasami, H.; Nakashima, K. Effect of Zinc Administration on Pancreatic Regeneration After 80% Pancreatectomy. Pancreas 1997, 14, 158–165. [Google Scholar] [CrossRef]
- Sato, R.; Kishiwada, M.; Kuriyama, N.; Azumi, Y.; Mizuno, S.; Usui, M.; Sakurai, H.; Tabata, M.; Yamada, T.; Isaji, S. Paradoxical impact of the remnant pancreatic volume and infectious complications on the development of nonalcoholic fatty liver disease after pancreaticoduodenectomy. J. Hepato-Biliary-Pancreat. Sci. 2014, 21, 562–572. [Google Scholar] [CrossRef]
- Okano, K.; Murakami, Y.; Nakagawa, N.; Uemura, K.; Sudo, T.; Hashimoto, Y.; Kondo, N.; Takahashi, S.; Sueda, T. Remnant pancreatic parenchymal volume predicts postoperative pancreatic exocrine insufficiency after pancreatectomy. Surgery 2016, 159, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Tsunematsu, M.; Gocho, T.; Yanagaki, M.; Shirai, Y.; Haruki, K.; Furukawa, K.; Yasuda, J.; Onda, S.; Sakamoto, T.; Ikegami, T. The impact of postoperative exocrine index on non-alcoholic fatty liver disease following pancreaticoduodenectomy. Ann. Gastroenterol. Surg. 2022, 6, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Asano, Y.; Ito, M.; Arakawa, S.; Shimura, M.; Koike, D.; Hayashi, C.; Kamio, K.; Kawai, T.; Horiguchi, A. Significant positive impact of duodenum-preserving pancreatic head resection on the prevention of postoperative nonalcoholic fatty liver disease and acute cholangitis. Ann. Gastroenterol. Surg. 2022, 6, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Higashiguchi, T.; Kato, H.; Yasuoka, H.; Ito, M.; Asano, Y.; Kawabe, N.; Arakawa, S.; Shimura, M.; Koike, D.; Hayashi, C. A preserved pancreatic exocrine function after pancreatectomy may be a crucial cause of pancreatic fistula: Paradoxical results of the 13C-trioctanoin breath test in the perioperative period. Surg. Today 2022, 52, 580–586. [Google Scholar] [CrossRef]
- Horiguchi, A.; Miyakawa, S.; Ishihara, S.; Ito, M.; Asano, Y.; Furusawa, K.; Shimizu, T.; Yamamoto, T. Surgical design and outcome of duodenum-preserving pancreatic head resection for benign or low-grade malignant tumors. J. Hepatobiliary Pancreat. Sci. 2010, 17, 792–797. [Google Scholar] [CrossRef]
- Siu, F.K.; Lam, I.P.; Chu, J.Y.; Chow, B.K. Signaling mechanisms of secretin receptor. Regul. Pept. 2006, 137, 95–104. [Google Scholar] [CrossRef]
- Ito, K. Duodenum preservation in pancreatic head resection to maintain pancreatic exocrine function (determined by pancreatic function diagnostant test and cholecystokinin secretion). J. Hepatobiliary Pancreat. Surg. 2005, 12, 123–128. [Google Scholar] [CrossRef]
- Chamokova, B.; Bastati, N.; Poetter-Lang, S.; Bican, Y.; Hodge, J.C.; Schindl, M.; Matos, C.; Ba-Ssalamah, A. The clinical value of secretin-enhanced MRCP in the functional and morphological assessment of pancreatic diseases. Br. J. Radiol. 2018, 91, 20170677. [Google Scholar] [CrossRef]
- Schneider, A.R.; Hammerstingl, R.; Heller, M.; Povse, N.; Murzynski, L.; Vogl, T.J.; Caspary, W.F.; Stein, J. Does secretin-stimulated MRCP predict exocrine pancreatic insufficiency?: A comparison with noninvasive exocrine pancreatic function tests. J. Clin. Gastroenterol. 2006, 40, 851–855. [Google Scholar] [CrossRef]
- Tsai, L.L.; Lee, K.S. Dynamic pancreatography with secretin-MRCP. Appl. Radiol. 2015, 44, 34–38. [Google Scholar] [CrossRef]
- Swensson, J.; Zaheer, A.; Conwell, D.; Sandrasegaran, K.; Manfredi, R.; Tirkes, T. Secretin-enhanced MRCP: How and why—AJR expert panel narrative review. Am. J. Roentgenol. 2021, 216, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Morifuji, M.; Murakami, Y.; Uemura, K.; Ohge, H.; Hayashidani, Y.; Sudo, T.; Sueda, T. Usefulness of a 13C-labeled mixed triglyceride breath test for assessing pancreatic exocrine function after pancreatic surgery. Surgery 2009, 145, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.; Layer, P.; Brückel, S.; Jahr, C.; Rosien, U. 13C-mixed triglyceride breath test for evaluation of pancreatic exocrine function in diabetes mellitus. Pancreas 2014, 43, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Miyakawa, S.; Hayakawa, M.; Horiguchi, A.; Mizuno, K.; Ishihara, S.; Niwamoto, N.; Miura, K. Estimation of fat absorption with the 13C-trioctanoin breath test after pancreatoduodenectomy or pancreatic head resection. World J. Surg. 1996, 20, 1024–1028, discussion 1028–1029. [Google Scholar] [CrossRef]
- Zhen, T.-T.; Li, S.-Z.; Pan, S.-T.; Yin, T.-Y.; Wang, M.; Guo, X.-J.; Zhang, H.; Qin, R.-Y. Nonalcoholic fatty liver disease following laparoscopic duodenum-preserving pancreatic total head resection vs laparoscopic pancreaticoduodenectomy: A retrospective cohort study. World J. Gastroenterol. 2025, 31, 104046. [Google Scholar] [CrossRef]
- Girish, B.N.; Rajesh, G.; Vaidyanathan, K.; Balakrishnan, V. Zinc status in chronic pancreatitis and its relationship with exocrine and endocrine insufficiency. JOP 2009, 10, 651–656. [Google Scholar]
- Lee, H.H.; Prasad, A.S.; Brewer, G.J.; Owyang, C. Zinc absorption in human small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 1989, 256, G87–G91. [Google Scholar] [CrossRef]
- Prasad, A.S. Zinc in human health: Effect of zinc on immune cells. Mol. Med. 2008, 14, 353–357. [Google Scholar] [CrossRef]
- McCall, K.A.; Huang, C.-c.; Fierke, C.A. Zinc and health: Current status and future directions. J. Nutr. 2000, 22, 1437–1446. [Google Scholar] [CrossRef]
- Wessells, K.R.; Brown, K.H. Estimating the global prevalence of zinc deficiency: Results based on zinc availability in national food supplies and the prevalence of stunting. PLoS ONE 2012, 7, e50568. [Google Scholar] [CrossRef]
- King, J.C. Zinc: An essential but elusive nutrient. Am. J. Clin. Nutr. 2011, 94, 679S–684S. [Google Scholar] [CrossRef]

| DPPHR (n = 23) | PD (n = 18) | p-Value | |
|---|---|---|---|
| Preoperative variables | |||
| Age (years) | 54.4 ± 15.8 | 70.0 ± 9.2 | 0.001 |
| Gender (male/female) | 11/12 | 14/4 | 0.051 |
| Body mass index | 20.3 ± 3.1 | 22.4 ± 3.0 | 0.110 |
| Primary disease | |||
| intraductal papillary mucinous adenoma | 12 | 10 | |
| intraductal papillary mucinous carcinoma (non-invasive) | 1 | 8 | |
| neuroendocrine neoplasm (WHO classification grade1) | 6 | 0 | |
| solid pseudopapillary neoplasm (borderline malignancy) | 3 | 0 | |
| serous cystic adenoma | 1 | 0 | |
| Albumin (g/dL) | 4.17 ± 0.35 | 4.27 ± 0.31 | 0.380 |
| Total lymphocyte count (/mm3) | 1775 ± 678 | 1554 ± 609 | 0.360 |
| Hemoglobin (g/dL) | 13.8 ± 1.0 | 13.6 ± 1.4 | 0.729 |
| Prognostic nutrition index | 47.8 ± 12.9 | 49.4 ± 5.1 | 0.377 |
| Aspartate aminotransferase (AST) (IU/L) | 21.5 ± 6.1 | 21.2 ± 14.9 | 0.872 |
| Alanine transaminase (ALT) (IU/L) | 19.2 ± 10.0 | 18.4 ± 6.1 | 0.797 |
| Platelet count (103/μL) | 20.2 ± 5.3 | 27.9 ± 14.4 | 0.076 |
| Intraoperative variables | |||
| Operative time (min) | 477 ± 87.3 | 438 ± 96 | 0.193 |
| Blood loss (g) | 379 ± 452 | 284 ± 267 | 0.448 |
| Postoperative variables | |||
| Clinically relevant pancreatic fistula (%) | 26.1% (6/23) | 27.8% (5/18) | 0.903 |
| Complication with Clavien-Dindo grade ≥3 (%) | 34.8% (8/23) | 33.3% (6/18) | 0.923 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunimura, Y.; Kato, H.; Arakawa, S.; Shimura, M.; Tashiro, T.; Koike, D.; Nagata, H.; Kondo, Y.; Yasuoka, H.; Higashiguchi, T.; et al. Clinical Influence of Bile Duct and Duodenum Preservation on Zinc Absorption and Remnant Pancreatic Volume in Duodenum-Preserving Pancreatic Head Resection for Low-Grade Malignant Pancreatic Tumors. Cancers 2025, 17, 2217. https://doi.org/10.3390/cancers17132217
Kunimura Y, Kato H, Arakawa S, Shimura M, Tashiro T, Koike D, Nagata H, Kondo Y, Yasuoka H, Higashiguchi T, et al. Clinical Influence of Bile Duct and Duodenum Preservation on Zinc Absorption and Remnant Pancreatic Volume in Duodenum-Preserving Pancreatic Head Resection for Low-Grade Malignant Pancreatic Tumors. Cancers. 2025; 17(13):2217. https://doi.org/10.3390/cancers17132217
Chicago/Turabian StyleKunimura, Yoshiki, Hiroyuki Kato, Satoshi Arakawa, Masahiro Shimura, Takahiro Tashiro, Daisuke Koike, Hidetoshi Nagata, Yuka Kondo, Hironobu Yasuoka, Takahiko Higashiguchi, and et al. 2025. "Clinical Influence of Bile Duct and Duodenum Preservation on Zinc Absorption and Remnant Pancreatic Volume in Duodenum-Preserving Pancreatic Head Resection for Low-Grade Malignant Pancreatic Tumors" Cancers 17, no. 13: 2217. https://doi.org/10.3390/cancers17132217
APA StyleKunimura, Y., Kato, H., Arakawa, S., Shimura, M., Tashiro, T., Koike, D., Nagata, H., Kondo, Y., Yasuoka, H., Higashiguchi, T., Tani, H., Horiguchi, K., Furukawa, M., Ito, M., Kato, Y., Hanai, T., & Horiguchi, A. (2025). Clinical Influence of Bile Duct and Duodenum Preservation on Zinc Absorption and Remnant Pancreatic Volume in Duodenum-Preserving Pancreatic Head Resection for Low-Grade Malignant Pancreatic Tumors. Cancers, 17(13), 2217. https://doi.org/10.3390/cancers17132217







