Genetic Polymorphisms in Base Excision Repair (BER) and Nucleotide Excision Repair (NER) Pathways as Potential Biomarkers for Gynecological Cancers: A Comprehensive Literature Review
Simple Summary
Abstract
1. Introduction
2. Causes of Gynecological Cancers
2.1. Menstruation and Inflammatory Processes Leading to DNA Damage
2.2. Estrogen Dominance Leading to DNA Damage
3. Methodology of the Systematic Review
4. SNPs in BER and NER Pathway Genes in Diagnostics and Therapy
SNPs Predict Susceptibility and Survival
| Protein Name | db SNP ID | Number of Cases/Controls | Gynecological Cancer | References |
|---|---|---|---|---|
| XPA | rs1800975 | 2338/2731 | breast cancer | [80] |
| rs1805348 | 371/420 | endometrial cancer | [81] | |
| rs2808667 | 783/795 | endometrial cancer | [82] | |
| XPC | rs2228000 | 400/400 | cervical cancer | [62] |
| rs2228001; rs2276466 | 210/200 | cervical cancer | [83] | |
| rs2227998 | 300/300 | breast cancer | [65] | |
| rs3731127 | 783/795 | endometrial cancer | [82] | |
| ERCC1 | rs1799793 | 25,446/41,106 | endometrial cancer | [73] |
| rs3212986 | 9896/11,027 | ovarian cancer | [84] | |
| ERCC2 | rs238406 | 400/400 | ovarian cancer | [85] |
| 1360/1320 | endometrial cancer | [86] | ||
| rs1799793 | 400/400 | cervical cancer | [62] | |
| rs13181 | 300/300 | breast cancer | [65] | |
| 610/610 | endometrial cancer | [87] | ||
| 510/510 | endometrial cancer | [63] | ||
| 1333/2691 | ovarian cancer | [18] | ||
| 430/430 | ovarian cancer | [88] | ||
| ERCC5 | rs4150386 | 783/795 | endometrial cancer | [82] |
| rs17655 | 478/922 | cervical cancer | [89] | |
| rs17655 | 4028/4953 | cervical cancer | [16] | |
| LIG1 | rs3730865 | 783/795 | endometrial cancer | [82] |
5. SNPs in Personalized Therapy
6. The Role of BER and NER Pathways in Tumor Regulation and Progression
7. Polymorphisms in BER and NER Pathway Genes as Epigenetic Markers
7.1. The Role of SNPs in Methylation Processes
7.2. Role of SNPs in miRNA-Binding Sites
8. Recommendations
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). Available online: https://www.who.int/ (accessed on 18 April 2025).[Green Version]
- International Agency for Research on Cancer (IARC). Available online: https://www.iarc.who.int/ (accessed on 18 April 2025).[Green Version]
- Soheili, M.; Keyvani, H.; Soheili, M.; Nasseri, S. Human Papilloma Virus: A Review Study of Epidemiology, Carcinogenesis, Diagnostic Methods, and Treatment of All HPV-Related Cancers. Med. J. Islam. Repub. Iran 2021, 35, 65. [Google Scholar] [CrossRef] [PubMed]
- Milne, R.L.; Antoniou, A.C. Modifiers of Breast and Ovarian Cancer Risks for BRCA1 and BRCA2 Mutation Carriers. Endocr. Relat. Cancer 2016, 23, T69–T84. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Lee, T.S. Associations between Metabolic Syndrome and Gynecologic Cancer. Obstet. Gynecol. Sci. 2020, 63, 215–224. [Google Scholar] [CrossRef]
- Ciebiera, M.; Esfandyari, S.; Siblini, H.; Prince, L.; Elkafas, H.; Wojtyła, C.; Al-Hendy, A.; Ali, M. Nutrition in Gynecological Diseases: Current Perspectives. Nutrients 2021, 13, 1178. [Google Scholar] [CrossRef] [PubMed]
- Katzke, V.A.; Kaaks, R.; Kühn, T. Lifestyle and Cancer Risk. Cancer J. 2015, 21, 104–110. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Zhu, B.; Gu, H.; Mao, Z.; Beeraka, N.M.; Zhao, X.; Anand, M.P.; Zheng, Y.; Zhao, R.; Li, S.; Manogaran, P.; et al. Global Burden of Gynaecological Cancers in 2022 and Projections to 2050. J. Glob. Health 2024, 14, 04155. [Google Scholar] [CrossRef]
- Kang, S.; Sun, H.-Y.; Zhou, R.-M.; Wang, N.; Hu, P.; Li, Y. DNA Repair Gene Associated with Clinical Outcome of Epithelial Ovarian Cancer Treated with Platinum-Based Chemotherapy. Asian Pac. J. Cancer Prev. 2013, 14, 941–946. [Google Scholar] [CrossRef]
- Choi, J.E.; Chung, W.-H. Synthetic Lethal Interaction between Oxidative Stress Response and DNA Damage Repair in the Budding Yeast and Its Application to Targeted Anticancer Therapy. J. Microbiol. 2019, 57, 9–17. [Google Scholar] [CrossRef]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA Damage Response in Cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef]
- Begum, Y.; Pandit, A.; Swarnakar, S. Insights Into the Regulation of Gynecological Inflammation-Mediated Malignancy by Metalloproteinases. Front. Cell Dev. Biol. 2021, 9, 780510. [Google Scholar] [CrossRef]
- Goswami, B.; Rajappa, M.; Sharma, M.; Sharma, A. Inflammation: Its Role and Interplay in the Development of Cancer, with Special Focus on Gynecological Malignancies. Int. J. Gynecol. Cancer 2008, 18, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Awasthi, S.; Horne, D.; Salgia, R.; Singhal, S.S. The Innate Effects of Plant Secondary Metabolites in Preclusion of Gynecologic Cancers: Inflammatory Response and Therapeutic Action. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2023, 1878, 188929. [Google Scholar] [CrossRef]
- Das, A.P.; Saini, S.; Tyagi, S.; Chaudhary, N.; Agarwal, S.M. Elucidation of Increased Cervical Cancer Risk Due to Polymorphisms in XRCC1 (R399Q and R194W), ERCC5 (D1104H), and NQO1 (P187S). Reprod. Sci. 2023, 30, 1118–1132. [Google Scholar] [CrossRef]
- Niwa, Y.; Matsuo, K.; Ito, H.; Hirose, K.; Tajima, K.; Nakanishi, T.; Nawa, A.; Kuzuya, K.; Tamakoshi, A.; Hamajima, N. Association of XRCC1 Arg399Gln and OGG1 Ser326Cys Polymorphisms with the Risk of Cervical Cancer in Japanese Subjects. Gynecol. Oncol. 2005, 99, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Pan, L.; Qin, X.; Chu, H.; Mu, H.; Wan, G. Association between ERCC2 Rs13181 Polymorphism and Ovarian Cancer Risk: An Updated Meta-Analysis with 4024 Subjects. Arch. Gynecol. Obstet. 2017, 296, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Jorge, S.; Chang, S.; Barzilai, J.J.; Leppert, P.; Segars, J.H. Mechanical Signaling in Reproductive Tissues: Mechanisms and Importance. Reprod. Sci. 2014, 21, 1093–1107. [Google Scholar] [CrossRef]
- Holdsworth-Carson, S.J.; Menkhorst, E.; Maybin, J.A.; King, A.; Girling, J.E. Cyclic Processes in the Uterine Tubes, Endometrium, Myometrium, and Cervix: Pathways and Perturbations. Mol. Hum. Reprod. 2023, 29, gaad012. [Google Scholar] [CrossRef]
- Sheets, E.E.; Yeh, J. The Role of Apoptosis in Gynaecological Malignancies. Ann. Med. 1997, 29, 121–126. [Google Scholar] [CrossRef]
- Cassidy, L.D.; Venkitaraman, A.R. Genome Instability Mechanisms and the Structure of Cancer Genomes. Curr. Opin. Genet. Dev. 2012, 22, 10–13. [Google Scholar] [CrossRef]
- Zhang, G.; Ren, J.; Luo, M.; Cui, J.; Du, Y.; Yang, D.; Cui, S.; Wang, X.; Wu, W.; Cao, J.; et al. Association of BER and NER Pathway Polymorphism Haplotypes and Micronucleus Frequencies with Global DNA Methylation in Benzene-Exposed Workers of China. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2019, 839, 13–20. [Google Scholar] [CrossRef]
- Beheshti, F.; Hassanian, S.M.; Khazaei, M.; Hosseini, M.; ShahidSales, S.; Hasanzadeh, M.; Maftouh, M.; Ferns, G.A.; Avan, A. Genetic Variation in the DNA Repair Pathway as a Potential Determinant of Response to Platinum-based Chemotherapy in Breast Cancer. J. Cell. Physiol. 2018, 233, 2752–2758. [Google Scholar] [CrossRef] [PubMed]
- Tomasova, K.; Cumova, A.; Seborova, K.; Horak, J.; Koucka, K.; Vodickova, L.; Vaclavikova, R.; Vodicka, P. DNA Repair and Ovarian Carcinogenesis: Impact on Risk, Prognosis and Therapy Outcome. Cancers 2020, 12, 1713. [Google Scholar] [CrossRef]
- Okoh, V.; Deoraj, A.; Roy, D. Estrogen-Induced Reactive Oxygen Species-Mediated Signalings Contribute to Breast Cancer. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2011, 1815, 115–133. [Google Scholar] [CrossRef]
- Tian, H.; Gao, Z.; Wang, G.; Li, H.; Zheng, J. Estrogen Potentiates Reactive Oxygen Species (ROS) Tolerance to Initiate Carcinogenesis and Promote Cancer Malignant Transformation. Tumor Biol. 2016, 37, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Maybin, J.A.; Critchley, H.O.D. Progesterone: A Pivotal Hormone at Menstruation. Ann. N. Y. Acad. Sci. 2011, 1221, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Whitcomb, B.W.; Mumford, S.L.; Perkins, N.J.; Wactawski-Wende, J.; Bertone-Johnson, E.R.; Lynch, K.E.; Schisterman, E.F. Urinary Cytokine and Chemokine Profiles across the Menstrual Cycle in Healthy Reproductive-Aged Women. Fertil. Steril. 2014, 101, 1383–1391.e2. [Google Scholar] [CrossRef]
- Roomruangwong, C.; Sirivichayakul, S.; Matsumoto, A.K.; Michelin, A.P.; De Oliveira Semeão, L.; De Lima Pedrão, J.V.; Barbosa, D.S.; Moreira, E.G.; Maes, M. Menstruation Distress Is Strongly Associated with Hormone-Immune-Metabolic Biomarkers. J. Psychosom. Res. 2021, 142, 110355. [Google Scholar] [CrossRef]
- Crona Guterstam, Y.; Strunz, B.; Ivarsson, M.A.; Zimmer, C.; Melin, A.; Jonasson, A.F.; Björkström, N.K.; Gidlöf, S.B. The Cytokine Profile of Menstrual Blood. Acta Obstet. Gynecol. Scand. 2021, 100, 339–346. [Google Scholar] [CrossRef]
- Li, T.; Li, R.H.W.; Ng, E.H.Y.; Yeung, W.S.B.; Chiu, P.C.N.; Chan, R.W.S. Interleukin 6 at Menstruation Promotes the Proliferation and Self-Renewal of Endometrial Mesenchymal Stromal/Stem Cells through the WNT/β-Catenin Signaling Pathway. Front. Immunol. 2024, 15, 1378863. [Google Scholar] [CrossRef]
- Maruyama, T.; Yoshimura, Y. Molecular and Cellular Mechanisms for Differentiation and Regeneration of the Uterine Endometrium. Endocr. J. 2008, 55, 795–810. [Google Scholar] [CrossRef]
- Hu, X.; Wu, H.; Yong, X.; Wang, Y.; Yang, S.; Fan, D.; Xiao, Y.; Che, L.; Shi, K.; Li, K.; et al. Cyclical Endometrial Repair and Regeneration: Molecular Mechanisms, Diseases, and Therapeutic Interventions. MedComm 2023, 4, e425. [Google Scholar] [CrossRef] [PubMed]
- Amano, T.; Murakami, A.; Murakami, T.; Chano, T. Antioxidants and Therapeutic Targets in Ovarian Clear Cell Carcinoma. Antioxidants 2021, 10, 187. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Gao, Y.; Feng, Z.; Zhang, B.; Na, Z.; Li, D. Reactive Oxygen Species and Ovarian Diseases: Antioxidant Strategies. Redox Biol. 2023, 62, 102659. [Google Scholar] [CrossRef] [PubMed]
- Criscuolo, D.; Avolio, R.; Parri, M.; Romano, S.; Chiarugi, P.; Matassa, D.S.; Esposito, F. Decreased Levels of GSH Are Associated with Platinum Resistance in High-Grade Serous Ovarian Cancer. Antioxidants 2022, 11, 1544. [Google Scholar] [CrossRef]
- Griñan-Lison, C.; Blaya-Cánovas, J.L.; López-Tejada, A.; Ávalos-Moreno, M.; Navarro-Ocón, A.; Cara, F.E.; González-González, A.; Lorente, J.A.; Marchal, J.A.; Granados-Principal, S. Antioxidants for the Treatment of Breast Cancer: Are We There Yet? Antioxidants 2021, 10, 205. [Google Scholar] [CrossRef]
- Luo, M.; Zhou, L.; Huang, Z.; Li, B.; Nice, E.C.; Xu, J.; Huang, C. Antioxidant Therapy in Cancer: Rationale and Progress. Antioxidants 2022, 11, 1128. [Google Scholar] [CrossRef]
- Zahra, K.F.; Lefter, R.; Ali, A.; Abdellah, E.-C.; Trus, C.; Ciobica, A.; Timofte, D. The Involvement of the Oxidative Stress Status in Cancer Pathology: A Double View on the Role of the Antioxidants. Oxid. Med. Cell. Longev. 2021, 2021, 9965916. [Google Scholar] [CrossRef]
- Scicchitano, S.; Vecchio, E.; Battaglia, A.M.; Oliverio, M.; Nardi, M.; Procopio, A.; Costanzo, F.; Biamonte, F.; Faniello, M.C. The Double-Edged Sword of Oleuropein in Ovarian Cancer Cells: From Antioxidant Functions to Cytotoxic Effects. Int. J. Mol. Sci. 2023, 24, 842. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Y.; Liu, Y.; Xing, Y.; Miao, C.; Zhao, Y.; Chang, X.; Zhang, Q. The Role of Oxidative Stress and Natural Antioxidants in Ovarian Aging. Front. Pharmacol. 2021, 11, 617843. [Google Scholar] [CrossRef]
- Lu, Y.; Shao, Y.; Cui, W.; Jia, Z.; Zhang, Q.; Zhao, Q.; Chen, Z.; Yan, J.; Chu, B.; Yuan, J. Excessive Lipid Peroxidation in Uterine Epithelium Causes Implantation Failure and Pregnancy Loss. Adv. Sci. 2024, 11, 2302887. [Google Scholar] [CrossRef] [PubMed]
- Błachnio-Zabielska, A.U.; Sadowska, P.; Zdrodowski, M.; Laudański, P.; Szamatowicz, J.; Kuźmicki, M. The Interplay between Oxidative Stress and Sphingolipid Metabolism in Endometrial Cancer. Int. J. Mol. Sci. 2024, 25, 10243. [Google Scholar] [CrossRef] [PubMed]
- Ashton, A.W.; Zhang, Y.; Cazzolli, R.; Honn, K.V. The Role and Regulation of Thromboxane A2 Signaling in Cancer-Trojan Horses and Misdirection. Molecules 2022, 27, 6234. [Google Scholar] [CrossRef] [PubMed]
- Kompella, P.; Vasquez, K.M. Obesity and Cancer: A Mechanistic Overview of Metabolic Changes in Obesity That Impact Genetic Instability. Mol. Carcinog. 2019, 58, 1531–1550. [Google Scholar] [CrossRef]
- Ferk, F.; Mišík, M.; Ernst, B.; Prager, G.; Bichler, C.; Mejri, D.; Gerner, C.; Bileck, A.; Kundi, M.; Langie, S.; et al. Impact of Bariatric Surgery on the Stability of the Genetic Material, Oxidation, and Repair of DNA and Telomere Lengths. Antioxidants 2023, 12, 760. [Google Scholar] [CrossRef]
- Abbas, M.; Srivastava, K.; Imran, M.; Banerjee, M. Genetic Polymorphisms in DNA Repair Genes and Their Association with Cervical Cancer. Br. J. Biomed. Sci. 2019, 76, 117–121. [Google Scholar] [CrossRef]
- Xu, X.-L.; Deng, S.-L.; Lian, Z.-X.; Yu, K. Estrogen Receptors in Polycystic Ovary Syndrome. Cells 2021, 10, 459. [Google Scholar] [CrossRef]
- Ring, K.L.; Mills, A.M.; Modesitt, S.C. Endometrial Hyperplasia. Obstet. Gynecol. 2022, 140, 1061–1075. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, H.; Li, J.; Bai, X.; Qi, R.; Li, Z.; Ge, Z.; Zhou, M.; Li, L. Association of Urinary Levels of Estrogens and Estrogen Metabolites with the Occurrence and Development of Endometrial Hyperplasia Among Premenopausal Women. Reprod. Sci. 2023, 30, 3027–3036. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, W.; Liu, H.; Zhang, D. Age at Menopause and Risk of Developing Endometrial Cancer: A Meta-Analysis. BioMed Res. Int. 2019, 2019, 8584130. [Google Scholar] [CrossRef]
- Andò, S.; Simões, B.M. Editorial: Adipokines and Hormone-Dependent Cancers. Front. Endocrinol. 2023, 14, 1340171. [Google Scholar] [CrossRef]
- Bujnakova Mlynarcikova, A.; Scsukova, S. The Role of the Environment in Hormone-Related Cancers. In Environmental Endocrinology and Endocrine Disruptors; Pivonello, R., Diamanti-Kandarakis, E., Eds.; Endocrinology; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–36. ISBN 978-3-030-38366-4. [Google Scholar]
- Hussain, M.S.; Mishra, A.K. Exploring the Connection: Endocrine Disruptors and Polycystic Ovarian Syndrome. Int. J. Basic Clin. Pharmacol. 2024, 13, 403–407. [Google Scholar] [CrossRef]
- Rajan, A.; Nadhan, R.; Latha, N.R.; Krishnan, N.; Warrier, A.V.; Srinivas, P. Deregulated Estrogen Receptor Signaling and DNA Damage Response in Breast Tumorigenesis. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2021, 1875, 188482. [Google Scholar] [CrossRef]
- Hsu, M.-S.; Yu, J.-C.; Wang, H.-W.; Chen, S.-T.; Hsiung, C.-N.; Ding, S.; Wu, P.-E.; Shen, C.-Y.; Cheng, C.-W. Synergistic Effects of Polymorphisms in DNA Repair Genes and Endogenous Estrogen Exposure on Female Breast Cancer Risk. Ann. Surg. Oncol. 2010, 17, 760–771. [Google Scholar] [CrossRef] [PubMed]
- PRISMA Guidelines 2020. Available online: https://www.prisma-statement.org/prisma-2020 (accessed on 25 April 2025).
- Chen, H.-W.; Kuo, W.-H.; Lu, Y.-S.; Chen, I.-C.; Hu, F.-C.; Wang, M.-Y.; Zahid, M.; Rogan, E.G.; Cheng, A.-L.; Lin, C.-H. Interaction of Base Excision Repair Gene Polymorphism and Estrogen-DNA Adducts in Breast Cancer Risk among East Asian Women. Breast Cancer Res. Treat. 2024, 208, 283–292. [Google Scholar] [CrossRef]
- Datkhile, K.D.; Patil, M.N.; Durgawale, P.P.; Joshi, S.A.; Korabu, K.S.; Kakade, S.V. Assessment of Role of Genetic Polymorphisms in XRCC1, XRCC2 and XRCC3 Genes in Cervical Cancer Susceptibility from a Rural Population: A Hospital Based Case-Control Study from Maharashtra, India. Int. J. Res. Med. Sci. 2018, 6, 3132. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Li, L. A Meta-Analysis of XRCC1 Single Nucleotide Polymorphism and Susceptibility to Gynecological Malignancies. Medicine 2021, 100, e28030. [Google Scholar] [CrossRef]
- Datkhile, K.D.; Durgawale, P.P.; Patil, M.N.; Gudur, R.A.; Gudur, A.K.; Patil, S.R. Impact of Polymorphism in Base Excision Repair and Nucleotide Excision Repair Genes and Risk of Cervical Cancer: A Case-Control Study. Asian Pac. J. Cancer Prev. 2022, 23, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Smolarz, B.; Romanowicz, H. Association between Single Nucleotide Polymorphism of DNA Repair Genes and Endometrial Cancer: A Case-Control Study. Int. J. Clin. Exp. Pathol. 2018, 11, 1732–1738. [Google Scholar]
- Kaur, J.; Sambyal, V.; Guleria, K.; Singh, N.R.; Uppal, M.S.; Manjari, M.; Sudan, M. Association of XRCC1, XRCC2 and XRCC3 Gene Polymorphism with Esophageal Cancer Risk. Clin. Exp. Gastroenterol. 2020, 13, 73–86. [Google Scholar] [CrossRef]
- Smolarz, B.; Michalska, M.M.; Samulak, D.; Romanowicz, H.; Wójcik, L. Polymorphism of DNA Repair Genes in Breast Cancer. Oncotarget 2019, 10, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wang, Q.; He, G.; Yu, H. Association between XRCC3 Thr241Met Polymorphism and Risk of Gynecological Malignancies: A Meta-Analysis. Cancer Genet. 2021, 254–255, 11–17. [Google Scholar] [CrossRef]
- Ye, F.; Wang, H.; Liu, J.; Cheng, Q.; Chen, X.; Chen, H. Association of SMUG1 SNPs in Intron Region and Linkage Disequilibrium with Occurrence of Cervical Carcinoma and HPV Infection in Chinese Population. J. Cancer 2019, 10, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Liu, J.; Wang, H.; Chen, X.; Cheng, Q.; Chen, H. Cervical Carcinoma Risk Associate with Genetic Polymorphisms of NEIL2 Gene in Chinese Population and Its Significance as Predictive Biomarker. Sci. Rep. 2020, 10, 5136. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Wang, H.; Liu, J.; Cheng, Q.; Chen, X.; Chen, H. Genetic Polymorphism (Rs246079) of the DNA Repair Gene Uracil N-Glycosylase Is Associated with Increased Risk of Cervical Carcinoma in a Chinese Population. Medicine 2018, 97, e13694. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, Z.; Chen, S.; Liang, Z.; Zhu, J.; Zhao, M.; Xu, C.; He, J.; Duan, P.; Zhang, A. The Association of Polymorphisms in Base Excision Repair Genes with Ovarian Cancer Susceptibility in Chinese Women: A Two-Center Case-Control Study. J. Cancer 2021, 12, 264–269. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Lu, H.; Zhou, H.; Xie, L.; Wu, M.; Lin, Z. Association Analysis between 8-Oxoguanine DNA Glycosylase Genetic Variants and Endometrial Cancer Susceptibility in Chinese Han Population. J. Pharm. Pharmacol. 2015, 67, 559–564. [Google Scholar] [CrossRef]
- Nagpal, A.; Verma, S.; Shah, R.; Bhat, G.; Bhat, A.; Bakshi, D.; Sharma, B.; Kaul, S.; Kumar, R. Genetic Polymorphism of hOGG1 Ser326cys and Its Association with Breast Cancer in Jammu and Kashmir. Indian J. Cancer 2020, 57, 187–189. [Google Scholar] [CrossRef]
- Das, A.P.; Chaudhary, N.; Tyagi, S.; Agarwal, S.M. Meta-Analysis of 49 SNPs Covering 25,446 Cases and 41,106 Controls Identifies Polymorphisms in Hormone Regulation and DNA Repair Genes Associated with Increased Endometrial Cancer Risk. Genes 2023, 14, 741. [Google Scholar] [CrossRef]
- Shi, Y.; Xu, W.; Zhang, X. Association of the hOGG1 Ser326Cys Polymorphism with Gynecologic Cancer Susceptibility: A Meta-Analysis. Biosci. Rep. 2020, 40, BSR20203245. [Google Scholar] [CrossRef]
- Sobczuk, A.; Poplawski, T.; Blasiak, J. Polymorphisms of DNA Repair Genes in Endometrial Cancer. Pathol. Oncol. Res. 2012, 18, 1015–1020. [Google Scholar] [CrossRef]
- Li, K.; Li, W. Association between Polymorphisms of XRCC1 and ADPRT Genes and Ovarian Cancer Survival with Platinum-Based Chemotherapy in Chinese Population. Mol. Cell. Biochem. 2013, 372, 27–33. [Google Scholar] [CrossRef]
- Singoprawiro, C.; Setianingsih, I.; Sutrisna, B.; Siregar, N.C.; Andrijono, A. A Prognostic Model Based on XRCC1 Gene Polymorphisms and Clinicopathological Factors to Predict One-Year Survival of Advanced Epithelial Ovarian Cancer Patients. Syntax. Lit. J. Ilm. Indones. 2022, 7, 75. [Google Scholar] [CrossRef]
- Przybylowska-Sygut, K.; Stanczyk, M.; Kusinska, R.; Kordek, R.; Majsterek, I. Association of the Arg194Trp and the Arg399Gln Polymorphisms of the XRCC1 Gene with Risk Occurrence and the Response to Adjuvant Therapy Among Polish Women with Breast Cancer. Clin. Breast Cancer 2013, 13, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Pachkowski, B.F.; Winkel, S.; Kubota, Y.; Swenberg, J.A.; Millikan, R.C.; Nakamura, J. XRCC1 Genotype and Breast Cancer: Functional Studies and Epidemiologic Data Show Interactions between XRCC1 Codon 280 His and Smoking. Cancer Res. 2006, 66, 2860–2868. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, Q.; Yin, X.; Zhu, X.; Zhao, L.; Zhang, Z.; Wei, R.; Wang, B.; Li, X. Association of XPA Polymorphism with Breast Cancer Risk: A Meta-Analysis. Medicine 2018, 97, e11276. [Google Scholar] [CrossRef]
- Weiss, J.M.; Weiss, N.S.; Ulrich, C.M.; Doherty, J.A.; Voigt, L.F.; Chen, C. Interindividual Variation in Nucleotide Excision Repair Genes and Risk of Endometrial Cancer. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2524–2530. [Google Scholar] [CrossRef] [PubMed]
- Doherty, J.A.; Weiss, N.S.; Fish, S.; Fan, W.; Loomis, M.M.; Sakoda, L.C.; Rossing, M.A.; Zhao, L.P.; Chen, C. Polymorphisms in Nucleotide Excision Repair Genes and Endometrial Cancer Risk. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1873–1882. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Naher, L.; Aka, T.D.; Aziz, M.A.; Shabnaz, S.; Shahriar, M.; Islam, M.S. The ECCR1 Rs11615, ERCC4 Rs2276466, XPC Rs2228000 and XPC Rs2228001 Polymorphisms Increase the Cervical Cancer Risk and Aggressiveness in the Bangladeshi Population. Heliyon 2021, 7, e05919. [Google Scholar] [CrossRef]
- Yang, F.; Mu, X.; Bian, C.; Zhang, H.; Yi, T.; Zhao, X.; Lin, X. Association of Excision Repair Cross-Complimentary Group 1 Gene Polymorphisms with Breast and Ovarian Cancer Susceptibility. J. Cell. Biochem. 2019, 120, 15635–15647. [Google Scholar] [CrossRef]
- Romanowicz, H.; Michalska, M.M.; Samulak, D.; Malinowski, J.; Szaflik, T.; Bieńkiewicz, J.; Smolarz, B. Association of R156R Single Nucleotide Polymorphism of the ERCC2 Gene with the Susceptibility to Ovarian Cancer. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 208, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Michalska, M.M.; Samulak, D.; Jabłoński, F.; Romanowicz, H.; Smolarz, B. The R156R ERCC2 Polymorphism as a Risk Factor of Endometrial Cancer. Tumor Biol. 2016, 37, 2171–2176. [Google Scholar] [CrossRef]
- Smolarz, B.; Michalska, M.M.; Samulak, D.; Wójcik, L.; Romanowicz, H. Studies of Correlations Between Single Nucleotide Polymorphisms of DNA Repair Genes and Endometrial Cancer in Polish Women. Anticancer Res. 2018, 38, 5223–5229. [Google Scholar] [CrossRef]
- Michalska, M.M.; Samulak, D.; Romanowicz, H.; Sobkowski, M.; Smolarz, B. An Association between Single Nucleotide Polymorphisms of Lys751Gln ERCC2 Gene and Ovarian Cancer in Polish Women. Adv. Med. 2015, 2015, 109593. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.; Yoon, K.-A.; Hayashi, T.; Kong, S.-Y.; Shin, H.-J.; Park, B.; Kim, Y.M.; Hwang, S.-H.; Kim, J.; Shin, A.; et al. Nucleotide Excision Repair Gene ERCC2 and ERCC5 Variants Increase Risk of Uterine Cervical Cancer. Cancer Res. Treat. 2016, 48, 708–714. [Google Scholar] [CrossRef]
- Aravantinou-Fatorou, A.; Georgakopoulou, V.; Dimopoulos, M.; Liontos, M. Precision Medicine in Gynecological Cancer (Review). Biomed. Rep. 2025, 22, 43. [Google Scholar] [CrossRef] [PubMed]
- Lliberos, C.; Richardson, G.; Papa, A. Oncogenic Pathways and Targeted Therapies in Ovarian Cancer. Biomolecules 2024, 14, 585. [Google Scholar] [CrossRef]
- Włodarczyk, M.; Ciebiera, M.; Nowicka, G.; Łoziński, T.; Ali, M.; Al-Hendy, A. Epigallocatechin Gallate for the Treatment of Benign and Malignant Gynecological Diseases—Focus on Epigenetic Mechanisms. Nutrients 2024, 16, 559. [Google Scholar] [CrossRef]
- Mozafaryan, M.J.; Rezaei, P.; Masoudpoor, F.; Bahri, A.; Samini, M.; Farkhondeh, T.; Pourhanifeh, M.H.; Samarghandian, S. Resveratrol in the Treatment of Gynecological Cancer: Mechanisms and Therapeutic Potential. Curr. Med. Chem. 2024, 31, 4430–4455. [Google Scholar] [CrossRef]
- Almalki, E.; Al-Amri, A.; Alrashed, R.; AL-Zharani, M.; Semlali, A. The Curcumin Analog PAC Is a Potential Solution for the Treatment of Triple-Negative Breast Cancer by Modulating the Gene Expression of DNA Repair Pathways. Int. J. Mol. Sci. 2023, 24, 9649. [Google Scholar] [CrossRef]
- Liblab, S.; Vusuratana, A.; Areepium, N. ERCC1, XRCC1, and GSTP1 Polymorphisms and Treatment Outcomes of Advanced Epithelial Ovarian Cancer Patients Treated with Platinum-Based Chemotherapy. Asian Pac. J. Cancer Prev. 2020, 21, 1925–1929. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.-D.; Lu, W.-G.; Ye, F.; Wan, X.-Y.; Xie, X. The Association of XRCC1gene Single Nucleotide Polymorphisms with Response to Neoadjuvant Chemotherapy in Locally Advanced Cervical Carcinoma. J. Exp. Clin. Cancer Res. 2009, 28, 91. [Google Scholar] [CrossRef] [PubMed]
- Adamowicz, M.; Hailstone, R.; Demin, A.A.; Komulainen, E.; Hanzlikova, H.; Brazina, J.; Gautam, A.; Wells, S.E.; Caldecott, K.W. XRCC1 Protects Transcription from Toxic PARP1 Activity during DNA Base Excision Repair. Nat. Cell Biol. 2021, 23, 1287–1298. [Google Scholar] [CrossRef]
- Huang, R.; Chen, H.; Liang, J.; Li, Y.; Yang, J.; Luo, C.; Tang, Y.; Ding, Y.; Liu, X.; Yuan, Q.; et al. Dual Role of Reactive Oxygen Species and Their Application in Cancer Therapy. J. Cancer 2021, 12, 5543–5561. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-R.; Cheng, X.-H.; Zhang, G.-N.; Wang, X.-X.; Huang, J.-M. Cardiac Safety Analysis of First-Line Chemotherapy Drug Pegylated Liposomal Doxorubicin in Ovarian Cancer. J. Ovarian Res. 2022, 15, 96. [Google Scholar] [CrossRef]
- Zheng, F.; Zhang, Y.; Chen, S.; Weng, X.; Rao, Y.; Fang, H. Mechanism and Current Progress of Poly ADP-Ribose Polymerase (PARP) Inhibitors in the Treatment of Ovarian Cancer. Biomed. Pharmacother. 2020, 123, 109661. [Google Scholar] [CrossRef]
- Bianchi, A.; Lopez, S.; Altwerger, G.; Bellone, S.; Bonazzoli, E.; Zammataro, L.; Manzano, A.; Manara, P.; Perrone, E.; Zeybek, B.; et al. PARP-1 Activity (PAR) Determines the Sensitivity of Cervical Cancer to Olaparib. Gynecol. Oncol. 2019, 155, 144–150. [Google Scholar] [CrossRef]
- Garg, V.; Oza, A.M. Treatment of Ovarian Cancer Beyond PARP Inhibition: Current and Future Options. Drugs 2023, 83, 1365–1385. [Google Scholar] [CrossRef]
- Chan, C.Y.; Tan, K.V.; Cornelissen, B. PARP Inhibitors in Cancer Diagnosis and Therapy. Clin. Cancer Res. 2021, 27, 1585–1594. [Google Scholar] [CrossRef]
- Curtin, N.J. PARP Inhibitors for Cancer Therapy. Expert Rev. Mol. Med. 2005, 7, 1–20. [Google Scholar] [CrossRef]
- Baquero, J.M.; Benítez-Buelga, C.; Fernández, V.; Urioste, M.; García-Giménez, J.L.; Perona, R.; CIMBA Consortium; Benítez, J.; Osorio, A. A Common SNP in the UNG Gene Decreases Ovarian Cancer Risk in BRCA2 Mutation Carriers. Mol. Oncol. 2019, 13, 1110–1120. [Google Scholar] [CrossRef] [PubMed]
- Popanda, O.; Seibold, P.; Nikolov, I.; Oakes, C.C.; Burwinkel, B.; Hausmann, S.; Flesch-Janys, D.; Plass, C.; Chang-Claude, J.; Schmezer, P. Germline Variants of Base Excision Repair Genes and Breast Cancer: A Polymorphism in DNA Polymerase Gamma Modifies Gene Expression and Breast Cancer Risk. Int. J. Cancer 2013, 132, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-Y.; Han, W.; Noh, D.-Y.; Kang, D.; Kwack, K. Impact of Genetic Polymorphisms in Base Excision Repair Genes on the Risk of Breast Cancer in a Korean Population. Gene 2013, 532, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Liu, H.; Qian, D.; Wang, X.; Moorman, P.G.; Luo, S.; Hwang, S.; Wei, Q. Genetic Variants of Genes in the NER Pathway Associated with Risk of Breast Cancer: A Large-Scale Analysis of 14 Published GWAS Datasets in the DRIVE Study. Int. J. Cancer 2019, 145, 1270–1279. [Google Scholar] [CrossRef]
- Ramachandran, D.; Dörk, T. Genomic Risk Factors for Cervical Cancer. Cancers 2021, 13, 5137. [Google Scholar] [CrossRef]
- Jariyal, H.; Weinberg, F.; Achreja, A.; Nagarath, D.; Srivastava, A. Synthetic Lethality: A Step Forward for Personalized Medicine in Cancer. Drug Discov. Today 2020, 25, 305–320. [Google Scholar] [CrossRef]
- Huang, A.; Garraway, L.A.; Ashworth, A.; Weber, B. Synthetic Lethality as an Engine for Cancer Drug Target Discovery. Nat. Rev. Drug Discov. 2020, 19, 23–38. [Google Scholar] [CrossRef]
- Lord, C.J.; Ashworth, A. PARP Inhibitors: Synthetic Lethality in the Clinic. Science 2017, 355, 1152–1158. [Google Scholar] [CrossRef]
- Ang, Y.L.E.; Tan, D.S.P. Development of PARP Inhibitors in Gynecological Malignancies. Curr. Probl. Cancer 2017, 41, 273–286. [Google Scholar] [CrossRef]
- Jezierska-Drutel, A.; Rosenzweig, S.A.; Neumann, C.A. Role of Oxidative Stress and the Microenvironment in Breast Cancer Development and Progression. In Advances in Cancer Research; Elsevier: Amsterdam, The Netherlands, 2013; Volume 119, pp. 107–125. ISBN 978-0-12-407190-2. [Google Scholar]
- Scanlon, S.E.; Glazer, P.M. Multifaceted Control of DNA Repair Pathways by the Hypoxic Tumor Microenvironment. DNA Repair 2015, 32, 180–189. [Google Scholar] [CrossRef]
- Mena, S.; Ortega, A.; Estrela, J.M. Oxidative Stress in Environmental-Induced Carcinogenesis. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2009, 674, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Jurkovicova, D.; Neophytou, C.M.; Gašparović, A.Č.; Gonçalves, A.C. DNA Damage Response in Cancer Therapy and Resistance: Challenges and Opportunities. Int. J. Mol. Sci. 2022, 23, 14672. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.; Costa, B.; Martins, C.; Gromicho, M.; Oliveira, N.; Guerreiro, P.; Rueff, J. DNA Repair and Resistance to Cancer Therapy. In New Research Directions in DNA Repair; Chen, C., Ed.; InTech: Rijeka, Croatia, 2013; ISBN 978-953-51-1114-6. [Google Scholar][Green Version]
- Fernandez, A.; Artola, M.; Leon, S.; Otegui, N.; Jimeno, A.; Serrano, D.; Calvo, A. Cancer Vulnerabilities Through Targeting the ATR/Chk1 and ATM/Chk2 Axes in the Context of DNA Damage. Cells 2025, 14, 748. [Google Scholar] [CrossRef]
- Kemp, M.G. DNA Damage-Induced ATM- and Rad-3-Related (ATR) Kinase Activation in Non-Replicating Cells Is Regulated by the XPB Subunit of Transcription Factor IIH (TFIIH). J. Biol. Chem. 2017, 292, 12424–12435. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.-H. Circadian Rhythm of NER and ATR Pathways. Biomolecules 2021, 11, 715. [Google Scholar] [CrossRef]
- Cheng, L.; Sturgis, E.M.; Eicher, S.A.; Spitz, M.R.; Wei, Q. Expression of Nucleotide Excision Repair Genes and the Risk for Squamous Cell Carcinoma of the Head and Neck. Cancer 2002, 94, 393–397. [Google Scholar] [CrossRef]
- Tian, Y.; Lin, X.; Yang, F.; Zhao, J.; Yao, K.; Bian, C. Contribution of Xeroderma Pigmentosum Complementation Group D Gene Polymorphisms in Breast and Ovarian Cancer Susceptibility: A Protocol for Systematic Review and Meta Analysis. Medicine 2020, 99, e20299. [Google Scholar] [CrossRef]
- Karahalil, B.; Bohr, V.A.; Wilson, D.M. Impact of DNA Polymorphisms in Key DNA Base Excision Repair Proteins on Cancer Risk. Hum. Exp. Toxicol. 2012, 31, 981–1005. [Google Scholar] [CrossRef]
- Wu, H.; Li, S.; Hu, X.; Qin, W.; Wang, Y.; Sun, T.; Wu, Z.; Wang, X.; Lu, S.; Xu, D.; et al. Associations of mRNA Expression of DNA Repair Genes and Genetic Polymorphisms with Cancer Risk: A Bioinformatics Analysis and Meta-Analysis. J. Cancer 2019, 10, 3593–3607. [Google Scholar] [CrossRef]
- Köberle, B.; Koch, B.; Fischer, B.M.; Hartwig, A. Single Nucleotide Polymorphisms in DNA Repair Genes and Putative Cancer Risk. Arch. Toxicol. 2016, 90, 2369–2388. [Google Scholar] [CrossRef]
- Wang, Q.; Peng, H.; Qi, X.; Wu, M.; Zhao, X. Targeted Therapies in Gynecological Cancers: A Comprehensive Review of Clinical Evidence. Signal Transduct. Target. Ther. 2020, 5, 137. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Nowsheen, S.; Deng, M. DNA Repair Deficiency Regulates Immunity Response in Cancers: Molecular Mechanism and Approaches for Combining Immunotherapy. Cancers 2023, 15, 1619. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, T.; Oda, T. DNA Damage Response in Multiple Myeloma: The Role of the Tumor Microenvironment. Cancers 2021, 13, 504. [Google Scholar] [CrossRef]
- Franzese, O.; Graziani, G. Role of PARP Inhibitors in Cancer Immunotherapy: Potential Friends to Immune Activating Molecules and Foes to Immune Checkpoints. Cancers 2022, 14, 5633. [Google Scholar] [CrossRef]
- Anadon, C.M.; Yu, X.; Hänggi, K.; Biswas, S.; Chaurio, R.A.; Martin, A.; Payne, K.K.; Mandal, G.; Innamarato, P.; Harro, C.M.; et al. Ovarian Cancer Immunogenicity Is Governed by a Narrow Subset of Progenitor Tissue-Resident Memory T Cells. Cancer Cell 2022, 40, 545–557.e13. [Google Scholar] [CrossRef]
- Chae, Y.K.; Anker, J.F.; Carneiro, B.A.; Chandra, S.; Kaplan, J.; Kalyan, A.; Santa-Maria, C.A.; Platanias, L.C.; Giles, F.J. Genomic Landscape of DNA Repair Genes in Cancer. Oncotarget 2016, 7, 23312–23321. [Google Scholar] [CrossRef] [PubMed]
- Samstein, R.M.; Krishna, C.; Ma, X.; Pei, X.; Lee, K.-W.; Makarov, V.; Kuo, F.; Chung, J.; Srivastava, R.M.; Purohit, T.A.; et al. Mutations in BRCA1 and BRCA2 Differentially Affect the Tumor Microenvironment and Response to Checkpoint Blockade Immunotherapy. Nat. Cancer 2021, 1, 1188–1203. [Google Scholar] [CrossRef]
- Kufel-Grabowska, J.; Wasąg, B. Diagnosis and Treatment of Patients with Breast Cancer and Mutation in the BRCA1/2 Genes. Oncol. Clin. Pract. 2024, 20, 222–228. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Z.; Li, Y.; Zhao, W.; Wu, J.; Zhang, Z. PD-1/PD-L1 Checkpoint Inhibitors in Tumor Immunotherapy. Front. Pharmacol. 2021, 12, 731798. [Google Scholar] [CrossRef]
- Santoro, A.; Angelico, G.; Inzani, F.; Arciuolo, D.; d’Amati, A.; Addante, F.; Travaglino, A.; Scaglione, G.; D’Alessandris, N.; Valente, M.; et al. The Emerging and Challenging Role of PD-L1 in Patients with Gynecological Cancers: An Updating Review with Clinico-Pathological Considerations. Gynecol. Oncol. 2024, 184, 57–66. [Google Scholar] [CrossRef]
- Feng, J.X.; Riddle, N.C. Epigenetics and Genome Stability. Mamm. Genome 2020, 31, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Lahtz, C.; Pfeifer, G.P. Epigenetic Changes of DNA Repair Genes in Cancer. J. Mol. Cell Biol. 2011, 3, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Karakaidos, P.; Karagiannis, D.; Rampias, T. Resolving DNA Damage: Epigenetic Regulation of DNA Repair. Molecules 2020, 25, 2496. [Google Scholar] [CrossRef]
- Reid, B.M.; Fridley, B.L. DNA Methylation in Ovarian Cancer Susceptibility. Cancers 2020, 13, 108. [Google Scholar] [CrossRef]
- Paweł, K.; Maria Małgorzata, S. CpG Island Methylator Phenotype—A Hope for the Future or a Road to Nowhere? Int. J. Mol. Sci. 2022, 23, 830. [Google Scholar] [CrossRef]
- Afifah, N.N.; Diantini, A.; Intania, R.; Abdulah, R.; Barliana, M.I. Genetic Polymorphisms and the Efficacy of Platinum-Based Chemotherapy: Review. Pharmacogenomics Pers. Med. 2020, 13, 427–444. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Sharma, V.; Nagpal, A.; Bhat, A.; Bhat, G.R.; Shah, R.; Wakhloo, A.; Suri, J.; Abrol, D.; Kaul, S.; et al. DNA Base Excision Repair Genes Variants Rs25487 (X-Ray Repair Cross-Complementing 1) and Rs1052133 (Human 8-Oxoguanine Glycosylase 1) with Susceptibility to Ovarian Cancer in the Population of the Jammu Region, India. J. Cancer Res. Ther. 2019, 15, 1270–1275. [Google Scholar] [CrossRef]
- Hartnett, L.; Egan, L.J. Inflammation, DNA Methylation and Colitis-Associated Cancer. Carcinogenesis 2012, 33, 723–731. [Google Scholar] [CrossRef]
- Gilchrist, J.J.; Fang, H.; Danielli, S.; Tomkova, M.; Nassiri, I.; Ng, E.; Tong, O.; Taylor, C.; Muldoon, D.; Cohen, L.R.Z.; et al. Characterization of the Genetic Determinants of Context-Specific DNA Methylation in Primary Monocytes. Cell Genom. 2024, 4, 100541. [Google Scholar] [CrossRef]
- Cai, L.; Zhan, M.; Li, Q.; Li, D.; Xu, Q. DNA Methyltransferase DNMT1 Inhibits Lipopolysaccharide-induced Inflammatory Response in Human Dental Pulp Cells Involving the Methylation Changes of IL-6 and TRAF6. Mol. Med. Rep. 2019, 21, 959–968. [Google Scholar] [CrossRef]
- Tan, S.Y.X.; Zhang, J.; Tee, W.-W. Epigenetic Regulation of Inflammatory Signaling and Inflammation-Induced Cancer. Front. Cell Dev. Biol. 2022, 10, 931493. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J. The Role of the DNA Methyltransferase Family and the Therapeutic Potential of DNMT Inhibitors in Tumor Treatment. Curr. Oncol. 2025, 32, 88. [Google Scholar] [CrossRef]
- Solt, L.A.; May, M.J. The IkappaB Kinase Complex: Master Regulator of NF-kappaB Signaling. Immunol. Res. 2008, 42, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Jin, W. Role of JAK/STAT3 Signaling in the Regulation of Metastasis, the Transition of Cancer Stem Cells, and Chemoresistance of Cancer by Epithelial-Mesenchymal Transition. Cells 2020, 9, 217. [Google Scholar] [CrossRef]
- Gong, F.; Miller, K.M. Histone Methylation and the DNA Damage Response. Mutat. Res./Rev. Mutat. Res. 2019, 780, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Kanekura, K.; Nishi, H.; Isaka, K.; Kuroda, M. MicroRNA and Gynecologic Cancers. J. Obstet. Gynaecol. 2016, 42, 612–617. [Google Scholar] [CrossRef]
- Miśkiewicz, J.; Mielczarek-Palacz, A.; Gola, J.M. MicroRNAs as Potential Biomarkers in Gynecological Cancers. Biomedicines 2023, 11, 1704. [Google Scholar] [CrossRef]
- Di Fiore, R.; Suleiman, S.; Pentimalli, F.; O’Toole, S.A.; O’Leary, J.J.; Ward, M.P.; Conlon, N.T.; Sabol, M.; Ozretić, P.; Erson-Bensan, A.E.; et al. Could MicroRNAs Be Useful Tools to Improve the Diagnosis and Treatment of Rare Gynecological Cancers? A Brief Overview. Int. J. Mol. Sci. 2021, 22, 3822. [Google Scholar] [CrossRef]
- Szatkowska, M.; Krupa, R. Regulation of DNA Damage Response and Homologous Recombination Repair by microRNA in Human Cells Exposed to Ionizing Radiation. Cancers 2020, 12, 1838. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Ahmad, A.; Zubair, H.; Miree, O.; Singh, S.; Rocconi, R.P.; Scalici, J.; Singh, A.P. MicroRNAs in Gynecological Cancers: Small Molecules with Big Implications. Cancer Lett. 2017, 407, 123–138. [Google Scholar] [CrossRef]
- Manikandan, M.; Munirajan, A.K. Single Nucleotide Polymorphisms in MicroRNA Binding Sites of Oncogenes: Implications in Cancer and Pharmacogenomics. OMICS J. Integr. Biol. 2014, 18, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.J.; Mishra, P.J.; Banerjee, D.; Bertino, J.R. MiRSNPs or MiR-Polymorphisms, New Players in microRNA Mediated Regulation of the Cell: Introducing microRNA Pharmacogenomics. Cell Cycle 2008, 7, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Palomba, G.; Atzori, F.; Budroni, M.; Ombra, M.; Cossu, A.; Sini, M.; Pusceddu, V.; Massidda, B.; Frau, B.; Notari, F.; et al. ERCC1 Polymorphisms as Prognostic Markers in T4 Breast Cancer Patients Treated with Platinum-Based Chemotherapy. J. Transl. Med. 2014, 12, 272. [Google Scholar] [CrossRef] [PubMed]
- Pardini, B.; Rosa, F.; Barone, E.; Di Gaetano, C.; Slyskova, J.; Novotny, J.; Levy, M.; Garritano, S.; Vodickova, L.; Buchler, T.; et al. Variation within 3′-UTRs of Base Excision Repair Genes and Response to Therapy in Colorectal Cancer Patients: A Potential Modulation of microRNAs Binding. Clin. Cancer Res. 2013, 19, 6044–6056. [Google Scholar] [CrossRef]
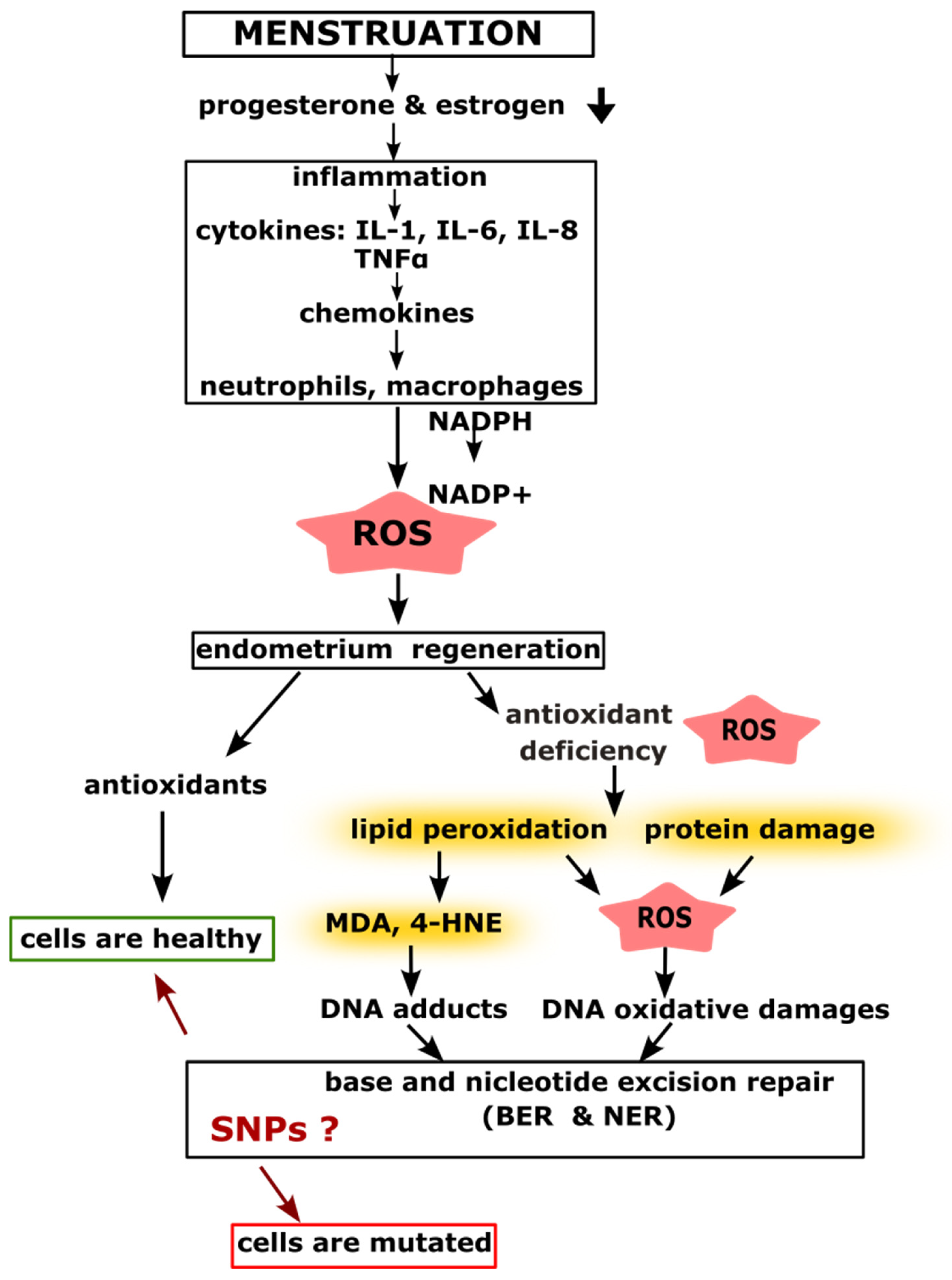


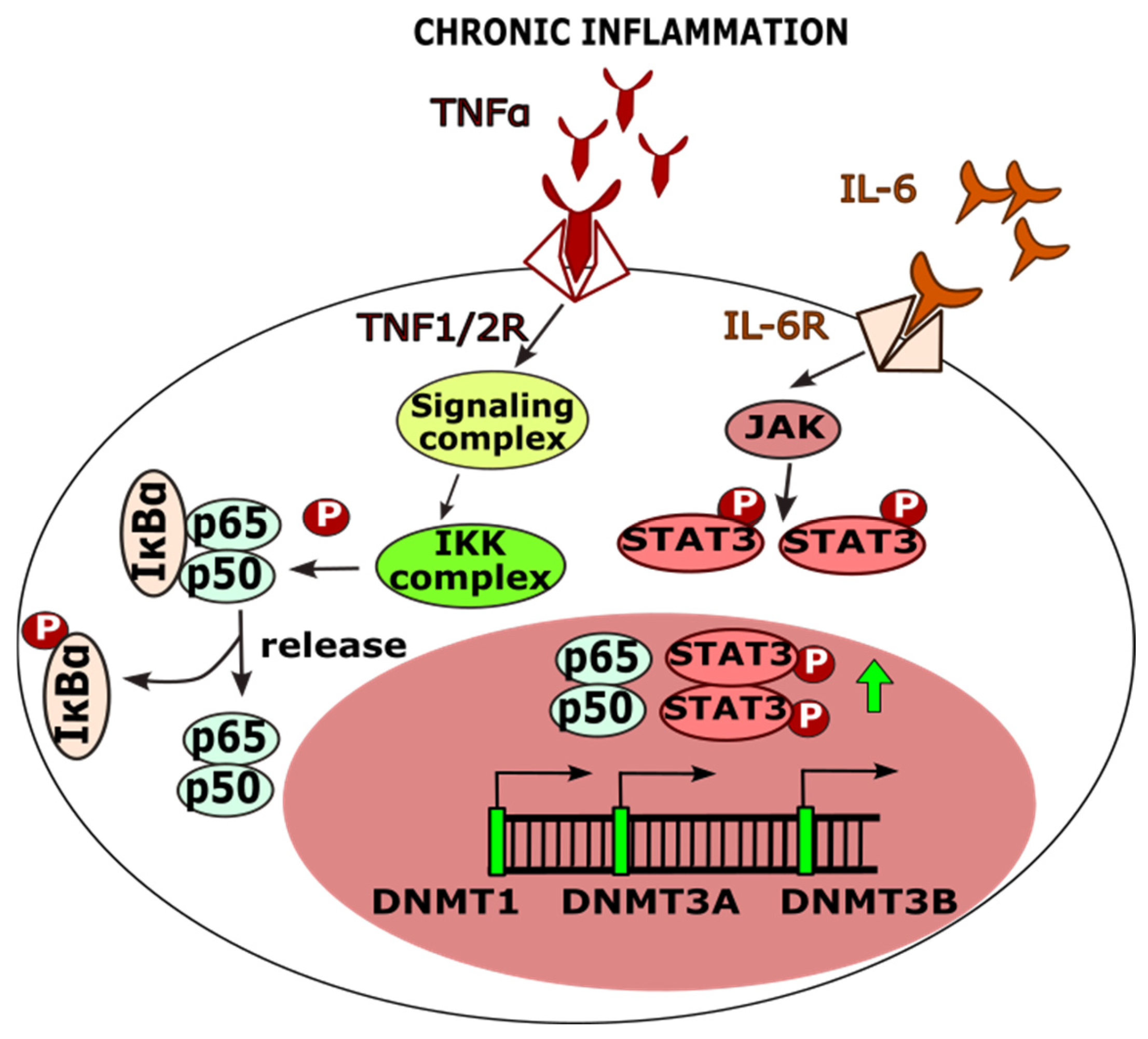
| Protein Name | db SNP ID | Number of Cases/Controls | Gynecological Cancer | References |
|---|---|---|---|---|
| XRCC1 | rs1799782 | 350/400 | cervical cancer | [60] |
| 530,000/260,000 | endometrial cancer | [61] | ||
| 525/265 | cervical cancer | [48] | ||
| rs25489; rs25487 | 400/400 | cervical cancer | [62] | |
| rs25487 | 510/510 | endometrial cancer | [63] | |
| 213/284 | endometrial cancer | [64] | ||
| 4028/4953 | cervical cancer | [16] | ||
| rs25486 | 300/300 | breast cancer | [65] | |
| XRCC3 | rs861539 | 5740/9931 | ovarian, cervical, and endometrial cancers | [66] |
| APE1 | rs1130409 | 176/177 | breast cancer | [59] |
| SMUG1 | rs3087404; rs2029167 | 400/1200 | cervical cancer | [67] |
| NEIL2 | rs804270; rs8191664 | 400/1200 | cervical cancer | [68] |
| UNG | rs246079 | 400/1200 | cervical cancer | [69] |
| rs293795 | 196/272 | ovarian cancer | [70] | |
| OGG1 | rs1052133 | 218/243 | endometrial cancer | [71] |
| 165/200 | breast cancer | [72] | ||
| 400/400 | cervical cancer | [62] | ||
| 25,446/41,106 | endometrial cancer | [73] | ||
| rs1052133 | 2712/3638 | breast, endometrial and ovarian cancer | [74] | |
| rs1052133 rs3764959 | 218/243 | endometrial cancer | [71] | |
| rs293795 | 196/272 | ovarian cancer | [70] | |
| PARP1 LIG3 | rs8679 rs4796030 | 196/272 | ovarian cancer | [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szatkowska, M.; Zdrada-Nowak, J. Genetic Polymorphisms in Base Excision Repair (BER) and Nucleotide Excision Repair (NER) Pathways as Potential Biomarkers for Gynecological Cancers: A Comprehensive Literature Review. Cancers 2025, 17, 2170. https://doi.org/10.3390/cancers17132170
Szatkowska M, Zdrada-Nowak J. Genetic Polymorphisms in Base Excision Repair (BER) and Nucleotide Excision Repair (NER) Pathways as Potential Biomarkers for Gynecological Cancers: A Comprehensive Literature Review. Cancers. 2025; 17(13):2170. https://doi.org/10.3390/cancers17132170
Chicago/Turabian StyleSzatkowska, Magdalena, and Julita Zdrada-Nowak. 2025. "Genetic Polymorphisms in Base Excision Repair (BER) and Nucleotide Excision Repair (NER) Pathways as Potential Biomarkers for Gynecological Cancers: A Comprehensive Literature Review" Cancers 17, no. 13: 2170. https://doi.org/10.3390/cancers17132170
APA StyleSzatkowska, M., & Zdrada-Nowak, J. (2025). Genetic Polymorphisms in Base Excision Repair (BER) and Nucleotide Excision Repair (NER) Pathways as Potential Biomarkers for Gynecological Cancers: A Comprehensive Literature Review. Cancers, 17(13), 2170. https://doi.org/10.3390/cancers17132170







