Correlation of CLDN18.2 and Tumor Microenvironment in Gastric Cancer: A Systematic Review
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. PICO Model
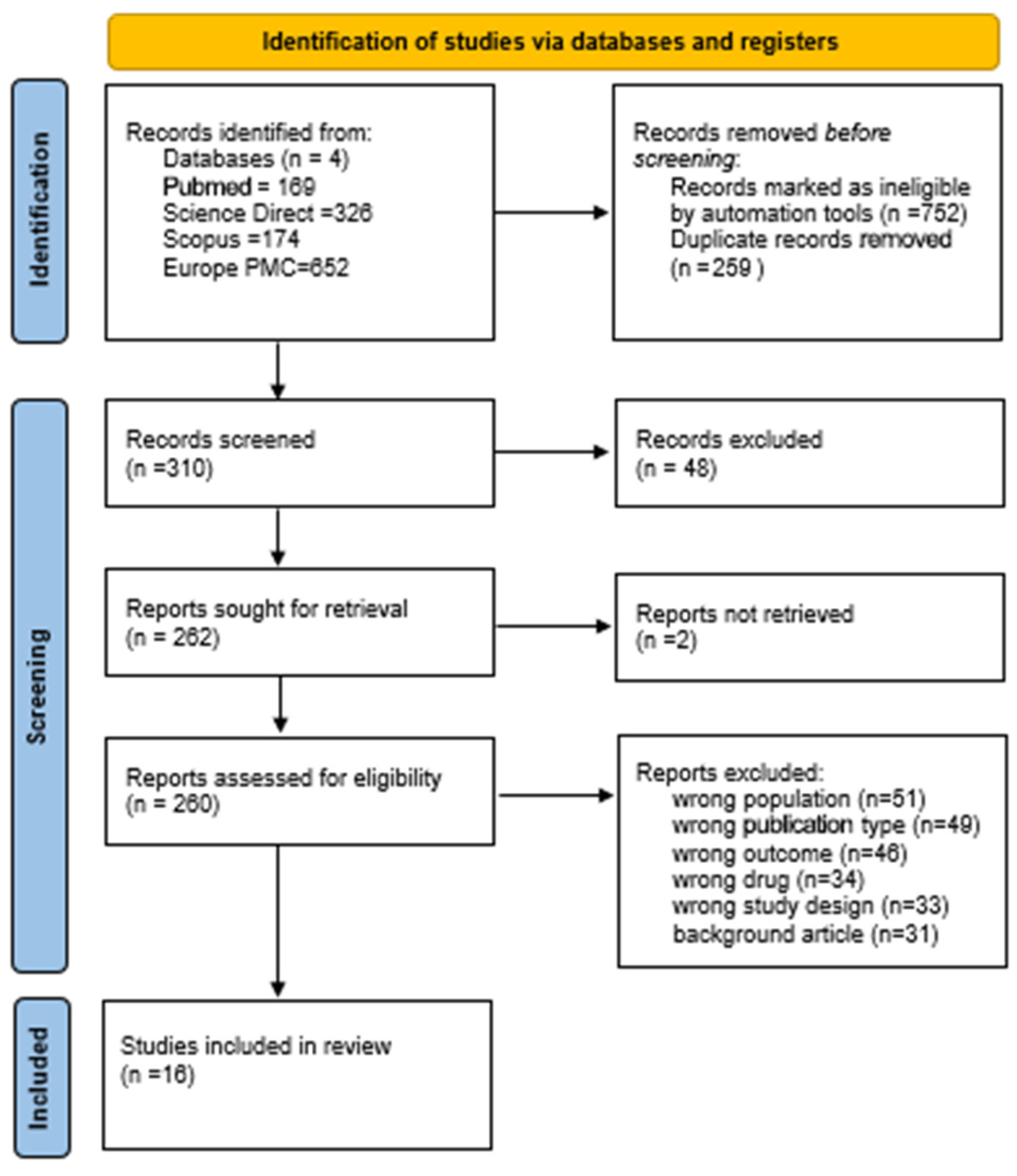
2.2. PRISMA
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to Build a Bridge from a Population-based to a More “Personalized” Approach to Cancer Staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Song, H.; Zhu, J.; Lu, D. Molecular-Targeted First-Line Therapy for Advanced Gastric Cancer. Cochrane Database Syst. Rev. 2016, 2016, CD011461. [Google Scholar] [CrossRef] [PubMed]
- Zaanan, A.; Bouche, O.; De La Fouchardiere, C.; Samalin-Scalzi, E.; Le Malicot, K.; Pernot, S.; Artru, P.; Lebrun, V.L.; Aldabbagh, K.; Akouz, F.K.; et al. LBA77 5-Fluorouracil and Oxaliplatin with or without Docetaxel in the First-Line Treatment of HER2 Negative Locally Advanced (LA) Unresectable or Metastatic Gastric or Gastro-Esophageal Junction (GEJ) Adenocarcinoma (GASTFOX-PRODIGE 51): A Randomized Phase III Trial Sponsored by the FFCD. Ann. Oncol. 2023, 34, S1318. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Ajani, J.A.; Moehler, M.; Shen, L.; Garrido, M.; Gallardo, C.; Wyrwicz, L.; Yamaguchi, K.; Cleary, J.M.; Elimova, E.; et al. First-Line Nivolumab Plus Chemotherapy for Advanced Gastric, Gastroesophageal Junction, and Esophageal Adenocarcinoma: 3-Year Follow-Up of the Phase III CheckMate 649 Trial. J. Clin. Oncol. 2024, 42, 2012–2020. [Google Scholar] [CrossRef] [PubMed]
- Rha, S.Y.; Oh, D.-Y.; Yañez, P.; Bai, Y.; Ryu, M.-H.; Lee, J.; Rivera, F.; Alves, G.V.; Garrido, M.; Shiu, K.-K.; et al. Pembrolizumab plus Chemotherapy versus Placebo plus Chemotherapy for HER2-Negative Advanced Gastric Cancer (KEYNOTE-859): A Multicentre, Randomised, Double-Blind, Phase 3 Trial. Lancet Oncol. 2023, 24, 1181–1195. [Google Scholar] [CrossRef]
- Qiu, M.-Z.; Oh, D.-Y.; Kato, K.; Arkenau, T.; Tabernero, J.; Correa, M.C.; Zimina, A.V.; Bai, Y.; Shi, J.; Lee, K.-W.; et al. Tislelizumab plus Chemotherapy versus Placebo plus Chemotherapy as First Line Treatment for Advanced Gastric or Gastro-Oesophageal Junction Adenocarcinoma: RATIONALE-305 Randomised, Double Blind, Phase 3 Trial. BMJ 2024, 385, e078876. [Google Scholar] [CrossRef]
- Lordick, F.; Kang, Y.-K.; Chung, H.-C.; Salman, P.; Oh, S.C.; Bodoky, G.; Kurteva, G.; Volovat, C.; Moiseyenko, V.M.; Gorbunova, V.; et al. Capecitabine and Cisplatin with or without Cetuximab for Patients with Previously Untreated Advanced Gastric Cancer (EXPAND): A Randomised, Open-Label Phase 3 Trial. Lancet Oncol. 2013, 14, 490–499. [Google Scholar] [CrossRef]
- Waddell, T.; Chau, I.; Cunningham, D.; Gonzalez, D.; Okines, A.F.C.; Wotherspoon, A.; Saffery, C.; Middleton, G.; Wadsley, J.; Ferry, D.; et al. Epirubicin, Oxaliplatin, and Capecitabine with or without Panitumumab for Patients with Previously Untreated Advanced Oesophagogastric Cancer (REAL3): A Randomised, Open-Label Phase 3 Trial. Lancet Oncol. 2013, 14, 481–489. [Google Scholar] [CrossRef]
- Ohtsu, A.; Shah, M.A.; Van Cutsem, E.; Rha, S.Y.; Sawaki, A.; Park, S.R.; Lim, H.Y.; Yamada, Y.; Wu, J.; Langer, B.; et al. Bevacizumab in Combination With Chemotherapy As First-Line Therapy in Advanced Gastric Cancer: A Randomized, Double-Blind, Placebo-Controlled Phase III Study. J. Clin. Oncol. 2011, 29, 3968–3976. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.S.; Tomasek, J.; Yong, C.J.; Dumitru, F.; Passalacqua, R.; Goswami, C.; Safran, H.; Dos Santos, L.V.; Aprile, G.; Ferry, D.R.; et al. Ramucirumab Monotherapy for Previously Treated Advanced Gastric or Gastro-Oesophageal Junction Adenocarcinoma (REGARD): An International, Randomised, Multicentre, Placebo-Controlled, Phase 3 Trial. Lancet 2014, 383, 31–39. [Google Scholar] [CrossRef]
- Kang, Y.-K.; Boku, N.; Satoh, T.; Ryu, M.-H.; Chao, Y.; Kato, K.; Chung, H.C.; Chen, J.-S.; Muro, K.; Kang, W.K.; et al. Nivolumab in Patients with Advanced Gastric or Gastro-Oesophageal Junction Cancer Refractory to, or Intolerant of, at Least Two Previous Chemotherapy Regimens (ONO-4538-12, ATTRACTION-2): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet 2017, 390, 2461–2471. [Google Scholar] [CrossRef]
- Ahn, S.; Kim, K.-M. PD-L1 Expression in Gastric Cancer: Interchangeability of 22C3 and 28-8 pharmDx Assays for Responses to Immunotherapy. Mod. Pathol. 2021, 34, 1719–1727. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.-J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in Combination with Chemotherapy versus Chemotherapy Alone for Treatment of HER2-Positive Advanced Gastric or Gastro-Oesophageal Junction Cancer (ToGA): A Phase 3, Open-Label, Randomised Controlled Trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Hollande, F.; Blanc, E.M.; Bali, J.P.; Whitehead, R.H.; Pelegrin, A.; Baldwin, G.S.; Choquet, A. HGF Regulates Tight Junctions in New Nontumorigenic Gastric Epithelial Cell Line. Am. J. Physiol.-Gastrointest. Liver Physiol. 2001, 280, G910–G921. [Google Scholar] [CrossRef]
- Zhu, G.; Foletti, D.; Liu, X.; Ding, S.; Witt, J.M.; Hasa-Moreno, A.; Rickert, M.; Holz, C.; Aschenbrenner, L.; Yang, A.H.; et al. Targeting CLDN18.2 by CD3 Bispecific and ADC Modalities for the Treatments of Gastric and Pancreatic Cancer. Sci. Rep. 2019, 9, 8420. [Google Scholar] [CrossRef]
- Xu, G.; Qian, N.; Liu, Y.; Li, H.; Yang, C.; Wang, J.; Wang, F.; Chen, L.; Bai, G.; Xu, Q.; et al. Preclinical Characterization of a Fab-like CD3/CLDN18.2 XFab® Bispecific Antibody against Solid Tumors. Immunobiology 2022, 227, 152283. [Google Scholar] [CrossRef]
- Ruan, D.-Y.; Liu, F.-R.; Wei, X.-L.; Luo, S.-X.; Zhuang, Z.-X.; Wang, Z.-N.; Liu, F.-N.; Zhang, Y.-Q.; Yang, J.-W.; Chen, Z.-D.; et al. Claudin 18.2-Targeting Antibody–Drug Conjugate CMG901 in Patients with Advanced Gastric or Gastro-Oesophageal Junction Cancer (KYM901): A Multicentre, Open-Label, Single-Arm, Phase 1 Trial. Lancet Oncol. 2025, 26, 227–238. [Google Scholar] [CrossRef]
- Brunner, J.; Ragupathy, S.; Borchard, G. Target Specific Tight Junction Modulators. Adv. Drug Deliv. Rev. 2021, 171, 266–288. [Google Scholar] [CrossRef]
- Schlingmann, B.; Molina, S.A.; Koval, M. Claudins: Gatekeepers of Lung Epithelial Function. Semin. Cell Dev. Biol. 2015, 42, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Turksen, K. Claudins and Cancer Stem Cells. Stem Cell Rev. Rep. 2011, 7, 797–798. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Fogg, V.C.; Margolis, B. Tight Junctions and Cell Polarity. Annu. Rev. Cell Dev. Biol. 2006, 22, 207–235. [Google Scholar] [CrossRef]
- Hashimoto, I.; Oshima, T. Claudins and Gastric Cancer: An Overview. Cancers 2022, 14, 290. [Google Scholar] [CrossRef] [PubMed]
- Zihni, C.; Mills, C.; Matter, K.; Balda, M.S. Tight Junctions: From Simple Barriers to Multifunctional Molecular Gates. Nat. Rev. Mol. Cell Biol. 2016, 17, 564–580. [Google Scholar] [CrossRef]
- Hayashi, D.; Tamura, A.; Tanaka, H.; Yamazaki, Y.; Watanabe, S.; Suzuki, K.; Suzuki, K.; Sentani, K.; Yasui, W.; Rakugi, H.; et al. Deficiency of Claudin-18 Causes Paracellular H+ Leakage, Up-Regulation of Interleukin-1β, and Atrophic Gastritis in Mice. Gastroenterology 2012, 142, 292–304. [Google Scholar] [CrossRef]
- Hagen, S.J. Unraveling a New Role for Claudins in Gastric Tumorigenesis. Cell. Mol. Gastroenterol. Hepatol. 2019, 8, 151–152. [Google Scholar] [CrossRef]
- Furuse, M.; Fujita, K.; Hiiragi, T.; Fujimoto, K.; Tsukita, S. Claudin-1 and -2: Novel Integral Membrane Proteins Localizing at Tight Junctions with No Sequence Similarity to Occludin. J. Cell Biol. 1998, 141, 1539–1550. [Google Scholar] [CrossRef]
- LaFemina, M.J.; Sutherland, K.M.; Bentley, T.; Gonzales, L.W.; Allen, L.; Chapin, C.J.; Rokkam, D.; Sweerus, K.A.; Dobbs, L.G.; Ballard, P.L.; et al. Claudin-18 Deficiency Results in Alveolar Barrier Dysfunction and Impaired Alveologenesis in Mice. Am. J. Respir. Cell Mol. Biol. 2014, 51, 550–558. [Google Scholar] [CrossRef]
- Li, G.; Flodby, P.; Luo, J.; Kage, H.; Sipos, A.; Gao, D.; Ji, Y.; Beard, L.L.; Marconett, C.N.; DeMaio, L.; et al. Knockout Mice Reveal Key Roles for Claudin 18 in Alveolar Barrier Properties and Fluid Homeostasis. Am. J. Respir. Cell Mol. Biol. 2014, 51, 210–222. [Google Scholar] [CrossRef]
- Niimi, T.; Nagashima, K.; Ward, J.M.; Minoo, P.; Zimonjic, D.B.; Popescu, N.C.; Kimura, S. Claudin-18, a Novel Downstream Target Gene for the T/EBP/NKX2.1 Homeodomain Transcription Factor, Encodes Lung- and Stomach-Specific Isoforms through Alternative Splicing. Mol. Cell. Biol. 2001, 21, 7380–7390. [Google Scholar] [CrossRef] [PubMed]
- Kyuno, D.; Zhao, K.; Bauer, N.; Ryschich, E.; Zöller, M. Therapeutic Targeting Cancer-Initiating Cell Markers by Exosome miRNA: Efficacy and Functional Consequences Exemplified for Claudin7 and EpCAM. Transl. Oncol. 2019, 12, 191–199. [Google Scholar] [CrossRef]
- Türeci, Ö.; Koslowski, M.; Helftenbein, G.; Castle, J.; Rohde, C.; Dhaene, K.; Seitz, G.; Sahin, U. Claudin-18 Gene Structure, Regulation, and Expression Is Evolutionary Conserved in Mammals. Gene 2011, 481, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Matsumoto, I.; Suzuki, K.; Tamura, A.; Shiraishi, A.; Kiyonari, H.; Kasamatsu, J.; Yamamoto, H.; Miyasaka, T.; Tanno, D.; et al. Deficiency of Lung-Specific Claudin-18 Leads to Aggravated Infection with Cryptococcus Deneoformans through Dysregulation of the Microenvironment in Lungs. Sci. Rep. 2021, 11, 21110. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Ding, L.; Lu, Z.; Lu, Q. The Claudin Family of Proteins in Human Malignancy: A Clinical Perspective. CMAR 2013, 5, 367–375. [Google Scholar] [CrossRef]
- Krause, G.; Winkler, L.; Mueller, S.L.; Haseloff, R.F.; Piontek, J.; Blasig, I.E. Structure and Function of Claudins. Biochim. Biophys. Acta (BBA)-Biomembr. 2008, 1778, 631–645. [Google Scholar] [CrossRef]
- Barbier, A.J.; Jiang, A.Y.; Zhang, P.; Wooster, R.; Anderson, D.G. The Clinical Progress of mRNA Vaccines and Immunotherapies. Nat. Biotechnol. 2022, 40, 840–854. [Google Scholar] [CrossRef]
- Sahin, U.; Koslowski, M.; Dhaene, K.; Usener, D.; Brandenburg, G.; Seitz, G.; Huber, C.; Türeci, Ö. Claudin-18 Splice Variant 2 Is a Pan-Cancer Target Suitable for Therapeutic Antibody Development. Clin. Cancer Res. 2008, 14, 7624–7634. [Google Scholar] [CrossRef]
- Shitara, K.; Lordick, F.; Bang, Y.-J.; Enzinger, P.; Ilson, D.; Shah, M.A.; Van Cutsem, E.; Xu, R.-H.; Aprile, G.; Xu, J.; et al. Zolbetuximab plus mFOLFOX6 in Patients with CLDN18.2-Positive, HER2-Negative, Untreated, Locally Advanced Unresectable or Metastatic Gastric or Gastro-Oesophageal Junction Adenocarcinoma (SPOTLIGHT): A Multicentre, Randomised, Double-Blind, Phase 3 Trial. Lancet 2023, 401, 1655–1668. [Google Scholar] [CrossRef]
- Yano, K.; Imaeda, T.; Niimi, T. Transcriptional Activation of the Human Claudin-18 Gene Promoter through Two AP-1 Motifs in PMA-Stimulated MKN45 Gastric Cancer Cells. Am. J. Physiol.-Gastrointest. Liver Physiol. 2008, 294, G336–G343. [Google Scholar] [CrossRef]
- Ito, T.; Kojima, T.; Yamaguchi, H.; Kyuno, D.; Kimura, Y.; Imamura, M.; Takasawa, A.; Murata, M.; Tanaka, S.; Hirata, K.; et al. Transcriptional Regulation of Claudin-18 via Specific Protein Kinase C Signaling Pathways and Modification of DNA Methylation in Human Pancreatic Cancer Cells. J. Cell. Biochem. 2011, 112, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Schuler, M.; Richly, H.; Bauer, S.; Krilova, A.; Dechow, T.; Jerling, M.; Utsch, M.; Rohde, C.; Dhaene, K.; et al. A Phase I Dose-Escalation Study of IMAB362 (Zolbetuximab) in Patients with Advanced Gastric and Gastro-Oesophageal Junction Cancer. Eur. J. Cancer 2018, 100, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Shah, M.A.; Lordick, F.; Van Cutsem, E.; Ilson, D.H.; Klempner, S.J.; Kang, Y.-K.; Lonardi, S.; Hung, Y.-P.; Yamaguchi, K.; et al. Zolbetuximab in Gastric or Gastroesophageal Junction Adenocarcinoma. N. Engl. J. Med. 2024, 391, 1159–1162. [Google Scholar] [CrossRef]
- Qi, C.; Gong, J.; Li, J.; Liu, D.; Qin, Y.; Ge, S.; Zhang, M.; Peng, Z.; Zhou, J.; Cao, Y.; et al. Claudin18.2-Specific CAR T Cells in Gastrointestinal Cancers: Phase 1 Trial Interim Results. Nat. Med. 2022, 28, 1189–1198. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Sun, W.; Lima, C.M.S.P.R.; Shah, S.; Scott, A.J.; Monga, D.K.; Kundranda, M.N.; Sher, A.F.; Gold, P.J.; Berlin, J.; et al. A Multi-Cohort Phase I/IIa Clinical Trial to Evaluate the Safety, Tolerability, and Pharmacokinetics of TST001 Administered as a Monotherapy, with Nivolumab or Standard of Care in Patients with Locally Advanced or Metastatic Solid Tumors: TransStar101. J. Clin. Oncol. 2023, 41, TPS4176. [Google Scholar] [CrossRef]
- Tao, D.; Guan, B.; Li, Z.; Jiao, M.; Zhou, C.; Li, H. Correlation of Claudin18.2 Expression with Clinicopathological Characteristics and Prognosis in Gastric Cancer. Pathol. Res. Pract. 2023, 248, 154699. [Google Scholar] [CrossRef]
- Kumar, V.; Ramnarayanan, K.; Sundar, R.; Padmanabhan, N.; Srivastava, S.; Koiwa, M.; Yasuda, T.; Koh, V.; Huang, K.K.; Tay, S.T.; et al. Single-Cell Atlas of Lineage States, Tumor Microenvironment, and Subtype-Specific Expression Programs in Gastric Cancer. Cancer Discov. 2022, 12, 670–691. [Google Scholar] [CrossRef]
- Zeng, D.; Li, M.; Zhou, R.; Zhang, J.; Sun, H.; Shi, M.; Bin, J.; Liao, Y.; Rao, J.; Liao, W. Tumor Microenvironment Characterization in Gastric Cancer Identifies Prognostic and Immunotherapeutically Relevant Gene Signatures. Cancer Immunol. Res. 2019, 7, 737–750. [Google Scholar] [CrossRef]
- Kayikcioglu, E.; Yüceer, R.O.; Cetin, B.; Yüceer, K.; Karahan, N. Prognostic Value of Claudin 18.2 Expression in Gastric Adenocarcinoma. World J. Gastrointest. Oncol. 2023, 15, 343–351. [Google Scholar] [CrossRef]
- Hong, J.Y.; An, J.Y.; Lee, J.; Park, S.H.; Park, J.O.; Park, Y.S.; Lim, H.Y.; Kim, K.-M.; Kang, W.K.; Kim, S.T. Claudin 18.2 Expression in Various Tumor Types and Its Role as a Potential Target in Advanced Gastric Cancer. Transl. Cancer Res. TCR 2020, 9, 3367–3374. [Google Scholar] [CrossRef]
- Baek, J.H.; Park, D.J.; Kim, G.Y.; Cheon, J.; Kang, B.W.; Cha, H.J.; Kim, J.G. Clinical Implications of Claudin18.2 Expression in Patients With Gastric Cancer. Anticancer. Res. 2019, 39, 6973–6979. [Google Scholar] [CrossRef]
- Dai, J.; Zheng, H.; Jin, J.; Cheng, Y.; Xu, H. Claudin18.2 Expression and Clinicopathological Features in Cytology Effusion Specimens from Gastric Adenocarcinoma: A Comparative Study with Tissue Specimens. Cancer Cytopathol. 2023, 131, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, Y.; Chen, J.; Wang, Y.; Pang, C.; Liang, C.; Yuan, L.; Ma, Y. CLDN18.2 Expression and Its Impact on Prognosis and the Immune Microenvironment in Gastric Cancer. BMC Gastroenterol. 2023, 23, 283. [Google Scholar] [CrossRef] [PubMed]
- Jia, K.; Chen, Y.; Sun, Y.; Hu, Y.; Jiao, L.; Ma, J.; Yuan, J.; Qi, C.; Li, Y.; Gong, J.; et al. Multiplex Immunohistochemistry Defines the Tumor Immune Microenvironment and Immunotherapeutic Outcome in CLDN18.2-Positive Gastric Cancer. BMC Med. 2022, 20, 223. [Google Scholar] [CrossRef]
- Kim, T.-Y.; Kwak, Y.; Nam, S.K.; Han, D.; Oh, D.-Y.; Im, S.-A.; Lee, H.S. Clinicopathological Analysis of Claudin 18.2 Focusing on Intratumoral Heterogeneity and Survival in Patients with Metastatic or Unresectable Gastric Cancer. ESMO Open 2024, 9, 104000. [Google Scholar] [CrossRef]
- Wu, J.; Lu, J.; Chen, Q.; Chen, H.; Zheng, Y.; Cheng, M. Pan-Cancer Analysis of CLDN18.2 Shed New Insights on the Targeted Therapy of Upper Gastrointestinal Tract Cancers. Front. Pharmacol. 2024, 15, 1494131. [Google Scholar] [CrossRef]
- Kubota, Y.; Kawazoe, A.; Mishima, S.; Nakamura, Y.; Kotani, D.; Kuboki, Y.; Bando, H.; Kojima, T.; Doi, T.; Yoshino, T.; et al. Comprehensive Clinical and Molecular Characterization of Claudin 18.2 Expression in Advanced Gastric or Gastroesophageal Junction Cancer. ESMO Open 2023, 8, 100762. [Google Scholar] [CrossRef]
- Hossain, M.A. A Comprehensive Review of Immune Checkpoint Inhibitors for Cancer Treatment. Int. Immunopharmacol. 2024, 143, 113365. [Google Scholar] [CrossRef]
- Matsuishi, A.; Nakajima, S.; Saito, M.; Saito, K.; Fukai, S.; Tsumuraya, H.; Kanoda, R.; Kikuchi, T.; Nirei, A.; Kaneta, A.; et al. The Impact of CLDN18.2 Expression on Effector Cells Mediating Antibody-Dependent Cellular Cytotoxicity in Gastric Cancer. Sci. Rep. 2024, 14, 17916. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Z.; Jiang, L.; Zhang, M.; Zhang, C.; Shen, L. Claudin-18.2 Mediated Interaction of Gastric Cancer Cells and Cancer-Associated Fibroblasts Drives Tumor Progression. Cell Commun. Signal 2024, 22, 27. [Google Scholar] [CrossRef]
- Ogawa, H.; Abe, H.; Yagi, K.; Seto, Y.; Ushiku, T. Claudin-18 Status and Its Correlation with HER2 and PD-L1 Expression in Gastric Cancer with Peritoneal Dissemination. Gastric Cancer 2024, 27, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Pellino, A.; Brignola, S.; Riello, E.; Niero, M.; Murgioni, S.; Guido, M.; Nappo, F.; Businello, G.; Sbaraglia, M.; Bergamo, F.; et al. Association of CLDN18 Protein Expression with Clinicopathological Features and Prognosis in Advanced Gastric and Gastroesophageal Junction Adenocarcinomas. J. Pers. Med. 2021, 11, 1095. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Chong, X.; Zhou, T.; Ma, M.; Gong, J.; Zhang, M.; Li, J.; Xiao, J.; Peng, X.; Liu, Z.; et al. Clinicopathological Significance and Immunotherapeutic Outcome of Claudin 18.2 Expression in Advanced Gastric Cancer: A Retrospective Study. Chin. J. Cancer Res. 2024, 36, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Waters, R.; Sewastjanow-Silva, M.; Yamashita, K.; Abdelhakeem, A.; Iwata, K.K.; Moran, D.; Elsouda, D.; Guerrero, A.; Pizzi, M.; Vicentini, E.R.; et al. Retrospective Study of Claudin 18 Isoform 2 Prevalence and Prognostic Association in Gastric and Gastroesophageal Junction Adenocarcinoma. JCO Precis. Oncol. 2024, 8, e2300543. [Google Scholar] [CrossRef]
- Kwak, Y.; Kim, T.-Y.; Nam, S.K.; Hwang, H.J.; Han, D.; Oh, H.J.; Kong, S.-H.; Park, D.J.; Oh, D.-Y.; Lee, H.-J.; et al. Clinicopathologic and Molecular Characterization of Stages II-IV Gastric Cancer with Claudin 18.2 Expression. Oncologist 2025, 30, oyae238. [Google Scholar] [CrossRef]
- Mousavi, P.; Ahmadi, A.; Behzadifar, S.; Mohammadnejad, J.; Mohammad Hosseini, S. New Approaches in Gastric Cancer Immunotherapy. In Gastric Cancer—Progress and Challenges in the Era of Precision Medicine; Cornelia Lazar, D., Ed.; IntechOpen: Rijeka, Croatia, 2024; ISBN 978-0-85466-443-6. [Google Scholar]
- Zhang, D.; He, W.; Wu, C.; Tan, Y.; He, Y.; Xu, B.; Chen, L.; Li, Q.; Jiang, J. Scoring System for Tumor-Infiltrating Lymphocytes and Its Prognostic Value for Gastric Cancer. Front. Immunol. 2019, 10, 71. [Google Scholar] [CrossRef]
- Park, Y.; Seo, A.N.; Koh, J.; Nam, S.K.; Kwak, Y.; Ahn, S.-H.; Park, D.J.; Kim, H.-H.; Lee, H.S. Expression of the Immune Checkpoint Receptors PD-1, LAG3, and TIM3 in the Immune Context of Stage II and III Gastric Cancer by Using Single and Chromogenic Multiplex Immunohistochemistry. OncoImmunology 2021, 10, 1954761. [Google Scholar] [CrossRef]
- Nakazawa, T.; Tanaka, H.; Kikuchi, A.; Rashid, R.; Avery, K.N.; Qi, J.; Nisthal, A.; Shimazaki, M.; Shirasuna, K. Abstract 2962: ASP2138, a Novel 2+1 Format, Claudin 18.2 x CD3 Bispecific Antibody, Demonstrates Selectivity and Activity in Preclinical Cancer Models. Cancer Res. 2023, 83, 2962. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, Y.; Peng, L.; Chen, N.; Chen, W.; Lv, Y.; Mao, F.; Zhang, J.; Cheng, P.; Teng, Y.; et al. Tumour-Activated Neutrophils in Gastric Cancer Foster Immune Suppression and Disease Progression through GM-CSF-PD-L1 Pathway. Gut 2017, 66, 1900–1911. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, H.; Yuan, X.; Jiang, P.; Qian, H.; Xu, W. Tumor-Derived Exosomes Induce N2 Polarization of Neutrophils to Promote Gastric Cancer Cell Migration. Mol. Cancer 2018, 17, 146. [Google Scholar] [CrossRef] [PubMed]
- de Moraes, F.C.A.; Sobreira, L.E.R.; Souza, M.E.C.; Burbano, R.M.R. The Role of CLDN18.2 in Gastric Cancer Prognosis: A Systematic Review and Meta-Analysis. Biomarkers 2024, 29, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Mao, F.; Kong, H.; Zhao, Y.; Peng, L.; Chen, W.; Zhang, J.; Cheng, P.; Wang, T.; Lv, Y.; Teng, Y.; et al. Increased Tumor-Infiltrating CD45RA−CCR7− Regulatory T-Cell Subset with Immunosuppressive Properties Foster Gastric Cancer Progress. Cell Death Dis. 2017, 8, e3002. [Google Scholar] [CrossRef] [PubMed]
- Kindlund, B.; Sjöling, Å.; Yakkala, C.; Adamsson, J.; Janzon, A.; Hansson, L.-E.; Hermansson, M.; Janson, P.; Winqvist, O.; Lundin, S.B. CD4+ Regulatory T Cells in Gastric Cancer Mucosa Are Proliferating and Express High Levels of IL-10 but Little TGF-β. Gastric Cancer 2017, 20, 116–125. [Google Scholar] [CrossRef]
- Qu, Y.; Wang, X.; Bai, S.; Niu, L.; Zhao, G.; Yao, Y.; Li, B.; Li, H. The Effects of TNF-α/TNFR2 in Regulatory T Cells on the Microenvironment and Progression of Gastric Cancer. Int. J. Cancer 2022, 150, 1373–1391. [Google Scholar] [CrossRef]
- Pernot, S.; Terme, M.; Radosevic-Robin, N.; Castan, F.; Badoual, C.; Marcheteau, E.; Penault-Llorca, F.; Bouche, O.; Bennouna, J.; Francois, E.; et al. Infiltrating and Peripheral Immune Cell Analysis in Advanced Gastric Cancer According to the Lauren Classification and Its Prognostic Significance. Gastric Cancer 2020, 23, 73–81. [Google Scholar] [CrossRef]
- Ishigami, S.; Natsugoe, S.; Tokuda, K.; Nakajo, A.; Che, X.; Iwashige, H.; Aridome, K.; Hokita, S.; Aikou, T. Prognostic Value of Intratumoral Natural Killer Cells in Gastric Carcinoma. Cancer 2000, 88, 577–583. [Google Scholar] [CrossRef]
- Gambardella, V.; Castillo, J.; Tarazona, N.; Gimeno-Valiente, F.; Martínez-Ciarpaglini, C.; Cabeza-Segura, M.; Roselló, S.; Roda, D.; Huerta, M.; Cervantes, A.; et al. The Role of Tumor-Associated Macrophages in Gastric Cancer Development and Their Potential as a Therapeutic Target. Cancer Treat. Rev. 2020, 86, 102015. [Google Scholar] [CrossRef]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-Associated Macrophages as Treatment Targets in Oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, C.; Zhang, R.; Xu, J.; Zhang, Y.; Yuan, L.; Zhang, S.; Pan, S.; Cao, M.; Qin, J.; et al. The Role of Macrophages in Gastric Cancer. Front. Immunol. 2023, 14, 1282176. [Google Scholar] [CrossRef]
- Zhu, Q.; Wu, X.; Tang, M.; Wu, L. Observation of Tumor-Associated Macrophages Expression in Gastric Cancer and Its Clinical Pathological Relationship. Medicine 2020, 99, e19839. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. Type 2 Cytokines: Mechanisms and Therapeutic Strategies. Nat. Rev. Immunol. 2015, 15, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S. Alternative Activation of Macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef] [PubMed]
| P | Patients with gastric cancer not receiving anti-CLDN18.2 therapy |
| I | Assessment of CLDN18.2 expression levels using immunohistochemistry (IHC), quantitative PCR, or other molecular techniques. |
| C | Tumor microenvironment components |
| O | Establish the correlation between CLDN18.2 expression and components of tumor microenvironment |
| Study | Way of Assesment | Tumor Immune Microenvironment | Immune Checkpoints |
|---|---|---|---|
| Wang et al., 2023 [54] | IHC (H-score ≥ 6) TIME, TIMER database | ↑ CD8+ T cells (p = 0.021); CD3 (p = 0.05), B cells (p < 0.001) | No correlation with Foxp3 Positive correlation with PD-L1 |
| CD4 T cells (p = 0.6), B cells (p = 0.112) | |||
| Jia et al., 2022 [55] | IHC (≥2, ≥40%) TCGA database | ↑ CD8+ T cells (p = 0.023), Non-depleted CD8+ T cells (p < 0.05), CD4 T cells (p = 0.045), effector CD4 T cells (p = 0.026), Neutrophils (p = 0.031) | No correlation with PD-L1 |
| - Depleted CD8 T cells (p = 0.71), Tregs (p = 0.47), B cells (p = 0.25), M1 (p = 0.5), M2 (p = 0.71) | |||
| Kubota et al., 2023 [58] | IHC (>2, ≥75%) | ↑ Macrophages (p = 0.037) | No correlation with PD-L1 |
| - CD8+ T cells (p = 0.808), CD56 (p = 0.789), CD3 (p = 0.457) | |||
| ↓ CD16 | |||
| Matsuishi et al., 2024 [60] | IHC (>2, ≥75%) | NK cells (CD16, CD56, CD56dimCD16+, CD56brightCD16-), Monocytes (classical, intermediate, non-classical), Macrophages | No correlation with PD-L1(CPS: 1) Positive correlation with PD-L1(CPS: 5) |
| Liu et al., 2024 [61] | ↑ CAFs (p < 0.01) | No | |
| Tao et al., 2023 [46] | TCGA database | ↑ CD8+ T cells (p < 0.01), Th17 (p < 0.01), aDC (p < 0.01), iDC (p < 0.001), Mast cells (p < 0.001), Neutrophils (p < 0.05). | No |
| ↓ Tγδ (p < 0.05), NK cells (p < 0.05), MDSC (p < 0.001) | |||
| - Tfh, Th1, Th2, Treg, B cells, Macrophages | |||
| Ogawa et al., 2024 [62] | IHC (≥2, >40%) | No | No correlation with PD-L1 |
| Pellino et al., 2021 [63] | IHC (>0, >0% and >2, >75%) | No | No correlation with PD-L1 (CPS: 1, CPS: 5) |
| Qi et al., 2024 [64] | IHC (>2, >40%) | No | Negative correlation with PD-L1 (CPS: 1, CPS: 5, CPS: 10) |
| Waters et al., 2024 [65] | IHC (>2, >50% and >75%) | No | No correlation with PD-L1(CPS > 1) |
| Kwak et al., 2025 [66] | IHC (>2, >75%) | No | Positive correlation with PD-L1 (CPS: 5) No correlation with PD-L1 (CPS: 1, CPS: 10) |
| Wu et al., 2024 [57] | AuCell (Xcell markers) | ↓ NK, CD4+ T cells, Th2 cells, CD8+ T cells, CD4+ memory T cells, Th1 cells | No |
| ↑ Macrophages, NKT, Macrophages M2, Eosinophils | |||
| Kim et al., 2025 [56] | IHC (>2, >75%) | ↑ CD8+ T cells (center) (p = 0.041), CD4 T cells (periphery) (p = 0.04), CD3 (periphery) (p = 0.009) | No correlation with PD-L1 (CPS: 1, CPS: 5, CPS: 10) |
| - CD8+ T cells (periphery) (p = 0.329), Foxp3 cells (center (p = 0.158) or periphery (p = 0.950)), CD4 (center) (p = 0.202) CD3 (center) (p = 0.140) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarampouka, K.; Tsiantas, C.; Stavropoulou, M.A.; Efthymiadis, K.; Theotokis, P.; Gargani, S.; Vrettou, E.; Koletsa, T.; Manthou, M.E.; Meditskou, S. Correlation of CLDN18.2 and Tumor Microenvironment in Gastric Cancer: A Systematic Review. Cancers 2025, 17, 2120. https://doi.org/10.3390/cancers17132120
Zarampouka K, Tsiantas C, Stavropoulou MA, Efthymiadis K, Theotokis P, Gargani S, Vrettou E, Koletsa T, Manthou ME, Meditskou S. Correlation of CLDN18.2 and Tumor Microenvironment in Gastric Cancer: A Systematic Review. Cancers. 2025; 17(13):2120. https://doi.org/10.3390/cancers17132120
Chicago/Turabian StyleZarampouka, Katerina, Christos Tsiantas, Maria Athanasia Stavropoulou, Konstantinos Efthymiadis, Paschalis Theotokis, Sofia Gargani, Eleni Vrettou, Triantafyllia Koletsa, Maria Eleni Manthou, and Soultana Meditskou. 2025. "Correlation of CLDN18.2 and Tumor Microenvironment in Gastric Cancer: A Systematic Review" Cancers 17, no. 13: 2120. https://doi.org/10.3390/cancers17132120
APA StyleZarampouka, K., Tsiantas, C., Stavropoulou, M. A., Efthymiadis, K., Theotokis, P., Gargani, S., Vrettou, E., Koletsa, T., Manthou, M. E., & Meditskou, S. (2025). Correlation of CLDN18.2 and Tumor Microenvironment in Gastric Cancer: A Systematic Review. Cancers, 17(13), 2120. https://doi.org/10.3390/cancers17132120









