Screening in Gastrointestinal Malignancies—Recent Trials and Advancements
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
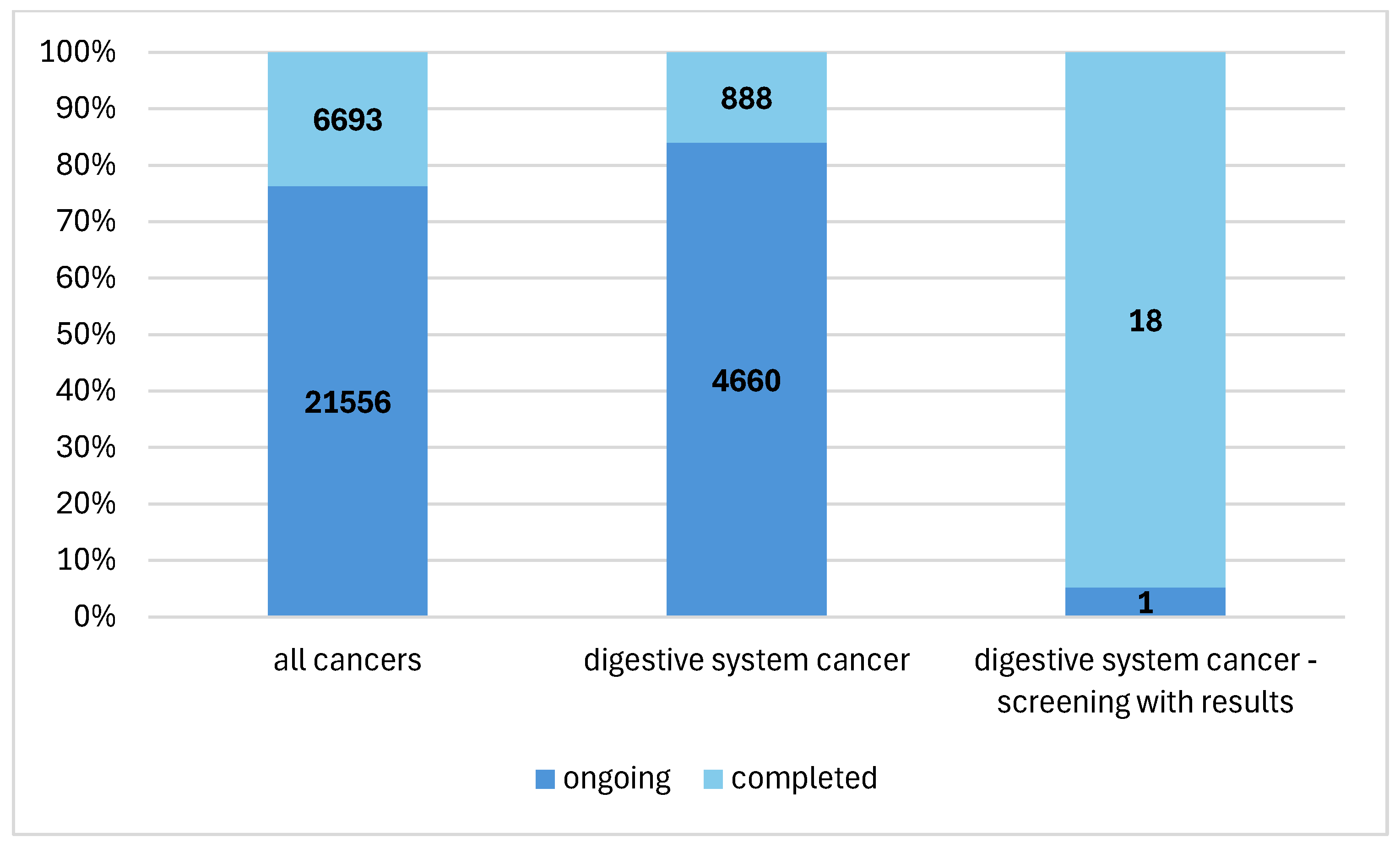
3. Results
3.1. Behavioral Interventions
3.2. Diagnosing
4. Discussion
| No. | Group | Title | n | Status | Study | Population | Effect Strength |
|---|---|---|---|---|---|---|---|
| 1. | Interventions | Paired Promotion of Colorectal Cancer and Social Determinants of Health Screening [10] | 26 | completed | interventional | staff of community health centers | performance of colorectal cancer screening COR = 0.068 for the intervention analyzed, COR = 0.028 for the standard procedure |
| 2. | behavioral | Multilevel Intervention Based on Colorectal Cancer (CRC) and Cervical Cancer Self-screening in Rural, Segregated Areas [11] | 48 | completed | interventional | women 50–65 years old | 75% of patients who performed an immunochemical test using the kit they received vs. 1% of patients who underwent testing only upon recommendation |
| 3. | Scaling CRC Screening Through Outreach, Referral, and Engagement (SCORE) [12] | 4318 | completed | interventional | persons 45–75 years old | 29.9% vs. 9.6% of patients who participated in the diagnosis following the intervention in the 50–75 years group; 33.5% vs. 28.5% of patients who participated in the diagnosis following the intervention in the 45–49 years group; | |
| 4. | Mailed FIT Outreach 2022 [13] | 5460 | completed | interventional | adults 50–74 years old | 21.2% and 20.3% of patients who participated in the screening after reminders were sent electronically or by letter vs. 14.6–18.0% of patients who participated in the screening despite not receiving reminders | |
| 5. | Effectiveness and Implementation of mPATH-CRC [14] | 77,145 | completed | interventional | primary healthcare professionals, patients aged 18 and over, patients 50–74 years old with indications of a colorectal cancer screening | 0.9% of patients who completed a higher intensity program vs. 1.0% of patients who completed a lower intensity program | |
| 6. | Engaging Patients in Colon Cancer Screening Decisions During COVID-19 [15] | 800 | completed | interventional | adults 45–75 years old | 35.1% of patients who underwent colonoscopy in the shared decision-making group vs. 22.8% in the control group. | |
| 7. | Helping Patients and Providers Make Better Decisions About Colorectal Cancer Screening [16] | 1111 | completed | interventional | adults 50–75 years old | 35.1% of individuals who made a screening decision in the group of patients that received personalized screening messages vs. 31.0% of those who made a screening decision in the group of patients that only received information material | |
| 8. | Evaluating the Shared Decision Making Process Scale in Cancer Screening Decisions [17] | 240 | completed | observational | adults 35–80 years old | Variation in mean values on the decision participation scale between the colorectal diagnosis group and the prostate cancer diagnosis group with Cohen’s effect strength d = 0.90 | |
| 9. | Test Up Now Education Programme [19] | 115 | completed | interventional | adults of African-American descent 45–64 years old | 64.4% of patients in the group who met with the counsellor completed the study vs. 64.6% of patients in the control group | |
| 10. | e-Motivación: Developing and Pilot Testing an App to Improve Latinos’ Screening Colonoscopy Rates [20] | 42 | completed | interventional | adults of Latin-American descent | 86.4% of patients who presented for colonoscopy in the intervention group vs. 95.0% in the control group | |
| 11. | Audio and Video Brochures for Increasing Colorectal Cancer Screening Among Adults Living in Appalachia [21] | 94 | completed | interventional | adults aged 50–64 residing in remote, hard-to-reach mountain towns | 28.1% of those who took the immunochemical test supplied with video materials, 9.5% of those who took the test provided with audio materials, 15.0% of those who took the test without additional materials | |
| 12. | Virtual Human Delivered Nutrition Module for Colorectal Cancer Prevention [22] | 139 | completed | interventional | adults aged 45–73 | Variation in mean values on the intention to take part in diagnostic tests scale between the group using the website and the group using the virtual assistant with a minimal effect strength—Cohen’s d = 0.13. | |
| 13. | Health Service Intervention for the Improvement of Access and Adherence to Colorectal Cancer Screening [23] | 183 | completed | interventional | adults aged 50–75 | 84.0% of patients rated the materials and assistance in performing an immunochemical test or colonoscopy as helpful | |
| 14. | The Prevent Anal Cancer Self-Swab Study [24] | 253 | completed | interventional | persons aged 25 or more, men who had anal sex with men | 65.0% of men who received a home HPV test performed the test (vs. 52.5% of men who performed the HPV test in a medical facility); 63.4% of men who could perform the HPV test in a medical facility (vs. 51.2% of men who received the test at home) reported for an anoscopy | |
| 15. | Diagnosing | The Prevent Anal Cancer Palpation Study [25] | 718 | completed | interventional | persons aged 25 or more, men who had anal sex with men | concordance between the findings on palpation performed by the patients post-training and that performed by a medical professional of at least 95% and concordance between the findings on palpation performed by the patients’ partners post-training and that performed by a medical professional of at least 88.5% |
| 16. | Patient Acceptance and Preference Among Screening Modalities for Detection of Barrett’s Esophagus [26] | 24 | completed | interventional | adults with gastroesophageal reflux disease or Barrett’s Esophagus | Variation in mean values on the pain analogue scale (VAS) between the group in which transnasal endoscopy was performed compared with the group in which cytosponge was utilized and the group in which traditional gastroscopy was performed with a very high Cohen’s d strength, d = 1.51 and d = 2.01, respectively. Percentage of patients willing to repeat the examination in the future: 87.5% in the cohort where transnasal endoscopy was performed and 100% in the other two groups | |
| 17. | Clinical Outcomes for Offering Genetic Testing in a Tiered Approach [27] | 6 | completed | interventional | oncologists | post-training 25/48 patients were correctly referred, pre-training 29/100 patients | |
| 18. | Endoscopic Optical Coherence Tomography for Screening and Diagnosis of Colorectal Precancerous and Malignant Polyps [28] | 36 | ongoing | interventional | adults aged 40 or more | the concordance between OCT-guided colonoscopy and biopsy results measured by Cohen’s k coefficient was high at =0.85. | |
| 19. | Colorectal Cancer and Pre-Cancerous Adenoma Non-Invasive Detection Test Study [29] | 14,263 | completed | interventional | adults aged 45 or more | The sensitivity of the test analyzed against colonoscopy results was 92.6%. |
5. Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2024; Available online: https://gco.iarc.who.int/today (accessed on 20 November 2024).
- ECIS—European Cancer Information System. Available online: https://ecis.jrc.ec.europa.eu/explorer.php?$0-2$1-POOL$2-All$4-1,2$3-12,16,9,13,10$6-0,14$5-2000,2007$7-1$CRelativeSurvivalAgeGroup$X0_14-$X0_15-RSC$CRelativeSurvivalFollow$X1_14-$X1_-1-$X1_15-RSC (accessed on 15 December 2024).
- American Cancer Society. Colorectal Cancer Risk Factors. Available online: https://www.cancer.org/cancer/types/colon-rectal-cancer/causes-risks-prevention/risk-factors.html (accessed on 15 December 2024).
- Tran, T.H.; Myung, S.-K.; Trinh, T.T.K. Proton pump inhibitors and risk of gastrointestinal cancer: A meta-analysis of cohort studies. Oncol. Lett. 2024, 27, 28. [Google Scholar] [CrossRef] [PubMed]
- US Preventive Services Task Force. Colorectal Cancer: Screening. Available online: https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening (accessed on 10 December 2024).
- Nishihara, R.; Wu, K.; Lochhead, P.; Morikawa, T.; Liao, X.; Qian, Z.R.; Inamura, K.; Kim, S.A.; Kuchiba, A.; Yamauchi, M.; et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N. Engl. J. Med. 2013, 369, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Atkin, W.; Wooldrage, K.; Parkin, D.M.; Kralj-Hans, I.; MacRae, E.; Shah, U.; Duffy, S.; Cross, A.J. Long term effects of once-only flexible sigmoidoscopy screening after 17 years of follow-up: The UK Flexible Sigmoidoscopy Screening randomised controlled trial. Lancet 2017, 389, 1299–1311. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.M.; Chen, S.L.; Yen, A.M.; Chiu, S.Y.; Fann, J.C.; Lee, Y.C.; Pan, S.L.; Wu, M.S.; Liao, C.S.; Chen, H.H.; et al. Effectiveness of fecal immunochemical testing in reducing colorectal cancer mortality from the One Million Taiwanese Screening Program. Cancer 2015, 121, 3221–3229. [Google Scholar] [CrossRef] [PubMed]
- Clinical Trials Gov. Available online: https://clinicaltrials.gov/ (accessed on 20 November 2024).
- Clinical Trials Gov. Available online: https://clinicaltrials.gov/study/NCT04585919?titles=Paired%20Promotion%20of%20Colorectal%20Cancer%20and%20Social%20Determinants%20of%20Health%20Screening&rank=1 (accessed on 26 November 2024).
- Clinical Trials Gov. Available online: https://clinicaltrials.gov/study/NCT04471194?titles=Multilevel%20Intervention%20Based%20on%20Colorectal%20Cancer%20(CRC)%20and%20Cervical%20Cancer%20Self-screening%20in%20Rural,%20Segregated%20Areas&rank=1 (accessed on 26 November 2024).
- Clinical Trials Gov. Available online: https://clinicaltrials.gov/study/NCT04406714?titles=Scaling%20CRC%20Screening%20Through%20Outreach,%20Referral,%20and%20Engagement%20(SCORE)&rank=1 (accessed on 26 November 2024).
- Clinical Trials Gov. Available online: https://clinicaltrials.gov/study/NCT05341622?titles=Mailed%20FIT%20Outreach%202022&rank=1 (accessed on 28 November 2024).
- Clinical Trials Gov. Available online: https://clinicaltrials.gov/study/NCT03843957?titles=Effectiveness%20and%20Implementation%20of%20mPATH-CRC&rank=1 (accessed on 28 November 2024).
- Clinical Trials Gov. Available online: https://clinicaltrials.gov/study/NCT04548531?titles=Engaging%20Patients%20in%20Colon%20Cancer%20Screening%20Decisions%20During%20COVID-19&rank=1 (accessed on 28 November 2024).
- Clinical Trials Gov. Available online: https://clinicaltrials.gov/study/NCT04683731?titles=Helping%20Patients%20and%20Providers%20Make%20Better%20Decisions%20About%20Colorectal%20Cancer%20Screening&rank=1 (accessed on 30 November 2024).
- Clinical Trials Gov. Available online: https://clinicaltrials.gov/study/NCT04601272?titles=Evaluating%20the%20Shared%20Decision%20Making%20Process%20Scale%20in%20Cancer%20Screening%20Decisions&rank=1 (accessed on 30 November 2024).
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Luque, J.S.; Matthew, O.O.; Jackson, D.R.; Vargas, M.A.; Austin, T.; Ali, A.; Kiros, G.E.; Harris, C.M.; Tawk, R.; Gwede, C.K.; et al. Assessing the effectiveness of a community health advisor plus screen to save educational intervention on stool-based testing adherence in an African American safety net clinic population: Study protocol for a randomized pragmatic trial. Trials 2022, 23, 151. [Google Scholar] [CrossRef] [PubMed]
- Clinical Trials Gov. Available online: https://clinicaltrials.gov/study/NCT04987788?titles=e-Motivaci%C3%B3n:%20Developing%20and%20Pilot%20Testing%20an%20App%20to%20Improve%20Latinos%27%20Screening%20Colonoscopy%20Rates&rank=1 (accessed on 30 November 2024).
- Clinical Trials Gov. Available online: https://clinicaltrials.gov/study/NCT05810714?titles=Audio%20and%20Video%20Brochures%20for%20Increasing%20Colorectal%20Cancer%20Screening%20Among%20Adults%20Living%20in%20Appalachia&rank=1 (accessed on 30 November 2024).
- Clinical Trials Gov. Available online: https://clinicaltrials.gov/study/NCT04192071?titles=Virtual%20Human%20Delivered%20Nutrition%20Module%20for%20Colorectal%20Cancer%20Prevention&rank=1 (accessed on 30 November 2024).
- Clinical Trials Gov. Available online: https://clinicaltrials.gov/study/NCT04607291?titles=Health%20Service%20Intervention%20for%20the%20Improvement%20of%20Access%20and%20Adherence%20to%20Colorectal%20Cancer%20Screening&rank=1 (accessed on 30 November 2024).
- Clinical Trials Gov. Available online: https://clinicaltrials.gov/study/NCT03489707?titles=The%20Prevent%20Anal%20Cancer%20Self-Swab%20Study&rank=1 (accessed on 1 December 2024).
- Clinical Trials Gov. Available online: https://clinicaltrials.gov/study/NCT04090060?titles=The%20Prevent%20Anal%20Cancer%20Palpation%20Study&rank=1 (accessed on 1 December 2024).
- Clinical Trials Gov. Available online: https://clinicaltrials.gov/study/NCT04301986?titles=Patient%20Acceptance%20and%20Preference%20Among%20Screening%20Modalities%20for%20Detection%20of%20Barrett%27s%20Oesophagus&rank=1 (accessed on 1 December 2024).
- Clinical Trials Gov. Available online: https://clinicaltrials.gov/study/NCT04902144?titles=Clinical%20Outcomes%20for%20Offering%20Genetic%20Testing%20in%20a%20Tiered%20Approach&rank=1 (accessed on 1 December 2024).
- Clinical Trials Gov. Available online: https://clinicaltrials.gov/study/NCT05179837?titles=Endoscopic%20Optical%20Coherence%20Tomography%20for%20Screening%20and%20Diagnosis%20of%20Colorectal%20Precancerous%20and%20Malignant%20Polyps&rank=1 (accessed on 1 December 2024).
- Clinical Trials Gov. Available online: https://clinicaltrials.gov/study/NCT04739722?titles=Colorectal%20Cancer%20and%20Pre-Cancerous%20Adenoma%20Non-Invasive%20Detection%20Test%20Study&rank=1 (accessed on 1 December 2024).
- Mourato, M.B.; Pratas, N.; Branco Pereira, A.; Taré, F.; Chança, R.; Fronteira, I.; Dinis, R.; Areia, M. Effectiveness of Gastric Cancer Endoscopic Screening in Intermediate-Risk Countries: Protocol for a Systematic Review and Meta-Analysis. JMIR Res. Protoc. 2025, 14, e56791. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Fan, X.; Huang, B.; Pan, K.; Gerhard, M.; Mejías, L.R.; Zhang, Y. Gastric cancer detection based on cell-free DNA in blood: A systematic review and meta-analysis. Clin. Transl. Discov. 2024, 4, e329. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czerw, N.; Deptała, A.; Badowska-Kozakiewicz, A.; Czerw, A.; Partyka, O.; Pajewska, M.; Sygit, K.; Michalska, M.; Gąska, I.; Dykowska, G.; et al. Screening in Gastrointestinal Malignancies—Recent Trials and Advancements. Cancers 2025, 17, 975. https://doi.org/10.3390/cancers17060975
Czerw N, Deptała A, Badowska-Kozakiewicz A, Czerw A, Partyka O, Pajewska M, Sygit K, Michalska M, Gąska I, Dykowska G, et al. Screening in Gastrointestinal Malignancies—Recent Trials and Advancements. Cancers. 2025; 17(6):975. https://doi.org/10.3390/cancers17060975
Chicago/Turabian StyleCzerw, Natalia, Andrzej Deptała, Anna Badowska-Kozakiewicz, Aleksandra Czerw, Olga Partyka, Monika Pajewska, Katarzyna Sygit, Magdalena Michalska, Izabela Gąska, Grażyna Dykowska, and et al. 2025. "Screening in Gastrointestinal Malignancies—Recent Trials and Advancements" Cancers 17, no. 6: 975. https://doi.org/10.3390/cancers17060975
APA StyleCzerw, N., Deptała, A., Badowska-Kozakiewicz, A., Czerw, A., Partyka, O., Pajewska, M., Sygit, K., Michalska, M., Gąska, I., Dykowska, G., Sienkiewicz, Z., Grochans, E., Grochans, S., Cybulska, A., Schneider-Matyka, D., Bandurska, E., Ciećko, W., Drobnik, J., Pobrotyn, P., ... Kozlowski, R. (2025). Screening in Gastrointestinal Malignancies—Recent Trials and Advancements. Cancers, 17(6), 975. https://doi.org/10.3390/cancers17060975









